Attenuation Law of Performance of Concrete Anti-Corrosion Coating under Long-Term Salt Corrosion
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of the Multi-Layer Anticorrosion Coating System
2.3. Test Methods
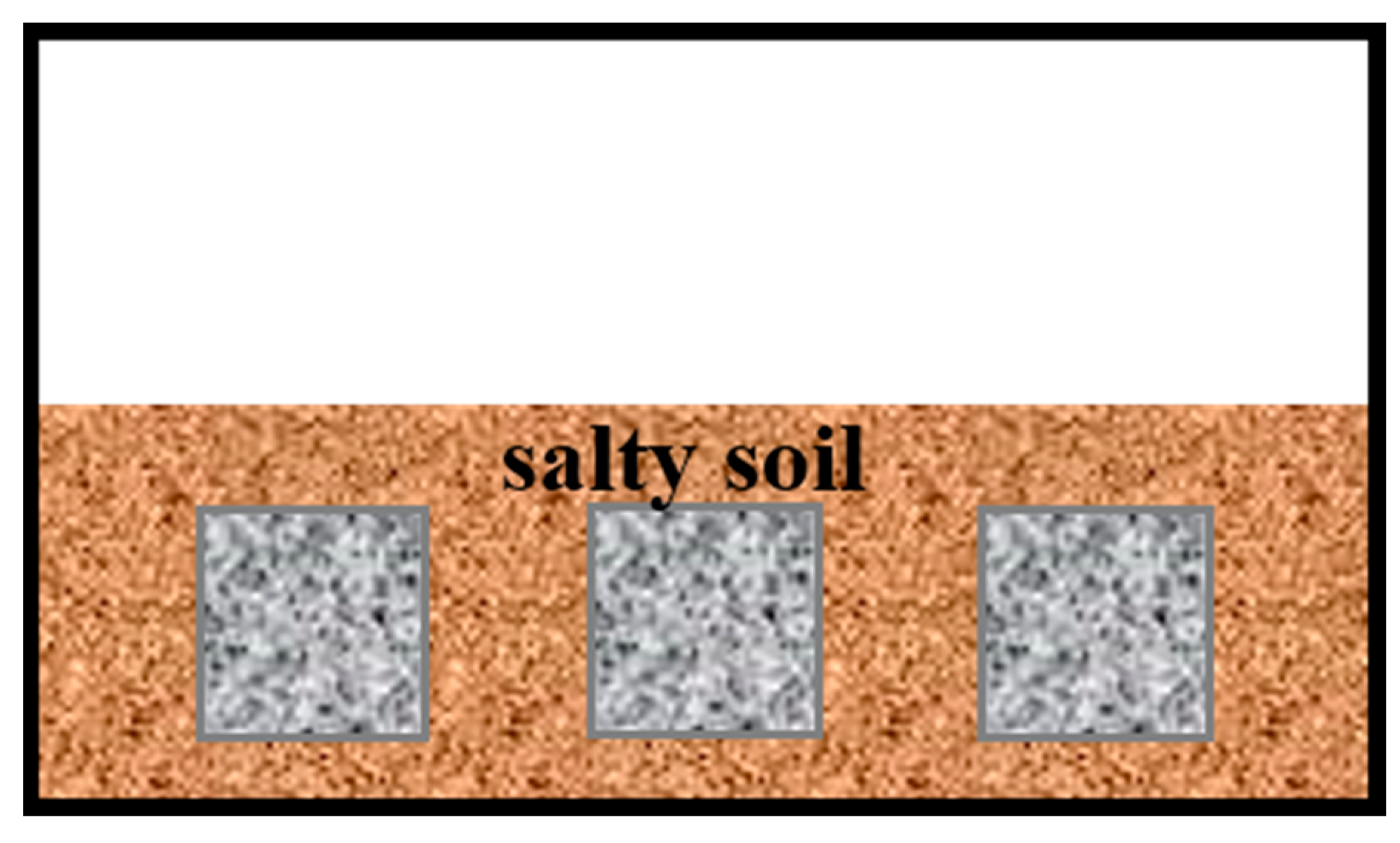
2.3.1. Long-Term Burial Salt Corrosion Test
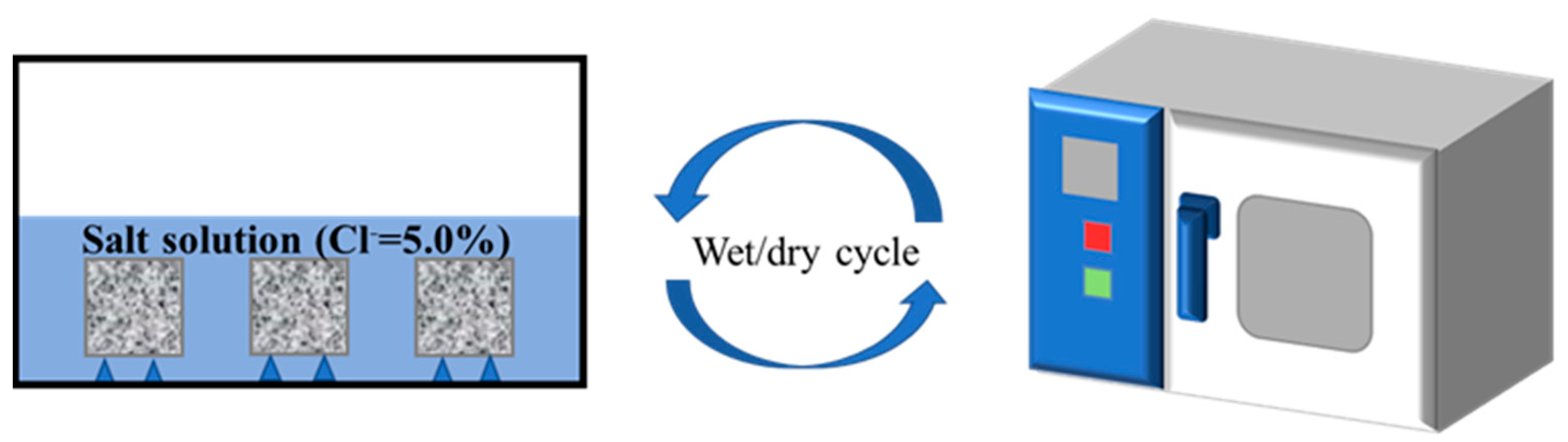
2.3.2. Wet–Dry Cycle Accelerated Salt Corrosion Test
2.4. Determination of Chloride Ion Content
2.5. Surface Characterization
2.6. Weighted Analysis Method of Coating Life
3. Results and Discussions
3.1. The Morphology of the Concrete Coating
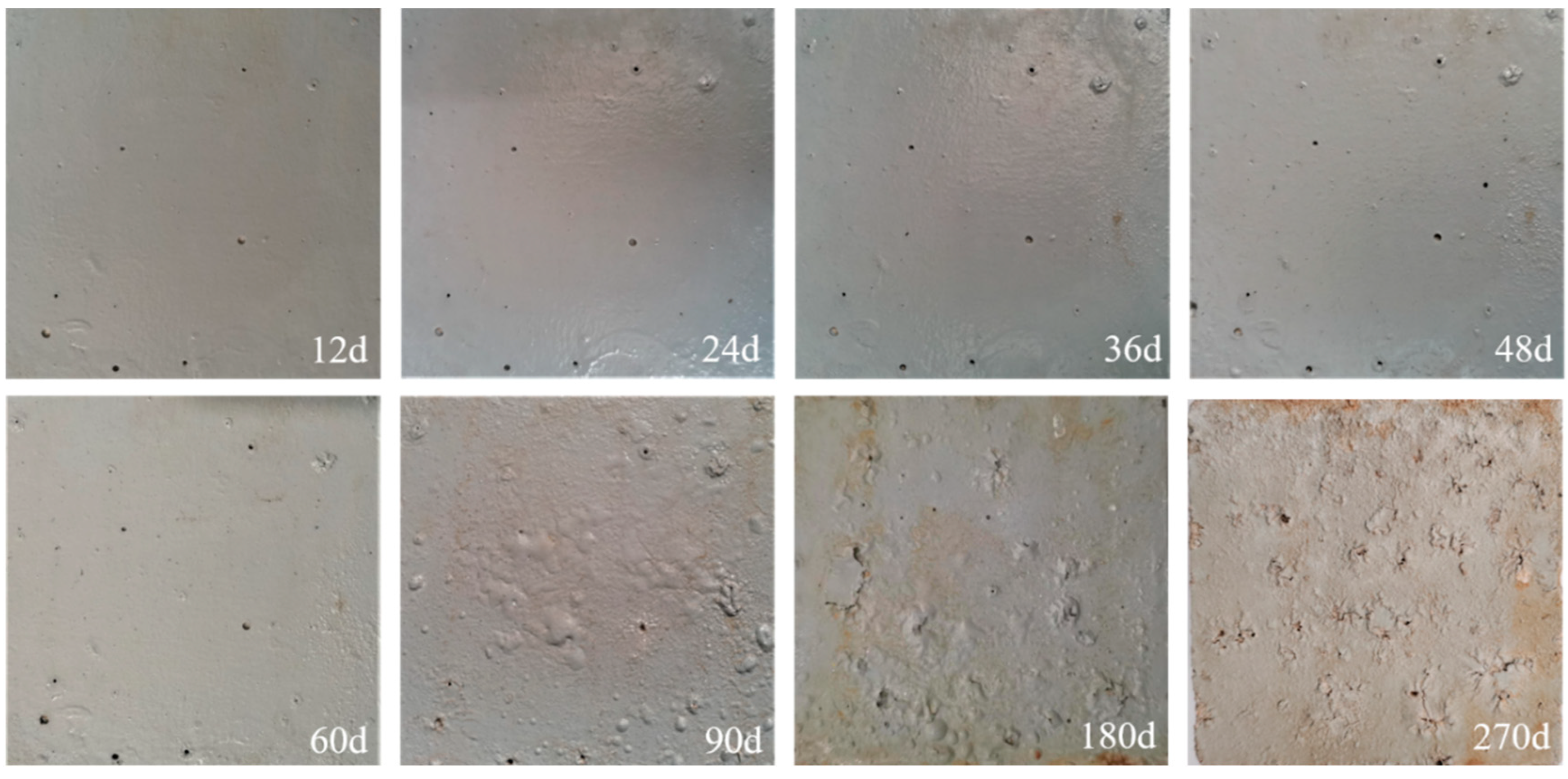
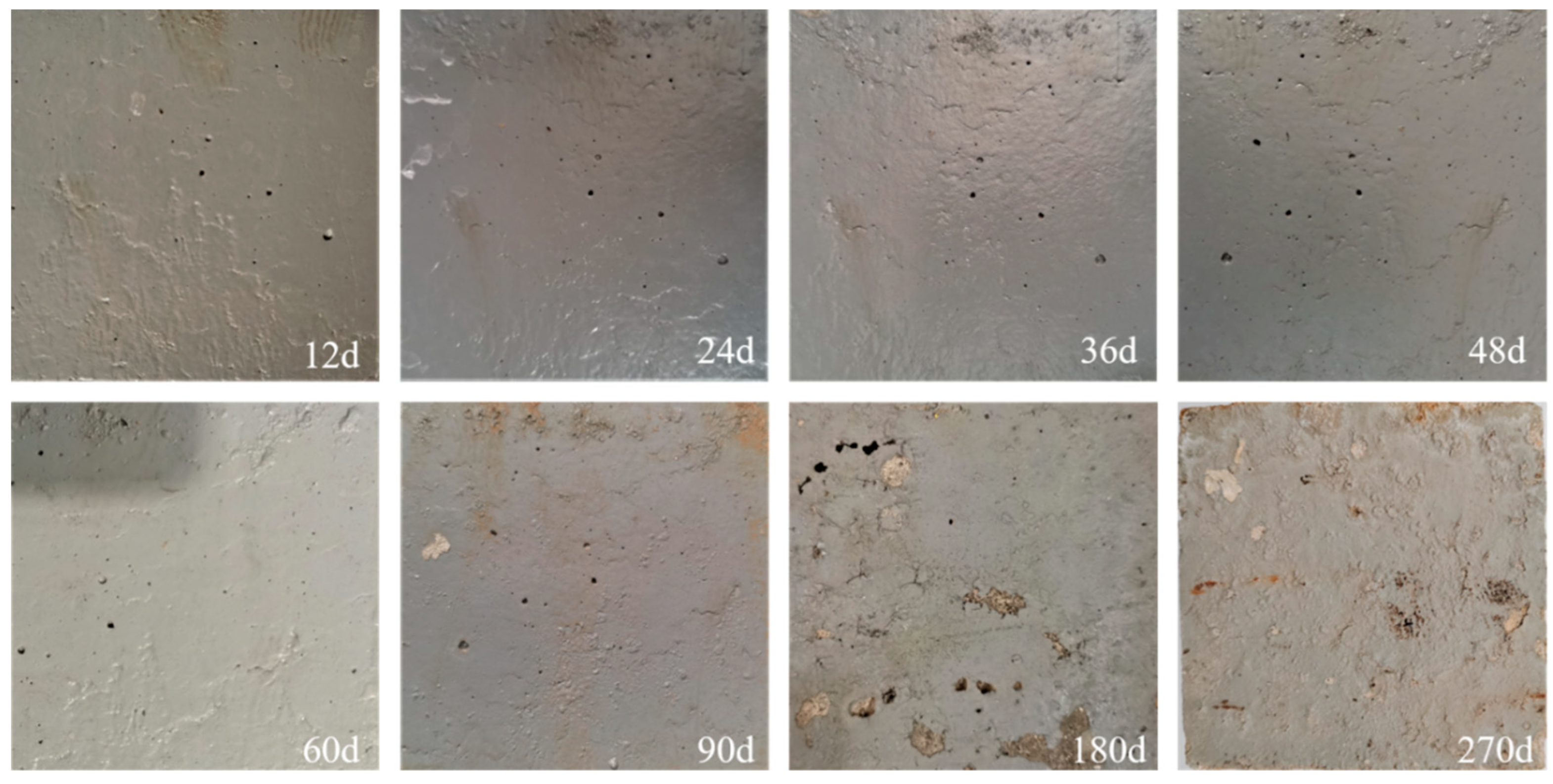
3.1.1. Macroscopic Topography of the Coating for Long-Term Salt Corrosion
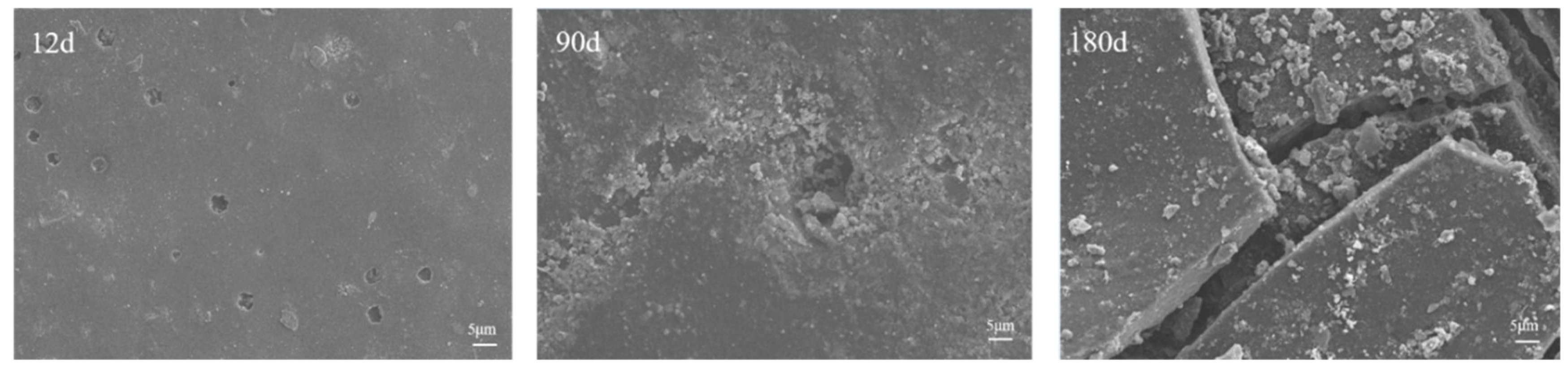
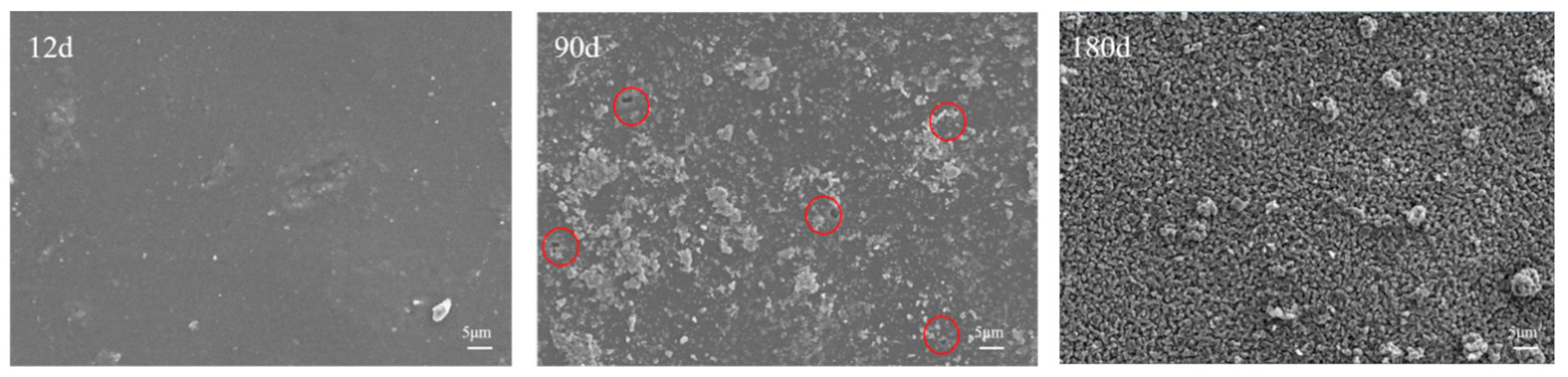
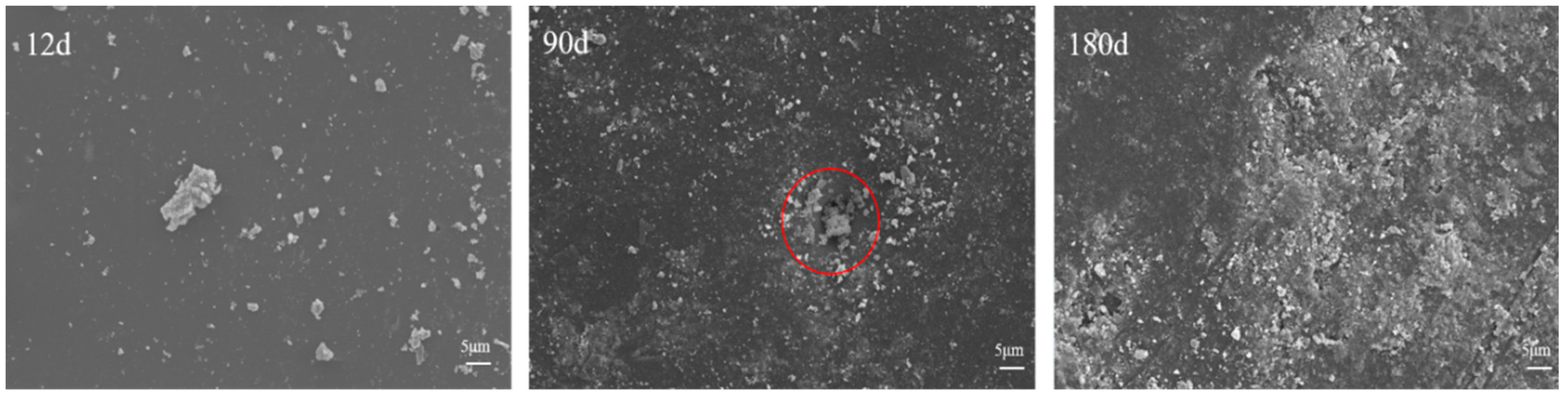
3.1.2. Microscopic Morphology of Long-Term Salt Corrosion Coatings
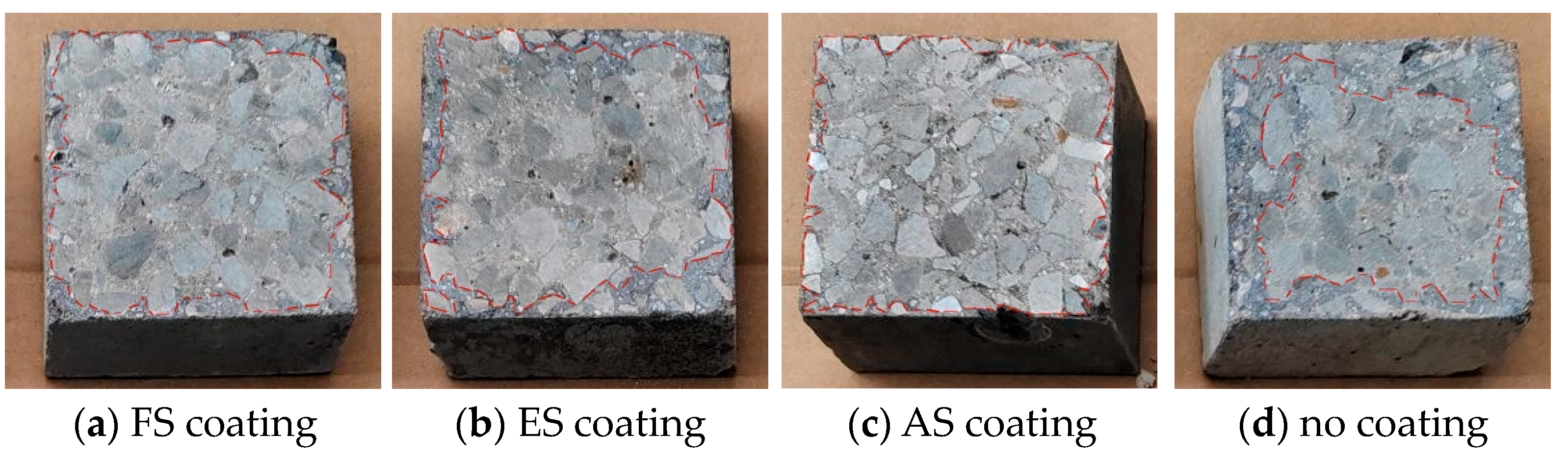
3.1.3. Macroscopic Discoloration Boundary of Coated Concrete under Salt Corrosion
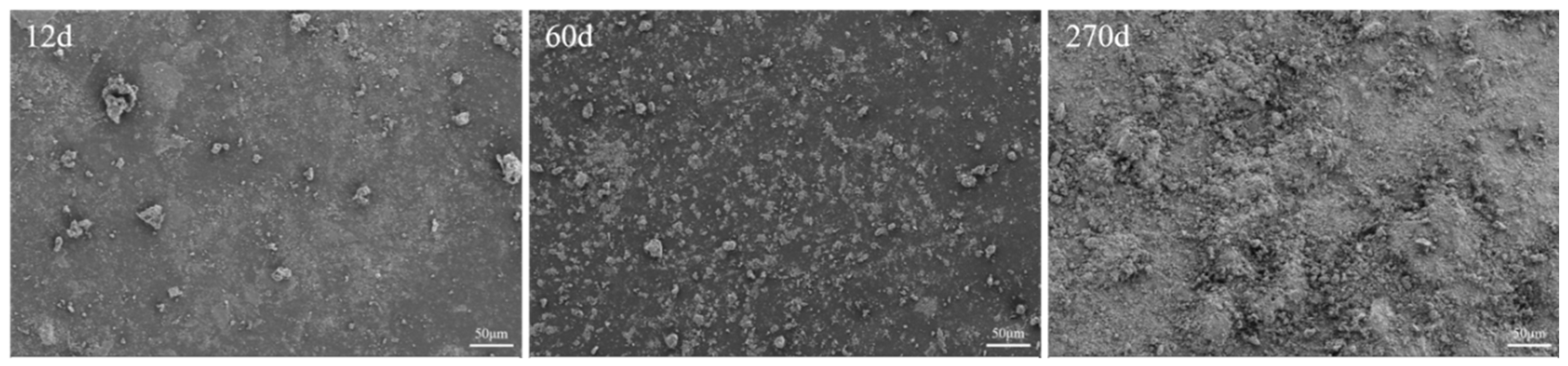
3.1.4. Micromorphology of Coated Concrete Buried by Saline Soil
3.2. Performance Change of Coating under Salt Corrosion
3.2.1. Glossiness of the Coating under Long-Term Salt Corrosion
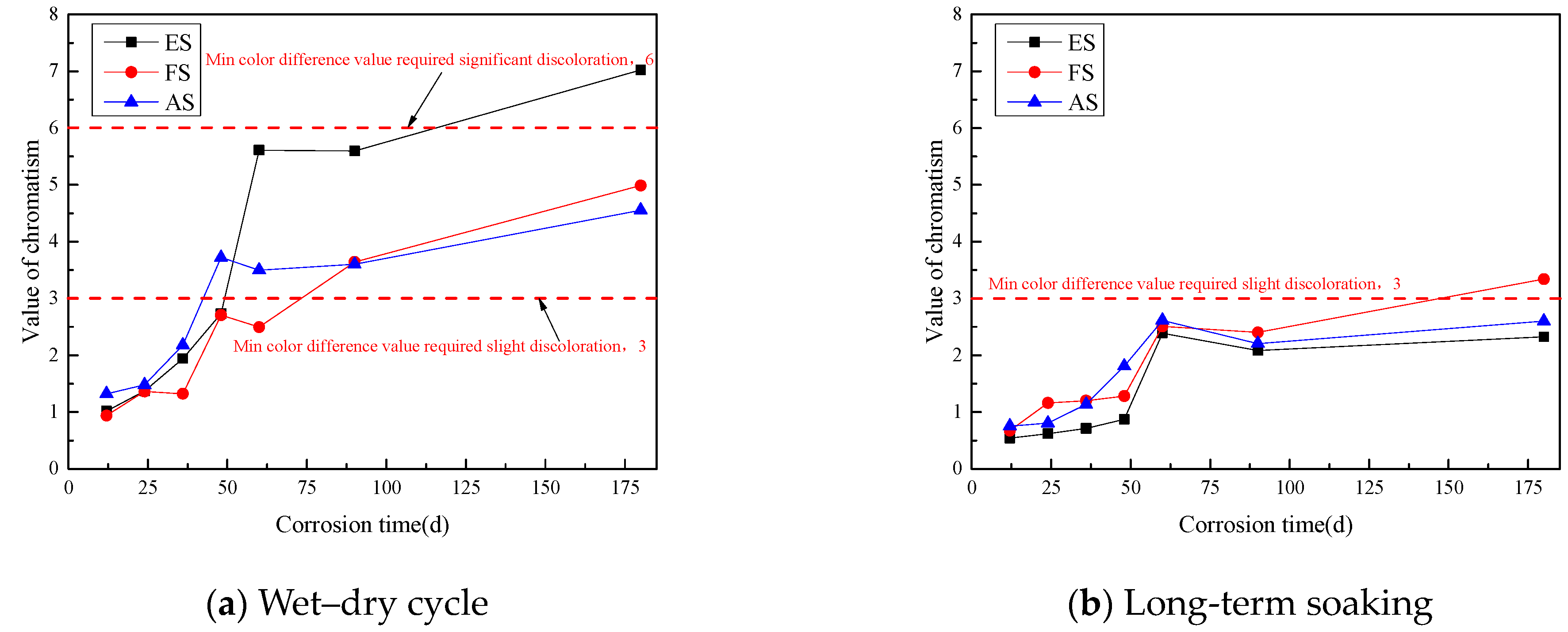
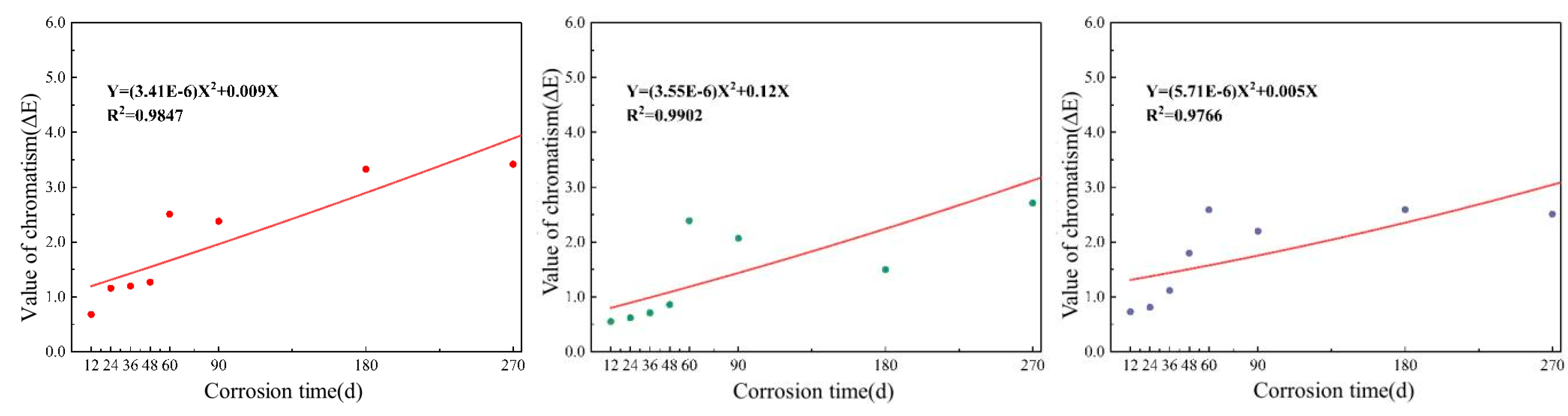
3.2.2. Color Difference Value of the Coating under Long-Term Salt Corrosion Conditions
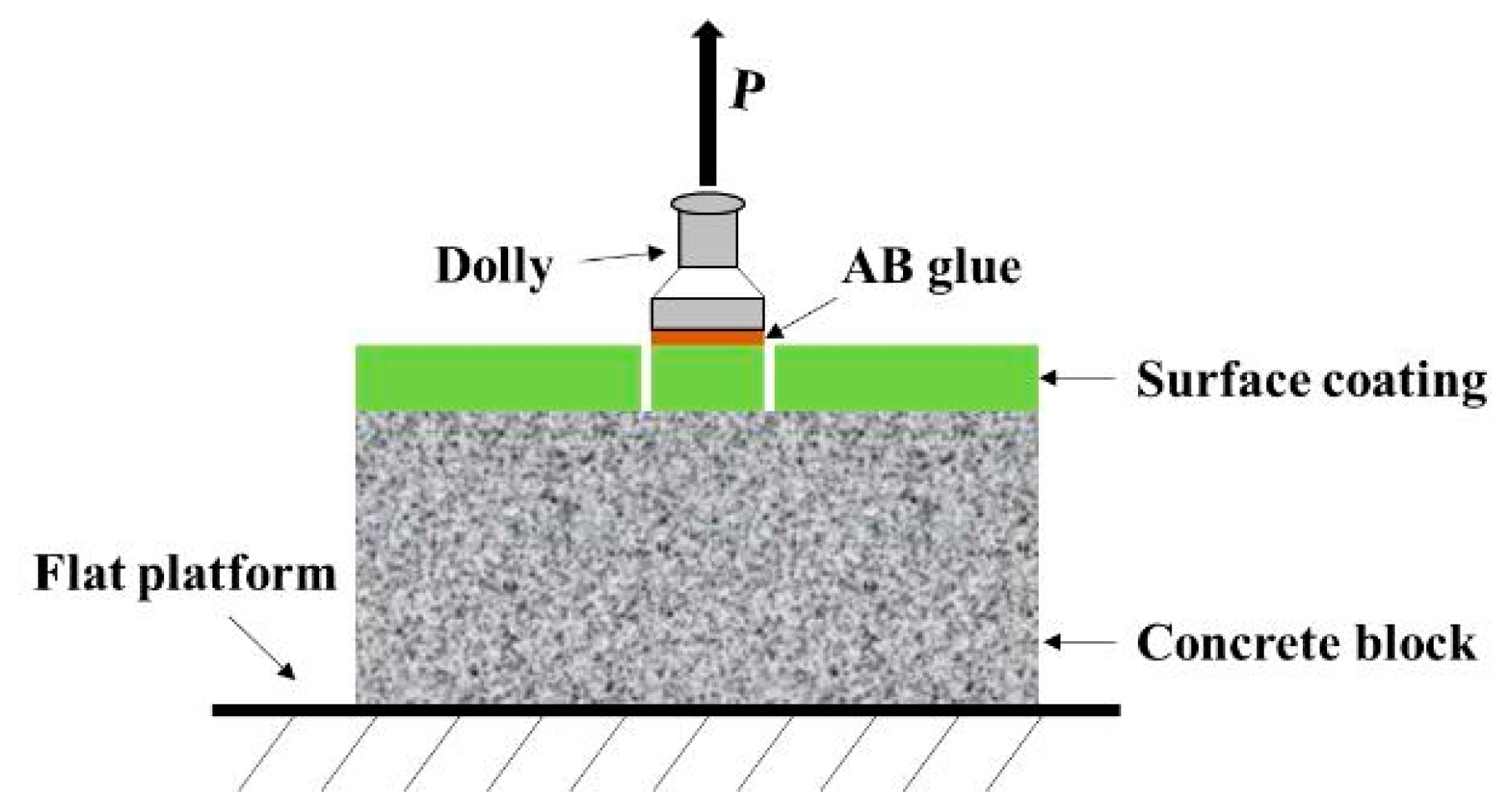
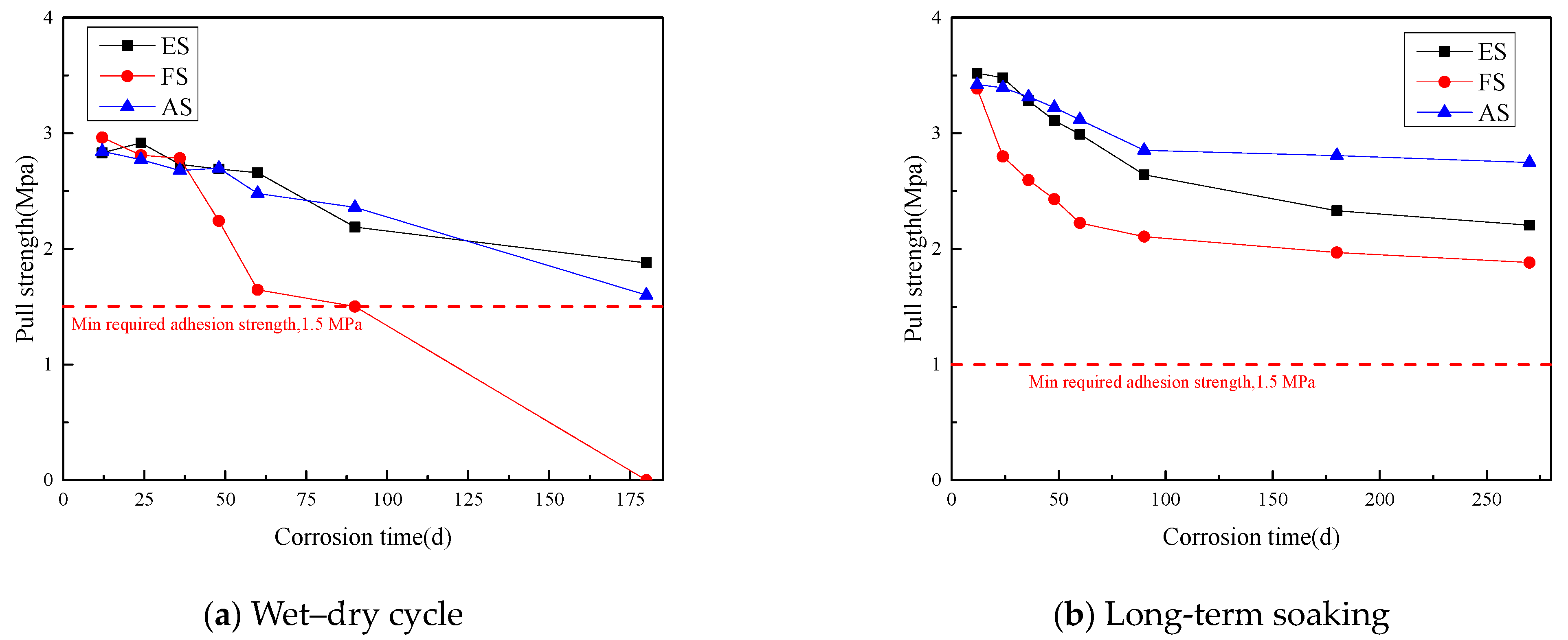
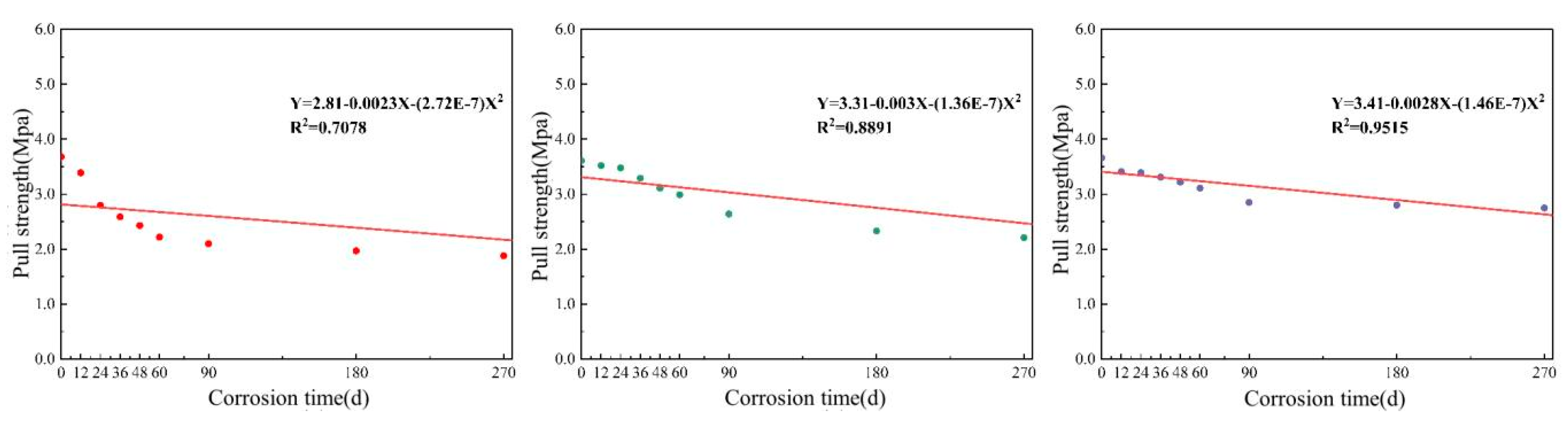
3.2.3. Adhesion of the Coating under Long-Term Salt Corrosion
3.3. Chloride Ion Resistance Characteristics of the Coating
3.4. Computation for Mechanism Analysis
3.4.1. Analysis of Coating Service Life Based on Gloss Variation Pattern
3.4.2. Analysis of Service Life of Long-Term Soaking Coating Based on Color Variation Rule
3.4.3. Analysis of the Service Life of the Long-Term Immersion Coating Based on the Law of Adhesion Change
3.4.4. Prediction Model of Chloride Ion Resistance Erosion Life of Coated Concrete
3.4.5. Calculation of Service Life of Coating under Salt Shell Environment
4. Conclusions
- (1)
- The corrosion erosion patterns of the three anti-corrosion coatings used in this study were basically consistent in the accelerated salt corrosion test under wet–dry cycles. The coating pores gradually expanded, followed by the loss of filler components inside the pores, forming “pits”, and eventually resulting in coating layer delamination. Compared to the other two water-based coatings, the hydrofluorocarbon (FS) coating was more prone to damage. There were more holes in the FS coating, which make the corrosive medium, such as water and chloride, rapidly permeate into the interior.
- (2)
- During the salt corrosion process under salt-shell environment, all coatings exhibited three stages: the adhesion of tiny saline soil particles on the coating surface, an increase in the size and area of attached saline soil particles, and the uneven coverage of multiple layers of saline soil resulting in surface irregularities. The corrosion caused by buried saline soil still required the infiltration of microscopic pores into the substrate surface, leading to coating failure.
- (3)
- The color difference values on the surfaces of the three coatings gradually increased under the conditions of wet–dry cycles and long-term immersion tests. At 180 days, the glossiness of all three coating systems reached a severe loss, with the FS coating completely losing its glossiness, while the color difference still does not reach the grade of slight discoloration. The pores and cracks expanded in the process of coating corrosion, which caused the gradual loss of paint particles, thus causing color difference.
- (4)
- The adhesion of the three coatings gradually decreased as the wet–dry cycle period increased. The loss of adhesion was mainly due to the enlargement of internal gaps within the coatings and the continuous expansion of localized microcracks.
- (5)
- In the salt corrosion test, the chloride ion content inside the concrete specimens decreased with increasing depth at the same corrosion time, while the chloride ion content within the concrete at the same depth increased with time. The coloration boundary of the uncoated concrete section moved closer to the concrete core as the corrosion time prolonged, with the brown area gradually approaching the concrete core.
- (6)
- Logistic curve, S-shaped curve, and quadratic function curve were used to analyze and fit the glossiness, color difference, and adhesion of the coatings, respectively. The predicted coating lifetimes weighted by glossiness, color difference, and adhesion were generally consistent with the actual results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, Y.; Yan, C.; Niu, P. Hysteretic model of reinforced concrete bridge piers based on earthquake damage and corrosion from saline soil. Soil Dynam. Earthq. Eng. 2023, 166, 107732. [Google Scholar] [CrossRef]
- Katiyar, P.K.; Randhawa, N.S. Corrosion behavior of WC-Co tool bits in simulated (concrete, soil, and mine) solutions with and without chloride additions. Int. J. Refract. Met. Hard Mater. 2019, 85, 105062. [Google Scholar] [CrossRef]
- Duan, M.; Qin, Y.; Li, Y.; Zhou, H. Durability and damage model of polyacrylonitrile fiber reinforced concrete under freeze–thaw and erosion. Construct. Build. Mater. 2023, 394, 132238. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Chen, Y.; Han, Y.; Zhang, X.; Liu, Y. Evolution process of the microstructure of saline soil with different compaction degrees during freeze-thaw cycles. Eng. Geol. 2022, 304, 106699. [Google Scholar] [CrossRef]
- Xue, Q.; Zhang, L.; Mei, K.; Li, X.; Newell, P.; Wang, Y.; Cheng, X.; Zheng, W. CO2-induced evolution of chemical, structural and mechanical properties of reinforced concrete: A review. Construct. Build. Mater. 2022, 353, 129069. [Google Scholar] [CrossRef]
- Sadati, S.; Arezoumandi, M.; Shekarchi, M. Long-term performance of concrete surface coatings in soil exposure of marine environments. Construct. Build. Mater. 2015, 94, 656–663. [Google Scholar] [CrossRef]
- Pasupathy, K.; Berndt, M.; Sanjayan, J.; Rajeev, P.; Cheema, D.S. Durability of low-calcium fly ash based geopolymer concrete culvert in a saline environment. Cem. Concr. Res. 2017, 100, 297–310. [Google Scholar] [CrossRef]
- Chen, Q.; Rong, H.; Tao, G.; Nimbalkar, S.; Xie, K. Fatigue characteristics of nano-SiO2 cemented soil under coupled effects of dry-wet cycle and seawater corrosion. Construct. Build. Mater. 2023, 401, 132579. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Liu, R.; Zhu, P.; Liu, H.; Wang, X.; Yu, J.; Chen, Y. Chloride penetration of concrete exposed to dry-wet cycle with various dry-wet ratios and temperature. Construct. Build. Mater. 2023, 400, 132883. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, G.; Ye, H.; Zeng, Q.; Zhang, Z.; Tian, Z.; Jin, X.; Jin, N.; Chen, Z.; Wang, J. Corrosion of steel rebar in concrete induced by chloride ions under natural environments. Construct. Build. Mater. 2023, 369, 130504. [Google Scholar] [CrossRef]
- Merachtsaki, D.; Tsardaka, E.; Anastasiou, E.; Zouboulis, A. Anti-corrosion properties of magnesium oxide/magnesium hydroxide coatings for application on concrete surfaces (sewerage network pipes). Construct. Build. Mater. 2021, 312, 125441. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, H.; Phalé Zeze, A.L.; Liu, X.; Tao, M. Coating performance, durability and anti-corrosion mechanism of organic modified geopolymer composite for marine concrete protection. Cem. Concr. Compos. 2022, 129, 104495. [Google Scholar] [CrossRef]
- Berndt, M.L. Evaluation of coatings, mortars and mix design for protection of concrete against sulphur oxidising bacteria. Construct. Build. Mater. 2011, 25, 3893–3902. [Google Scholar] [CrossRef]
- Aguirre-Guerrero, A.M.; Robayo-Salazar, R.A.; de Gutiérrez, R.M. A novel geopolymer application: Coatings to protect reinforced concrete against corrosion. Appl. Clay Sci. 2017, 135, 437–446. [Google Scholar] [CrossRef]
- Deshmukh, K.; Parsai, R.; Anshul, A.; Singh, A.; Bharadwaj, P.; Gupta, R.; Mishra, D.; Amritphale, S.S. Studies on fly ash based geopolymeric material for coating on mild steel by paint brush technique. Int. J. Adhesion Adhes. 2017, 75, 139–144. [Google Scholar] [CrossRef]
- Kuenzel, C.; Vandeperre, L.J.; Donatello, S.; Boccaccini, A.R.; Cheeseman, C. Ambient temperature drying shrinkage and cracking in metakaolin-based geopolymers. J. Am. Ceram. Soc. 2012, 95, 3270–3277. [Google Scholar] [CrossRef]
- Lee, N.K.; Jang, J.G.; Lee, H.K. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Z.; Liu, S.; Luo, H.; Fan, W.; Liu, Z.; Liu, F.; Wang, H. Anti-corrosion and wear-resistant coating of waterborne epoxy resin by concrete-like three-dimensional functionalized framework fillers. Chem. Eng. Sci. 2021, 242, 116748. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, G.; Xie, Q.; Ma, C.; Zhang, G. High performance epoxy coating with cross-linkable solvent via Diels-Alder reaction for anti-corrosion of concrete. Prog. Org. Coat. 2020, 139, 105473. [Google Scholar] [CrossRef]
- Kiwa, T.; Hayashi, T.; Kawasaki, Y.; Yamada, H.; Tsukada, K. Magnetic thickness gauge using a Fourier transformed eddy current technique. NDT E Int. 2009, 42, 606–609. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, M.; Zhong, X.; Zhu, Z.; Deng, P.; Liu, H. Hydrophobic composite coatings with photocatalytic self-cleaning properties by micro/nanoparticles mixed with fluorocarbon resin. Ceram. Int. 2015, 41, 5341–5347. [Google Scholar] [CrossRef]
- Choudalakis, G.; Gotsis, A.D. Morphology and gas transport properties of acrylic resin/bentonite nanocomposite coatings. Prog. Org. Coat. 2014, 77, 845–852. [Google Scholar] [CrossRef]
- Bagherzadeh, M.R.; Ghasemi, M.; Mahdavi, F.; Shariatpanahi, H. Investigation on anticorrosion performance of nano and micro polyaniline in new water-based epoxy coating. Prog. Org. Coat. 2011, 72, 348–352. [Google Scholar] [CrossRef]
- Liu, X.; Liu, H.; Meng, X.; Sun, C.; Wang, M.; Qi, L.; Shi, G.; Wu, S. Effects of aging treatment on microstructure and tribological properties of nickel-based high-temperature self-lubrication wear resistant composite coatings by laser cladding. Mater. Chem. Phys. 2014, 143, 616–621. [Google Scholar] [CrossRef]
- Bhargava, S.; Kubota, M.; Lewis, R.D.; Advani, S.G.; Prasad, A.K.; Deitzel, J.M. Ultraviolet, water, and thermal aging studies of a waterborne polyurethane elastomer-based high reflectivity coating. Prog. Org. Coat. 2015, 79, 75–82. [Google Scholar] [CrossRef]
- Elkebir, Y.; Mallarino, S.; Trinh, D.; Touzain, S. Effect of physical ageing onto the water uptake in epoxy coatings. Electrochim. Acta 2020, 337, 135766. [Google Scholar] [CrossRef]
- Nguyen Dang, D.; Peraudeau, B.; Cohendoz, S.; Mallarino, S.; Feaugas, X.; Touzain, S. Effect of mechanical stresses on epoxy coating ageing approached by Electrochemical Impedance Spectroscopy measurements. Electrochim. Acta 2014, 124, 80–89. [Google Scholar] [CrossRef]
- Coudun, C.; Gégout, J. The derivation of species response curves with Gaussian logistic regression is sensitive to sampling intensity and curve characteristics. Ecol. Monogr. 2006, 199, 164–175. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rukzon, S.; Sirivivatnanon, V. Effect of carbon dioxide on chloride penetration and chloride ion diffusion coefficient of blended Portland cement mortar. Construct. Build. Mater. 2008, 22, 1701–1707. [Google Scholar] [CrossRef]
- Li, B.; Mao, J.; Nawa, T.; Liu, Z. Mesoscopic chloride ion diffusion model of marine concrete subjected to freeze-thaw cycles. Construct. Build. Mater. 2016, 125, 337–351. [Google Scholar] [CrossRef]
- Guo, L.; Chen, T.; Gao, X. Transient meshless boundary element method for prediction of chloride diffusion in concrete with time dependent nonlinear coefficients. Eng. Anal. Bound. Elem. 2012, 36, 104–111. [Google Scholar] [CrossRef]
- Park, S.; Kwon, S.; Jung, S. Analysis technique for chloride penetration in cracked concrete using equivalent diffusion and permeation. Construct. Build. Mater. 2012, 29, 183–192. [Google Scholar] [CrossRef]










| Chemical Composition (%) | SiO2 | Al2O3 | CaO | MgO | SO3 | Fe2O3 | Na2O | K2O | Loss |
| 24.53 | 8.38 | 56.93 | 2.52 | 1.79 | 4.27 | 0.43 | 0.80 | 1.09 |
| Specific Surface Area (m2/kg) | Cement Stability (Boiling Method) | Setting Time (min) | Resistance Strength (MPa) | Compressive Strength (MPa) | |||
|---|---|---|---|---|---|---|---|
| Initial Setting Time | Final Setting Time | 3 d | 28 d | 3 d | 28 d | ||
| 300 | No crack and deformation for 3 h | 45 | 600 | 3.5 | 6.5 | 17.0 | 42.5 |
| Aggregate | Type | Grain Size (mm) | Apparent Density (kg/m3) | Stacking Density (kg/m3) | Crushing Value (%) | Sediment Percentage (%) | Fineness Modulus |
|---|---|---|---|---|---|---|---|
| Coarse aggregate | Limestone | 5~25 | 2700 | — | 16.8 | 1.0 | — |
| Fine aggregate | River sand | 0–4.75 | 2600 | 1500 | — | 0.9 | 2.9 |
| Water Cement Ratio | Amount of Material Used per Cubic Meter of Concrete (kg/m3) | Sand to Aggregate Ratio | |||
|---|---|---|---|---|---|
| Cement | Fine Aggregate | Coarse Aggregate | Water | ||
| 0.6 | 325 | 656 | 1274 | 195 | 0.36 |
| Coating System | Primer | Thickness (μm) | Intermediate Coat | Thickness (μm) | Topcoat | Thickness (μm) | Aggregate Thickness (μm) |
|---|---|---|---|---|---|---|---|
| Hydrofluorocarbon coating (FS) | Water-based epoxy sealing primer | 50 | Water-based epoxy intermediate coat | 150 | Water-based fluorocarbon topcoat | 60 | 260 |
| Aqueous epoxy system (ES) | Water-based epoxy sealing primer | 50 | Water-based epoxy intermediate coat | 150 | Water-based epoxy topcoat | 60 | 260 |
| Waterborne acrylic acid system (AS) | Waterborne acrylic closure primer | 50 | Water-based acrylic resin intermediate coat | 120 | Water-based acrylic resin topcoat | 80 | 250 |
| Concrete Specimen | Corrosion Time (d) | ES | AS | FS | No Coating | Number of Replication |
|---|---|---|---|---|---|---|
| Wet-dry cycle accelerated salt corrosion test (100 * 100 * 100 mm) | 12 | ES-A-1 | AS-A-1 | FS-A-1 | US-A-1 | 3 |
| 24 | ES-A-2 | AS-A-2 | FS-A-2 | US-A-2 | 3 | |
| 36 | ES-A-3 | AS-A-3 | FS-A-3 | US-A-3 | 3 | |
| 48 | ES-A-4 | AS-A-4 | FS-A-4 | US-A-4 | 3 | |
| 60 | ES-A-5 | AS-A-5 | FS-A-5 | US-A-5 | 3 | |
| 90 | ES-A-6 | AS-A-6 | FS-A-6 | US-A-6 | 3 | |
| 180 | ES-A-7 | AS-A-7 | FS-A-7 | US-A-7 | 3 | |
| 270 | ES-A-8 | AS-A-8 | FS-A-8 | US-A-8 | 3 | |
| long-term burial salt corrosion test (100 * 100 * 100 mm) | 12 | ES-B-1 | AS-B-1 | FS-B-1 | US-B-1 | 3 |
| 24 | ES-B-2 | AS-B-2 | FS-B-2 | US-B-2 | 3 | |
| 36 | ES-B-3 | AS-B-3 | FS-B-3 | US-B-3 | 3 | |
| 48 | ES-B-4 | AS-B-4 | FS-B-4 | US-B-4 | 3 | |
| 60 | ES-B-5 | AS-B-5 | FS-B-5 | US-B-5 | 3 | |
| 90 | ES-B-6 | AS-B-6 | FS-B-6 | US-B-6 | 3 | |
| 180 | ES-B-7 | AS-B-7 | FS-B-7 | US-B-7 | 3 | |
| 270 | ES-B-8 | AS-B-8 | FS-B-8 | US-B-8 | 3 |
| Chemical Composition (mg/kg) | Total Soluble Salt Content (mg/kg) | |||||
|---|---|---|---|---|---|---|
| CO32− | HCO3−. | Cl−. | SO42− | Na+ | K+ | 16,940 |
| 0 | 170 | 3980 | 9120 | 3000 | 670 | |
| Chemical Composition (mg/kg) | Total Soluble Salt Content (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| CO32− | HCO3− | Cl−. | SO42− | Ca2+ | Mg2+ | Na+ | K+ | 815,700 |
| 264 | 0 | 504,892 | 25,048 | 1381 | 7996 | 270,058 | 5,237,926 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, T.; Wu, Y.; Yang, M.; Xu, P.; Li, Y.; Wang, L.; Chen, H. Attenuation Law of Performance of Concrete Anti-Corrosion Coating under Long-Term Salt Corrosion. Coatings 2024, 14, 1249. https://doi.org/10.3390/coatings14101249
Fan T, Wu Y, Yang M, Xu P, Li Y, Wang L, Chen H. Attenuation Law of Performance of Concrete Anti-Corrosion Coating under Long-Term Salt Corrosion. Coatings. 2024; 14(10):1249. https://doi.org/10.3390/coatings14101249
Chicago/Turabian StyleFan, Tao, Yongchang Wu, Mingda Yang, Peng Xu, Yongqing Li, Laifa Wang, and Huaxin Chen. 2024. "Attenuation Law of Performance of Concrete Anti-Corrosion Coating under Long-Term Salt Corrosion" Coatings 14, no. 10: 1249. https://doi.org/10.3390/coatings14101249





