Aluminide Coatings by Means of Slurry Application: A Low Cost, Versatile and Simple Technology
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Materials
2.2. Coating Application
2.3. Characterisation
3. Results and Discussion
3.1. Method of Deposition and Importance of Diffusion Heat Treatment
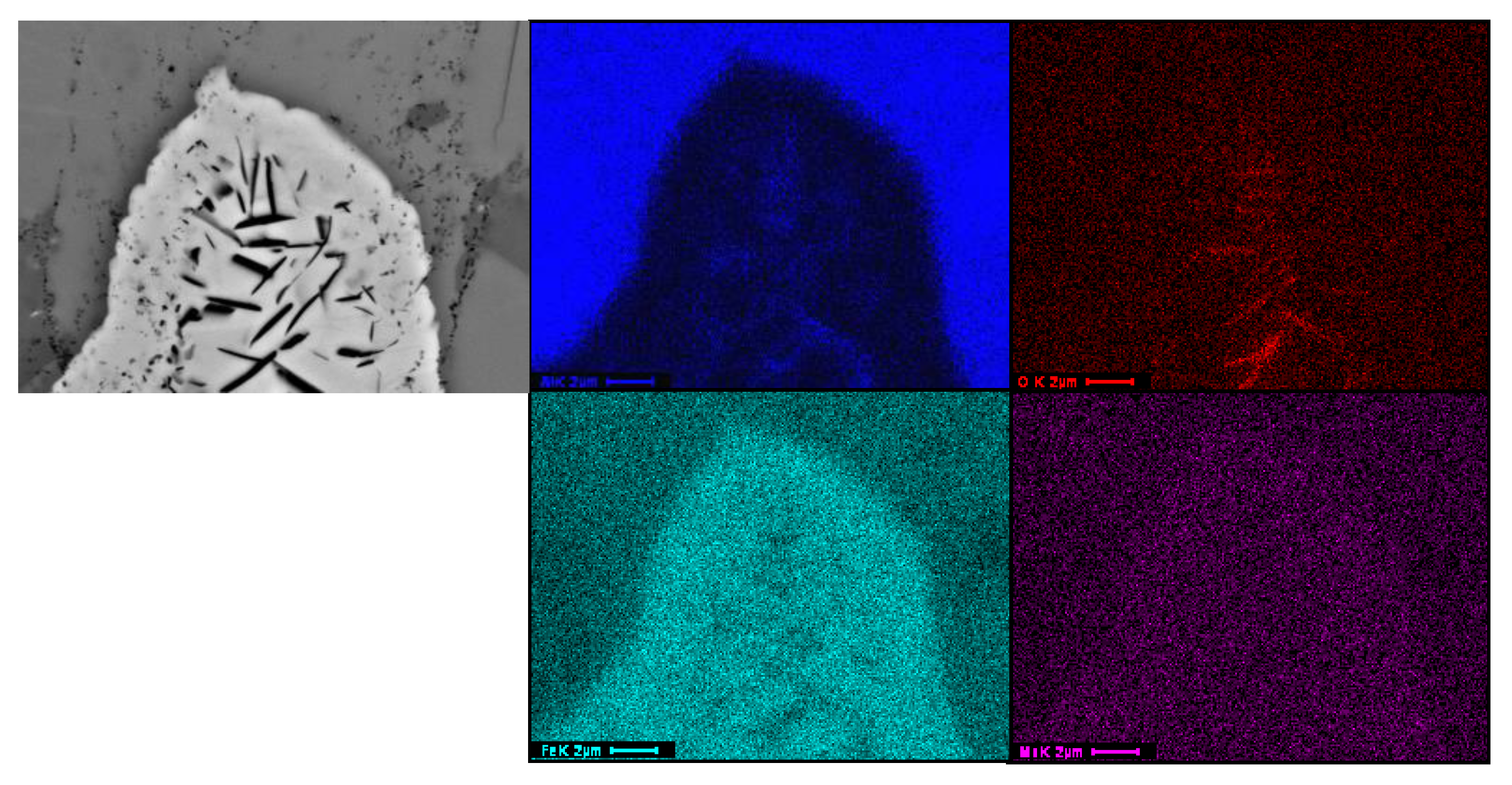
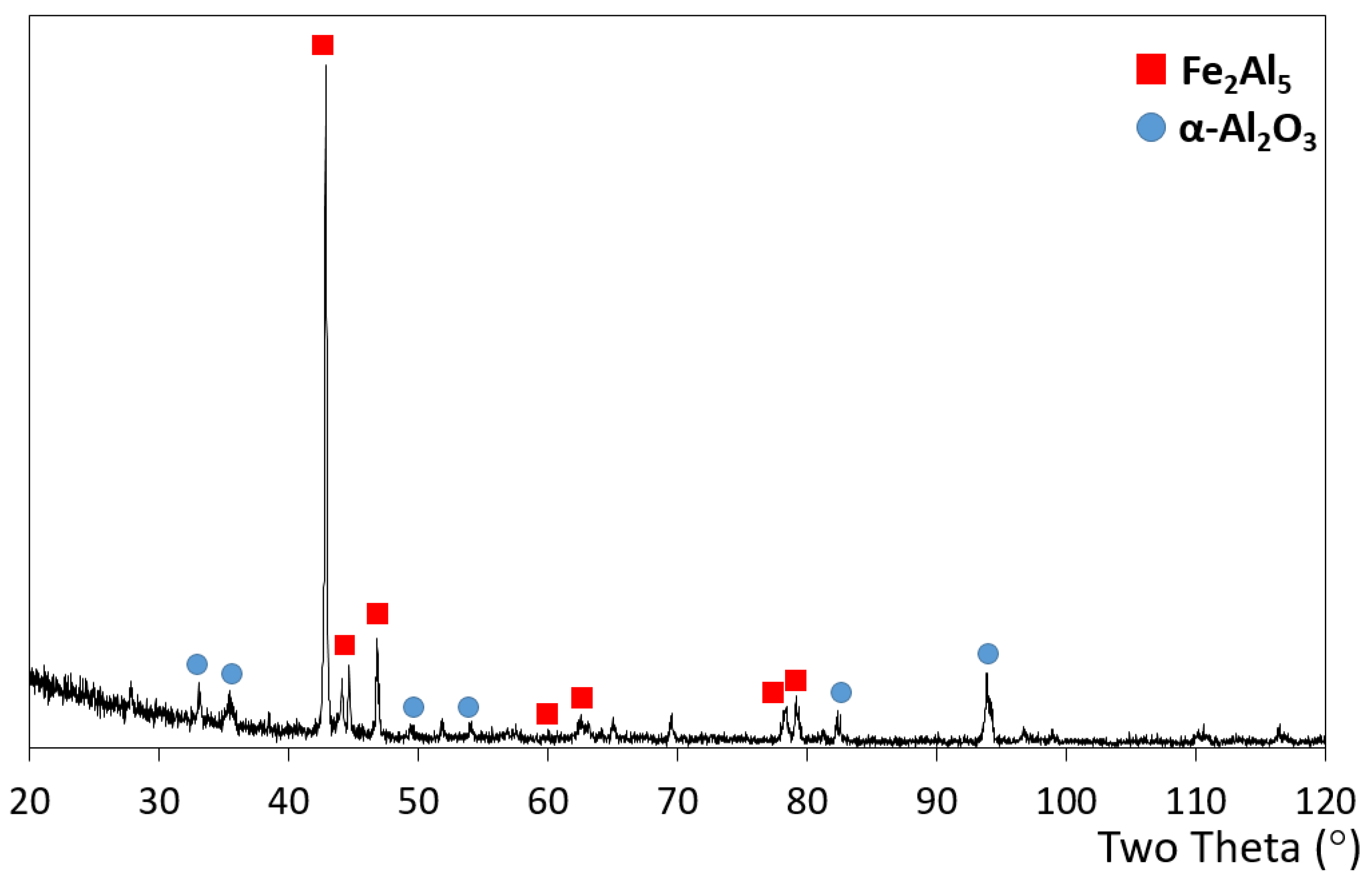
3.2. Microstructure of the Slurry Aluminide Coating Applied on Carbon Steel A516
3.3. Microstructure of the Slurry Aluminide Coating Applied on Austenitic Steel 310H
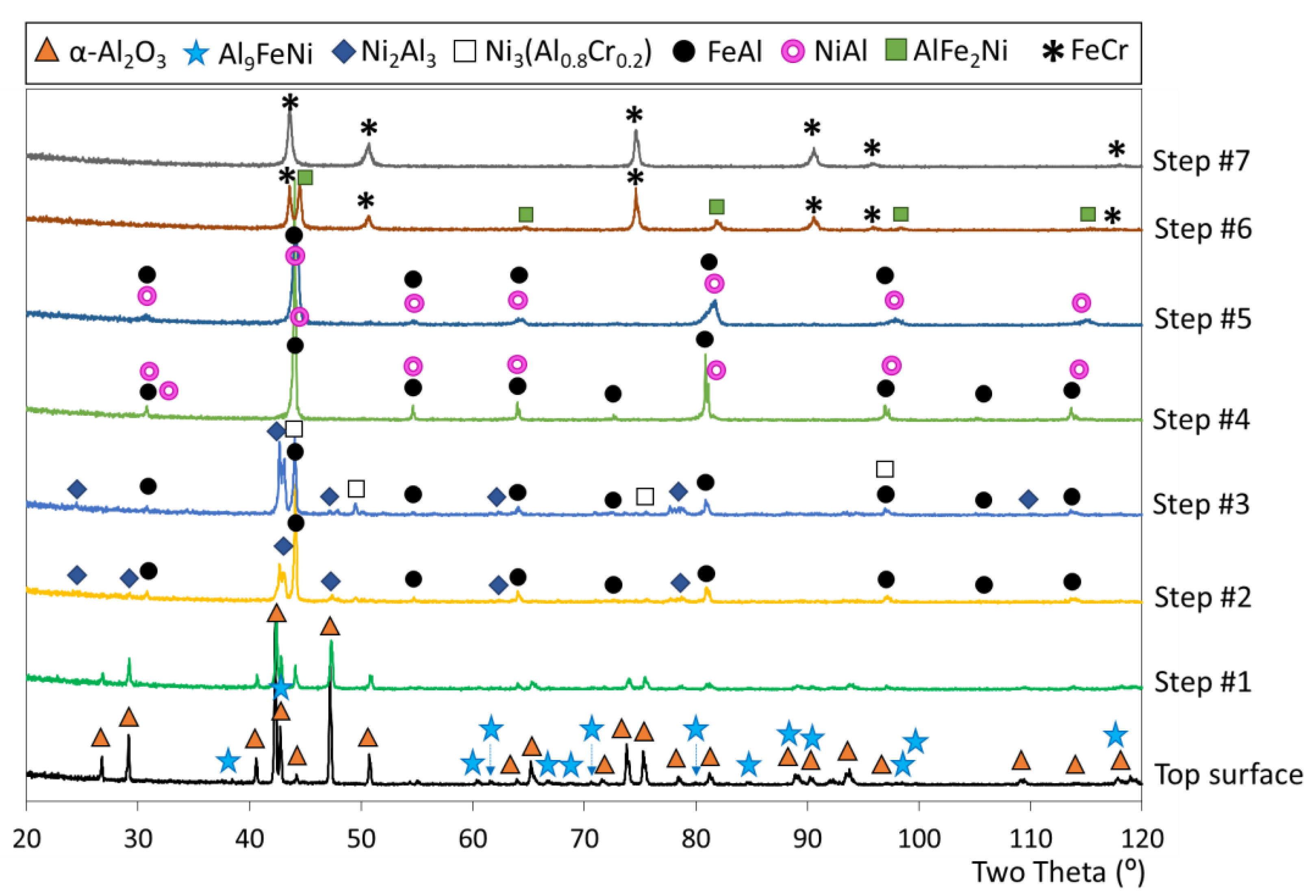
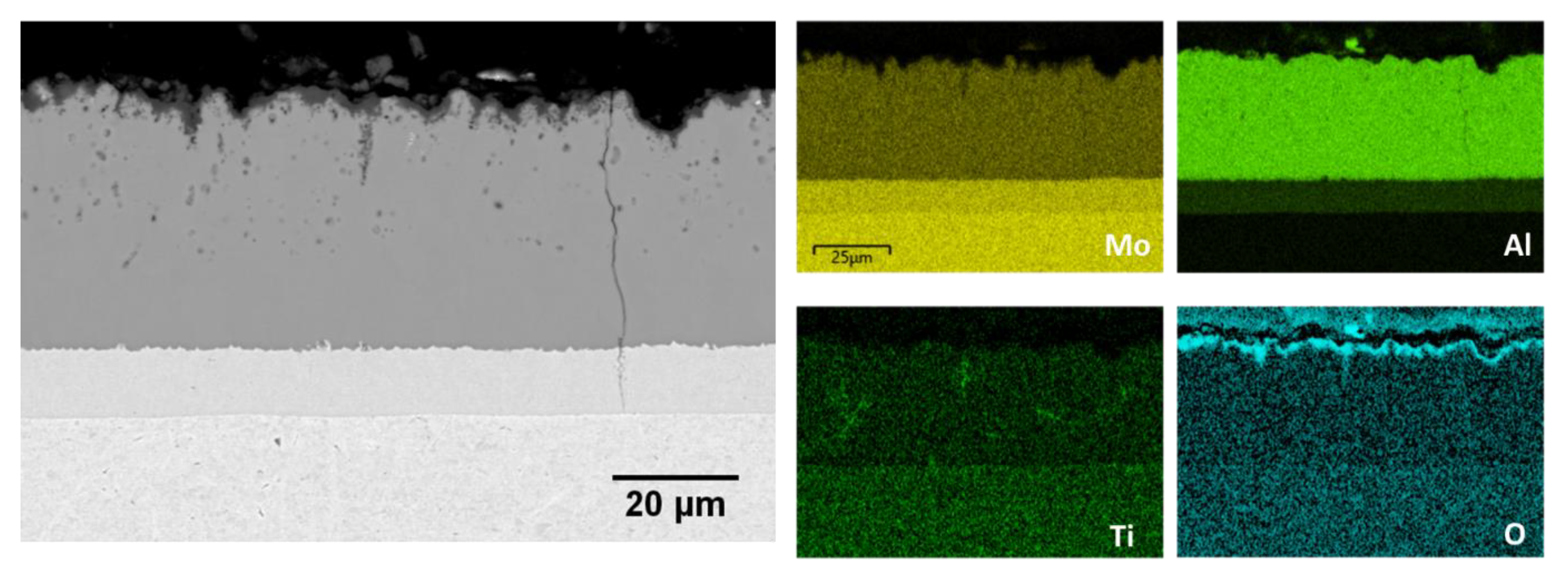
3.4. Microstructure of the Slurry Aluminide Coating Applied on Ti Alloy Ti6246
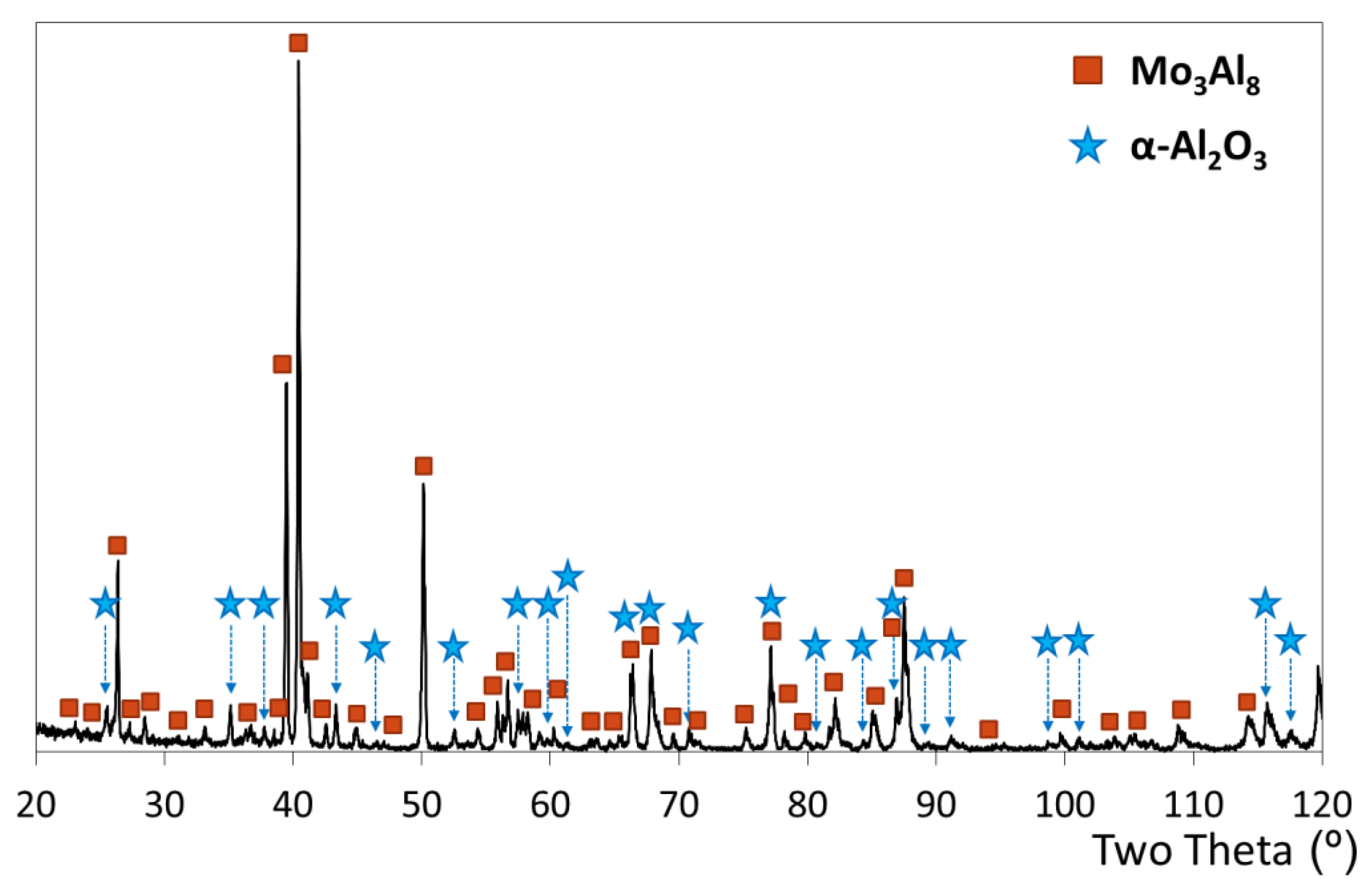
3.5. Microstructure of the Slurry Aluminide Coating Applied on Mo Alloy TZM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y. Aluminide Coatings for Power-Generation Applications; Oak Ridge National Lab. (ORNL): Oak Ridge, TN, USA, 2003. [Google Scholar]
- Grilli, M.L.; Valerini, D.; Slobozeanu, A.E.; Postolnyi, B.O.; Balos, S.; Rizzo, A.; Piticescu, R.R. Critical Raw Materials Saving by Protective Coatings under Extreme Conditions: A Review of Last Trends in Alloys and Coatings for Aerospace Engine Applications. Materialsed 2021, 14, 1656. [Google Scholar] [CrossRef] [PubMed]
- Pint, B.A.; Zhang, Y.; Walker, L.R.; Wright, I.G. Long-term performance of aluminide coatings on Fe-base alloys. Surf. Coat. Technol. 2007, 202, 637–642. [Google Scholar] [CrossRef]
- Dosta, S.; Betancor, L.; Barreneche, C. Overview of surface engineering technology to improve the energy efficiency in concentrated solar power (CSP) plants. Sol. Energy Mater. Sol. Cells 2024, 277, 113090. [Google Scholar] [CrossRef]
- Goral, M.; Swadzba, L.; Moskal, G.; Jarczyk, G.; Aguilar, J. Diffusion aluminide coatings for TiAl intermetallic turbine blades. Intermetallics 2011, 19, 744–747. [Google Scholar] [CrossRef]
- Zeng, S.; Li, F. Research Status of Aluminum Base Coating on Titanium Alloy. Coatings 2023, 13, 1525. [Google Scholar] [CrossRef]
- Pint, B.A.; Zhang, Y. Performance of Al-rich oxidation resistant coatings for Fe-base alloys. Mater. Corros. 2011, 62, 549–560. [Google Scholar] [CrossRef]
- Deevi, S.C. Advanced intermetallic iron aluminide coatings for high temperature applications. Prog. Mater. Sci. 2021, 118, 100769. [Google Scholar] [CrossRef]
- Goward, G.W.; Boone, D.H. Mechanisms of formation of diffusion aluminide coatings on nickel-base superalloys. Oxid. Met. 1971, 3, 475–495. [Google Scholar] [CrossRef]
- Nicholls, J. Advances in coating design for high-performance gas turbines. Mrs Bull. 2003, 28, 659–670. [Google Scholar] [CrossRef]
- Ren, W.; Li, Q.; Song, J.; Xiao, C.; Xu, Z.; He, L.; Cao, C. Oxidation and microstructure evolution of cobalt aluminide coatings on directionally solidified superalloys during long term exposure at 1000 °C. Mater. Res. Innov. 2014, 18 (Suppl. S4), S4-945–S944-951. [Google Scholar] [CrossRef]
- Feng, K.; Li, M.; Chen, M.; Li, Z.; Sha, J.; Zhou, C. Cyclic oxidation behavior of Al-Si coating on new γ′-strengthened cobalt-based superalloy: Experimental study and first-principles calculation. Corros. Sci. 2021, 185, 109422. [Google Scholar] [CrossRef]
- Choi, K.; Yang, W.; Baik, K.-H.; Kim, Y.; Lee, S.; Park, J.S. Growth Kinetics and Isothermal Oxidation Behavior of Aluminide Pack Coatings on a Multiphase Mo–Si–B Alloy. Oxid. Met. 2019, 92, 423–437. [Google Scholar] [CrossRef]
- Karpe, B.; Prijatelj, K.; Bizjak, M.; Kosec, T. Corrosion properties of aluminized 16Mo3 steel. J. Min. Metall. Sect. B-Metall. 2023, 59, 91–100. [Google Scholar] [CrossRef]
- Filipovic, J.; Beck, K.; Kontermann, C.; Galetz, M.; Oechsner, M. Mechanical Characterization Potentials of Aluminide Diffusion Coatings on Molybdenum Substrates. Adv. Eng. Mater. 2024, 26, 2302027. [Google Scholar] [CrossRef]
- Pomeroy, M.J. Coatings for gas turbine materials and long term stability issues. Mater. Des. 2005, 26, 223–231. [Google Scholar] [CrossRef]
- Audigié, P.; Put, A.R.-V.; Malié, A.; Monceau, D. High-temperature cyclic oxidation behaviour of Pt-rich γ-γ’ coatings. Part I: Oxidation kinetics of coated AM1 systems after very long-term exposure at 1100 °C. Corros. Sci. 2018, 144, 127–135. [Google Scholar] [CrossRef]
- Guo, L.; He, W.; Chen, W.; Xue, Z.; He, J.; Guo, Y.; Wu, Y.; Gao, L.; Li, D.; Zhang, Z.; et al. Progress on high-temperature protective coatings for aero-engines. Surf. Sci. Technol. 2023, 1, 6. [Google Scholar] [CrossRef]
- Agüero, A.; Gutiérrez, M.; Korcakova, L.; Nguyen, T.T.M.; Hinnemann, B.; Saadi, S. Metal Dusting Protective Coatings. A Literature Review. Oxid. Met. 2011, 76, 23–42. [Google Scholar] [CrossRef]
- Agüero, A.; Baraibar, I.; Muelas, R.; Oskay, C.; Galetz, M.; Korner, E. Analysis of an aluminide coating on austenitic steel 800HT exposed to metal dusting conditions: Lessons from an industrial hydrogen production plant. Int. J. Press. Vessel. Pip. 2020, 186, 104129. [Google Scholar] [CrossRef]
- Madloch, S.; Galetz, M.C. Microstructural evolution of germanium modified AlSi-slurry coatings on alloy 600 at 620°C in metal dusting environment. Surf. Coat. Technol. 2017, 315, 335–341. [Google Scholar] [CrossRef]
- Boulesteix, C.; Pedraza, F.; Proy, M.; Lasanta, I.; de Miguel, T.; Illana, A.; Pérez, F.J. Steam Oxidation Resistance of Slurry Aluminum and Aluminum/Silicon Coatings on Steel for Ultrasupercritical Steam Turbines. Oxid. Met. 2017, 87, 469–479. [Google Scholar] [CrossRef]
- Agüero, A.; Baraibar, I.; Gutierrez, M.; Tuurna, S.; Toivonen, A.; Pennila, S.; Auerkari, P. Steam Oxidation of Aluminide-Coated and Uncoated TP347HFG Stainless Steel under Atmospheric and Ultra-Supercritical Steam Conditions at 700 °C. Coatings 2020, 10, 839. [Google Scholar] [CrossRef]
- Agüero, A.; Audigié, P.; Rodríguez, S.; Encinas-Sánchez, V.; de Miguel, M.T.; Pérez, F.J. Protective coatings for high temperature molten salt heat storage systems in solar concentration power plants. AIP Conf. Proc. 2018, 2033, 090001. [Google Scholar]
- Audigie, P.; Encinas-Sanchez, V.; Rodriguez, S.; Perez, F.J.; Agüero, A. High temperature corrosion beneath carbonate melts of aluminide coatings for CSP application. Sol. Energy Mater. Sol. Cells 2020, 210, 110514. [Google Scholar] [CrossRef]
- Meißner, T.M.; Oskay, C.; Bonk, A.; Grégoire, B.; Donchev, A.; Solimani, A.; Galetz, M.C. Improving the corrosion resistance of ferritic-martensitic steels at 600 °C in molten solar salt via diffusion coatings. Sol. Energy Mater. Sol. Cells 2021, 227, 111105. [Google Scholar] [CrossRef]
- Kochmanska, A. Aluminide coatings on Inconel 617 obtained by slurry method with inorganic binder. J. Achiev. Mater. Manuf. Eng. 2017, 2, 49–55. [Google Scholar] [CrossRef]
- Berry, D.; Meelu, M.C.; McMordie, B.G.; Kircher, T.A. Enhancing Performance of Silicon-Modified Slurry Aluminides on Turbine Components Operating in Marine Environments. In Proceedings of the ASME 1995 International Gas Turbine and Aeroengine Congress and Exposition, Houston, TX, USA, 5–8 June 1995. [Google Scholar]
- Kircher, T.A.; McMordie, B.G.; McCarter, A. Performance of a silicon-modified aluminide coating in high temperature hot corrosion test conditions. Surf. Coat. Technol. 1994, 68–69, 32–37. [Google Scholar] [CrossRef]
- Agueero, A.; Gutierrez, M.; Muelas, R.; Spiradek-Hahn, K. Overview of steam oxidation behaviour of Al protective oxide precursor coatings on P92. Surf. Eng. 2018, 34, 30–39. [Google Scholar] [CrossRef]
- Kochmańska, A.; Kochmański, P. Aluminide Protective Coatings Obtained by Slurry Method. Mater. Sci. Forum 2014, 782, 590–593. [Google Scholar] [CrossRef]
- Agüero, A.; Østergård, M.J.L.; Hansson, A.N.; Gutierrez, M. Thermal cyclic resistance and long term inter-diffusion properties of slurry aluminide coatings modified with Si. Results Surf. Interfaces 2022, 6, 100042. [Google Scholar] [CrossRef]
- Das, D.K.; Trivedi, S.P. Microstructure of diffusion aluminide coatings on Ti-base alloy IMI-834 and their cyclic oxidation behaviour at 650 °C. Mater. Sci. Eng. A 2004, 367, 225–233. [Google Scholar] [CrossRef]
- Kochmańska, A.E.; Jarlaczyńska, A.; Baranowska, J. Formation of Silicide and Silicide-Aluminide Coatings on Molybdenum Alloy during Slurry Cementation Process: Influence of Slurry Volume. Materials 2021, 14, 6940. [Google Scholar] [CrossRef] [PubMed]
- Agüero, A.; Audigié, P.; Rodríguez, S. 10,000 h molten salt corrosion testing on IN617, uncoated and aluminide ferritic steels at 580 °C. AIP Conf. Proc. 2020, 2303, 150002. [Google Scholar]
- Wang, W.; Zhu, Z.; Yang, L.; Lu, J.; Huang, J.; Tan, J.; Kuang, W. Superior corrosion resistance of a slurry FeAl coating on 316LN stainless steel in 550 °C liquid lead-bismuth eutectic. Corros. Sci. 2024, 227, 111757. [Google Scholar] [CrossRef]
- Pedraza, F.; Boulesteix, C.; Proy, M.; Lasanta, I.; de Miguel, T.; Illana, A.; Perez, F.J. Behavior of Slurry Aluminized Austenitic Stainless Steels under Steam at 650 and 700 °C. Oxid. Met. 2017, 87, 443–454. [Google Scholar] [CrossRef]
- ArcelorMittal Asturias en Avilés | ArcelorMittal España. Available online: https://spain.arcelormittal.com/ (accessed on 4 September 2024).
- Alleima® 7RE10—Alleima. Available online: https://www.alleima.com/en/products/ (accessed on 4 September 2024).
- 6246—Aubert & Duval English. Available online: www.aubertduval.com (accessed on 4 September 2024).
- Molybdenum Alloys | Goodfellow—TZM Molybdenum Alloy | Material: TZM Molybdenum Alloy. Available online: https://www.fonlinkmetal.com/products/molybdenum/tzm_alloy_and_piercing_plug.html?gad_source=1&gclid=EAIaIQobChMI1ezQy77fiAMVpqpmAh2PFTD9EAAYAyAAEgJqXvD_BwE (accessed on 4 September 2024).
- Gutierrez, M.; Illana, A.; Bahillo, A.; Benito, M.J.; Garcia-Martin, G.; Perez, F.J.; Aguero, A. Comparison between pilot and lab scale testing of aluminide coated and uncoated ferritic steels under oxy-fuel and coal/thistle co-firing conditions. Surf. Coat. Technol. 2022, 450, 128982. [Google Scholar] [CrossRef]
- Palacios, A.; Navarro, M.E.; Jiang, Z.; Avila, A.; Qiao, G.; Mura, E.; Ding, Y. High-temperature corrosion behaviour of metal alloys in commercial molten salts. Sol. Energy 2020, 201, 437–452. [Google Scholar] [CrossRef]
- Applicability & Allowable Stress of ASTM A516 Gr.70. Available online: www.metalspiping.com (accessed on 3 August 2022).
- Ladkany, S.G.; Culbreth, W.; Loyd, N. Molten Salts and Applications II: 565 °C Molten Salt Solar Energy Storage Design, Corrosion, and Insulation. J. Energy Power Eng. 2018, 12, 517–532. [Google Scholar]
- Agüero, A.; Spiradek, K.; Höfinger, S.; Gutiérrez, M.; Muelas, R. Microstructural Evolution of Slurry Fe Aluminide Coatings during High Temperature Steam Oxidation. Mater. Sci. Forum 2008, 595–598, 251–259. [Google Scholar] [CrossRef]
- Du, H.; Tan, N.; Fan, L.; Zhuang, J.; Qiu, Z.; Lei, Y. Formation Mechanism of Aluminide Diffusion Coatings on Ti and Ti-6Al-4V Alloy at the Early Stages of Deposition by Pack Cementation. Materialsed 2019, 12, 3097. [Google Scholar] [CrossRef]
- Madhavan, N.; Brooks, G.A.; Rhamdhani, M.A.; Rout, B.K.; Schrama, F.N.H.; Overbosch, A. General mass balance for oxygen steelmaking. Ironmak. Steelmak. 2021, 48, 40–54. [Google Scholar] [CrossRef]
- Acero inoxidable 310/310S/310H, aleación 310 (UNS S31000/S31008/S31009) (allianzsteel.com). Available online: https://es.allianzsteel.com/serve/stainless-steel-310-310s-310h.html (accessed on 1 September 2024).
- Audigié, P.; Rodríguez, S.; Agüero, A. Improved Corrosion Resistance of Slurry Coated Austenitic Steel in Molten Carbonates Doped with Nanoparticles for Thermal Energy Storage Technologies. Poster Commun. 2024. [Google Scholar]
- Chumak, I.; Richter, K.W.; Ipser, H. The Fe–Ni–Al phase diagram in the Al-rich (>50at.% Al) corner. Intermetallics 2007, 15, 1416–1424. [Google Scholar] [CrossRef]
- Kochmańska, A.E. Microstructure of Al-Si Slurry Coatings on Austenitic High-Temperature Creep Resisting Cast Steel. Adv. Mater. Sci. Eng. 2018, 2018, 5473079. [Google Scholar] [CrossRef]
- Boyer, R.R. Titanium for aerospace: Rationale and applications. Adv. Perform. Mater. 1995, 2, 349–368. [Google Scholar] [CrossRef]
- Boyer, R.R. An overview on the use of titanium in the aerospace industry. Mater. Sci. Eng. A 1996, 213, 103–114. [Google Scholar] [CrossRef]
- Peters, M.; Kumpfert, J.; Ward, C.H.; Leyens, C. Titanium alloys for aerospace applications. Adv. Eng. Mater. 2003, 5, 419–427. [Google Scholar] [CrossRef]
- Najafizadeh, M.; Yazdi, S.; Bozorg, M.; Ghasempour-Mouziraji, M.; Hosseinzadeh, M.; Zarrabian, M.; Cavaliere, P. Classification and applications of titanium and its alloys: A review. J. Alloys Compd. Commun. 2024, 3, 100019. [Google Scholar] [CrossRef]
- Berthaud, M.; Popa, I.; Chassagnon, R.; Heintz, O.; Lavková, J.; Chevalier, S. Study of titanium alloy Ti6242S oxidation behaviour in air at 560 °C: Effect of oxygen dissolution on lattice parameters. Corros. Sci. 2020, 164, 108049. [Google Scholar] [CrossRef]
- Školáková, A.; Salvetr, P.; Leitner, J.; Lovaši, T.; Novák, P. Formation of Phases in Reactively Sintered TiAl3 Alloy. Molecules 2020, 25, 1912. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, Y.; Yu, X. Interfacial microstructure evolution mechanism of high temperature oxidation-resistant Al-based coating on Ti alloy surface. Mater. Res. Express 2019, 6, 086472. [Google Scholar] [CrossRef]
- Smialek, J.L. Oxidation behaviour of TiAl3 coatings and alloys. Corros. Sci. 1993, 35, 1199–1208. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, Z.; Yao, Y.; Li, L. Finite element analysis of thermal stresses in Ti-Al3Ti metal-intermetallic laminated composites. Results Phys. 2019, 15, 102706. [Google Scholar] [CrossRef]
- Wang, J.; Kong, L.; Li, T.; Xiong, T. A novel TiAl3/Al2O3 composite coating on γ-TiAl alloy and evaluating the oxidation performance. Appl. Surf. Sci. 2016, 361, 90–94. [Google Scholar] [CrossRef]
- Sharma, I.G.; Chakraborty, S.P.; Suri, A.K. Preparation of TZM alloy by aluminothermic smelting and its characterization. J. Alloys Compd. 2005, 393, 122–127. [Google Scholar] [CrossRef]
- Smolik, G.R.; Petti, D.A.; Schuetz, S.T. Oxidation and volatilization of TZM alloy in air. J. Nucl. Mater. 2000, 283–287, 1458–1462. [Google Scholar] [CrossRef]
- Alam, M.Z.; Venkataraman, B.; Sarma, B.; Das, D.K. MoSi2 coating on Mo substrate for short-term oxidation protection in air. J. Alloys Compd. 2009, 487, 335–340. [Google Scholar] [CrossRef]
- Agüero, A.; Muelas, R.; Pastor, A.; Osgerby, S. Long exposure steam oxidation testing and mechanical properties of slurry aluminide coatings for steam turbine components. Surf. Coat. Technol. 2005, 200, 1219–1224. [Google Scholar] [CrossRef]
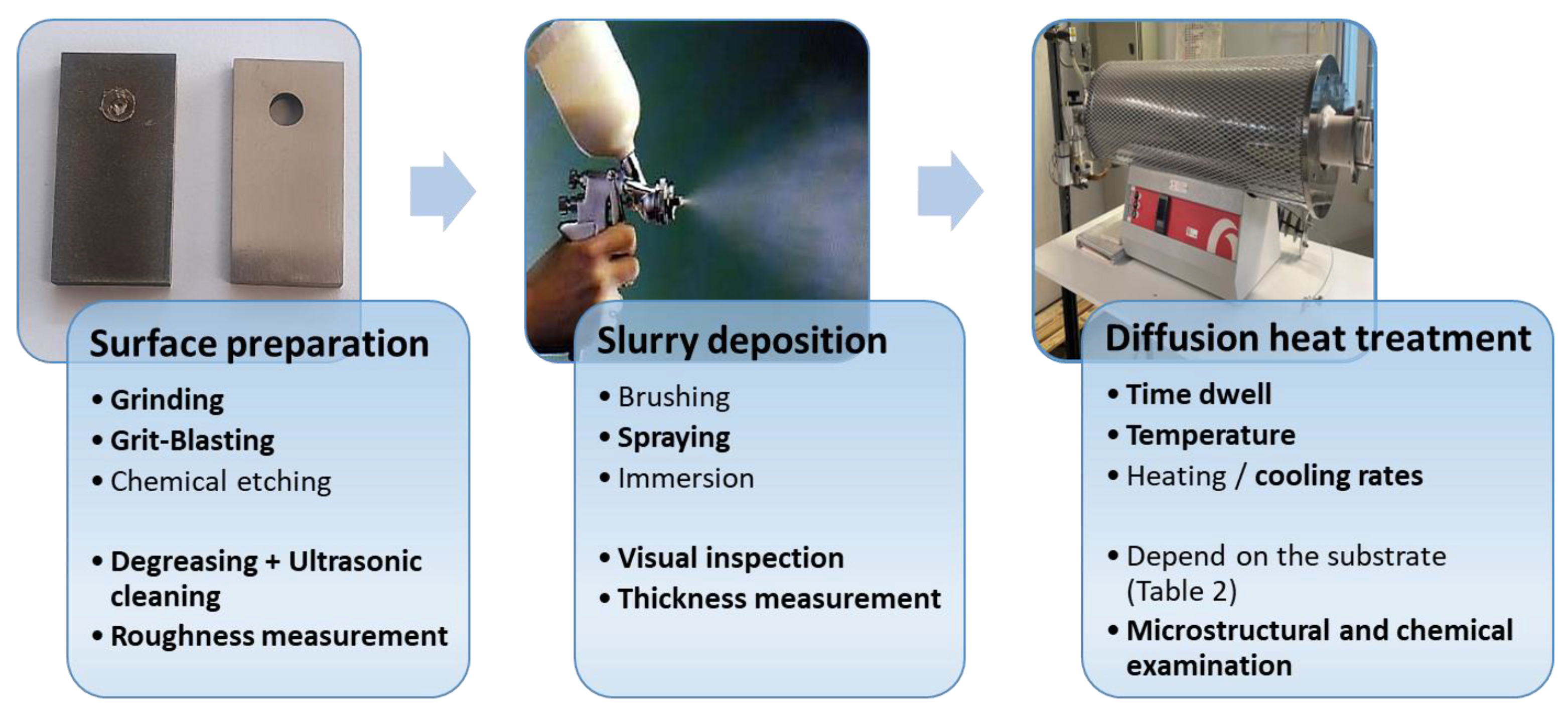


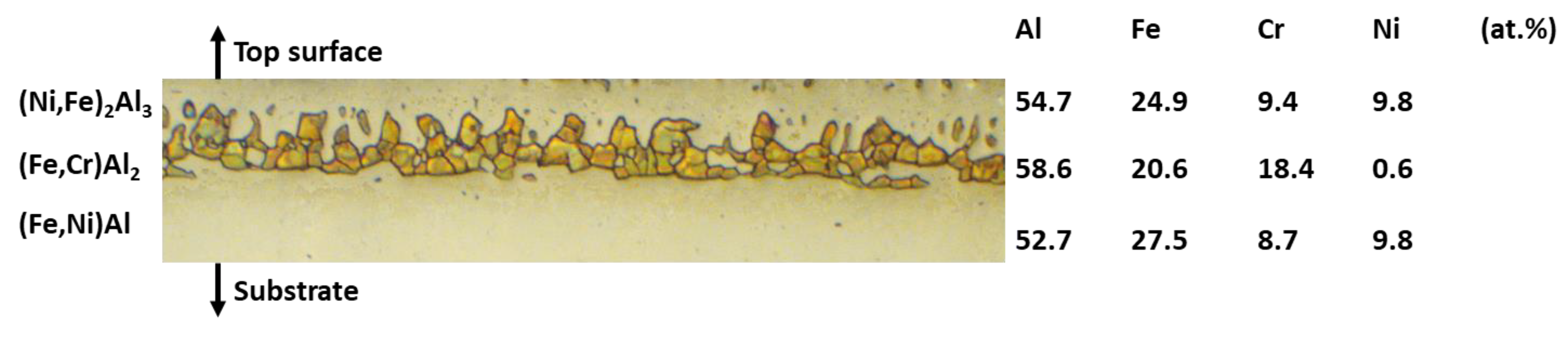
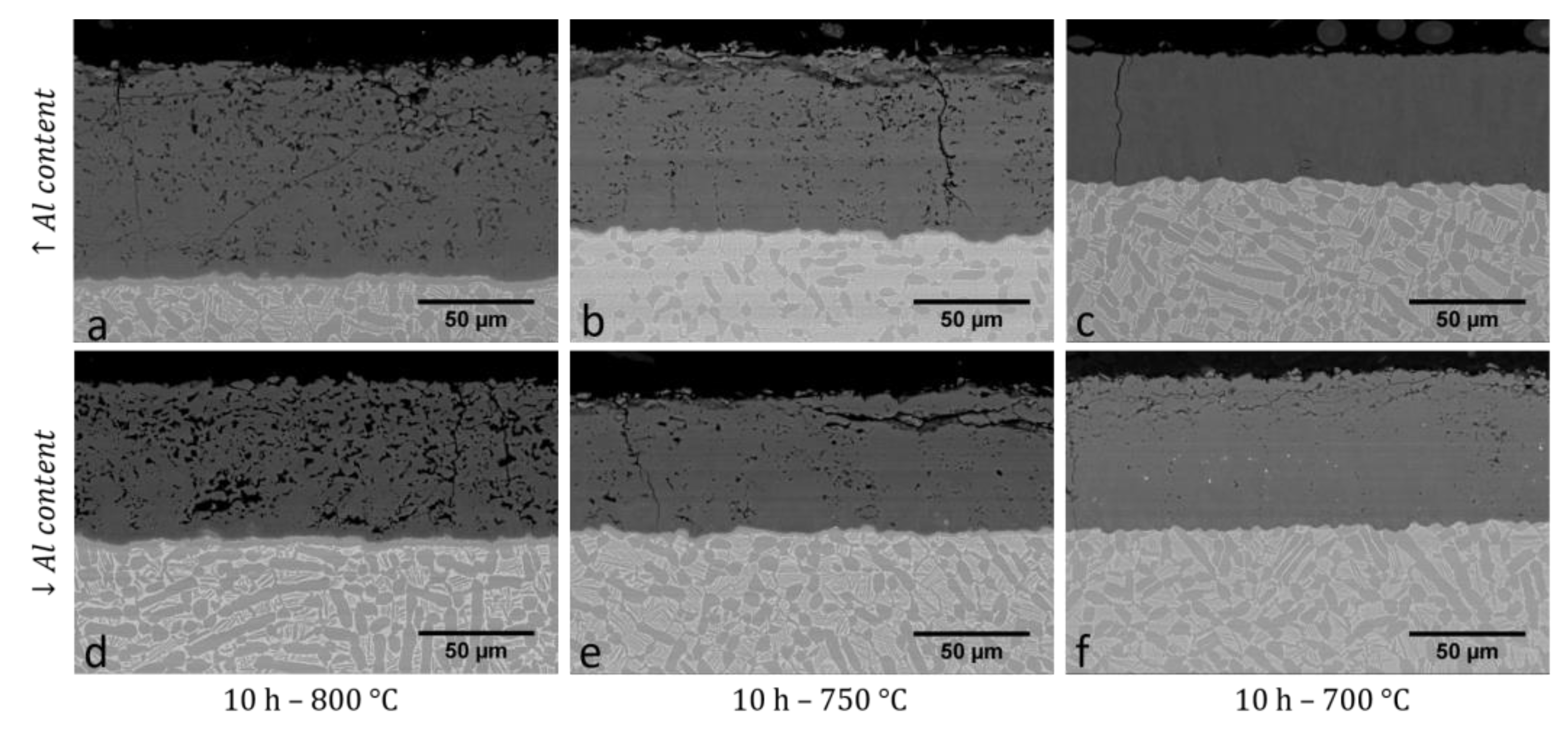
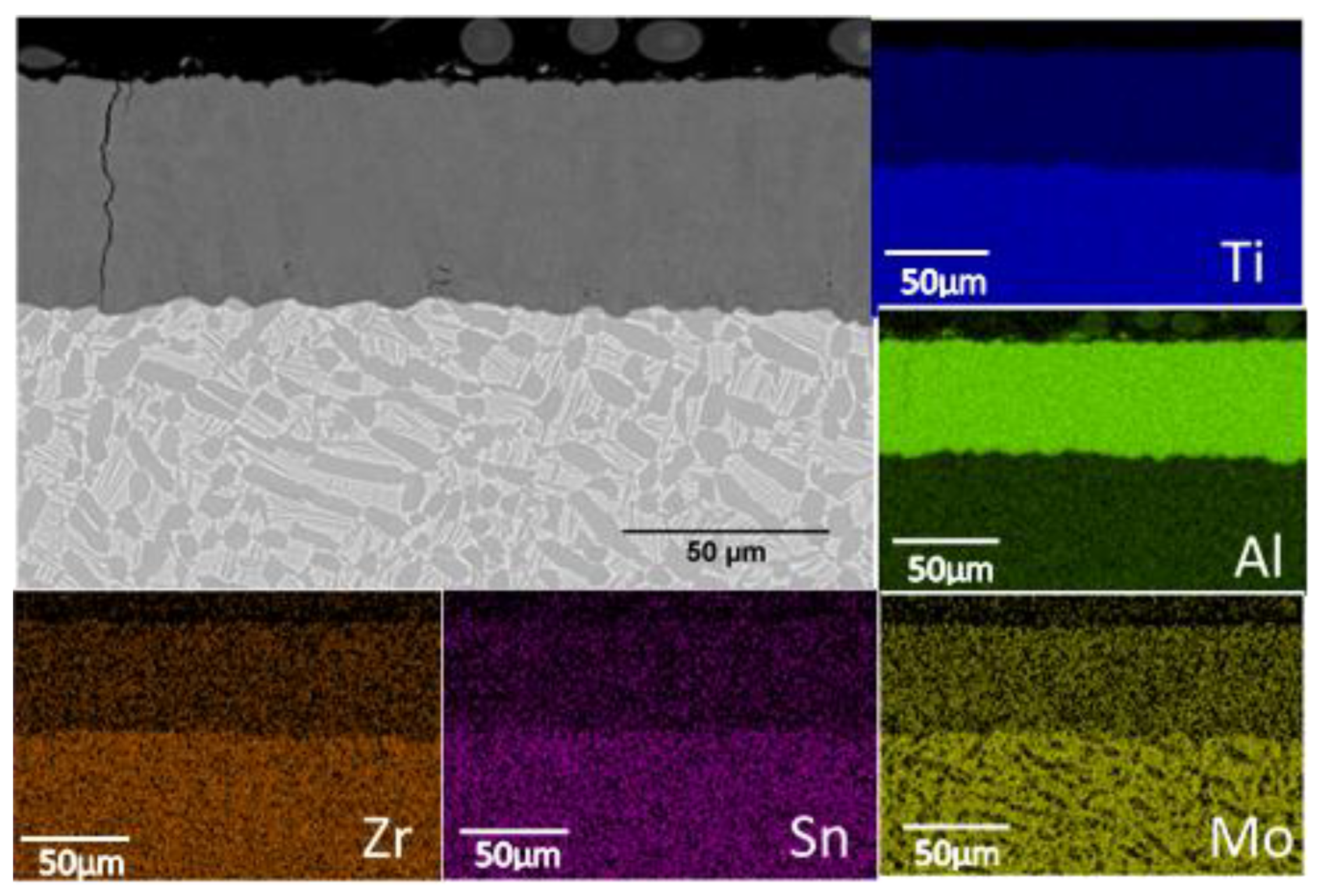


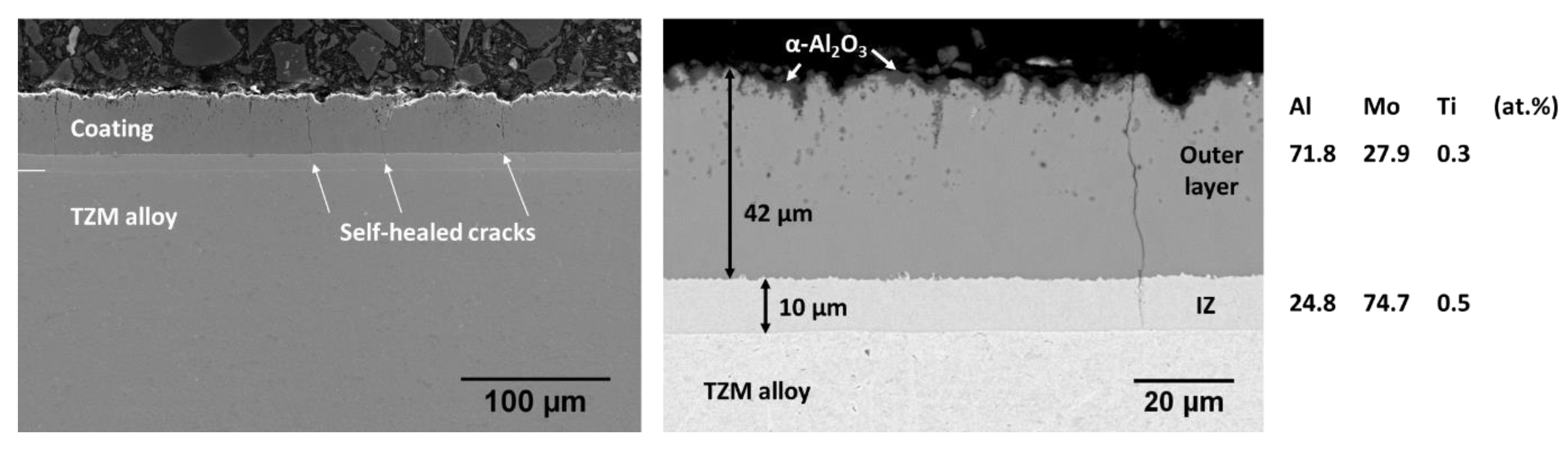
| Fe | Cr | Ni | Si | V | S | Mo | C | Co | Nb | N | W | Mn | Cu | Ti | Al | Sn | Zr | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A516 | Bal. | 0.30 | 0.30 | 0.40 | 0.02 | 0.03 | 0.08 | 0.20 | - | - | - | - | 1.40 | 0.30 | - | - | - | - | - |
| 310H | Bal. | 25 | 19 | 0.5 | - | 0.0006 | 0.4 | 0.04 | - | - | - | - | 1.5 | - | - | - | - | - | 0.023 |
| Ti6246 | - | - | - | - | - | - | 6 | - | - | - | - | - | - | - | Bal. | 6 | 2 | 4 | - |
| TZM | - | - | - | - | - | - | Bal. | - | - | - | - | - | - | - | 0.5 | - | - | 0.1 | - |
| Alloy | Surface Preparation | Slurry Deposition Method | Diffusion Heat Treatment | |
|---|---|---|---|---|
| Ra (µm) | ||||
| A516 | 1. Grit blasting (F46 corundum) 2. Grinding (P180 SiC paper) 3. Ultrasonic cleaning | 0.319 | Spraying | 10 h—700 °C Argon |
| 310H | 1. Grinding (P180 SiC paper) 2. Ultrasonic cleaning | 0.524 | Spraying | 2 h—700 °C + 2 h—900 °C Argon |
| Ti6246 | 1. Grinding (P180 SiC paper) 2. Ultrasonic cleaning | 0.407 | Spraying | 10 h—700 °C Argon |
| TZM | 1. Grit blasting (GL80 angular grit) 2. Ultrasonic cleaning | 3.823 | Spraying | 2 h—1200 °C Argon |
| TZM optimised process | 1. Grinding (P180 SiC paper) 2. Ultrasonic cleaning | 0.587 | Spraying | 1 h—1150 °C Argon |
| Point | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| O | 5.50 | 4.94 | 5.51 | 6.09 |
| Al | 69.64 | 70.15 | 69.86 | 69.19 |
| Ti | 23.60 | 23.32 | 23.07 | 23.22 |
| Zr | 0.58 | 0.57 | 0.61 | 0.54 |
| Mo | 0.65 | 1.02 | 0.94 | 0.88 |
| Sn | 0.04 | 0 | 0.02 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agüero, A.; Audigié, P.; Gutiérrez, M.; Lorente, C.; Mora, J.; Rodríguez, S. Aluminide Coatings by Means of Slurry Application: A Low Cost, Versatile and Simple Technology. Coatings 2024, 14, 1243. https://doi.org/10.3390/coatings14101243
Agüero A, Audigié P, Gutiérrez M, Lorente C, Mora J, Rodríguez S. Aluminide Coatings by Means of Slurry Application: A Low Cost, Versatile and Simple Technology. Coatings. 2024; 14(10):1243. https://doi.org/10.3390/coatings14101243
Chicago/Turabian StyleAgüero, Alina, Pauline Audigié, Marcos Gutiérrez, Cristina Lorente, Julio Mora, and Sergio Rodríguez. 2024. "Aluminide Coatings by Means of Slurry Application: A Low Cost, Versatile and Simple Technology" Coatings 14, no. 10: 1243. https://doi.org/10.3390/coatings14101243
APA StyleAgüero, A., Audigié, P., Gutiérrez, M., Lorente, C., Mora, J., & Rodríguez, S. (2024). Aluminide Coatings by Means of Slurry Application: A Low Cost, Versatile and Simple Technology. Coatings, 14(10), 1243. https://doi.org/10.3390/coatings14101243






