Sunlight-Driven Photodegradation of RB49 Dye Using TiO2-P25 and TiO2-UV100: Performance Comparison
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Product Characterization
2.3. Adsorption Kinetics
- qe (mg/g−1): quantity adsorbed at equilibrium;
- Ci (mg/L−1): initial dye concentration;
- Ce (mg/L−1): dye concentration at equilibrium;
- V (L): volume of solution;
- m (g): mass of adsorbent in solution.
- Ct: the dye concentration after an illumination time t;
- Kapp: the apparent first-order constant rate.
| Dye | Chemical Structure | Family | Molecular Mass (g.mol−1) | Maximum Absorption λmax (nm) |
|---|---|---|---|---|
| C.I. Reactive Blue 49 (RB49) |  | Azoic | 882.2 | 590 |
2.4. Photocatalysis under Solar Irradiation
2.5. Total Organic Carbon Determination
3. Results and Discussion
3.1. Characterization of TiO2-Based Catalysts by XRD
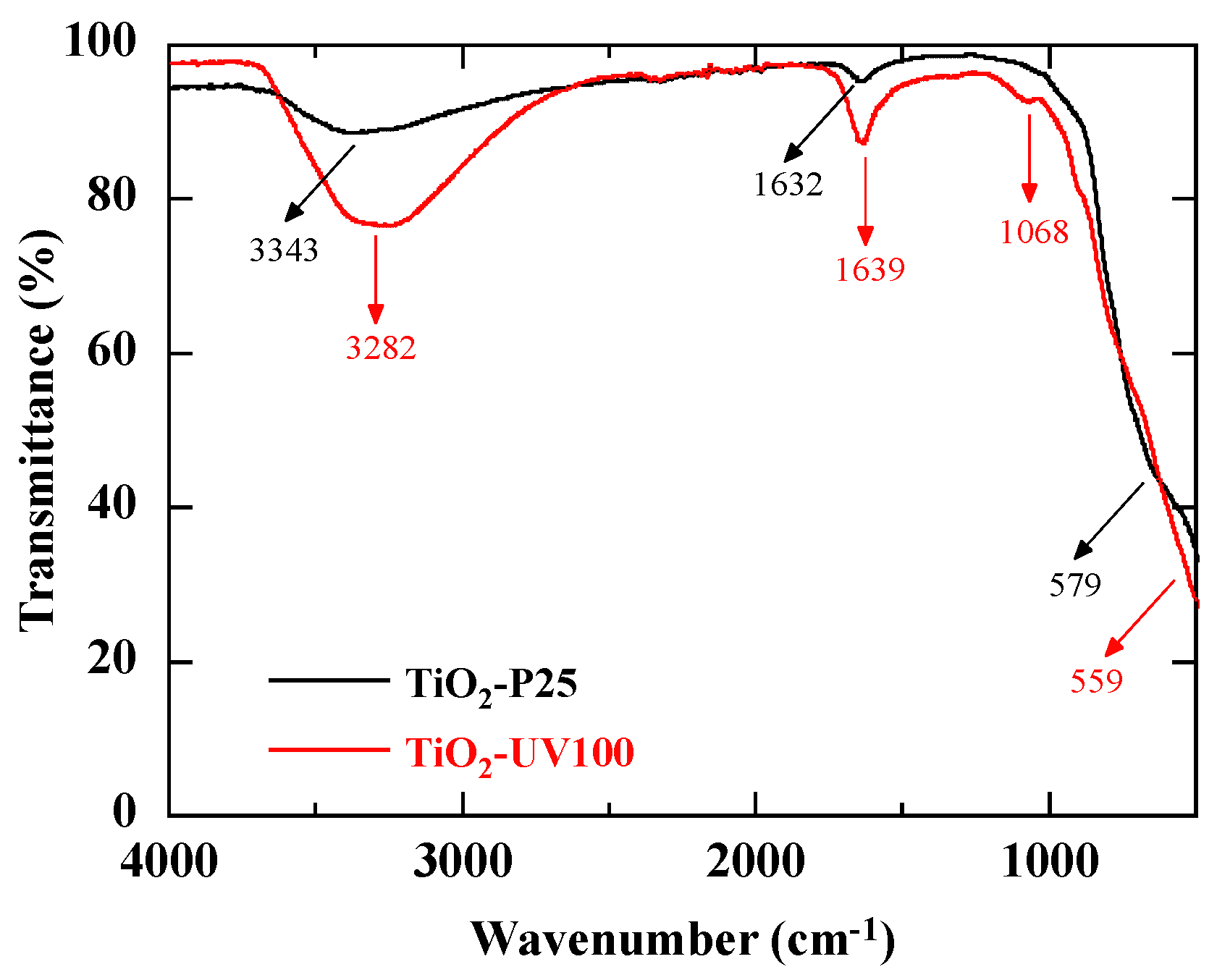
3.2. Characterization by FTIR
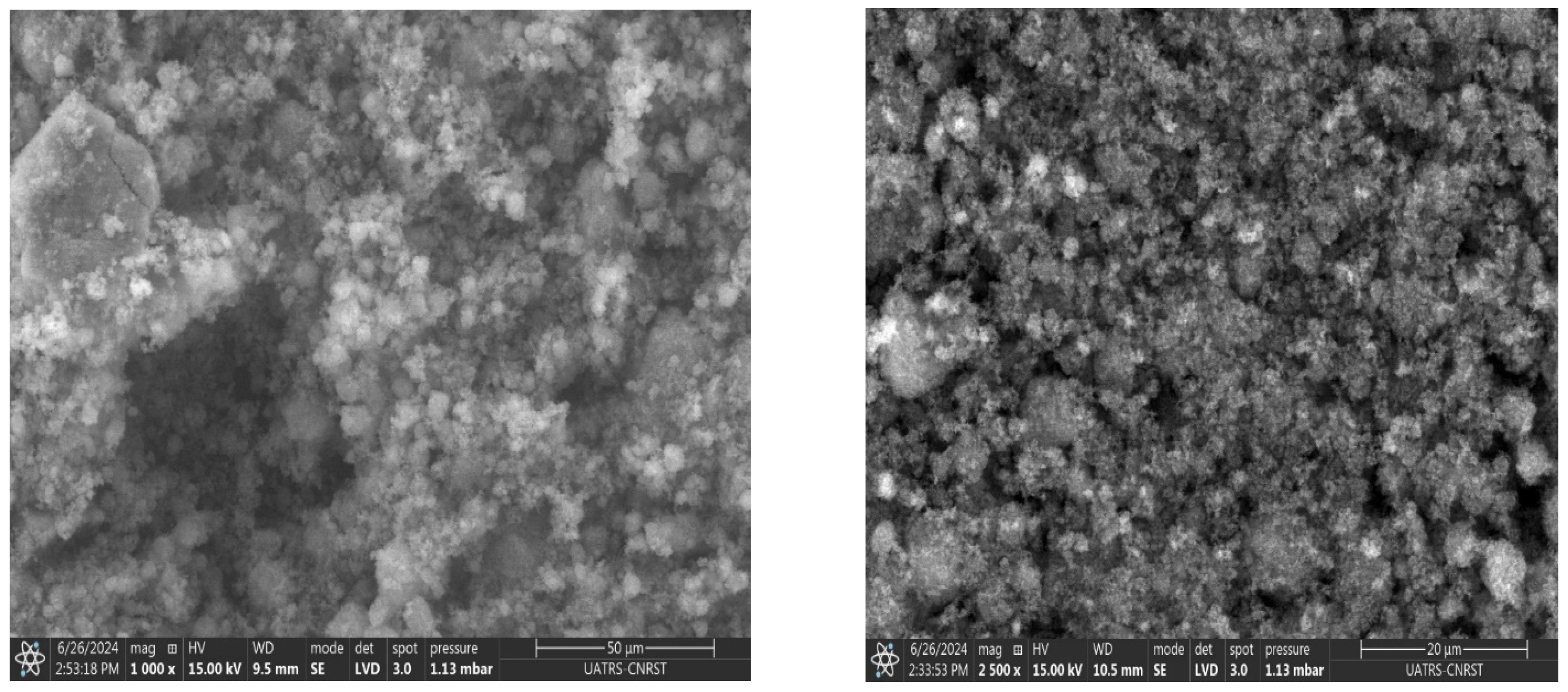
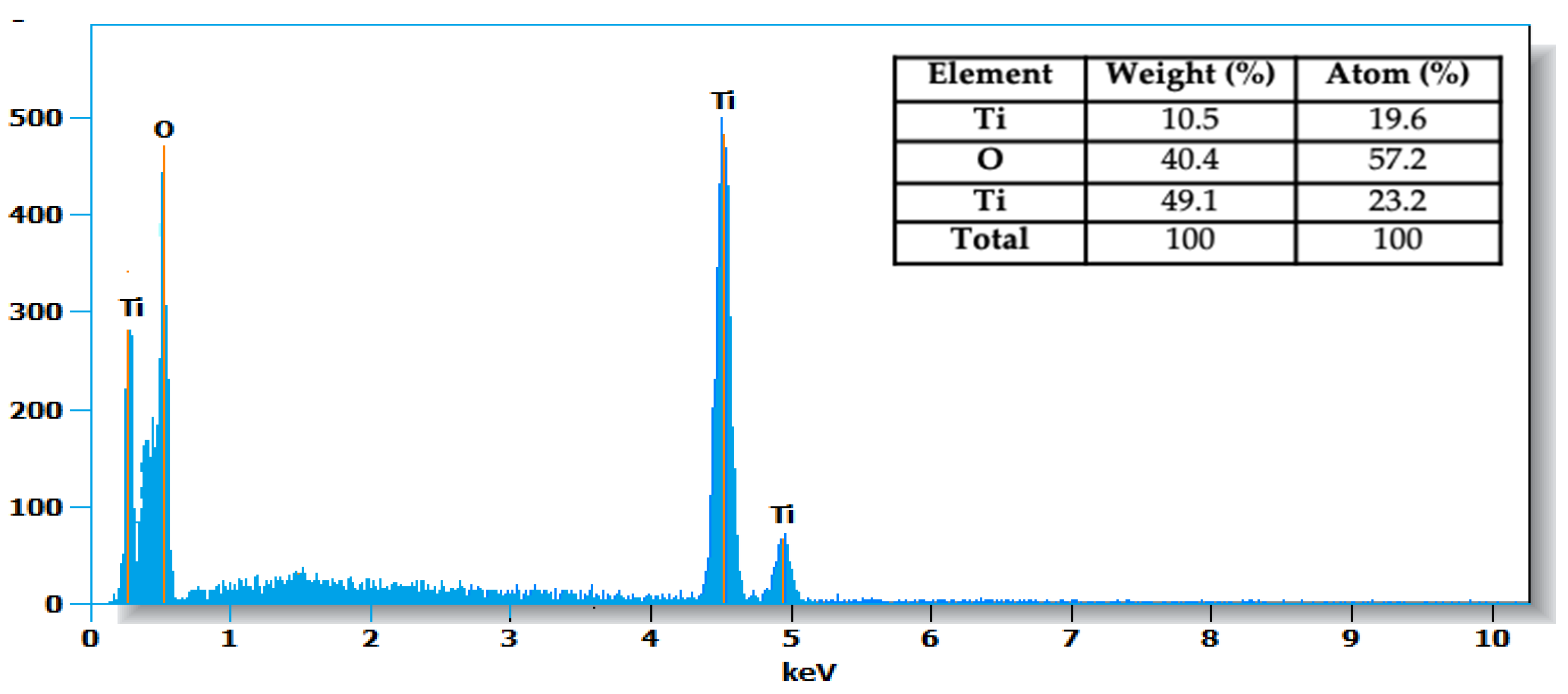
3.3. Morphology and EDS Analysis
3.4. Adsorption Kinetics of RB49
3.5. Adsorption Isotherm
3.6. Photolysis and Photocatalysis
3.7. Effect of Photocatalyst Loading
3.8. Effect of Dye Solution Concentration
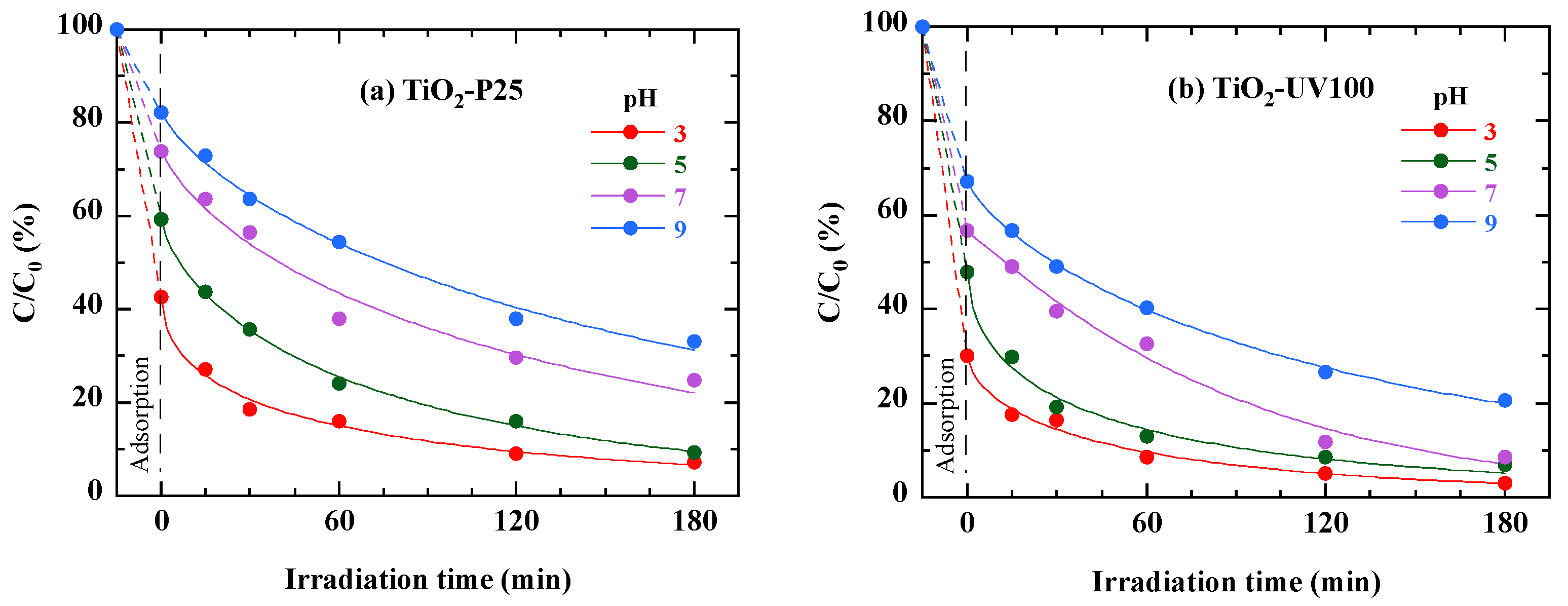
3.9. Effect of the Solution pH
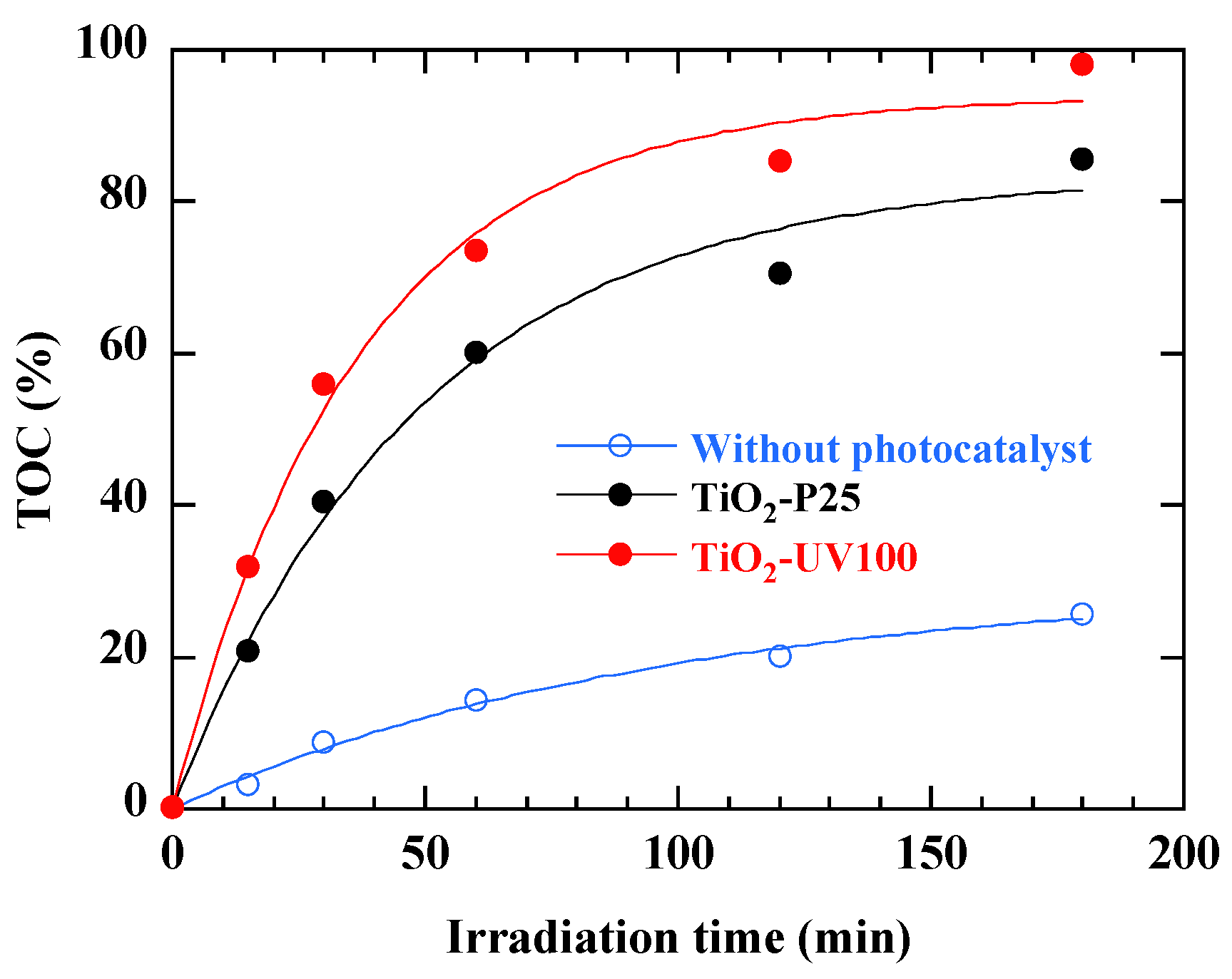
3.10. Kinetics Study of Total Organic Carbon (TOC) Removal
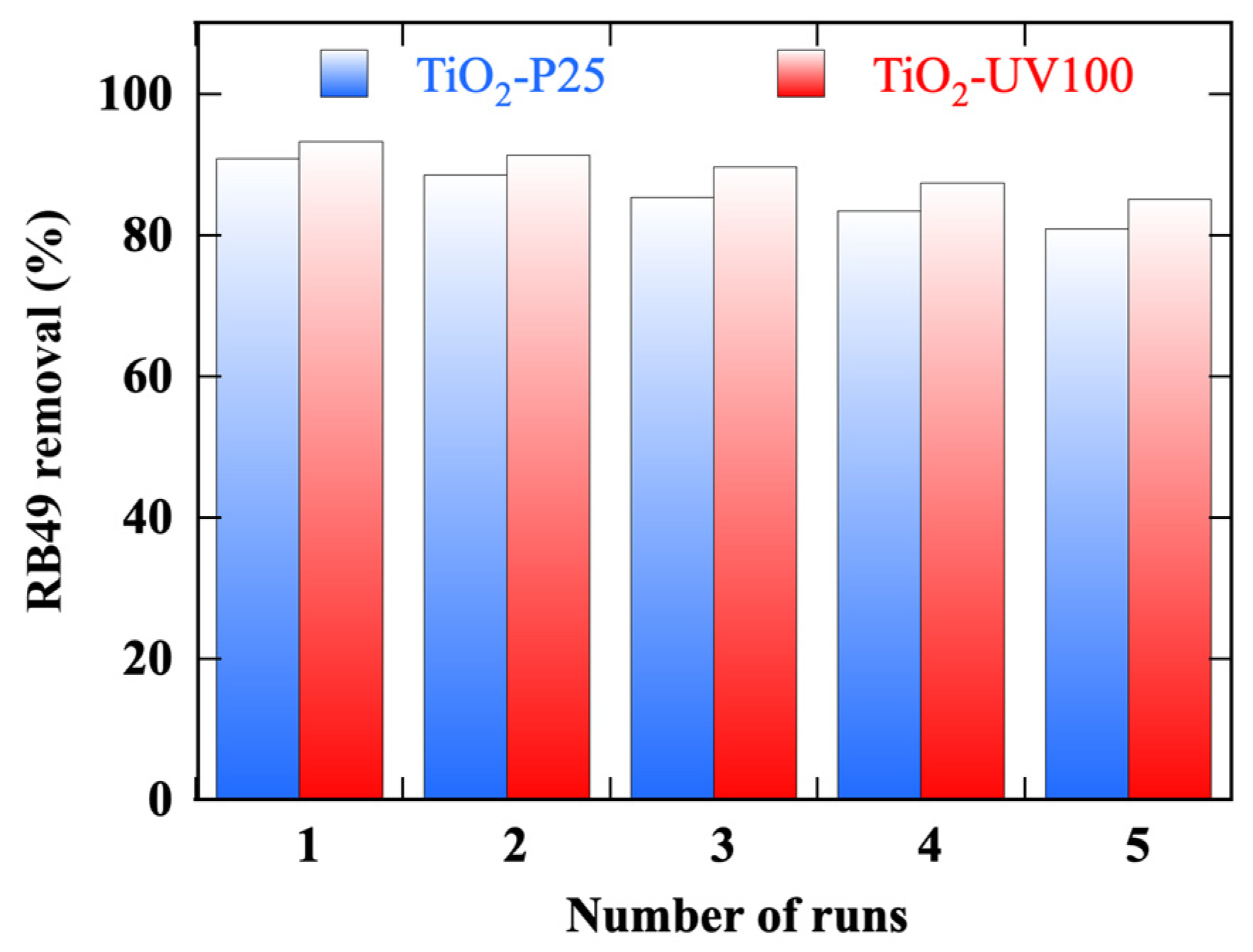
3.11. Reusability
- (i)
- Often, a first cycle is required as a step to activate the photocatalyst surface. In our case, both photocatalysts are activated in the first cycle.
- (ii)
- Over the five photocatalytic cycles, TiO2-UV100 maintains a more stable and higher-efficiency photocatalytic performance.
- (iii)
- After up to five cycles, there is a slight decrease in the photocatalytic efficiency of 8% for TiO2-UV100, compared to 11% for TiO2-P25.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khiari, M.; Gilliot, M.; Lejeune, M.; Lazar, F.; Hadjadj, A. Effects of ag nanoparticles on zinc oxide photocatalytic performance. Coatings 2021, 11, 400. [Google Scholar] [CrossRef]
- Sahu, A.; Poler, J.C. Removal and degradation of dyes from textile industry wastewater: Benchmarking recent advancements, toxicity assessment and cost analysis of treatment processes. J. Environ. Chem. Eng. 2024, 12, 113754. [Google Scholar] [CrossRef]
- Dutta, S.; Adhikary, S.; Bhattacharya, S.; Roy, D.; Chatterjee, S.; Chakraborty, A.; Banerjee, D.; Ganguly, A.; Nanda, S.; Rajak, P. Contamination of textile dyes in aquatic environment: Adverse impacts on aquatic ecosystem and human health, and its management using bioremediation. J. Environ. Manag. 2024, 353, 120103. [Google Scholar] [CrossRef] [PubMed]
- Köktürk, M.; Altindağ, F.; Ozhan, G.; Çalimli, M.H.; Nas, M.S. Textile dyes Maxilon blue 5G and Reactive blue 203 induce acute toxicity and DNA damage during embryonic development of Danio rerio. Comp. Biochem. Physiol. Part C 2021, 242, 108947. [Google Scholar] [CrossRef]
- Khiari, M.; Lejeune, M.; Gilliot, M.; Lazar, F.; Hadjadj, A. Photocatalytic Performance of ZnO/Ag(NPs) Nanocomposite Thin Films under Natural Conditions. Coatings 2022, 12, 1782. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Abdullah, E.A. Band edge positions as a key parameter to a systematic design of heterogeneous photocatalyst. Eur. J. Chem. 2019, 10, 82–94. [Google Scholar] [CrossRef]
- Yu, S.; Che Mohamad, N.A.R.; Kim, M.; Nah, Y.; Marques Mota, F.; Kim, D.H. Plasmon-Enhanced Electrocatalysis, Chapter 9. In Plasmonic Catalysis: From Fundamentals to Applications; Camargo, P.H.C., Cortés, E., Eds.; Wily: Hoboken, NJ, USA, 2021; pp. 261–293. [Google Scholar] [CrossRef]
- Gilliot, M.; Eypert, C.; Hadjadj, A. Dielectric function of sol-gel prepared nano-granular zinc oxide by spectroscopic ellipsometry. J. Appl. Phys. 2013, 114, 183513. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Ajmal, N.; Saraswat, K.; Bakht, M.A.; Riadi, Y.; Ahsan, M.J.; Noushad, M. Cost-effective and eco-friendly synthesis of titanium dioxide (TiO2) nanoparticles using fruit’s peel agro-waste extracts: Characterization, in vitro antibacterial, antioxidant activities. Green Chem. Lett. Rev. 2019, 12, 244–254. [Google Scholar] [CrossRef]
- Nabi, G.; Majid, A.; Riaz, A.; Alharbi, T.; Arshad Kamran, M.; Al-Habardi, M. Green synthesis of spherical TiO2 nanoparticles using Citrus Limetta extract: Excellent photocatalytic water decontamination agent for RhB dye. Inorg. Chem. Commun. 2021, 129, 108618. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; Rodríguez-Lorenzo, L.; El Hamidi, A.; Santaballa, J.A.; Canle, M. Cobalt Impregnation on Titania Photocatalysts Enhances Vis Phenol Photodegradation. Materials 2023, 16, 4134. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Tellez, A.; Masson, R.; Robert, D.; Keller, N.; Keller, V. Comparison of Hombikat UV100 and P25-TiO2 performance in gas-phase photocatalytic oxidation reactions. J. Photochem. Photobiol. A 2012, 250, 58–65. [Google Scholar] [CrossRef]
- Bresolin, B.M.; Balayeva, N.O.; Granone, L.I.; Dillert, R.; Bahnemann, D.W.; Sillanpää, M. Anchoring lead-free halide Cs3Bi2I9 perovskite on UV100–TiO2 for enhanced photocatalytic performance. Sol. Energy Mater. Sol. Cells 2020, 204, 110214. [Google Scholar] [CrossRef]
- Doudrick, K.; Monzoόn, O.; Mangonon, A.; Hristovski, K.; Westerhoff, P.K. Nitrate reduction in water using commercial titanium dioxide photocatalysts (P25 P90, and Hombikat UV100). ASCE J. Environ. Eng. 2012, 138, 852–861. [Google Scholar] [CrossRef]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why is anatase a better photocatalyst than rutile?—Model studies on epitaxial TiO2 films. Sci. Rep. 2015, 4, 4043. [Google Scholar] [CrossRef]
- Krupková, O.; Dušek, L.; Cuhorka, J.; Soares, G.; Kuchtová, G.; Mikulášek, P.; Bendová, H. Removal of textile dye reactive blue 49 from wastewater and dye baths by membrane separation and subsequent photo-Fenton reaction, UV-C and UV-C/H2O2. J. Water Process Eng. 2024, 65, 105735. [Google Scholar] [CrossRef]
- Zaaboul, F.; Kaichouh, G.; Haoufazane, C.; Abuelizz, H.A.; Karrouchi, K.; Zarrouk, A.; El Hourch, A. Adsorption of reactive blue day 49 from aqueous solution on commercial activated carbon and polyaniline electrochemically deposited on carbon felt: Kinetic modeling and equilibrium isotherm analysis. Int. J. Electrochem. Sci. 2024, 19, 100713. [Google Scholar] [CrossRef]
- Wang, X.; Pehkonen, S.O.; Rämö, J.; Väänänen, M.; Highfield, J.G.; Laasonen, K. Experimental and computational studies of nitrogen doped Degussa P25 TiO2: Application to visible-light driven photo-oxidation of As(iii). Catal. Sci. Technol. 2012, 2, 784–793. [Google Scholar] [CrossRef]
- Sadia, S.I.; Shishir, M.K.H.; Ahmed, S.; Aidid, A.R.; Islam, M.M.; Rana, M.M.; Al-Reza, S.M.; Alam, M.A. Crystallographic biography on nanocrystalline phase of polymorphs titanium dioxide (TiO2): A perspective static review. S. Afr. J. Chem. Eng. 2024, 50, 51–64. [Google Scholar] [CrossRef]
- Siah, W.R.; Lintang, H.O.; Shamsuddin, M.; Yuliati, L. High photocatalytic activity of mixed anatase-rutile phases on commercial TiO2 nanoparticles. Mater. Sci. Eng. 2016, 107, 012005. [Google Scholar] [CrossRef]
- Sanchez-Lorenzo, A.; Calbó, J.; Wild, M. Global and diffuse solar radiation in Spain: Building a homogeneous dataset and assessing their trends. Glob. Planet. Change 2013, 100, 343–352. [Google Scholar] [CrossRef]
- Kusic, H.; Koprivanac, N.; Horvat, S.; Bakija, S.; Bozic, A.L. Modeling dye degradation kinetic using dark- and photo-Fenton type processes. Chem. Eng. J. 2009, 155, 144–154. [Google Scholar] [CrossRef]
- Becerra-Ruiz, J.D.; Rangel-Vazquez, I.; Jauregui-Correa, J.C.; del Angel-Montes, G.A. Photo-catalytic water splitting: TiO2–GO for water splitting. In Proceedings of the CONIIN 2021—17th International Engineering Congress, Queretaro, Mexico, 14–18 June 2021. [Google Scholar] [CrossRef]
- Srinivasan, M.; Venkatesan, M.; Arumugam, V.; Natesan, G. Green synthesis and characterization of titanium dioxide nanoparticles (TiO2 NPs) using Sesbania grandiflora and evaluation of toxicity in zebrafish embryos. Process Biochem. 2019, 80, 197–202. [Google Scholar] [CrossRef]
- Huseynov, E.M.; Huseynova, E.A. Infrared spectroscopy of nanocrystalline anatase (TiO2) particles under the neutron irradiation. Opt. Mater. 2023, 144, 114351. [Google Scholar] [CrossRef]
- Jain, K.; Jain, S.K.; Tripathi, B. Structural, optical and morphological study of sol-gel synthesized titanium dioxide incorporated with transition metal elements (silver and cobalt). Interactions 2024, 245, 124. [Google Scholar] [CrossRef]
- León, A.; Reuquen, P.; Garín, C.; Segura, R.; Vargas, P.; Zapata, P.; Orihuela, P.A. FTIR and Raman characterization of TiO2 nanoparticles coated with polyethylene glycol as carrier for 2-methoxyestradiol. Appl. Sci. 2017, 7, 49. [Google Scholar] [CrossRef]
- Maanane, F.; El Yadini, A.; El Alouani, M.; Mabrouki, J.; Saufi, H.; Tabyaoui, M. Green Development of Titanium Dioxide Using Astragalus boeticus for the Degradation of Cationic and Anionic Dyes in an Aqueous Environment. Water 2023, 15, 3471. [Google Scholar] [CrossRef]
- Goñi-ciaurriz, L.; Senosiain-nicolay, M.; Vélaz, I. Aging studies on food packaging films containing β-cy-clodextrin-grafted TiO2 nanoparticles. Int. J. Mol. Sci. 2021, 22, 2257. [Google Scholar] [CrossRef]
- Connor, P.A.; Dobson, K.D.; James McQuillan, A. Infrared spectroscopy of the TiO2/aqueous solution interface. Langmuir 1999, 15, 2402–2408. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Zhang, Q.; Yang, J.; Deng, N. Efficient photodegradation of 4,4’-(propane-2,2-diyl)diphenol over biomolecule modified titanium dioxide under visible light irradiation. Catalys. Commun. 2011, 16, 7–10. [Google Scholar] [CrossRef]
- Belekbir, S.; El Azzouzi, M.; El Hamidi, A.; Rodríguez-Lorenzo, L.; Arturo Santaballa, J.; Canle, M. Improved Photocatalyzed Degradation of Phenol, as a Model Pollutant, over Metal-Impregnated Nanosized TiO2. Nanomaterials 2020, 10, 996. [Google Scholar] [CrossRef] [PubMed]
- Azeez, F.; Al-Hetlani, E.; Arafa, M.; Abdelmonem, Y.; Nazeer, A.A.; Amin, M.O.; Madkour, M. The effect of surface charge on photocatalytic degradation of methylene blue dye using chargeable titania nanoparticles. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tolosana-Moranchel, A.; Montejano, A.; Casas, J.A.; Bahamonde, A. Elucidation of the photocatalytic-mechanism of phenolic compounds. J. Environ. Chem. Eng. 2018, 6, 5712–5719. [Google Scholar] [CrossRef]
- Salahshoor, S.; Fahes, M.; Teodoriu, C. A review on the effect of confinement on phase behavior in tight formations. J. Nat. Gas Sci. Eng. 2018, 51, 89–103. [Google Scholar] [CrossRef]
- Sahbaz, D.A.; Dandil, S.; Acikgoz, C. Adsorption of reactive blue 49 onto cross-linked chitosan-based composites containing waste mussel shell and waste active sludge char. Water Sci. Technol. 2021, 83, 715–726. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Iqbal, M.; Nadeem, M.A.; Hussain, I.; Yousaf, S.; Mustafa, G.; Zafar, M.I.; Nadeem, M.A. Photocatalytic degradation of textile dyes on Cu2O-CuO/TiO2 anatase powders. J. Environ. Chem. Eng. 2016, 4, 2138–2146. [Google Scholar] [CrossRef]
- Baral, S.C.; Maneesha, P.; Datta, S.; Dukiya, K.; Sasmal, D.; Samantaray, K.S.; Krupa, B.V.; Dasgupta, A.; Sen, S. Enhanced photocatalytic degradation of organic pollutants in water using copper oxide (CuO) nanosheets for environmental application. JCIS Open 2024, 13, 100102. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH dependent surface charging and points of zero charge. Update. Adv. Colloid Interface Sci. 2023, 319, 102973. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef]
- Mir, N.A.; Khan, A.; Dar, A.A.; Muneer, M. Photocatalytic study of two azo dye derivatives, ponceau bs and reactive blue 160 in aqueous suspension of TiO2: Adsorption isotherm and decolorization kinetics. IJIRSET 2014, 3, 933–9348. [Google Scholar]
- Velmurugan, R.; Krishnakumar, B.; Kumar, R.; Swaminathan, M. Solar active nano-TiO2 for mineralization of Reactive Red 120 and Trypan Blue. Arab. J. Chem. 2012, 5, 447–452. [Google Scholar] [CrossRef]
- Alahiane, S.; Qourzal, S.; El Ouardi, M.; Belmouden, M.; Assabbane, A.; Ait-Ichou, Y. Adsorption and photocatalytic degradation of indigo carmine dye in aqueous solutions using TiO2/UV/O2. J. Mater. Environ. Sci. 2013, 4, 239–250. [Google Scholar]


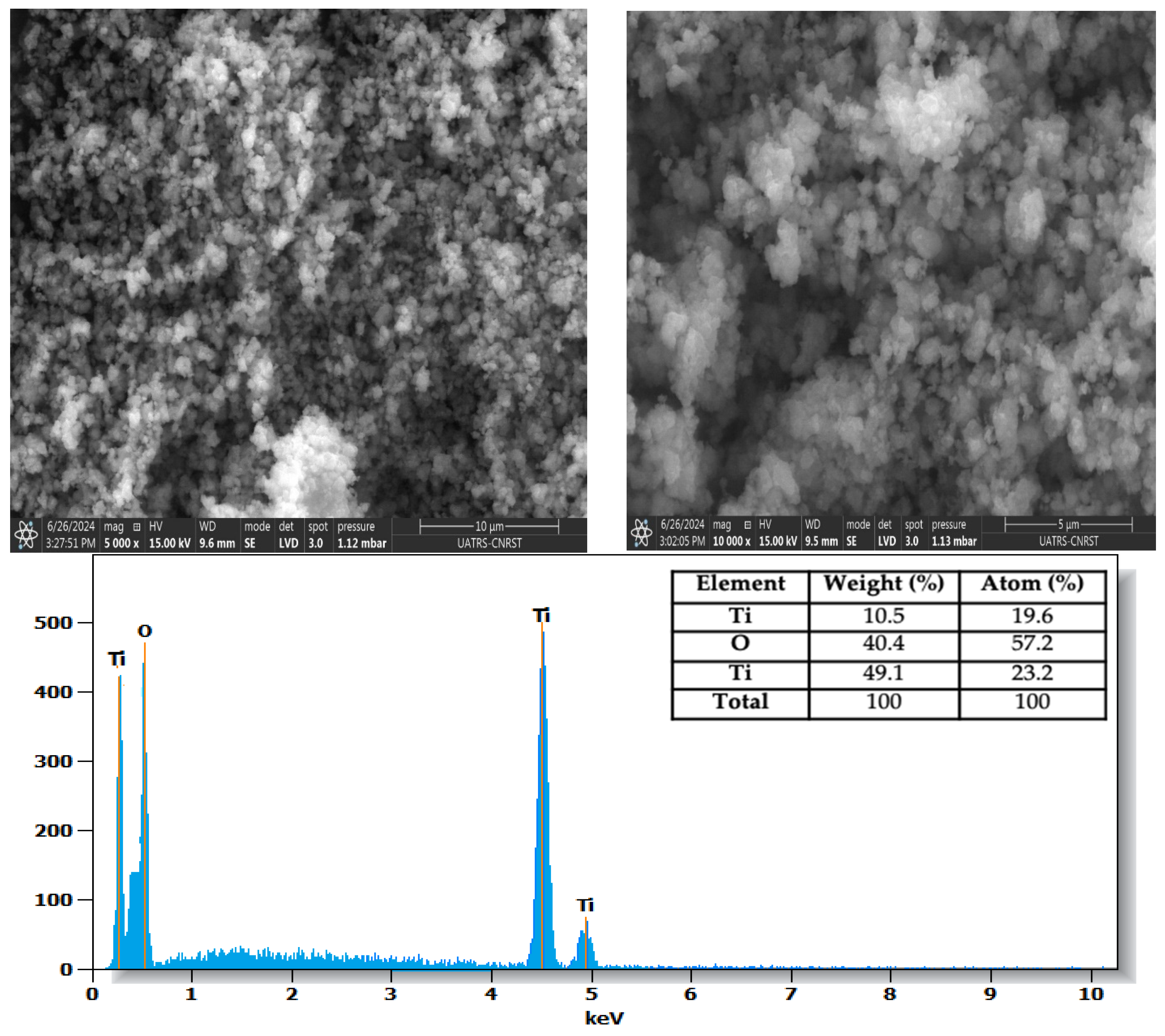
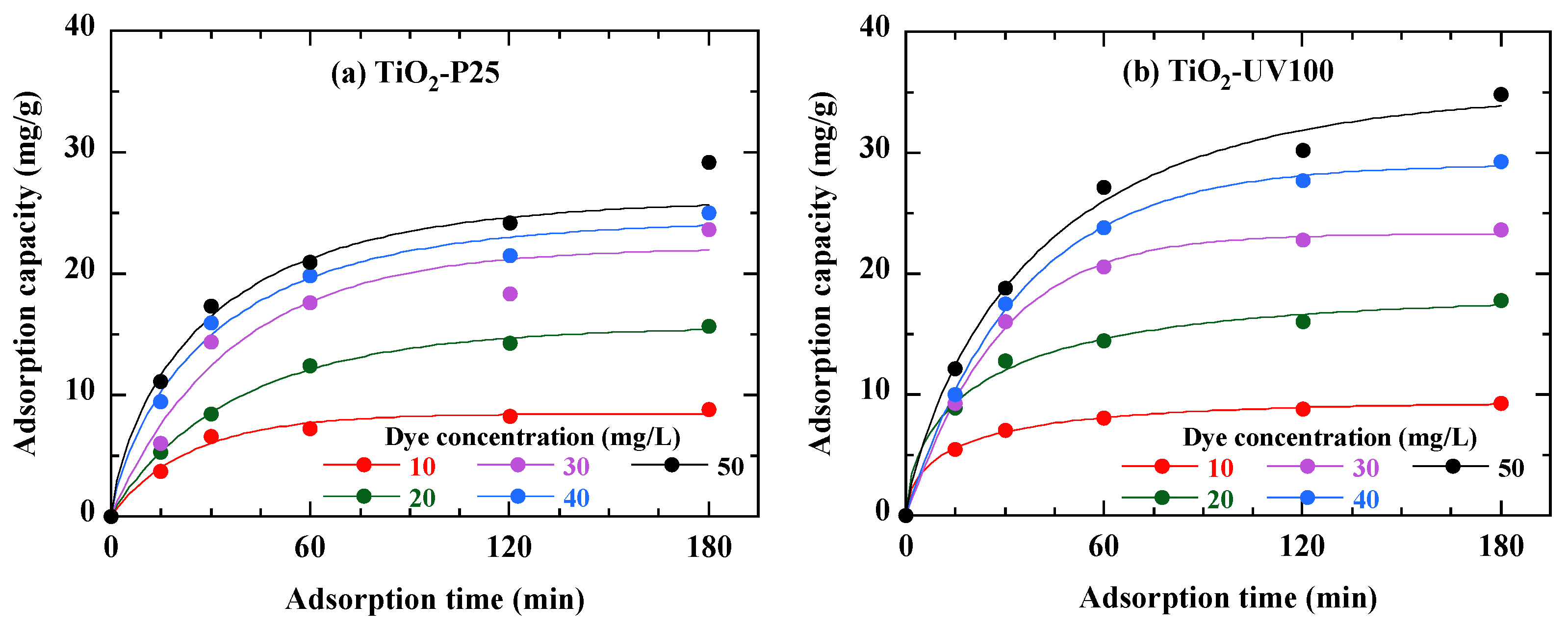


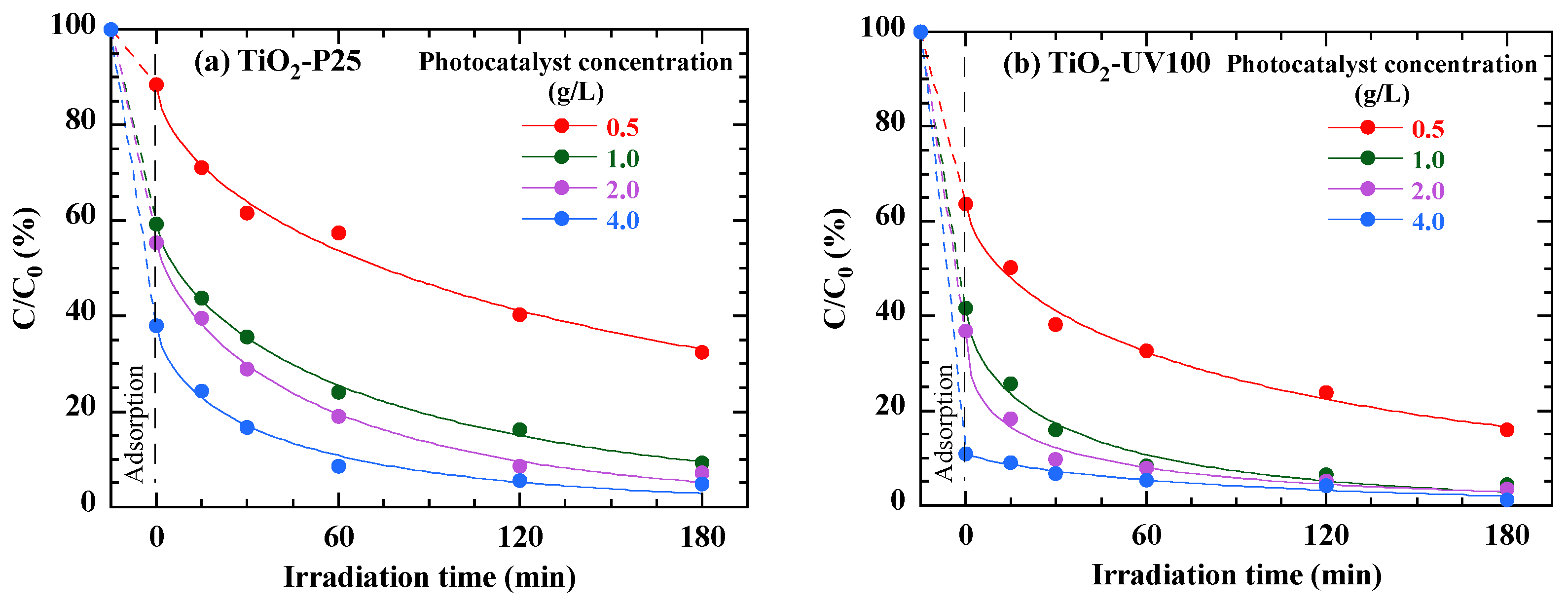
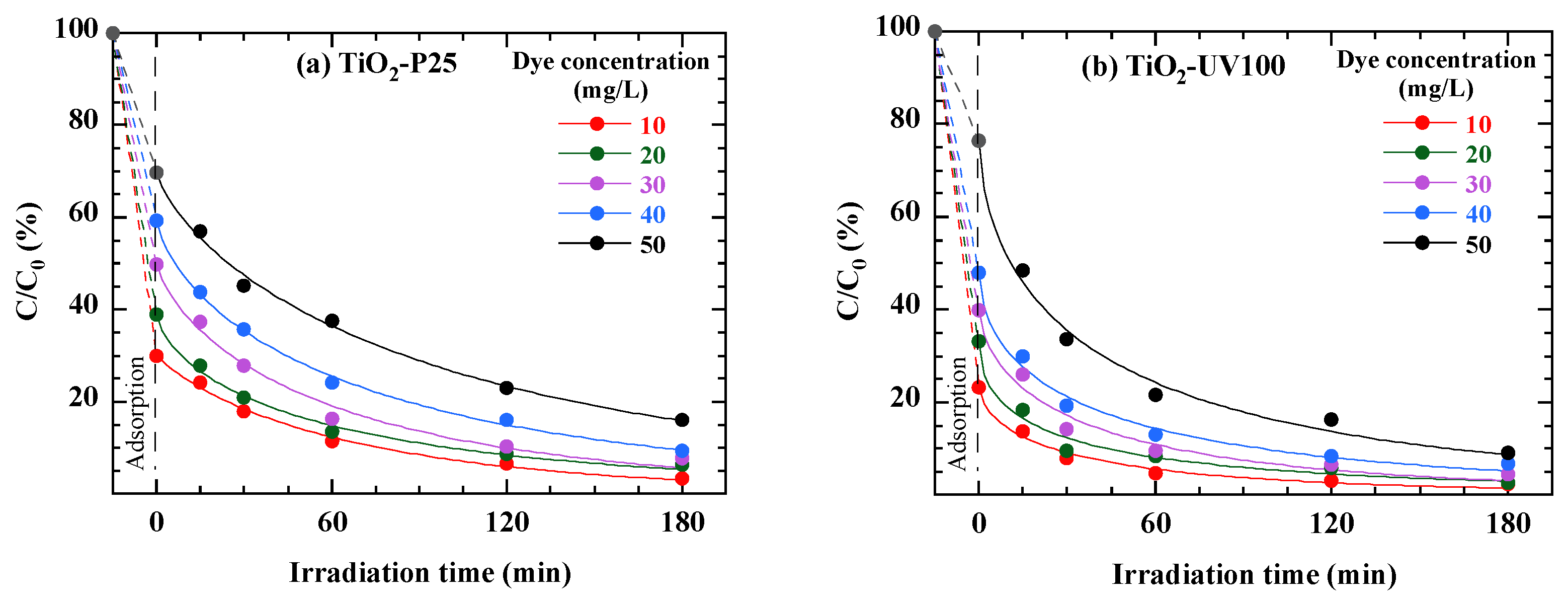
| Parameter | TiO2-P25 | TiO2-UV100 |
|---|---|---|
| qm (mg/g) | 51 ± 2 | 103 ± 1 |
| KL (L/mg) | 0.023 ± 0.006 | 0.010 ± 0.002 |
| Photocatalyst | Pollutant | Irradiation | Duration | Efficiency | Ref. |
|---|---|---|---|---|---|
| TiO2-P25 | 2,4-dichlorophenoxyacetic acid | UV | 60 min | 83% | [22] |
| Reactive Blue 160 | UV-light | 20 min | 70.04% | [43] | |
| Ponceau BS | UV-light | 15 min | 82.04% | [43] | |
| Red 120 | Sunlight | 90 min | 81% | [44] | |
| RB49 | Sunlight | 180 min | 85% | This study | |
| TiO2-UV100 | 2,4-dichlorophenoxyacetic acid | UV | 60 min | 73% | [22] |
| Reactive Blue 160 | UV | 20 min | 63.78% | [43] | |
| Ponceau BS | UV | 15 min | 75.49% | [43] | |
| Indigo Carmine | UV | 330 min | 80% | [45] | |
| RB49 | Sunlight | 180 min | 98% | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaaboul, F.; Canle, M.; Haoufazane, C.; Santaballa, J.A.; Hammouti, B.; Azzaoui, K.; Jodeh, S.; Hadjadj, A.; El Hourch, A. Sunlight-Driven Photodegradation of RB49 Dye Using TiO2-P25 and TiO2-UV100: Performance Comparison. Coatings 2024, 14, 1270. https://doi.org/10.3390/coatings14101270
Zaaboul F, Canle M, Haoufazane C, Santaballa JA, Hammouti B, Azzaoui K, Jodeh S, Hadjadj A, El Hourch A. Sunlight-Driven Photodegradation of RB49 Dye Using TiO2-P25 and TiO2-UV100: Performance Comparison. Coatings. 2024; 14(10):1270. https://doi.org/10.3390/coatings14101270
Chicago/Turabian StyleZaaboul, Fatima, Moisés Canle, Chaimaa Haoufazane, Juan Arturo Santaballa, Belkheir Hammouti, Khalil Azzaoui, Shehdeh Jodeh, Aomar Hadjadj, and Abderrahim El Hourch. 2024. "Sunlight-Driven Photodegradation of RB49 Dye Using TiO2-P25 and TiO2-UV100: Performance Comparison" Coatings 14, no. 10: 1270. https://doi.org/10.3390/coatings14101270
APA StyleZaaboul, F., Canle, M., Haoufazane, C., Santaballa, J. A., Hammouti, B., Azzaoui, K., Jodeh, S., Hadjadj, A., & El Hourch, A. (2024). Sunlight-Driven Photodegradation of RB49 Dye Using TiO2-P25 and TiO2-UV100: Performance Comparison. Coatings, 14(10), 1270. https://doi.org/10.3390/coatings14101270













