A Review on Preparation of Palladium Oxide Films
Abstract
:1. General Considerations on the Growth of PdO Films and Early Research as the Background of the Present Study
1.1. General Considerations on the Growth of PdO Films
1.2. Early Research as the Background of the Present Study
- (1)
- Preparation of a Pd metal coating on a substrate, followed by oxidation.
- (2)
- Preparation of a PdO film by thermal decomposition of salts.
- (3)
- Preparation of the PdO oxide films by reactive methods.
- (i)
- Betteridge and Rhys [17] demonstrated that, when the Pd metal is heated to 700 °C in air, a PdO film forms on its surface. The electrode was not stable, and the utility of the thermal oxidation method for these purposes was reconsidered.
- (ii)
- Grubb and King [14] used chemical oxidation of the metal surface. The Pd metal surface, after cleaning in aqua regia for 20 s and roughening, was dipped into a 50% aqueous solution of NaOH and dried for 10 min in a stream of dry nitrogen. The coated Pd wire was heated in a flow of oxygen at 800 °C for 20 min. The portion covered with NaOH resulted in formation of a black PdO layer. The oxide layer was uniform, nonporous, and its thickness was of about 6 µm. Kinoshita et al. [18] expanded this research and studied different parameters, such as oxidation temperature in the range of 600 and 870 °C. They found that the best results were obtained for the electrodes processed at 750 °C after having been immersed in a 50% NaOH solution.
- (iii)
- An alternative method was proposed, namely, the electrochemical oxidation. Kinoshita et al. [18] oxidized the metal electrodes by treating them in 0.2 M NaOH for 5 min at 2.7 V and for 17 h at 0.74 V. Electrodes lost their near-Nernstian response to pH after a few acid–base cycles. A longer lifetime of less than 6 days was determined for an electrode fabricated by Liu et al. [13]. The authors used a molten salt electrolyte of NaNO3/LiCl for oxidation, with a current density of 20 mA·mm−2 at 5.9–6.2 V for 90 s. In [24], the authors applied electrolysis waveforms containing various levels of the ac and dc components for a processing time up to 24 h. The pH sensor’s response depends on the processing conditions. The authors also observed surface morphology changes.
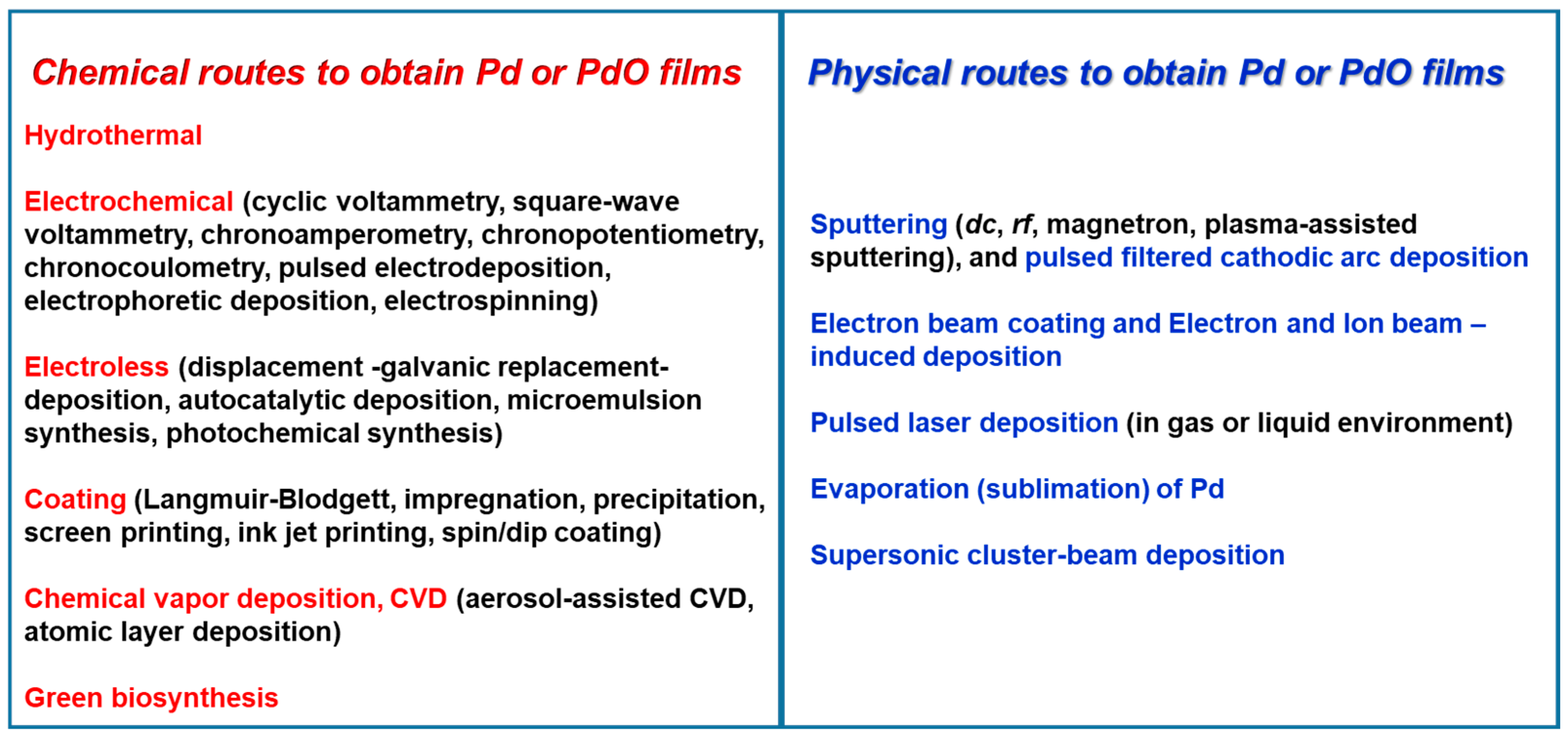
2. Chemical Routes of PdO and Pd Precursor Films’ Growth
2.1. Hydrothermal Method
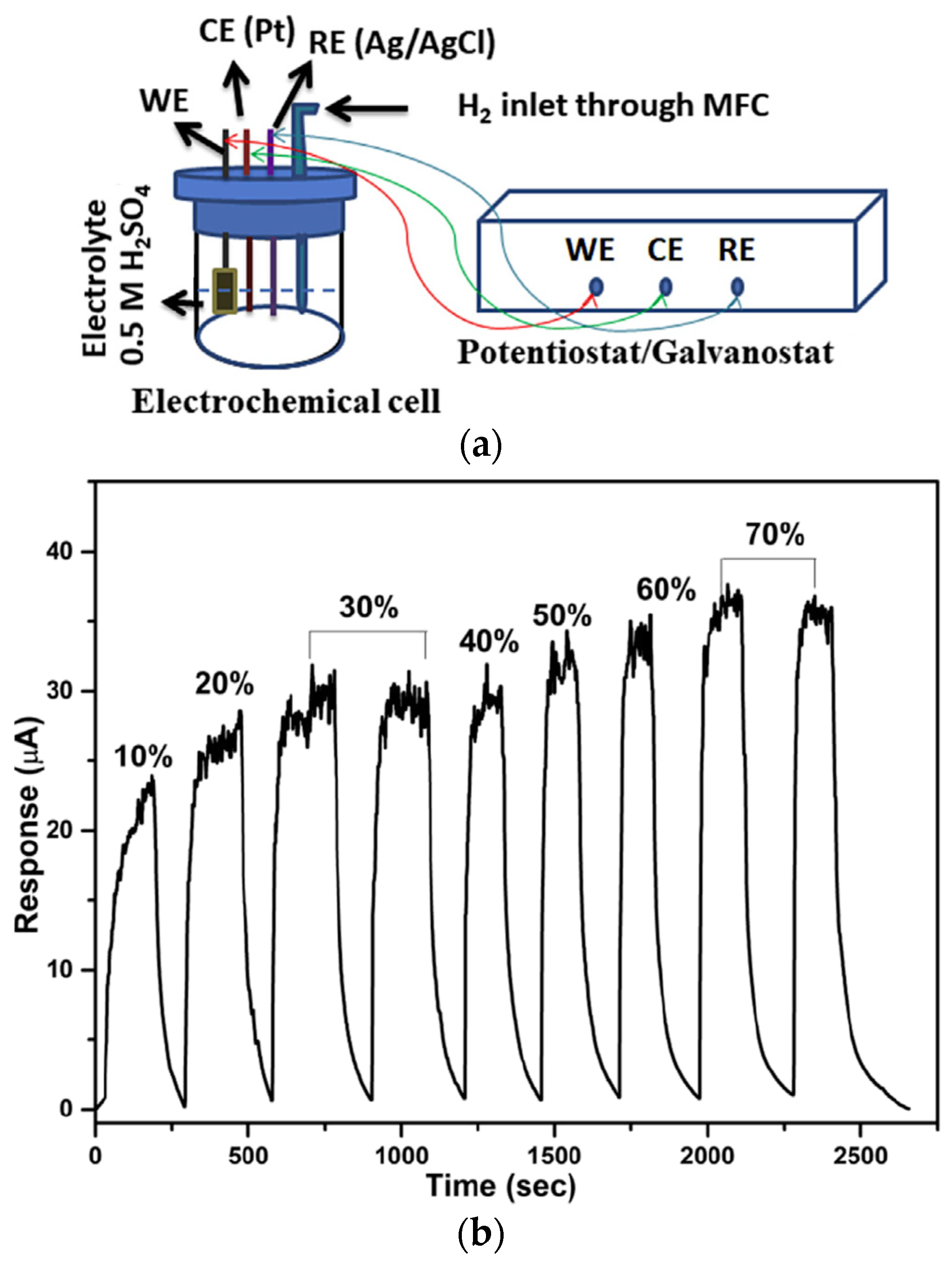
2.2. Electrochemical Deposition
2.3. Electroless Deposition
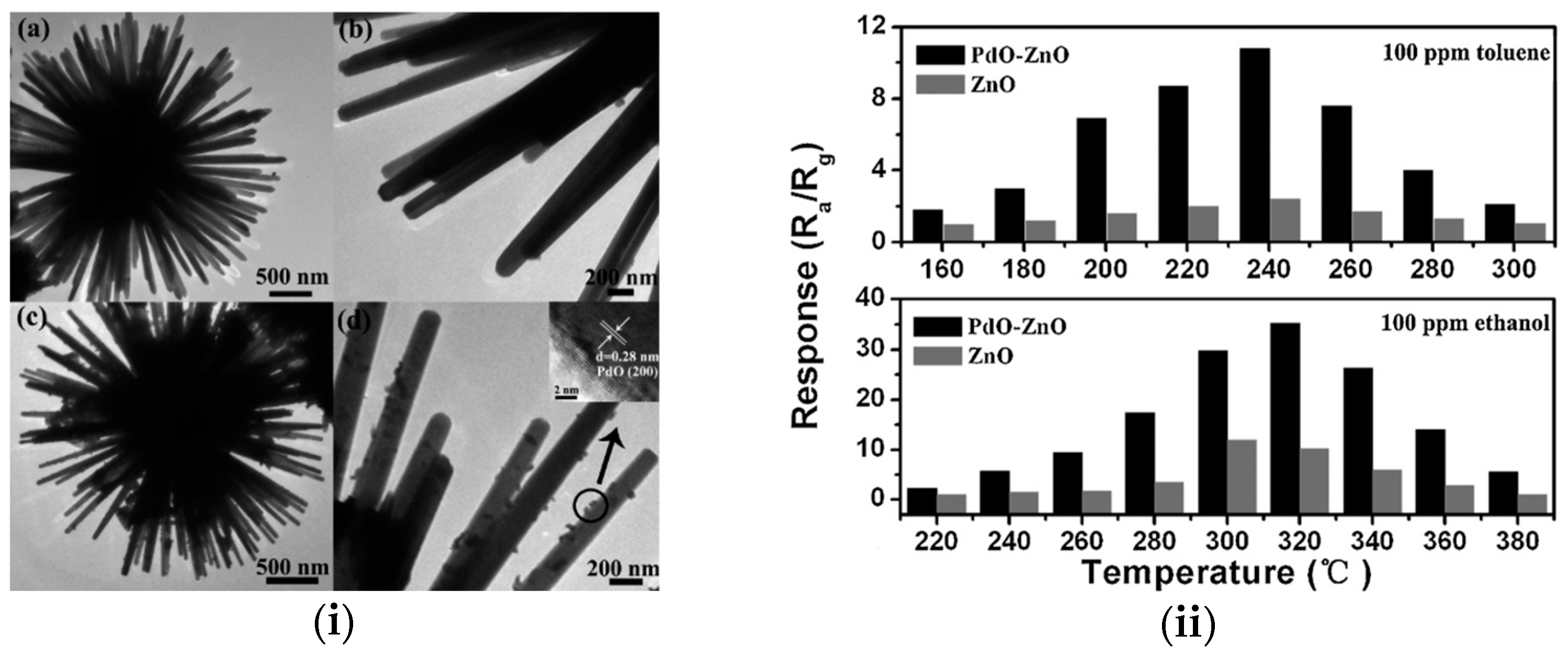
2.4. Coating Techniques
2.5. Chemical Vapor Deposition
2.6. Green Biosynthesis
3. Physical Routes of Pd and PdO Thin Films’ Growth
3.1. Sputtering and Cathodic Arc Deposition
3.2. Electron Beam Coating and Electron and Ion Beam-Induced Deposition
3.3. Pulsed Laser Deposition
3.4. Evaporation (Sublimation) of Pd
3.5. Supersonic Cluster-Beam Deposition
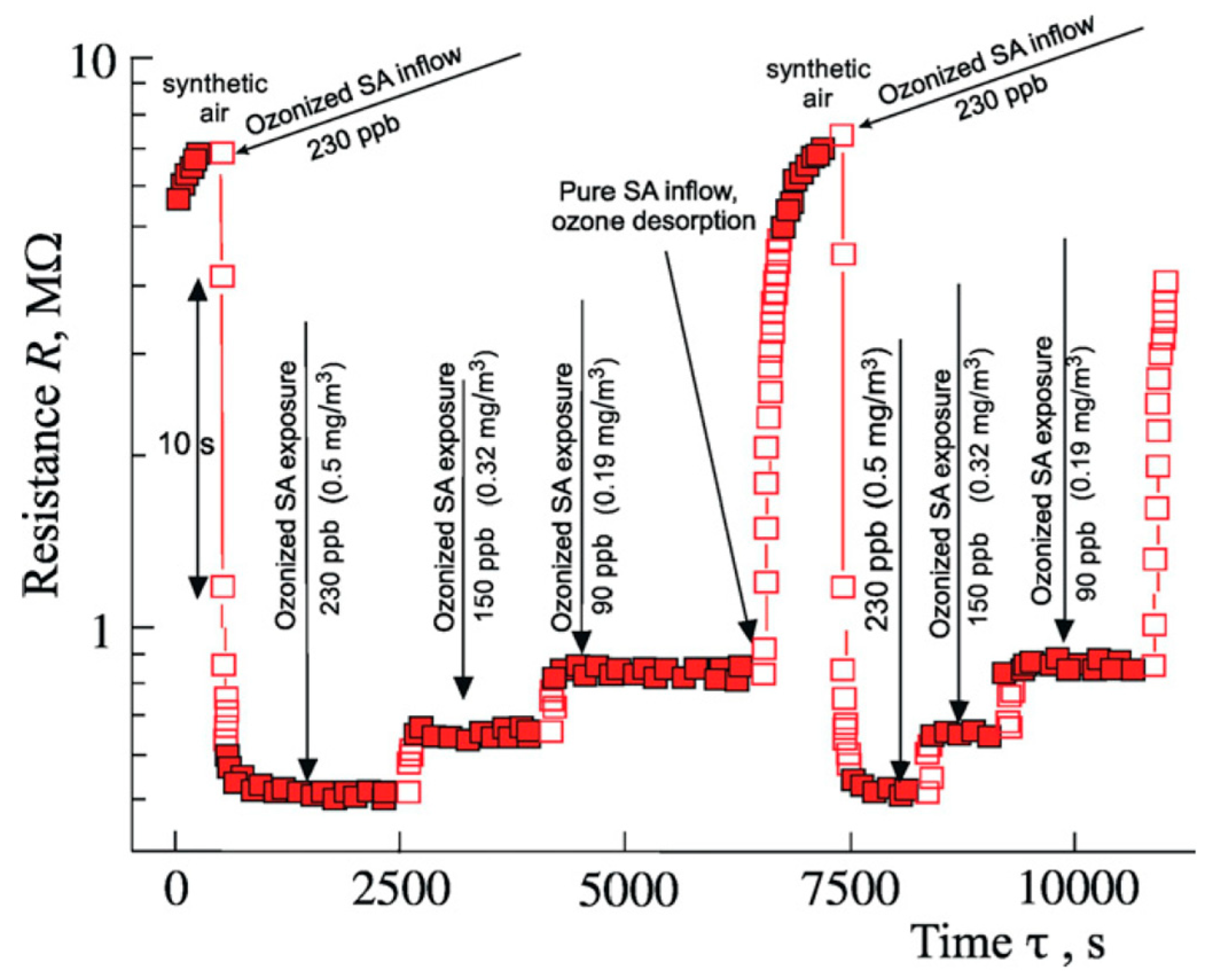
4. Some Practical Aspects of Pd Films’ Oxidation Toward PdO and Specific Issues Concerning Applications of the Films
- (i)
- Kinetics of the Pd oxidation (in the mbar pressure range) depends on the crystal surface: oxidation of Pd(110) proceeds at ~100 K lower temperatures than Pd(111) [213].
- (ii)
- The PdO layer formed as a skin on Pd is green when relatively thin [23]. When thicker (annealed in air at 800 °C), it turns gray.
- (iii)
- (iv)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IUPAC. Compendium of Chemical Terminology (The “Gold Book”), 2nd ed.; McNaught, A.D., Wilkinson, A., Eds.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar] [CrossRef]
- Swanson, H.E.; Tatge, E. Standard X-ray Diffraction Powder Patterns. Natl. Bur. Stand. Circ. 1955, 539, 27. [Google Scholar]
- Saldan, I.; Semenyuk, Y.; Marchuk, I.; Reshetnyak, O. Chemical synthesis and application of palladium nanoparticles. J. Mater. Sci. 2015, 50, 2337–2354. [Google Scholar] [CrossRef]
- Yamaguchi, S. Zur bildung von nichtstöchiometrischem palladiumoxyd. Mater. Chem. 1980, 5, 257–266. [Google Scholar] [CrossRef]
- Ismail, E.; Khenfouch, M.; Dhlamini, M.; Dube, S.; Maaza, M. Green palladium and palladium oxide nanoparticles synthesized via Aspalathus linearis natural extract. J. Alloys Compd. 2017, 695, 3632–3638. [Google Scholar] [CrossRef]
- Powers, D.C.; Ritter, T. Palladium (III) in Synthesis and Catalysis in Higher Oxidation State Organopalladium and Platinum Chemistry; Canty, A.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 129–156. [Google Scholar]
- Chen, W.Z.; Shimada, S.; Tanaka, M. Synthesis and structure of formally hexavalent palladium complexes. Science 2002, 295, 308–310. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, R.H. A new oxidation state for Pd? Science 2002, 295, 288–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hu, P. Identifying the general trend of activity of non-stoichiometric metal oxide phase for CO oxidation on Pd(111). Sci. China Chem. 2019, 62, 784–789. [Google Scholar] [CrossRef]
- Samoylov, A.M.; Gvarishvili, L.J.; Ivkov, S.A.; Pelipenko, D.I.; Badica, P. Two-stage synthesis of palladium (II) oxide nanocrystalline powders for gas sensor application. Res. Dev. Mater. Sci. 2018, 8, 857–863. [Google Scholar] [CrossRef]
- Edström, H.; Schaefer, A.; Jacobse, L.; von Allmen, K.; Hagman, B.; Carlsson, P.-A.; Gustafson, J. Alloying and oxidation of PdAu thin films. Thin Solid Film. 2024, 790, 140212. [Google Scholar] [CrossRef]
- Kreider, K.G.; Tarlov, M.J.; Cline, J.P. Sputtered thin-film pH electrodes of platinum. palladium, ruthenium, and iridium oxides. Sens. Actuators 1995, 28, 167–172. [Google Scholar] [CrossRef]
- Liu, C.-C.; Bocchicchio, D.B.; Overmyer, P.A.; Neuman, M.R. A Palladium–Palladium Oxide Miniature pH electrode. Science 1980, 207, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Grubb, W.T.; King, L.H. Palladium-Palladium oxide pH electrodes. Anal. Chem. 1980, 52, 270–273. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 1, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.-H. Pd-PdO pH microprobe for local pH measurement. Biotechnol. Bioeng. 1989, 34, 131–136. [Google Scholar] [CrossRef]
- Betteridge, W.; Rhys, D.W. First International Congress on Metallic Corrosion; Butterworths: London, UK; Woburn, MA, USA, 1962; pp. 186–192. [Google Scholar]
- Kinoshita, E.; Ingman, F.; Edwall, G. An examination of the Palladium/Palladium oxide system and its utility for pH-sensing electrodes. Electrochim. Acta 1986, 31, 29–38. [Google Scholar] [CrossRef]
- Eryürek, M.; Karadag, Y.; Taşaltın, N.; Kılınç, N.; Kiraz, A. Optical sensor for hydrogen gas based on a palladium-coated polymer microresonator. Sens. Actuators B Chem. 2015, 212, 78–83. [Google Scholar] [CrossRef]
- Lee, J.; Noh, J.S.; Lee, S.H.; Song, B.; Jung, H.; Kim, W.; Lee, W. Cracked palladium films on an elastomeric substrate for use as hydrogen sensors. Int. J. Hydrogen Energy 2012, 37, 7934–7939. [Google Scholar] [CrossRef]
- Si, L.; Yu, P.; Huang, J.; Zhao, Z.; Huang, M.; He, S.; Liu, H.; Wang, X.; Liu, W. Advances in gas-sensitive materials based on polyurethane film, foam, and fiber. Mater. Today Comm. 2024, 38, 108528. [Google Scholar] [CrossRef]
- Zhang, Y.-N.; Peng, H.; Qian, X.; Zhang, Y.; An, G.; Zhao, Y. Recent advancements in optical fiber hydrogen sensors. Sens. Actuators B 2017, 244, 393–416. [Google Scholar] [CrossRef]
- Coughlin, J.P. Contributions to the data on theoretical metallurgy XIII. Heats and free energies of formation of inorganic oxides,, US Bur. Mines Bull. 1954, 542, 35. [Google Scholar]
- Bloor, L.J.; Malcolme-Lawes, D.J. An electrochemical preparation of palladium oxide pH sensors. J. Electroanal. Chem. Interfacial Electrochem. 1990, 278, 161–173. [Google Scholar] [CrossRef]
- Chen, A.; Ostrom, C. Palladium-based materials: Synthesis and electrochemical applications. Chem. Rev. 2018, 115, 11999–12044. [Google Scholar] [CrossRef] [PubMed]
- Tri, P.N.; Ouellet-Plamondon, C.; Rtimi, S.; Assadi, A.A.; Nguyen, T.A. Methods for synthesis of hybrid nanoparticles. In Noble Metal—Metal Oxide Hybrid nanoparticles: Fundamentals and Applications; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 3; pp. 51–63. [Google Scholar]
- Sarkar, S.; Pal, T. Theoretical aspects of synthesis for controlled morphological Nanostructures. In Noble Metal—Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 2; pp. 7–50. [Google Scholar]
- Hu, T.; Wang, Y.; Liu, Q.; Zhang, L.; Wang, H.; Tang, T.; Chen, W.; Zhao, M.; Jia, J. In-situ synthesis of palladium-base binary metal oxide nanoparticles with enhanced electrocatalytic activity for ethylene glycol and glycerol oxidation. Int. J. Hydrogen Energy 2017, 42, 25951–25959. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Y.; Liu, Q.; Zhang, L.; Tang, T.; Xiao, H.; Chen, W.; Zhao, M.; Jia, J.; Zhu, H. Facile synthesis of PdO-doped Co3O4 nanoparticles as an efficient bifunctional oxygen electrocatalyst. Appl. Catal. B Environ. 2019, 243, 175–182. [Google Scholar] [CrossRef]
- Chung, M.; Maalouf, J.H.; Adams, J.S.; Jiang, C.; Román-Leshkov, Y.; Manthiram, K. Direct propylene epoxidation via water activation over Pd-Pt electrocatalysts. Science 2024, 383, 49–55. [Google Scholar] [CrossRef]
- Zhang, N.; He, C.; Jing, Y.; Qian, Y.; Toyao, T.; Shimizu, K.-I. Enhanced N2O decomposition on Rh/ZrO2 catalysts through the promotional effect of palladium. Surf. Interfaces 2024, 46, 104120. [Google Scholar] [CrossRef]
- Konrad, M.; Popescu-Pelin, G.; Socol, G.; Mardare, A.I.; Hassel, A.W. Combinatorial Analysis of Silver-Palladium Alloy Thin Film Libraries. Phys. Status Solidi A 2024, 2400355. [Google Scholar] [CrossRef]
- Wang, T.-J.; Sun, L.-B.; Ai, X.; Chen, P.; Chen, Y.; Wang, X. Boosting Formate Electrooxidation by Heterostructured PtPd Alloy and Oxides Nanowires. Adv. Mater. 2024, 36, 2403664. [Google Scholar] [CrossRef]
- Lokteva, E.S.; Pesotskiy, M.D.; Golubina, E.V.; Maslakov, K.I.; Kharlanov, A.N.; Shishova, V.V.; Kaplin, I.Y. Effect of Iron Content in Alumina-Supported Palladium Catalysts and Their Reduction Conditions on Diclofenac Hydrodechlorination in an Aqueous Medium. Kinet. Catal. 2024, 65, 133–154. [Google Scholar] [CrossRef]
- Huang, J.; Klahn, M.; Tian, X.; Bartling, S.; Zimina, A.; Radtke, M.; Rockstroh, N.; Naliwajko, P.; Steinfeldt, N.; Peppel, T.; et al. Fundamental Structural and Electronic Understanding of Palladium Catalysts on Nitride and Oxide Supports. Angew. Chem. Int. Ed. 2024, 63, e202400174. [Google Scholar] [CrossRef]
- Karczmarska, A.; Adamek, M.; El Houbbadi, S.; Kowalczyk, P.; Laskowska, M. Carbon-supported noble-metal nanoparticles for catalytic applications—A review. Crystals 2022, 12, 584. [Google Scholar] [CrossRef]
- Rodríguez-Otamendi, D.I.; Bizarro, M.; Meza-Laguna, V.; Álvarez-Zauco, E.; Rudolf, P.; Basiuk, V.A.; Basiuk, E.V. Eco-friendly synthesis of graphene oxide–palladium nanohybrids. Mater. Today Commun. 2023, 35, 106007. [Google Scholar] [CrossRef]
- Leve, Z.; Ross, N.; Pokpas, K.; Carleschi, E.; Doyle, B.P.; Sanga, N.A.; Mokwebo, K.V.; Iwuoha, E. Synthesis and Characterization of Palladium/Silver Modified Reduced Graphene Oxide–Nanocomposite Platform for Electrochemical Sensors. ChemistrySelect 2024, 9, e202400606. [Google Scholar] [CrossRef]
- Li, Z.; Xing, X.; Feng, D.; Du, L.; Tian, Y.; Chen, X.; Yang, D. Nitrogen-doped carbon microfibers decorated with palladium and palladium oxide nanoparticles for high-concentration hydrogen sensing. Ceram. Int. 2024, 50, 21519–21525. [Google Scholar] [CrossRef]
- Renjini, S.; Pillai, A.M.; Abraham, P.; Pavitha, P.A. Electrochemical synthesis of graphene oxide/palladium composite for the detection of norepinephrine in the presence of ascorbic acid and uric acid. Ionics 2024. [Google Scholar] [CrossRef]
- Feng, Y.; Cheng, G.; Wang, Z.; Wu, K.; Deng, A.; Li, J. Electrochemiluminescence immunosensor based on tin dioxide quantum dots and palladium-modified graphene oxide for the detection of zearalenone. Talanta 2024, 271, 125740. [Google Scholar] [CrossRef]
- Ndlovu, L.; Ndlwana, L.; Mishra, A.K.; Nxumalo, E.; Mishra, S.B. Imobilizing palladium nanoparticles in beta-cyclodextrin-grafted graphene oxide modified polyvinylidene fluoride mixed matrix membranes for the removal of anionic azo dyes. Chem. Eng. Res. Des. 2024, 203, 149–164. [Google Scholar] [CrossRef]
- Brusko, V.V.; Prytkova, A.; Kirsanova, M.; Vakhitov, I.; Sabirova, A.; Tayurskii, D.; Kadirov, M.; Dimiev, A.M. A copper–palladium/reduced graphene oxide composite as a catalyst for the oxygen reduction reaction. New J. Chem. 2024, 48, 4126–4136. [Google Scholar]
- Chauhan, A.S.; Kumar, A.; Bains, R.; Kumar, M.; Das, P. A comprehensive study of palladium-based catalysts on different supports for the hydrogenolysis of 5-hydroxymethylfurfural (HMF) to 2,5-dimethylfuran (DMF) biofuel. Biomass Bioenergy 2024, 185, 107209. [Google Scholar] [CrossRef]
- Pocklanová, R.; Warkad, I.R.; Prucek, R.; Balzerová, A.; Panácek, A.; Kadam, R.G.; Kvítek, L.; Gawande, M.B. Nanodiamond Supported Ultra-Small Palladium Nanoparticles as an Efficient Catalyst for Suzuki Cross-Coupling Reactions. Catalysts 2024, 14, 53. [Google Scholar] [CrossRef]
- van Oossanena, R.; Maierc, A.; Godarta, J.; Pignola, J.-P.; Denkovab, A.G.; van Rhoon, G.C.; Djanashvili, K. Magnetic hybrid Pd/Fe-oxide nanoparticles meet the demands for ablative thermo-brachytherapy. Int. J. Hyperth. 2024, 41, 2299480. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.C.-F.; Xuan, S. Noble metal-iron oxide hybrid nanomaterials: Emerging applications. Chem. Rec. 2016, 16, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, L.-X.; Luo, Y.; Zeng, Y.; Wang, S.; Wang, H. PdO/Pd-CeO2 holow spheres with fresh Pd surface for enhancing formic acid oxidation. Chem. Eng. J. 2018, 347, 193–201. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, W.; Zhao, M.; Xu, H.; Wang, J.; Chen, Y. Silicate-induced high-temperature-resistant small-crystallite ceria support enhancing palladium-catalyzed low-concentration methane combustion. Sep. Purif. Technol. 2025, 353, 128385. [Google Scholar] [CrossRef]
- Sivasankaran, S.; Kumar, M.J.K. Sonochemical synthesis of Pd-metal oxide hybrid nanoparticles. In Noble Metal—Metal Oxide Hybrid Nanoparticles: Fundamentals and Applications; Mohapatra, S., Nguyen, T.A., Nguyen-Tri, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 10; pp. 189–194. [Google Scholar]
- Veerakumar, P.; Sangili, A.; Chen, S.-M.; Kumar, R.S.; Arivalagan, G.; Firdhouse, M.J.; Hameed, K.S.; Sivakumar, S. Photocatalytic degradation of phenolic pollutants over palladium-tungsten trioxide nanocomposite. Chem. Eng. J. 2024, 489, 151127. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, N.; Bei, S.; Bao, K.; Xiang, M.; Yu, C.; Dong, S.; Qin, H. Single-atom palladium on nonstoichiometric tungsten oxide as bifunctional electrocatalyst for zinc-air battery. Electrochim. Acta 2024, 476, 143768. [Google Scholar] [CrossRef]
- Rani, R.; Sharma, M.; Sharma, S.; Chandra, R.; Malik, V.K. Palladium enhanced electrochemical supercapacitive performance of chromium oxide thin films synthesized by sputtering process. Thin Solid Film. 2024, 799, 140379. [Google Scholar] [CrossRef]
- Chen, Y.; Rana, R.; Zhang, Y.; Hoffman, A.S.; Huang, Z.; Vila, F.D.; Perez-Aguilar, J.E.; Hong, J.; Li, X.; Zeng, J.; et al. Dynamic structural evolution of MgO-supported palladium catalysts: From metal to metal oxide nanoparticles to surface then subsurface atomically dispersed cations. Chem. Sci. 2024, 15, 6454–6464. [Google Scholar] [CrossRef]
- Rüzgar, A.; Şener, L.; Karataş, Y.; Gülcan, M. Palladium (0) nanoparticles distributed on lanthanum (III) oxide as an effective catalyst for the methanolysis of hydrazine-borane to produce hydrogen. Turk. J. Chem. 2024, 48, 137–151. [Google Scholar] [CrossRef]
- Bekmezci, M.; Cibo, M.C.; Akin, M.; Poyraz, H.B.; Kaya, G.; Sen, F. Kaolin and zinc oxide supported PdCu catalysts as a superior catalyst in methanol oxidation reaction. Int. J. Hydrogen Energy 2024, 82, 456–463. [Google Scholar] [CrossRef]
- Hua, Y.; Vikrant, K.; Kim, K.-H.; Heynderickx, P.M.; Boukhvalov, D.W. Room temperature thermocatalytic removal of formaldehyde in air using a copper manganite spinel-supported palladium catalyst with ultralow noble metal content. Sep. Purif. Technol. 2025, 354, 128863. [Google Scholar] [CrossRef]
- Feng, M.; Wang, M.-Y.; Wang, F.; Xu, J.; Xue, B. Soft-templating synthesis of mesoporous MnSiOx composites as catalytic supports for Pd nanoparticles towards solvent-free oxidation of benzyl alcohol under atmospheric pressure O2. Appl. Catal. A Gen. 2024, 683, 119829. [Google Scholar] [CrossRef]
- Ibrahim, O.M. A Comparative Study of Platinum- Versus Palladium-Based Catalysts on FeCrAl-Sintered Metal Fiber Filter Substrate for Reducing Gaseous Diesel Engine Emissions. Emiss. Control. Sci. Technol. 2024. [Google Scholar] [CrossRef]
- Choi, J.; Nguyen, Q.T.; Park, S.; Ghule, B.G.; Park, J.H.; Park, J.R.; Nakate, U.T.; Jang, J.-H.; Kim, D.-W.; Park, S. Interfacially engineered palladium nanoparticle-decorated nickel oxide nanostructured electrocatalysts for high-performance hydrogen evolution reaction. Chem. Eng. J. 2024, 497, 154407. [Google Scholar] [CrossRef]
- Bhalothia, D.; Yan, C.; Hiraoka, N.; Ishii, H.; Liao, Y.-F.; Dai, S.; Chen, P.-C.; Chen, T.-Y. Iridium Single Atoms to Nanoparticles: Nurturing the Local Synergy with Cobalt-Oxide Supported Palladium Nanoparticles for Oxygen Reduction Reaction. Adv. Sci. 2024, 11, 2404076. [Google Scholar] [CrossRef]
- Yao, P.-C. Study of a New Hydrogen Sensor Based on the Synthesis of a Sputtered In–Sn–Zn-O Thin Film and Evaporated Palladium Nanoparticles. IEEE Trans. Electron Devices 2024, 71, 2612–2617. [Google Scholar] [CrossRef]
- Alrashdi, K.S.; Munshi, A.M.; Alrefaee, S.H.; Alalawy, A.I.; Abumelha, H.M.; Alamoudi, W.M. Miniaturization of Palladium and Gold in Nanosize to Hold Immense Potentiality for Application in Wool Functional Finishing. Fibers Polym. 2024, 25, 2555–2568. [Google Scholar] [CrossRef]
- Formenti, M.; Casaletto, M.P.; Barone, G.; Pagliaro, M.; Della Pina, C.; Butera, V.; Ciriminna, R. GrafeoPlad Palladium: Insight on Structure and Activity of a New Catalyst Series of Broad Scope. Adv. Sustain. Syst. 2024, 8, 2300643. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Z.; Xiao, M.; Zhang, C.; Ruan, L.; Zhang, Y.; Zhong, Y.; Yan, Y.; Yu, Y.; He, H. Catalytic performance of Pd catalyst supported on CeO2 or ZrO2 modified beta zeolite for methane oxidation. J. Environ. Sci. 2025, 152, 248–261. [Google Scholar] [CrossRef]
- Ni, F.; Lewis, R.J.; López-Martín, Á.; Smith, L.R.; Morgan, D.J.; Davies, T.E.; Taylor, S.H.; Hutchings, G.J. The direct synthesis of H2O2 and in situ oxidation of methane: An investigation into the role of the support. Catal. Today 2024, 442, 114910. [Google Scholar] [CrossRef]
- Catalano, M.; Carlino, E.; Tagliente, M.A.; Licciulli, A.; Tapfer, L. Microstructural and Microanalytical Characterization of Pd Clusters in ORMOCER Matrix. Microsc. Microanal. Microstruct. 1995, 6, 611–619. [Google Scholar] [CrossRef]
- Xiong, Y.; Xia, Y. Shape-controlled synthesis of metal nanostructures: The case of Palladium. Adv. Mater. 2007, 19, 3385–3391. [Google Scholar] [CrossRef]
- Choudhury, S.; Betty, C.A.; Bhattacharyya, K.; Saxena, V.; Bhattacharya, D. Nanostructured PdO Thin Film from Langmuir–Blodgett Precursor for Room-Temperature H2 Gas Sensing. ACS Appl. Mater. Interfaces 2016, 8, 16997–17003. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Wang, T.; Li, X.; Li, Q.; Zhang, X.; Cao, T.; Li, Y.; Zhang, L.; Guo, L.; Fu, Y. Pd-Decorated PdO Hollow Shells: A H2-Sensing System in Which Catalyst Nanoparticle and Semiconductor Support are Interconvertible. ACS Appl. Mater. Interfaces 2020, 12, 42971–42981. [Google Scholar] [CrossRef] [PubMed]
- Mhlongo, G.H.; Motaung, D.E.; Cummings, F.R.; Swart, H.C.; Ray, S.S. A highly responsive NH3 sensor based on Pd-loaded ZnO nanoparticles prepared via a chemical precipitation approach. Sci. Rep. 2019, 9, 9881. [Google Scholar] [CrossRef]
- Lupan, C.; Khaledialidusti, R.; Mishra, A.K.; Postica, V.; Terasa, M.-I.; Magariu, N.; Pauporté, T.; Viana, B.; Drewes, J.; Vahl, A.; et al. Pd-Functionalized ZnO:Eu Columnar Films for Room-Temperature Hydrogen Gas Sensing: A Combined Experimental and Computational Approach. ACS Appl. Mater. Interfaces 2020, 12, 24951–24964. [Google Scholar] [CrossRef]
- Xuan, J.; Zhao, G.; Sun, M.; Jia, F.; Wang, X.; Zhou, T.; Yin, G.; Liu, B. Low-temperature operating ZnO-based NO2 sensors: A review. RSC Adv. 2020, 10, 39786–39807. [Google Scholar] [CrossRef]
- Goodarzi, M.T.; Ranjbar, M. Atmospheric flame vapor deposition of WO3 thin films for hydrogen detection with enhanced sensing characteristics. Ceram. Int. 2020, 46, 21248–21255. [Google Scholar] [CrossRef]
- Castillo, C.; Cabello, G.; Chornik, B.; Huentupil, Y.; Buono-Core, G.E. Characterization of photochemically grown Pd loaded WO3 thin films and its evaluation as ammonia gas sensor. J. Alloys Compd. 2020, 825, 154166. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Mishra, Y.K.; Lin, L. Functional gas sensing nanomaterials: A panoramic view. Appl. Phys. Rev. 2020, 7, 021301. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, K.; Sun, X.; Duan, X.; Zhang, C.; Xu, X. Electrochemical sensor to environmental pollutant of acetone based on Pd-loaded on mesoporous In2O3 architecture. Sens. Actuators B Chem. 2019, 290, 217–225. [Google Scholar] [CrossRef]
- Zappa, D.; Galstyan, V.; Kaur, N.; Munasinghe Arachchige, H.M.M.; Sisman, O.; Comini, E. Metal oxide—Based heterostructures for gas sensors—A review. Anal. Chim. Acta 2018, 1039, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Huang, S.; Men, G.; Han, D.; Gu, F. Sensitization of Pd loading for remarkably enhanced hydrogen sensing performance of 3DOM WO3. Sens. Actuators B Chem. 2018, 262, 577–587. [Google Scholar] [CrossRef]
- Tian, J.; Wang, J.; Hao, Y.; Du, H.; Li, X. Toluene sensing properties of porous Pd-loaded flower-like SnO2 microspheres. Sens. Actuators B Chem. 2014, 202, 795–802. [Google Scholar] [CrossRef]
- Van Toan, N.; Chien, N.V.; Van Duy, N.; Hong, H.S.; Nguyen, H.; Hoa, N.D.; Van Hieu, N. Fabrication of highly sensitive and selective H2 gas sensor based on SnO2 thin film sensitized with microsized Pd islands. J. Hazard. Mater. 2016, 301, 433–442. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, J.; Zhang, Y.; Ma, S.; Xin, L.; Zhu, L.; Xu, C. Optical properties and photocatalytic performance of Pd modified ZnO samples. J. Phys. Chem. C 2009, 113, 18761–18767. [Google Scholar] [CrossRef]
- Hu, J.; Gao, F.; Sang, S.; Li, P.; Deng, X.; Zhang, W.; Chen, Y.; Lian, K. Optimization of Pd content in ZnO microstructures for high-perfomance gas detection. J. Mater. Sci. 2015, 50, 1935–1942. [Google Scholar] [CrossRef]
- Khan, T.M. Into the nature of Pd-dopant induced local phonon modes and associated disorders in ZnO based on spatial correlation model. Mater. Chem. Phys. 2015, 153, 248–255. [Google Scholar] [CrossRef]
- Mane, A.; Suryawanshi, M.; Kim, J.; Moholkar, A. Superior selectivity and enhanced response characteristics of Palladium sensitized Vanadium Pentoxide nanorods for detection of Nitrogen Dioxide gas. J. Colloid. Interf. Sci. 2017, 495, 53–60. [Google Scholar] [CrossRef]
- Trung, D.D.; Hoa, N.D.; Van Tong, P.; Van Duy, N.; Dao, T.; Chung, H.; Nagao, T.; Van Hieu, N. Effective decoration of Pd nanoparticles on the surface of SnO2 nanowires for enhancement of CO gas-sensing performance. Hazard. Mater. 2014, 265, 124–132. [Google Scholar] [CrossRef]
- Yi, Z.; Lou, Z.; Wang, L.; Zou, B. Enhanced Ammonia sensing performances of Pd-sensitized flowerlike ZnO nanostructure. Sens. Actuators B Chem. 2011, 156, 395–400. [Google Scholar]
- Jiao, M.; Van Duy, N.; Chien, N.V.; Hoa, N.D.; Van Hieu, N.; Hjort, K.; Nguyen, H. On-chip growth of patterned ZnO nanorod sensors with PdO decoration for enhancement of Hydrogen-sensing performance. Int. J. Hydrogen Energy 2017, 42, 16294–16304. [Google Scholar] [CrossRef]
- Ma, N.; Suematsu, K.; Yuasa, M.; Shimanoe, K. Pd Size effect on the gas sensing properties of Pd-loaded SnO2 in humid atmosphere. ACS Appl. Mater. Interfaces 2015, 7, 15618–15625. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhu, S.; Wu, G.; Jiao, F.; Li, W.; Wang, X.; An, Y.; Hu, Y.; Sun, J.; Dong, X.; et al. Highly sensitive H2 sensor based on PdO-decorated WO3 nanospindle p-n heterostructure. Int. J. Hydrogen Energy 2020, 45, 31327–31340. [Google Scholar] [CrossRef]
- Liu, M.; Cui, F.; Ma, Q.; Xu, L.; Zhang, J.; Zhang, R.; Cui, T. Janus coordination polymer derived PdO/ZnO nanoribbons for efficient 4-nitrophenol reduction. New J. Chem. 2020, 44, 4042–4048. [Google Scholar] [CrossRef]
- Rao, F.; Zhu, G.; Wang, M.; Zubairu, S.M.; Peng, J.; Gao, J.; Hojamberdiev, M. Constructing the Pd/PdO/β-Bi2O3 microspheres with enhanced photocatalytic activity for Bisphenol A degradation and NO removal. J. Chem. Technol. Biotechnol. 2020, 95, 862–874. [Google Scholar] [CrossRef]
- Lupan, O.; Postica, V.; Hoppe, M.; Wolff, N.; Polonskyi, O.; Pauporté, T.; Viana, B.; Majérus, O.; Kienle, L.; Faupel, F.; et al. PdO/PdO2 functionalized ZnO:Pd films for lower operating temperature H2 gas sensing. Nanoscale 2018, 10, 14107–14127. [Google Scholar] [CrossRef]
- Geng, X.; Luo, Y.; Zheng, B.; Zhang, C. Photon assisted room-temperature hydrogen sensors using PdO loaded WO3 nanohybrids. Int. J. Hydrogen Energy 2017, 42, 6425–6434. [Google Scholar] [CrossRef]
- Guo, P.; Wei, Z.; Ye, W.; Qin, W.; Wang, Q.; Guo, X.; Lu, C.; Zhao, X.S. Preparation and characterization of nanostructured Pd with high electrocatalytic activity. Colloids Surf. A 2012, 395, 75–81. [Google Scholar] [CrossRef]
- Adams, B.D.; Wu, G.; Nigro, S.; Chen, A. Facile Synthesis of Pd−Cd Nanostructures with High Capacity for Hydrogen Storage. J. Am. Chem. Soc. 2009, 131, 6930–6931. [Google Scholar] [CrossRef]
- Tahira, A.; Aftab, U.; Solangi, M.Y.; Gradone, A.; Morandi, V.; Medany, S.S.; Kasry, A.; Infantes-Molina, A.; Nafady, A.; Ibupoto, Z.H. Facile deposition of palladium oxide (PdO) nanoparticles on CoNi2S4 microstructures towards enhanced oxygen evolution reaction. Nanotechnology 2022, 33, 275402. [Google Scholar] [CrossRef] [PubMed]
- Arora, K.; Puri, N.K. Electrophoretically deposited nanostructured PdO thin film for room temperature amperometric H2 sensing. Vacuum 2018, 154, 302–308. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Wang, R.; Fei, T.; Zhang, T. Ring-like PdO–NiO with lamellar structure for gas sensor application. J. Mater. Chem. 2012, 22, 12453. [Google Scholar] [CrossRef]
- Wang, H.; Xu, C.; Cheng, F.; Jiang, S. Pd nanowire arrays as electrocatalysts for ethanol electrooxidation. Electrochem. Commun. 2007, 9, 1212–1216. [Google Scholar] [CrossRef]
- Cui, C.-H.; Yu, J.-W.; Li, H.-H.; Gao, M.-R.; Liang, H.-W.; Yu, S.-H. Remarkable enhancement of electrocatalytic activity tuning the interface of Pd- Au bimetallic nanoparticle tubes. ACS Nano 2011, 5, 4211–4218. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yoo, S.-H.; Lee, S.A.; Park, S. Wet-chemical synthesis of Palladium nanosprings. Nano Lett. 2011, 11, 3979–3982. [Google Scholar] [CrossRef]
- Elezovic, N.R.; Lovic, J.D.; Jovic, B.M.; Zabinski, P.; Włoch, G.; Jovic, V.D. Synthesis and characterization of AgPd alloy coatings as beneficial catalysts for low temperature fuel cells application. Electrochim. Acta 2019, 307, 360–368. [Google Scholar] [CrossRef]
- Qiu, C.; Dong, X.; Huang, M.; Wang, S.; Ma, H. Facile fabrication of nanostructured Pd-Fe bimetallic thin films and their electrodechlorination activity. J. Mol. Catal. A Chem. 2011, 350, 56–63. [Google Scholar] [CrossRef]
- Ho, P.H.; Ambrosetti, M.; Groppi, G.; Tronconi, E.; Jaroszewicz, J.; Ospitali, F.; Rodríguez-Castellón, E.; Fornasari, G.; Vaccari, A.; Benito, P. One-step electrodeposition of Pd-CeO2 on high pore density foams for environmental catalytic processes. Catal. Sci. Technol. 2018, 8, 4678–4689. [Google Scholar] [CrossRef]
- Ryabtsev, S.V.; Ievlev, V.M.; Samoylov, A.M.; Kuschev, S.B.; Soldatenko, S.A. Microstructure and electrical properties of palladium oxide thin films for oxidizing gases detection. Thin Solid Film. 2017, 636, 751–759. [Google Scholar] [CrossRef]
- Chen, R.; Ruan, X.; Liu, W.; Stefanini, C. A reliable and fast hydrogen gas leakage detector based on irreversible cracking of decorated palladium nanolayer upon aligned polymer fibers. Int. J. Hydrogen Energy 2015, 40, 746–751. [Google Scholar] [CrossRef]
- Hu, R.-J.; Wang, J.; Zhu, H.-C. Preparation and Gas Sensing Properties of PdO. Au, CdO Coatings on SnO2 Nanofibers, Acta Phys.-Chim. Sin. 2015, 31, 1997–2004. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, H.-C.; Liu, J.; Huang, C.Z.; Xia, Y. Use of reduction rate as a quantitative knob for controlling the twin structure and shape of Palladium nanocrystals. Nano Lett. 2015, 15, 1445–1450. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Choi, S.-L.; Zhao, X.; Xie, S.; Peng, H.-C.; Chi, M.; Huang, C.Z.; Xia, Y. Polyol synthesis of ultrathin Pd nanowires via attachment-based growth and their enhanced activity towards formic acid oxidation. Adv. Funct. Mater. 2014, 24, 131–139. [Google Scholar] [CrossRef]
- Huang, X.; Tang, S.; Yang, J.; Tan, Y.; Zheng, N. Etching growth under surface confinement: An effective strategy to prepare mesocrystalline Pd nanocorolla. J. Am. Chem. Soc. 2011, 133, 15946–15949. [Google Scholar] [CrossRef]
- Raghuveer, V.; Ferreira, P.; Manthiram, A. Comparison of Pd-Co-Au electrocatalysts prepared by conventional borohydrideand microemulsion methods for oxygen reduction in fuel cells. Electrochem. Commun. 2006, 8, 807–814. [Google Scholar] [CrossRef]
- Yuasa, M.; Masaki, T.; Kida, T.; Shimanoe, K.; Yamazoe, N. Nano-sized PdO loaded SnO2 nanoparticles by reverse micelle method for highly sensitive CO gas sensor. Sens. Actuators B Chem. 2009, 136, 99–104. [Google Scholar] [CrossRef]
- Tian, M.; Malig, M.; Chen, S.; Chen, A. Synthesis and electrochemical study of TiO2-supported PdAu nanoparticles. Electrochem. Commun. 2011, 13, 370–373. [Google Scholar] [CrossRef]
- Wei, M.; Li, H.; Guo, G.; Liu, Y.; Zhang, D. Effects of PdO modification on the performance of La0.6Sr0.4Co0.2Fe0.8O3−δ cathodes for solid oxide fuel cells: A first principle study. Int. J. Hydrogen Energy 2017, 42, 23180–23188. [Google Scholar] [CrossRef]
- Qin, Y.; Alam, A.U.; Pan, S.; Howlander, M.M.R.; Ghosh, R.; Selvaganapathy, P.R.; Wu, Y.; Deen, M.J. Low-temperature solution processing of palladium/palladium oxide films and their pH sensing performance. Talanta 2016, 146, 517–524. [Google Scholar] [CrossRef]
- Kabcum, S.; Channel, D.; Tuantranont, A.; Wisitsoraat, A.; Liewhiran, C.; Phanichphant, S. Ultra-responsive hydrogen gas sensors based on PdO nanoparticle-decorated WO3 nanorods synthesized via precipitation and impregnation methods. Sens. Actuators B Chem. 2016, 226, 76–89. [Google Scholar] [CrossRef]
- Balamurugan, C.; Jeong, Y.J.; Lee, D.W. Enhanced H2S sensing performance of a p-type semiconducting PdO-NiO nanoscale heteromixture. Appl. Surf. Sci. 2017, 420, 638–650. [Google Scholar] [CrossRef]
- Jin, J.; Li, C.; Tsang, C.-W.; Xu, B.; Liang, C. Catalytic combustion of methane over Pd/Ce–Zr oxides washcoated monolithic catalysts under oxygen lean conditions. RSC Adv. 2015, 5, 102147–102156. [Google Scholar] [CrossRef]
- Wei, Y.; Ji, J.; Liang, F.; Ma, D.; Du, Y.; Pang, Z.; Wang, H.; Li, Q.; Shi, G.; Wang, Z. Adjusting active sites and metal-support interactions of ceramic-loaded Pd/P-CeO2-Al2O3 coating to optimize CO2 methanation pathways. J. Environ. Chem. Eng. 2023, 11, 110773. [Google Scholar] [CrossRef]
- Lou, Z.; Deng, J.; Wang, L.; Wang, L.; Fei, T.; Zhang, T. Toluene and ethanol sensing performances of pristine and PdO-decorated flower-like ZnO structures. Sens. Actuators B Chem. 2013, 176, 323–329. [Google Scholar] [CrossRef]
- Preus, A.; Korb, M.; Ruffer, T.; Bankwitz, J.; Geogi, C.; Jakob, A.; Schulz, S.E.; Lang, H. A β-ketoiminato palladium(II) complex for palladium deposition. Z. Für Naturforschung B 2019, 74, 901–912. [Google Scholar] [CrossRef]
- Kim, I.J.; Han, S.D.; Singh, I.; Lee, H.D.; Wang, J.S. Sensitivity enhancement for CO gas detection using a SnO2–CeO2–PdOx system. Sens. Actuators B Chem. 2005, 107, 825–830. [Google Scholar] [CrossRef]
- Qin, Y.; Alam, A.U.; Howlader, M.; Hu, N.-X.; Deen, M.J. Morphology and electrical properties of inkjet-printed palladium/palladium oxide. J. Mater. Chem. C 2017, 5, 1893–1902. [Google Scholar] [CrossRef]
- Garcia, J.R.V.; Goto, T. Chemical vapor deposition of iridium, platinum, rhodium, and palladium. Mater. Trans. 2003, 44, 1717. [Google Scholar] [CrossRef]
- Wang, L.; Griffin, G.L. Batch CVD Process for Depositing Pd Activation Layers. J. Electrochem. Soc. 2007, 154, D151. [Google Scholar] [CrossRef]
- Guerrero, R.M.; Hernández-Gordillo, A.; Santes, V.; García, J.R.V.; Escobar, J.; Díaz-García, L.; Arceo, L.D.B.; Febles, V.G. Monometallic Pd and Pt and Bimetallic Pd-Pt/Al2O3-TiO2 for the HDS of DBT: Effect of the Pd and Pt Incorporation Method. J. Chem. 2014, 2014, 679281. [Google Scholar]
- Belousov, O.V.; Tarabanko, V.E.; Borisov, R.V.; Simakova, I.L.; Zhyzhaev, A.M.; Tarabanko, N.; Isakova, V.G.; Parfenov, V.V.; Ponomarenko, I.V. Synthesis and catalytic hydrogenation activity of Pd and bimetallic Au–Pd nanoparticles supported on high-porosity carbon materials. React. Kinet. Catal. Lett. 2019, 127, 25–39. [Google Scholar] [CrossRef]
- Krisyuk, V.V.; Turgambaeva, A.E.; Mirzaeva, I.V.; Urkasymkyzy, S.; Koretskaya, T.P.; Trubin, S.V.; Sysoev, S.V.; Shubin, Y.V.; Maksimovskiy, E.A.; Petrova, N.I. MOCVD Pd–Cu alloy films from single source heterometallic precursors. Vacuum 2019, 166, 248–254. [Google Scholar] [CrossRef]
- Ehsan, M.A.; Sohail, M.; Jamil, R.; Hakeem, A.S. Single-step fabrication of nanostructured Palladium thin films via aerosol-assisted chemical vapor deposition (AACVD) for the electrochemical detection of hydrazine. Electrocatalysis 2019, 10, 214–221. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Roso, S.; Haddi, Z.; Vallejos, S.; Umek, P.; Bittencourt, C.; Blackman, C.; Vilic, T.; Llobet, E. p-Type PdO nanoparticles supported on n-type WO3 nanoneedles for hydrogen sensing. Thin Solid Film. 2016, 618, 238–245. [Google Scholar] [CrossRef]
- Annanouch, F.E.; Haddi, Z.; Ling, M.; Di Maggio, F.; Vallejos, S.; Vilic, T.; Zhu, Y.; Shujah, T.; Umek, P.; Bittencourt, C.; et al. Aerosol-Assisted CVD-Grown PdO Nanoparticle-Decorated Tungsten Oxide Nanoneedles Extremely Sensitive and Selective to Hydrogen. ACS Appl. Mater. Interfaces 2016, 8, 10413–10421. [Google Scholar] [CrossRef]
- Alvarado, M.; De La Flor, S.; Llobet, E.; Romero, A.; Ramírez, J.L. Performance of Flexible Chemoresistive Gas Sensors after Having Undergone Automated Bending Tests. Sensors 2019, 19, 5190. [Google Scholar] [CrossRef]
- Ling, M.; Blackman, C.S. Gas-phase synthesis of hybrid nanostructured materials. Nanoscale 2018, 10, 22981–22989. [Google Scholar] [CrossRef]
- Kapica, R.; Redzynia, W.; Tyczkowski, J. Characterization of Palladium-based Thin Films Prepared by Plasma-enhanced Metalorganic Chemical Vapor Deposition. Mater. Sci. 2012, 18, 128–131. [Google Scholar] [CrossRef]
- Lang, H.; Dietrich, S. Comprehensive Inorganic Chemistry II: From Elements to Applications, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 4, pp. 211–269. [Google Scholar]
- Hierso, J.-C.; Feurer, R.; Kalck, P. Platinum, palladium and rhodium complexes as volatile precursors for depositing materials. Coord. Chem. Rev. 1998, 178–180, 1811–1834. [Google Scholar] [CrossRef]
- Assim, K.; Melzer, M.; Korb, M.; Rüffer, T.; Jakob, A.; Noll, J.; Georgi, C.; Schulz, S.E.; Lang, H. Bis (β-diketonato)-and allyl-(β-diketonato)-palladium (II) complexes: Synthesis, characterization and MOCVD application. RSC Adv. 2016, 6, 102557–102569. [Google Scholar] [CrossRef]
- Gozum, J.E.; Pollina, D.M.; Jensen, J.A.; Girolami, G.S. “Tailored” organometallics as precursors for the chemical vapor deposition of high-purity palladium and platinum thin films. J. Am. Chem. Soc. 1988, 110, 2688–2689. [Google Scholar] [CrossRef]
- Henc, B.; Jolly, P.W.; Salz, R.; Stobbe, S.; Wilke, G.; Benn, R.; Mynott, R.; Seevogel, K.; Goddard, R.; Krüger, C. Transition metal allyls: IV. The (η3-allyl)2M complexes of nickel, palladium and platinum: Reaction with tertiary phosphines. J. Organomet. Chem. 1980, 191, 449–475. [Google Scholar] [CrossRef]
- Yuan, Z.; Puddephatt, R.J. Allyl(β-diketonato)palladium(II) complexes as precursors for palladium films. Adv. Mater. 1994, 6, 51–54. [Google Scholar] [CrossRef]
- Nikolaeva, N.S.; Kuratieva, N.V.; Vikulova, E.S.; Stabnikov, P.A.; Morozova, N.B. Volatile asymmetric fluorinated (O^N)-chelated palladium complexes: From ligand sources to MOCVD application. Polyhedron 2019, 171, 455–463. [Google Scholar] [CrossRef]
- Aaltonen, T.; Ritala, M.; Tung, Y.L.; Chi, Y.; Arstila, K.; Meinander, K.; Leskelä, M. Atomic layer deposition of noble metals: Exploration of the low limit of the deposition temperature. J. Mater. Res. 2004, 19, 3353–3358. [Google Scholar] [CrossRef]
- Liu, Y.H.; Cheng, Y.C.; Tung, Y.L.; Chi, Y.; Chen, Y.L.; Liu, C.S.; Peng, S.M.; Lee, G.H. Synthesis and characterization of fluorinated β-ketoiminate and imino-alcoholate Pd complexes: Pre-cursors for palladium chemical vapor deposition. J. Mater. Chem. 2003, 13, 135–142. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Stabnikov, P.A.; Baidina, I.A.; Smolentsev, A.I.; Tkachev, S.V. Synthesis, properties and crystal structures of volatile β-ketoiminate Pd complexes, precursors for palladium chemical vapor deposition. Polyhedron 2009, 28, 2307–2312. [Google Scholar] [CrossRef]
- Zharkova, G.I.; Sysoev, S.V.; Stabnikov, P.A.; Logvinenko, V.A.; Igumenov, I.K. Vapor pressure and crystal lattice energy of volatile palladium(II) β-iminoketonates. J. Therm. Anal. Calorim. 2011, 103, 381–385. [Google Scholar] [CrossRef]
- Vikulova, E.S.; Cherkasov, S.A.; Nikolaeva, N.S.; Smolentsev, A.I.; Sysoev, S.V.; Morozova, N.B. Thermal behavior of volatile palladium(II) complexes with tetradentate Schiff bases containing propylene-diimine bridge. J. Therm. Anal. Calorim. 2019, 135, 2573–2582. [Google Scholar] [CrossRef]
- Manjunatha, K.B.; Dileep, R.; Umesh, G.; Bhat, B.R. Nonlinear optical and all-optical switching studies of palladium(II) complex. Mater. Lett. 2013, 105, 173–176. [Google Scholar] [CrossRef]
- Tung, Y.-L.; Tseng, W.-C.; Lee, C.-Y.; Hsu, P.-F.; Chi, Y.; Peng, S.-M.; Lee, G.-H. Synthesis and Characterization of Allyl(β-ketoiminato)palladium(II) Complexes: New Precursors for Chemical Vapor Deposition of Palladium Thin Films. Organometallics 1999, 18, 864–869. [Google Scholar] [CrossRef]
- Giebelhaus, I.; Müller, R.; Tyrra, W.; Pantenburg, I.; Fischer, T.; Mathur, S. First air stable tin(II) β-heteroarylalkenolate: Synthesis. characterization and application in chemical vapor deposition. Inorg. Chim. Acta 2011, 372, 340–346. [Google Scholar] [CrossRef]
- Kuratieva, N.V.; Vikulova, E.S.; Shushanyan, A.D.; Nikolaeva, N.S.; Dorovskikh, S.I.; Mikhaleva, N.S.; Morozova, N.B. Structure of Cu(II) and Pd(II) complexes with 2-(2,2-dimethylhydrazone)pentanone-4. J. Struct. Chem. 2017, 58, 1004. [Google Scholar] [CrossRef]
- Senkevich, J.J.; Tang, F.; Rogers, D.; Drotar, J.T.; Jezewski, C.; Lanford, W.A.; Wang, G.-C.; Lu, T.-M. Substrate-independent palladium atomic layer deposition. Chem. Vap. Depos. 2003, 9, 258–264. [Google Scholar] [CrossRef]
- Goldstein, D.N.; George, S.M. Enhancing the nucleation of palladium atomic layer deposition on Al2O3 using trimethylaluminum to prevent surface poisoning by reaction products. Appl. Phys. Lett. 2009, 95, 143106. [Google Scholar] [CrossRef]
- Achari, I.; Ambrozik, S.; Dimitrov, N. Electrochemical Atomic Layer Deposition of Pd Ultrathin Films by Surface Limited Redox Replacement of Underpotentially Deposited H in a Single Cell. J. Phys. Chem. C 2017, 121, 4404–4411. [Google Scholar] [CrossRef]
- Nallan, H.C.; Yang, X.; Coffey, B.M.; Dolocan, A.; Ekerdt, J.G. Area-selective atomic layer deposition of palladium. J. Vac. Sci. Technol. A 2024, 42, 022401. [Google Scholar] [CrossRef]
- Cao, T.Y.; Wang, C.Y.; Shan, K.; Vohs, J.M.; Gorte, R.J. Investigation into support effects for Pt and Pd on LaMnO3. Appl. Catal. A-Gen. 2022, 646, 118873. [Google Scholar] [CrossRef]
- Lausecker, C.; Munoz-Rojas, D.; Webber, M. Atomic layer deposition (ALD) of palladium: From processes to applications. Crit. Rev. Solid State Mater. Sci. 2024, 49, 908–930. [Google Scholar] [CrossRef]
- Feng, J.; He, L.; Hui, J.Q.; Kavithaa, K. Synthesis of Bimetallic Palladium/Zinc Oxide Nanocomposites Using Crocus sativus and Its Anticancer Activity via the Induction of Apoptosis in Cervical Cancer. Appl. Biochem. Biotechnol. 2024. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekharan, B.; Sampatkumar, H.G.; George, D.; Antony, A.M.; Doddamani, S.V.; Sasidhar, B.S.; Balakrishna, R.G.; Patil, S.A. Palladium nanoparticle immobilized on coconut coir extract coated boron carbon nitride: A green and sustainable nanocatalyst for cross-coupling reactions and HER studies. Diam. Relat. Mater. 2024, 147, 111261. [Google Scholar] [CrossRef]
- Tiri, R.N.E.; Aygun, A.; Bekmezci, M.; Gonca, S.; Ozdemir, S.; Kaymak, G.; Karimi-Maleh, H.; Sen, F. Environmental Energy Production and Wastewater Treatment Using Synthesized Pd Nanoparticles with Biological and Photocatalytic Activity. Top. Catal. 2024, 67, 714–724. [Google Scholar] [CrossRef]
- Naveenkumar, S.; Kamaraj, C.; Prem, P.; Raja, R.K.; Priyadharsan, A.; Alrefaei, A.F.; Govindarajan, R.-V.K.; Thamarai, R.; Subramaniyan, V. Eco-friendly synthesis of palladium nanoparticles using Zaleya decandra: Assessing mosquito larvicidal activity, zebrafish embryo developmental toxicity, and impacts on freshwater sludge worm Tubifex tubifex. J. Environ. Chem. Eng. 2024, 12, 111912. [Google Scholar] [CrossRef]
- Griffith, W.P.; Robinson, S.D.; Swars, K. Palladium and Oxygen. In Pd Palladium. Gmelin Handbook of Inorganic Chemistry/Gmelin Handbuch der Anorganischen Chemie; Griffith, W.P., Swars, K., Eds.; Springer: Berlin/Heidelberg, Germany, 1989; Volume P-d/B/2. [Google Scholar] [CrossRef]
- Matsushima, T. Micro-Fabrication by Sputtering in Handbook of Sputtering Technology, 2nd ed.; Wasa, K., Kanno, I., Kotera, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 597–622. [Google Scholar]
- Rossnagel, S.M.; Hopwood, J. Magnetron sputter deposition with high levels of metal ionization. Appl. Phys. Lett. 1993, 63, 3285–3287. [Google Scholar] [CrossRef]
- Yoon, J.-H.; Kim, B.-J.; Kim, J.-S. Design and fabrication of micro hydrogen gas sensors using palladium thin film. Mater. Chem. Phys. 2012, 133, 987–991. [Google Scholar] [CrossRef]
- Joshi, R.K.; Krishnan, S.; Yoshimura, M.; Kumar, A. Pd nanoparticles and thin films for room temperature hydrogen sensor. Nanoscale Res. Lett. 2009, 4, 1191–1196. [Google Scholar] [CrossRef]
- Hao, M.; Wu, S.; Zhou, H.; Ye, W.; Wei, X.; Wang, X.; Chen, Z.; Li, S. Room-temperature and fast response hydrogen sensor based on annealed nanoporous palladium film. J. Mater. Sci. 2016, 51, 2420–2426. [Google Scholar] [CrossRef]
- Rafieerad, A.R.; Bushroa, A.R.; Nasiri-Tabrizi, B.; Vadivelu, J.; Baradaran, S.; Mesbah, M.; Zavareh, M.A. Mechanical properties, corrosion behavior and in-vitro bioactivity of nanostructured Pd/PdO coating on Ti–6Al–7Nb implant. Mater. Des. 2016, 103, 10–24. [Google Scholar] [CrossRef]
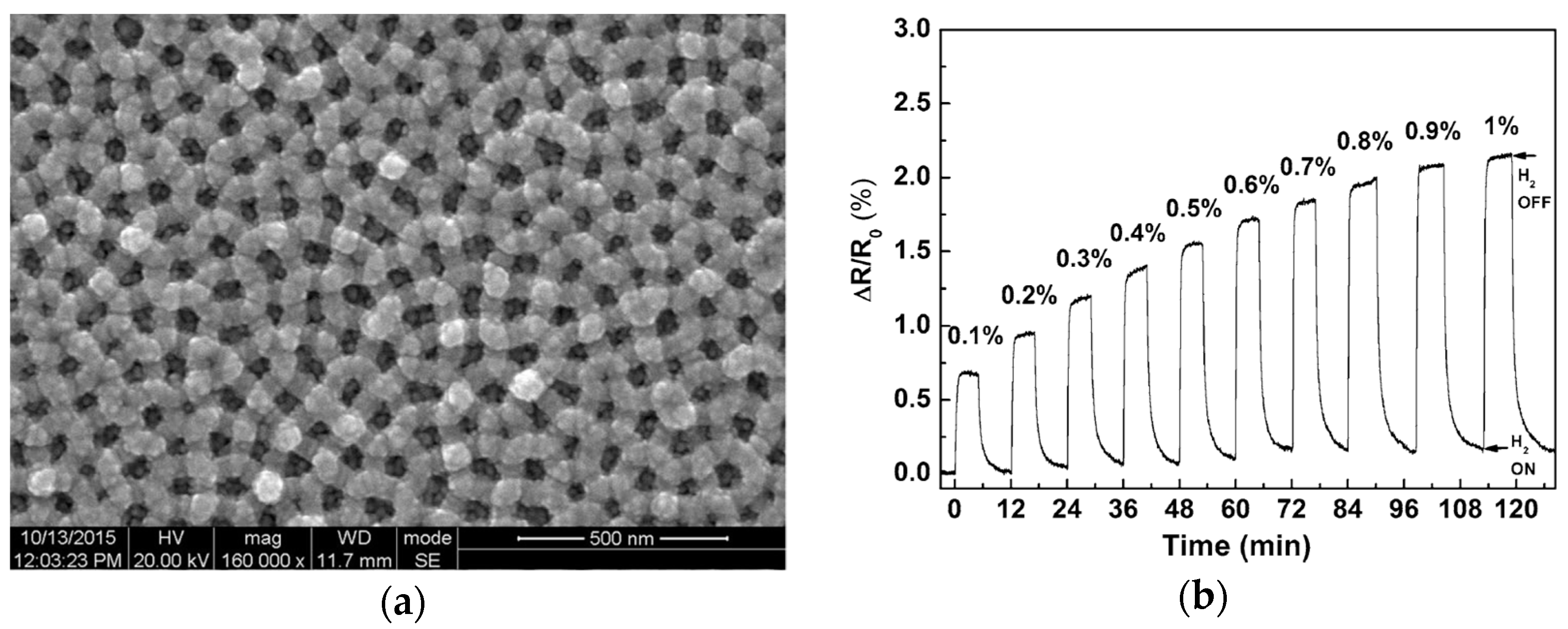
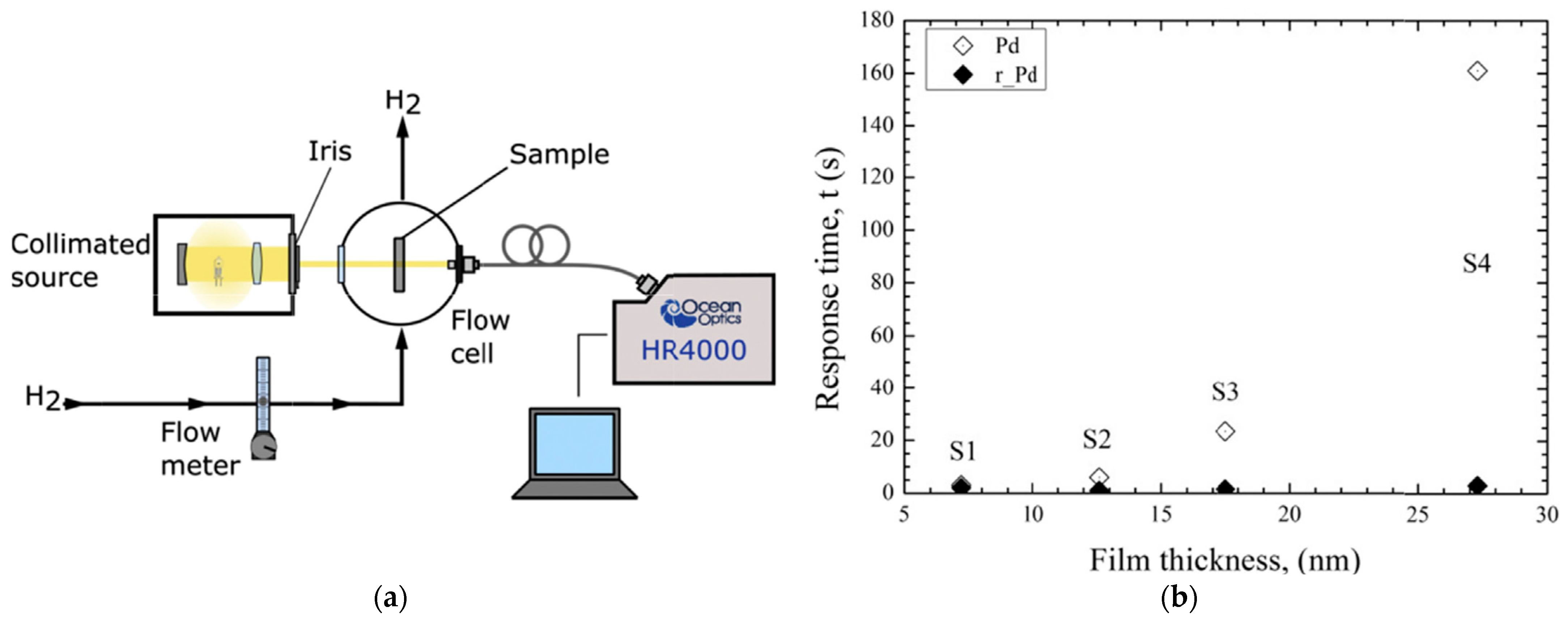
- Hassan, E.S.; Abd, A.N.; Habubi, N.F.; Mansour, H.L. Sensing properties controlled by thickness variable of palladium oxide synthesized by RF-reactive sputtering. Optik 2018, 174, 481–488. [Google Scholar] [CrossRef]
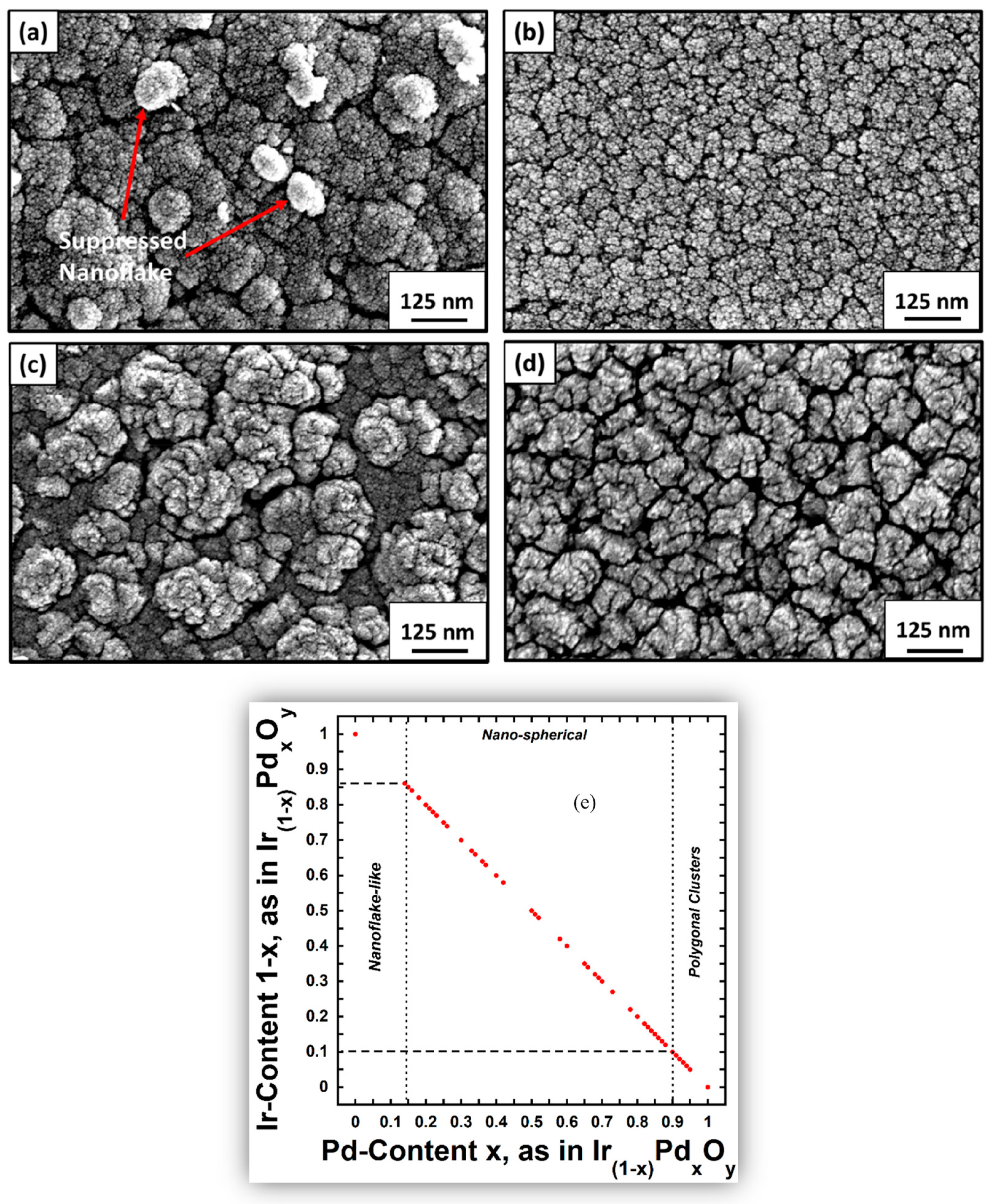
- Chiang, Y.-J.; Pan, F.-M. PdO nanoflake thin films for CO gas sensing at low temperatures. J. Phys. Chem. C 2013, 117, 15593–15601. [Google Scholar] [CrossRef]
- Arai, T.; Shima, T.; Nakano, T.; Tominaga, J. Thermally-induced optical property changes of sputtered PdOx films. Thin Solid Film. 2007, 515, 4774–4777. [Google Scholar] [CrossRef]
- Ketteler, G.; Ogletree, D.F.; Bluhm, H.; Liu, H.; Hebenstreit, E.L.; Salmeron, M. In Situ Spectroscopic Study of the Oxidation and Reduction of Pd(111). J. Am. Chem. Soc. 2005, 127, 18269–18273. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-J.; Li, K.-C.; Lin, Y.-C.; Pan, F.-M. A mechanistic study of hydrogen gas sensing by PdO nanoflake thin films at temperatures below 250 °C. Phys. Chem. Chem. Phys. 2015, 17, 3039–3049. [Google Scholar] [CrossRef]
- Thieu, C.-A.; Hong, J.; Kim, H.; Yoon, K.J.; Lee, J.-H.; Kim, B.-K.; Son, J.-W. Incorporation of a Pd catalyst at the fuel electrode of a thin-film-based solid oxide cell by multi-layer deposition and its impact on low-temperature co-electrolysis. J. Mater. Chem. A 2017, 5, 7433–7444. [Google Scholar] [CrossRef]
- Nguyen, D.-C.; Chu, C.-C.; Lee, C.-H.; Hsu, T.; Chang, C.-S. Fabrication and tailoring of the nano-scale textures of Pd films by selective doping for hydrogen gas sensing. Thin Solid Film. 2016, 616, 722–727. [Google Scholar] [CrossRef]
- Lee, Y.T.; Lee, J.M.; Kim, Y.J.; Joe, J.H.; Lee, W. Hydrogen gas sensing properties of PdO thin films with nano-sized cracks. Nanotechnology 2010, 21, 165503. [Google Scholar] [CrossRef]
- Sui, M.; Kunwar, S.; Pandey, P.; Zhang, Q.; Li, M.-Y. Fabrication and determination of growth regimes of various Pd NPs based on the control of deposition amount and temperature on c-plane GaN. J. Mater. Res. 2017, 32, 3593–3604. [Google Scholar] [CrossRef]
- Horwat, D.; Zakharov, D.I.; Endrino, J.L.; Soldera, F.; Anders, A.; Migot, S.; Karoum, R.; Vernoux, P.; Pierson, J.F. Chemistry, phase formation, and catalytic activity of thin palladium-containing oxide films synthesized by plasma-assisted physical vapor deposition. Surf. Coat. Technol. 2011, 205, S171–S177. [Google Scholar] [CrossRef]
- Anders, A. A review comparing cathodic arcs and high power impulse magnetron sput-tering (HiPIMS). Surf. Coat. Technol. 2014, 257, 308–325. [Google Scholar] [CrossRef]
- Amin-Ahmadi, B.; Idrissi, H.; Galceran, M.; Colla, M.S.; Raskin, J.P.; Pardoen, T.; Godet, S.; Schyvers, D. Effect of deposition rate on the microstructure of electron beam evaporated nanocrystalline palladium thin films. Thin Solid Film. 2013, 539, 145–150. [Google Scholar] [CrossRef]
- Barzola-Quiquia, J.; Schulze, S.; Esquinazi, P. Transport properties and atomic structure of ion-beam deposited W. Pd, and Pt nanostructures. Nanotechnology 2009, 20, 165704–165711. [Google Scholar] [CrossRef] [PubMed]
- Bhuvana, T.; Kulkarni, G.U. Highly conducting patterned Pd nanowires by direct-write electron-beam lithography. ACS Nano 2008, 2, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Samovat, F.; Mahmoodi, F.; Ahmad, P.T.; Samavat, M.F.; Tavakoli, M.H.; Hadidchi, S. Effect of annealing temperature on the optical properties of Palladium thin film. Open J. Phys. Chem. 2012, 2, 103–106. [Google Scholar] [CrossRef]
- Nakagawa, H.; Aoyagi, M.; Kurosawa, I.; Takada, S. Palladium thin-film resistors for Josephson LSI circuits. Jpn. J. Appl. Phys. 1992, 31, 2550–2553. [Google Scholar] [CrossRef]
- Corso, A.J.; Tessarolo, E.; Guidolin, M.; Gaspera, E.D.; Martucci, A.; Angiola, M.; Donazzan, A.; Pelizzo, M.G. Room-temperature optical detection of hydrogen gas using palladium nano-islands. Int. J. Hydrogen Energy 2018, 43, 5783–5792. [Google Scholar] [CrossRef]
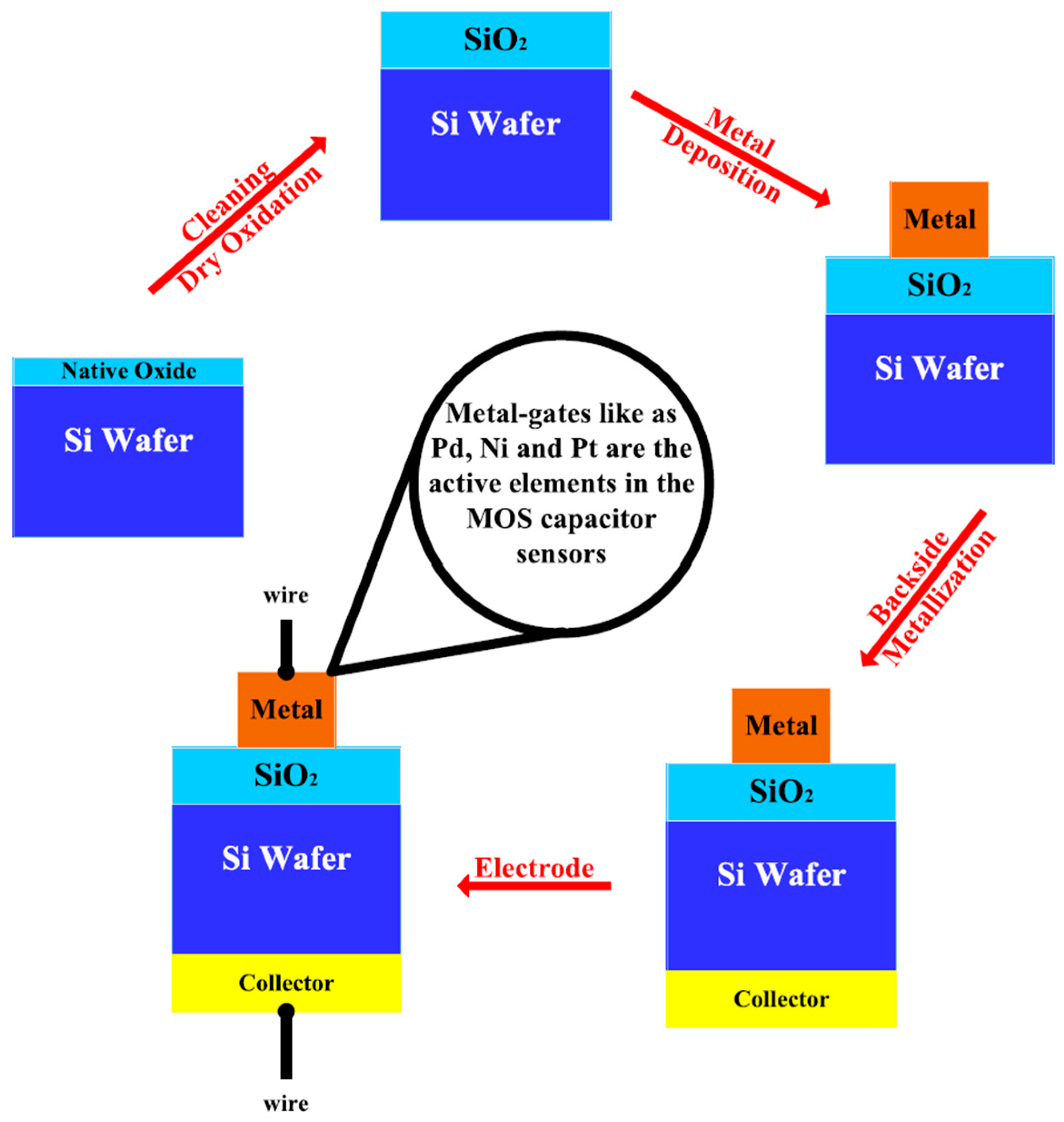
- Sharma, P.; Singh, R.; Sharma, R.; Mukhiya, R.; Awasthi, K.; Kumar, M. Palladium-oxide extended gate field effect transistor as pH sensor. Mater. Lett. X 2021, 12, 100102. [Google Scholar] [CrossRef]
- Guidoni, A.G.; Di Palma, T.M.; Teghil, R.; Marotta, V.; Ambrico, M.; Piccirillo, S.; Orlando, S. Pulsed lased deposition of Pd on amorphous alumina substrate. Surf. Coat. Technol. 1996, 80, 216–220. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. Development of Pd/TiO2 Porous Layers by Pulsed Laser Deposition for Surface Acoustic Wave H2 Gas Sensor. Nanomaterials 2020, 10, 760. [Google Scholar] [CrossRef]
- Bouhtiyya, S.; Roué, L. On the characteristics of Pd thin films prepared by pulsed laser deposition under different helium pressures. Int. J. Hydrogen Energy 2008, 33, 2912–2920. [Google Scholar] [CrossRef]
- Scarisoreanu, N.D.; Nicolae, I.; Grigoriu, C.; Dinescu, M.; Hirai, M.; Suzuki, T.; Yatsui, K. Pt, Pd, Ni metallic thin films deposited by pulsed laser ablation. In Proceedings of the SPIE 5581, ROMOPTO 2003: Seventh Conference on Optics, Constanta, Romania, 8–11 September 2004. [Google Scholar]
- Aggarwal, S.; Monga, A.P.; Perusse, S.R.; Ramesh, R.; Ballarotto, V.; Williams, E.D.; Chalamala, B.R.; Wei, Y.; Reuss, R.H. Spontaneous Ordering of Oxide Nanostructures. Science 2000, 287, 2235–2237. [Google Scholar] [CrossRef] [PubMed]
- Semaltianos, N.G.; Petkov, P.; Scholz, S.; Guetaz, L. Palladium or Palladium hydride nanoparticles synthesized by laser ablation od a bulk Palladium target in liquids. J. Colloid Interface Sci. 2013, 402, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Reddy, D.A.; Kim, Y.; Lee, S.; Ma, R.; Kim, T.K. Synthesis of Ultra-Small Palladium Nanoparticles Deposited on CdS Nanorods by Pulsed Laser Ablation in Liquid: Role of Metal Nanocrystal Size in the Photocatalytic Hydrogen Production. Chem. A Eur. J. 2017, 23, 13112–13119. [Google Scholar] [CrossRef] [PubMed]
- Amer, J.; Alkhawwam, A.; Jazmati, A.K. Activation of wood surface by Pd pulsed laser deposition for Ni electroless plating: Effects of wood morphology on coated films. Int. J. Struct. Integr. 2020, 12, 165–176. [Google Scholar] [CrossRef]
- Meyerheim, H.L.; Soyka, E.; Kirschner, J. Alloying and dealloying in pulsed laser deposited Pd films on Cu(100). Phys. Rev. B 2006, 74, 085405. [Google Scholar] [CrossRef]
- Shatokhin, A.N.; Putilin, F.N.; Safonova, O.V.; Rumyantseva, M.N. Sensor Properties of Pd-Doped SnO2 Films Deposited by Laser Ablation. Inorg. Mater. 2002, 38, 374–379. [Google Scholar] [CrossRef]
- Pereira, A.; Cultrera, L.; Dima, A.; Susu, M.; Perrone, A.; Du, H.L.; Volkov, A.O.; Cutting, R.; Datta, P.K. Pulsed laser deposition and characterization of textured Pd-doped-SnO2 thin films for gas sensing applications. Thin Solid Film. 2006, 497, 142–148. [Google Scholar] [CrossRef]
- Paillier, J.; Roue, L. Nanostructured Palladium Thin Films Prepared by Pulsed Laser Deposition. Structural Characterizations and Hydrogen Electrosorption Properties. J. Electrochem. Soc. 2005, 152, E1–E8. [Google Scholar] [CrossRef]
- Aggarwal, S.; Ogale, S.B.; Ganpule, C.S.; Shinde, S.R.; Novikov, V.A.; Monga, A.P.; Burr, M.R.; Ramesh, R. Oxide nanostructures through self-assembly. Appl. Phys. Lett. 2001, 78, 1442–1444. [Google Scholar] [CrossRef]
- Alexiadou, M. Pulsed laser deposition of ZnO thin films decorated with Au and Pd nanoparticles with enhanced acetone sensing performance. Appl. Phys. A 2017, 123, 262. [Google Scholar] [CrossRef]
- Heras, J.M.; Estiu, G.; Viscido, L. Aneealing behavior of clean and oxygen covered polycrystalline palladium films: A work function and electrical resistance study. Thin Solid Film. 1990, 188, 165–172. [Google Scholar] [CrossRef]
- Kalli, K.; Othonos, A.; Christofides, C. Temperature-induced reflectivity changes and activation of hydrogen sensitive optically thin palladium films on silicone oxide. Rev. Sci. Instrum. 1998, 69, 3331–3338. [Google Scholar] [CrossRef]
- Okamoto, H.; Aso, T. Formation of thin films of PdO and their electric properties. Jpn. J. Appl. Phys. 1967, 6, 779. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Y.-T. Hydrogen diffusion and solubility in Pd thin films. Int. J. Hydrogen Energy 1996, 21, 281–291. [Google Scholar] [CrossRef]
- Ievlev, V.M.; Ryabtsev, S.V.; Samoylov, A.M.; Shaposhninik, A.V.; Kuschev, S.B. Thin and ultrathin films of palladium oxide for oxidizing gases detection. Sens. Actuators B Chem. 2018, 255, 1335–1342. [Google Scholar] [CrossRef]
- Efimov, A.I.; Belokurova, L.P.; Vasilkova, I.V.; Chechev, V.P. Properties of inorganic compounds/Handbook; Khimiya: Leningrad, Russia, 1983; p. 392. (In Russian) [Google Scholar]
- Darling, A.S. Some properties and applications of the platinum group metals. Int. Metall. Rev. 1973, 18, 91–122. [Google Scholar] [CrossRef]
- Campbell, C.T.; Foyt, D.C.; White, J.M. Oxygen Penetration into the Bulk of Palladium. J. Phys. Chem. 1977, 81, 491–494. [Google Scholar] [CrossRef]
- Tafreshi, H.V.; Piseri, P.; Benedek, G.; Milani, P. The role of gas dynamics in operation conditions of a pulsed microplasma cluster source for nanostructured thin films deposition. J. Nanosci. Nanotechnol. 2006, 6, 1140–1149. [Google Scholar] [CrossRef]
- Cassina, V.; Gerosa, L.; Podesta, A.; Ferrari, G.; Sampietro, M.; Fiorentini, F.; Mazza, T.; Lenardi, C.; Milani, P. Nanoscale electrical properties of cluster-assembled palladium oxide thin films. Phys. Rev. B 2009, 79, 115422. [Google Scholar] [CrossRef]
- Garcia-Serrano, O.; Andraca-Adame, A.; Baca-Arroyo, R.; Pena-Sierra, R.; Romero-Peredes, R.G. Thermal oxidation of ultrathin palladium (Pd) foils at room conditions. In Proceedings of the CCE 2011—2011 8th International Conference on Electrical Engineering, Computing Science and Automatic Control, Program and Abstract Book, Merida City, Mexico, 26–28 October 2011. [Google Scholar]
- Kleykamp, H. Freie Bildungsenthalpie von Palladiumoxid. Z. Phys. Chemie. Neue Folge 1970, 71, 142–148. [Google Scholar] [CrossRef]
- Zemlyanov, D.; Klötzer, B.; Gabasch, H.; Smeltz, A.; Ribeiro, F.H.; Zafeiratos, S.; Teschner, D.; Schnörch, P.; Vass, E.; Hävecker, M.; et al. Kinetics of Palladium oxidation in the mbar pressure range: Ambient pressure XPS study. Top. Catal. 2013, 56, 885–895. [Google Scholar] [CrossRef]
- Samoylov, A.M.; Pelipenko, D.I.; Ivkov, S.A.; Tyulyanova, E.S.; Agapov, B. Thermal stability limit of of thin Palladium(II) oxide films. Inorg. Mater. 2022, 58, 48–55. (In Russian) [Google Scholar] [CrossRef]
- Peuckert, M. XPS study on surface and bulk palladium oxide, its thermal stability and a comparison with other noble metal oxides. J. Phys. Chem. 1985, 89, 2481–2486. [Google Scholar] [CrossRef]
- Rogal, J.; Reuter, K.; Scheffler, M. Thermodynamic stability of PdO surfaces. Phys. Rev. B 2004, 69, 075421. [Google Scholar] [CrossRef]
- Hellman, A.; Resta, A.; Martin, N.M.; Gustafson, J.; Trinchero, A.; Carlsson, P.A.; Balmes, O.; Felici, R.; Van Rijn, R.; Frenken, J.W.; et al. The active phase of palladium during methane oxidation. J. Phys. Chem. Lett. 2012, 3, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Martin, N.M.; Van Den Bossche, M.; Hellman, A.; Grönbeck, H.; Hakanoglu, C.; Gustafson, J.; Blomberg, S.; Johansson, N.; Liu, Z.; Axnanda, S.; et al. Intrinsic ligand effect governing the catalytic activity of Pd oxide thin films. ACS Catal. 2014, 4, 3330–3334. [Google Scholar] [CrossRef]
- Xie, G.; Sun, P.; Yan, X.; Du, X.; Jiang, Y. Fabrication of methane gas sensor by layer-by-layer self-assembly of polyaniline/PdO ultra-thin films on quartz crystal microbalance. Sens. Actuators B Chem. 2010, 145, 373–377. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.-M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.-F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and natural radiative forcing. In Climate Change 2013: The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgle, P.M., Eds.; Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar]
- Raub, E.; Plate, W. Über das Verhalten der Edelmetalle und ihrer Legierungen zu Sauerstoff bei hoher Temperatur im festen Zustand. Int. J. Mater. Res. 1957, 48, 529–539. [Google Scholar] [CrossRef]
- Jehn, H. High temperature behaviour of platinum group metals in oxidizing atmospheres. J. Less Common Met. 1984, 100, 321–339. [Google Scholar] [CrossRef]
- Usoltsev, O.; Stoian, D.; Skorynina, A.; Kozyr, E.; Njoroge, P.N.; Pellegrini, R.; Groppo, E.; van Bokhoven, J.A.; Bugaev, A. Restructuring of Palladium Nanoparticles during Oxidation by Molecular Oxygen. Small 2024, 2401184. [Google Scholar] [CrossRef]
- Nazarpour, S.; Chaker, M. Fractal analysis of Palladium hillocks generated due to oxide formation. Surf. Coat. Technol. 2012, 206, 2991–2997. [Google Scholar] [CrossRef]
- Pour, G.B.; Aval, L.F.; Sarvi, M.N.; Aval, S.F.; Fard, H.N. Hydrogen sensors: Palladium-based electrode. J. Mater. Sci. Mater. Electron. 2019, 30, 8145–8153. [Google Scholar] [CrossRef]
- Zou, Q.; Itoh, T.; Choi, P.G.; Masuda, Y.; Shin, W. Prediction of the effects of process informatics parameters on platinum, palladium, and gold-loaded tin oxide sensors with an artificial neural network. Sens. Actuators B Chem. 2024, 410, 135704. [Google Scholar] [CrossRef]
- Kafil, V.; Sreenan, B.; Hadj-Nacer, M.; Wang, Y.; Yoon, J.; Greiner, M.; Chu, P.; Wang, X.; Fadali, M.S.; Zhu, X. Review of noble metal and metal-oxide-semiconductor based chemiresistive hydrogen sensors. Sens. Actuators A Phys. 2024, 373, 115440. [Google Scholar] [CrossRef]
- Stetter, J.R.; Li, J. Amperometric gas sensors a review. Chem. Rev. 2008, 108, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Buttner, W.J.; Post, M.B.; Burgess, R.; Rivkin, C. An overview of hydrogen safety sensors and requirements. Int. J. Hydrogen Energy 2011, 36, 2462–2470. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Han, S.D.; Stetter, J.R. Review of electrochemical hydrogen sensors. Chem. Rev. 2009, 109, 1402–1433. [Google Scholar] [CrossRef]
- Zhao, Z.; Carpenter, M.A.; Xia, H.; Welch, D. All-optical hydrogen sensor based on a high alloy content palladium thin film. Sens. Actuators B Chem. 2006, 113, 532–538. [Google Scholar] [CrossRef]
- Hughes, R.C.; Schubert, W.K. Thin films of Pd/Ni alloys for detection of high hydrogen concentrations. J. Appl. Phys. 1992, 71, 542–544. [Google Scholar] [CrossRef]
- Ding, D.; Chen, Z.; Lu, C. Hydrogen sensing of nanoporous palladium films supported by anodic aluminum oxides. Sens. Actuators B Chem. 2006, 120, 182–186. [Google Scholar] [CrossRef]
- Ding, D.; Chen, Z.; Pyrolytic, A. Carbon-stabilized, nanoporous Pd film for wide-range H2 sensing. Adv. Mater. 2007, 19, 1996–1999. [Google Scholar] [CrossRef]
- Ding, D.; Chen, Z.; Rajaputra, S.; Singh, V. Hydrogen sensors based on aligned carbon nanotubes in an anodic aluminum oxide template with palladium as a top electrode. Sens. Actuators B Chem. 2007, 124, 12–17. [Google Scholar] [CrossRef]
- Zeng, X.Q.; Wang, Y.L.; Deng, H.; Latimer, M.L.; Xiao, Z.L.; Pearson, J.; Xu, T.; Wang, H.H.; Welp, U.; Crabtree, G.W.; et al. Networks of ultrasmall Pd/Cr nanowires as high-performance hydrogen sensors. ACS Nano 2011, 4, 7443–7452. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zach, M.P.; Xiao, Z.L.; Rosenmann, D.; Welp, U.; Kwok, W.K.; Crabtree, G.W. Self-assembled monolayer-enhanced hydrogen sensing with ultrathin palladium films. Appl. Phys. Lett. 2005, 86, 203104. [Google Scholar] [CrossRef]
- Favier, F.; Walter, E.C.; Zach, M.P.; Benter, T.; Penner, R.M. Hydrogen sensors and switches from electrodeposited palladium mesowire arrays. Science 2001, 293, 2227–2231. [Google Scholar] [CrossRef]
- Liu, N.; Tang, M.L.; Hentschel, M.; Giessen, H.; Alivisatos, A.P. Nanoantenna-enhanced gas sensing in a single tailored nanofocus. Nat. Mater. 2011, 10, 631–636. [Google Scholar] [CrossRef]
- Xie, B.; Zhang, S.; Liu, F.; Peng, X.; Song, F.; Wang, G.; Han, M. Response behavior of a palladium nanoparticle array based hydrogen sensor in hydrogen–nitrogen mixture. Sens. Actuators A Phys. 2012, 181, 20–24. [Google Scholar] [CrossRef]
- Lee, J.; Shim, W.; Noh, J.-S.; Lee, W. Design Rules for Nanogap-Based Hydrogen Gas Sensors. ChemPhysChem 2012, 13, 1395–1403. [Google Scholar] [CrossRef]
- Zhao, X.; Du, L.; Xing, X.; Tian, Y.; Li, Z.; Wang, C.; Feng, D.; Liu, H.; Yang, D. Palladium and palladium oxide enwrapped iron oxide shell/core nanoparticles for stable detection of ppb-level hydrogen. Chem. Eng. J. 2023, 457, 141258. [Google Scholar] [CrossRef]
- Guthe, S.; Mudhalwadkar, R. Thin film sensors for nitro aromatic explosive detection. Mater. Today Proc. 2017, 4, 10324–10327. [Google Scholar] [CrossRef]
- Taylor, G.; Shallenberger, J.; Tint, S.; Fones, A.; Hamilton, H.; Yu, L.; Amini, S.; Hettinger, J. Investigation of iridium; ruthenium; rhodium, and palladium binary metal oxide solid solution thin films for implantable neural interfacing applications. Surf. Coat. Technol. 2021, 426, 127803. [Google Scholar] [CrossRef]
- Salagare, S.; Adarakatti, P.S.; Venkataramanappa, Y. Electrochemical nitrite sensing employing palladium oxide–reduced graphene oxide (PdO-RGO) nanocomposites: Application to food and environmental samples. Ionics 2022, 28, 927–938. [Google Scholar] [CrossRef]
- Nguyen, P.H.; Pham, M.-T.; Nguyen, H.Q.; Cao, T.M.; Van Pham, V. Boosting visible-light-driven photocatalysis of nitrogen oxide degradation by Mott–Schottky Pd/TiO2 heterojunctions. Sep. Purif. Technol. 2025, 354, 129012. [Google Scholar] [CrossRef]
- El-Shafai, N.M.; Mostafa, Y.S.; Alamri, S.A.; El-Mehasseb, I.M. A nanoelectrode of hybrid nanomaterials of palladium oxide with cadmium sulfide based on 2D-carbon nanosheets for developing electron transfer efficiency for supercapacitor applications. New J. Chem. 2024, 48, 11932–11948. [Google Scholar] [CrossRef]
- El-Gohary, R.M.; El-Shafai, N.M.; El-Mehasseb, I.M.; Mostafa, Y.S.; Alamri, S.A.; Beltagi, A.M. Removal of pollutants through photocatalysis. adsorption, and electrochemical sensing by a unique plasmonic structure of palladium and strontium oxide nanoparticles sandwiched between 2D nanolayers. J. Environ. Manag. 2024, 363, 121257. [Google Scholar] [CrossRef] [PubMed]
- Karagonis, V.A.; Liu, C.C.; Neuman, M.R.; Romankiw, L.T.; Leary, P.W.; Cuomo, J.J. A Pd-Rd film potentiometric pH sensor. IEEE Trans. Biomed. Eng. BME 1986, 33, 113–116. [Google Scholar] [CrossRef]
- Tellez, V.C.; Portillo, M.C.; Santiesteban, H.J.; Castillo, M.P. Green synthesis of palladium mixed with PdO nanoparticles by chemical bath deposition. Opt. Mater. 2021, 112, 110747. [Google Scholar] [CrossRef]
- Brodyn, M.S.; Volkov, V.I.; Rudenko, V.I.; Liakhovetskyi, V.R.; Borshch, A.O. Large third-order optical nonlinearity in PdO thin films. J. Nonlinear Opt. Phys. Mater. 2017, 26, 1750037. [Google Scholar] [CrossRef]
- Liakhovetskyi, V.; Brodin, A.; Rudenko, V.; Brodyn, M.; Styopkin, V. High refractive nonlinearity of PdO films under femtosecond 800 nm laser pulses. J. Appl. Phys. 2020, 128, 013108. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badica, P.; Lőrinczi, A. A Review on Preparation of Palladium Oxide Films. Coatings 2024, 14, 1260. https://doi.org/10.3390/coatings14101260
Badica P, Lőrinczi A. A Review on Preparation of Palladium Oxide Films. Coatings. 2024; 14(10):1260. https://doi.org/10.3390/coatings14101260
Chicago/Turabian StyleBadica, Petre, and Adam Lőrinczi. 2024. "A Review on Preparation of Palladium Oxide Films" Coatings 14, no. 10: 1260. https://doi.org/10.3390/coatings14101260





