Characterization of Mechanical Properties and Surface Wettability of Epoxy Resin/Benzoxazine Composites in a Simulated Acid Rain Environment
Abstract
:1. Introduction
2. Materials and Methods
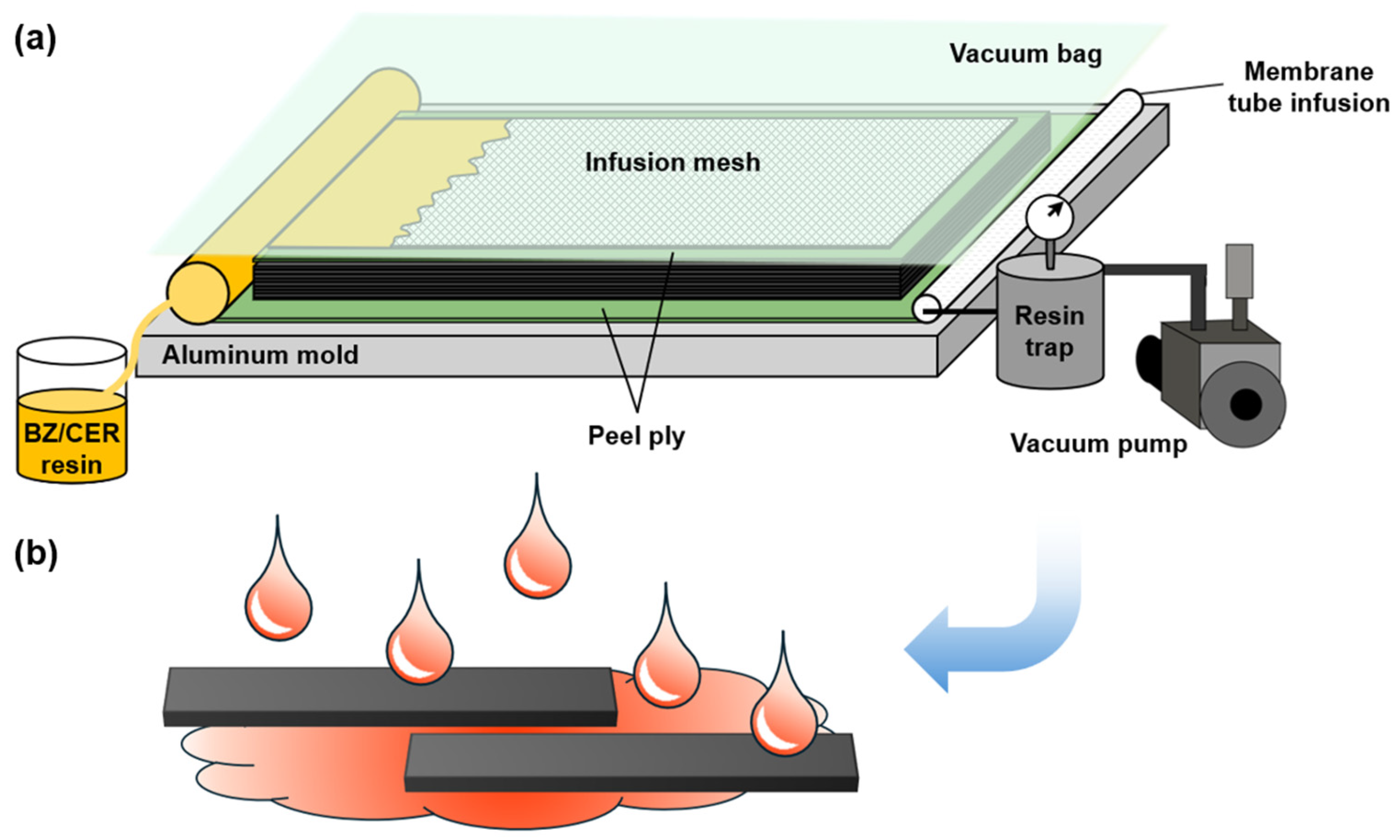
2.1. Composite Fabrication and Acid Rain Simulation
2.2. Weight Change
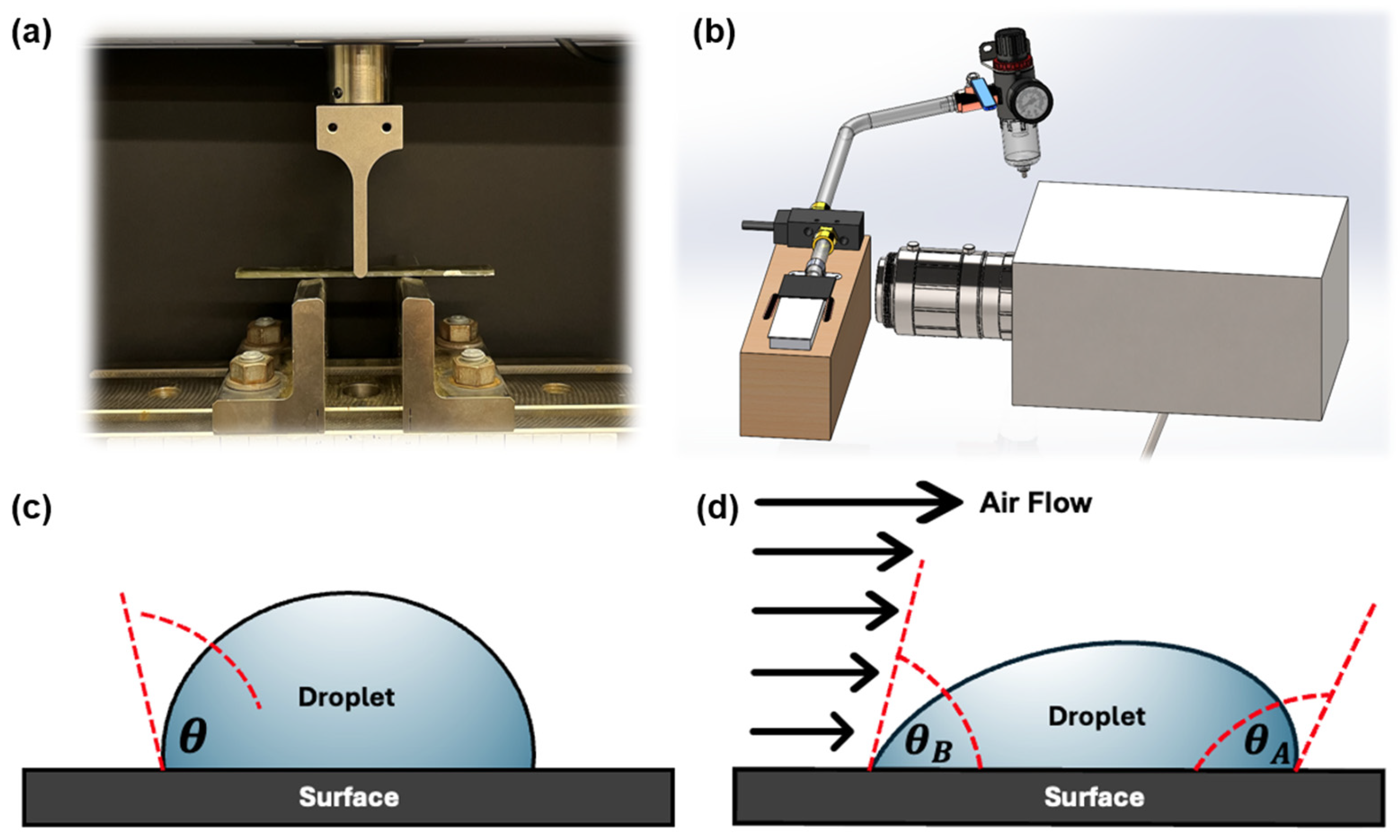
2.3. Characterization of Flexural and Short Beam Properties
2.4. Optical Microscopy
2.5. Characterization of Contact Angle and Contact Angle Hysteresis
3. Results and Discussion
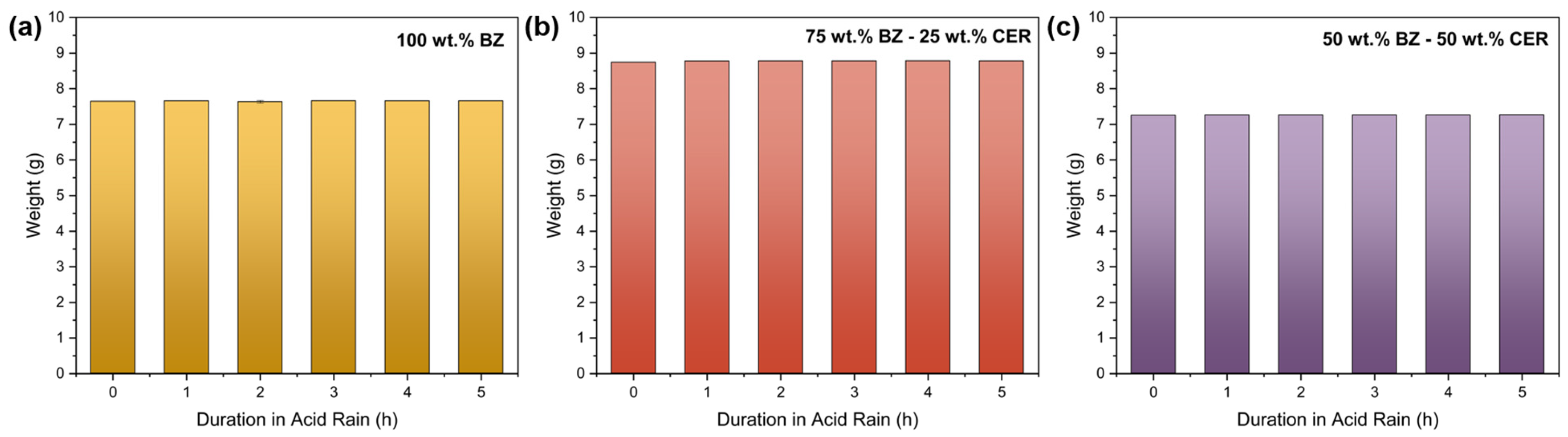
3.1. Weight Change
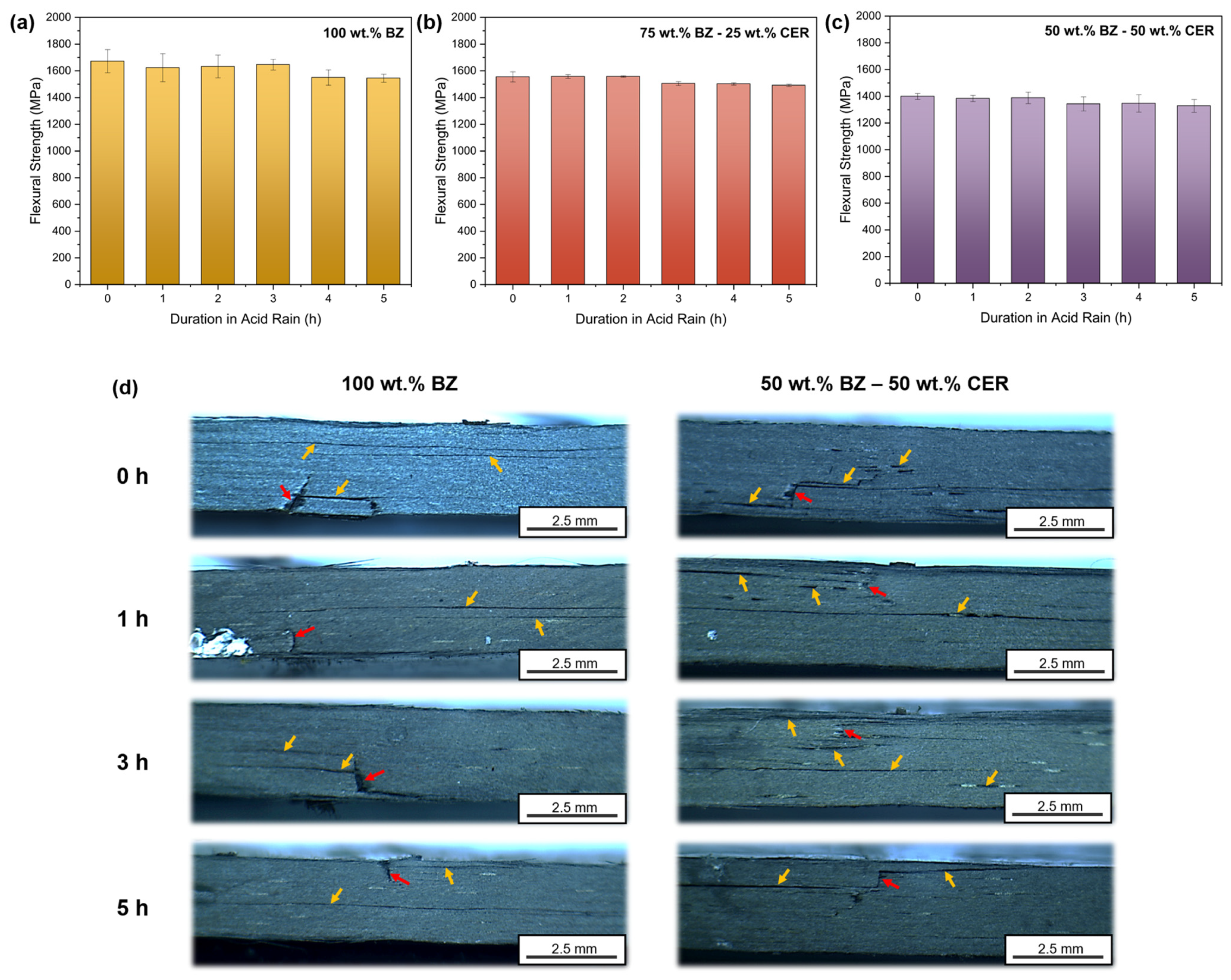
3.2. Flexural Properties
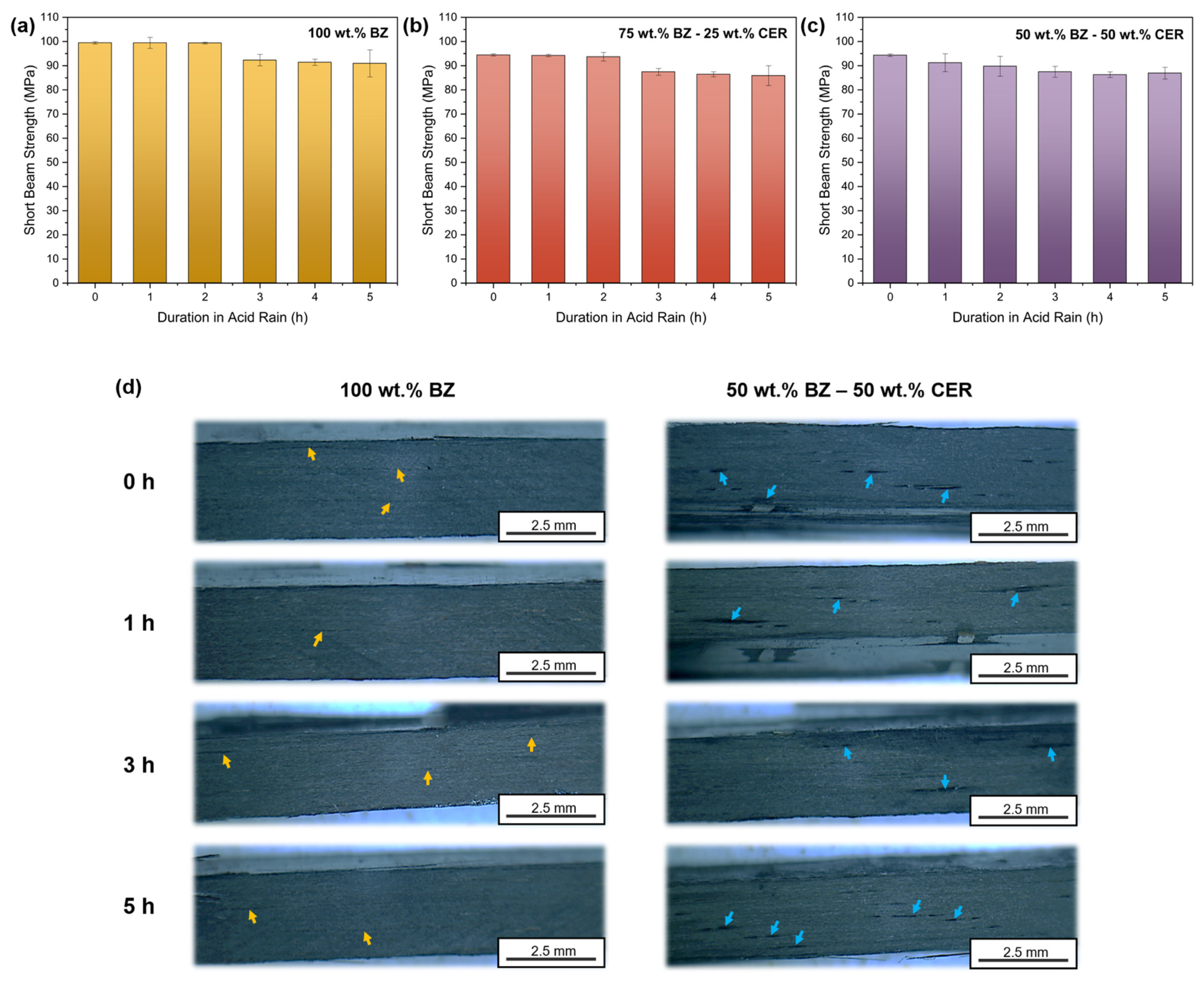
3.3. Short Beam Properties
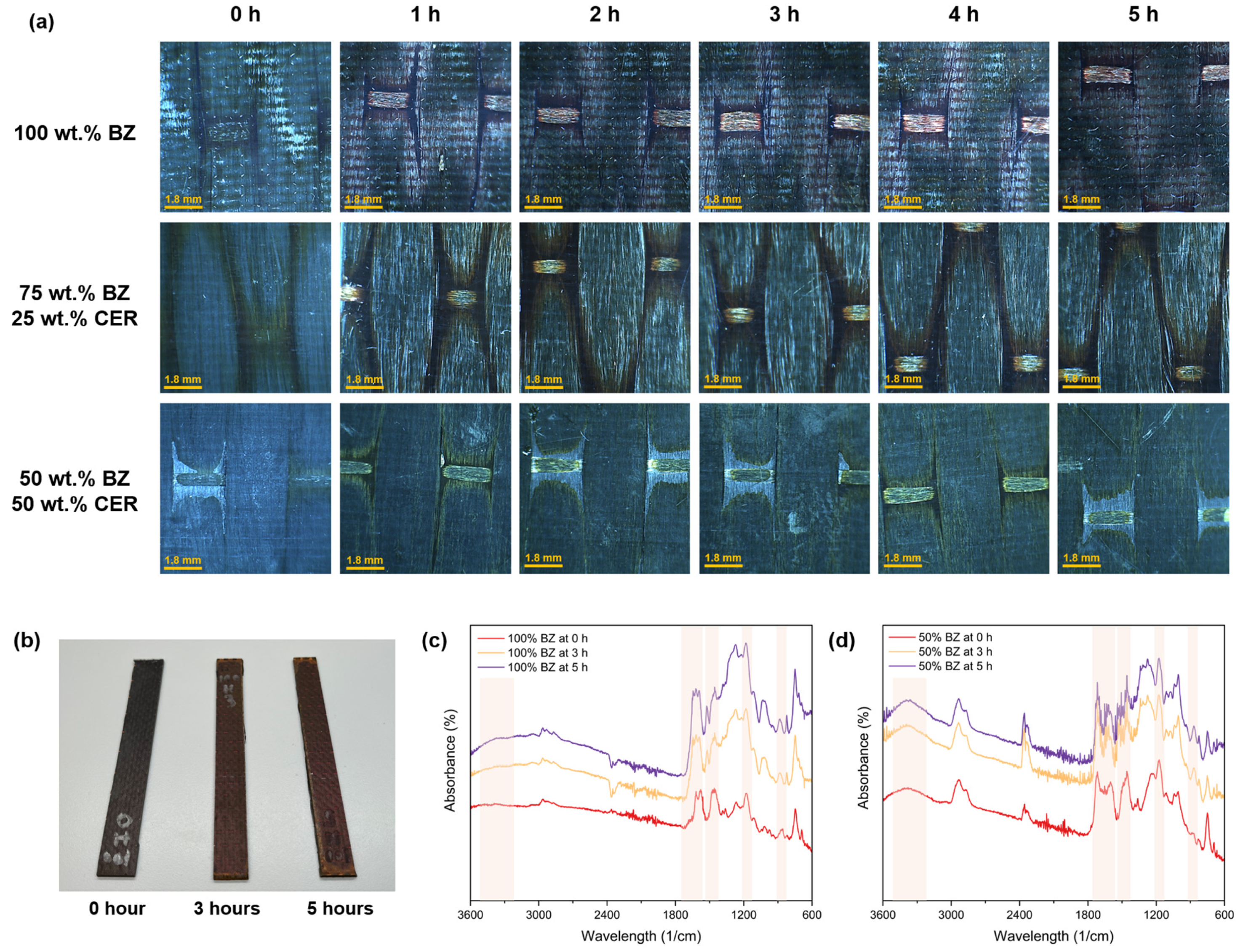
3.4. Surface Properties
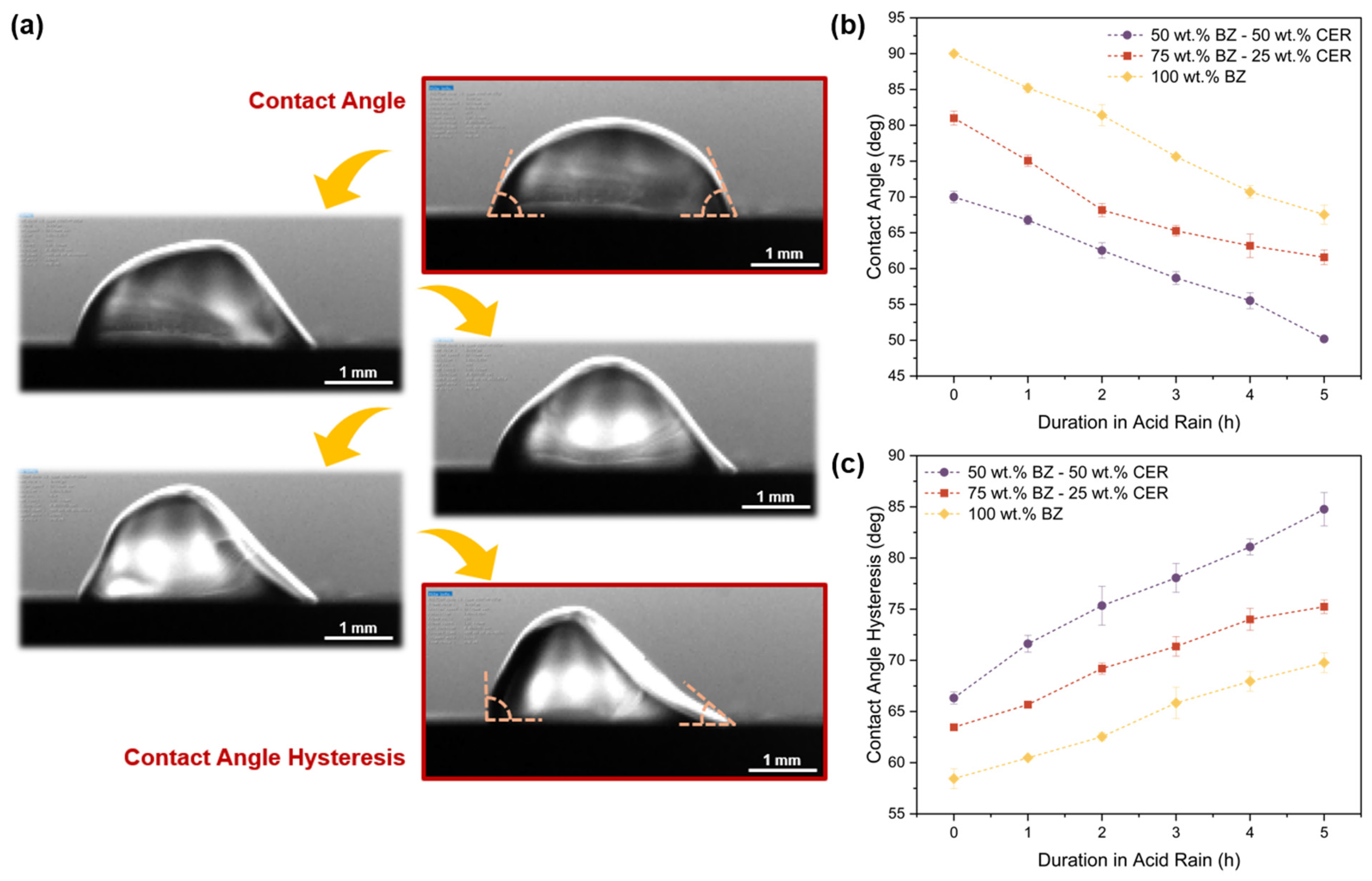
3.5. Contact Angle and Contact Angle Hysteresis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Lin, G.; Vaidya, U.; Wang, H. Past, present and future prospective of global carbon fibre composite developments and applications. Compos. B Eng. 2023, 250, 110463. [Google Scholar] [CrossRef]
- Isa, A.; Nosbi, N.; Ismail, M.C.; Akil, H.M.; Faiz Wan Ali, W.F.; Omar, M.F. A Review on Recycling of Carbon Fibres: Methods to Reinforce and Expected Fibre Composite Degradations. Materials 2022, 15, 4991. [Google Scholar] [CrossRef] [PubMed]
- Mangalgiri, P.D. Composite materials for aerospace applications. Bull. Mater. Sci. 1999, 22, 657–664. [Google Scholar] [CrossRef]
- Park, S.J.; Jin, F.L. Epoxy Resins: Recent Advances in Synthesis, Properties, and Applications. J. Ind. Eng. Chem. 2006, 12, 759–774. [Google Scholar]
- Hamerton, I.; Mooring, L. The Use of Thermosets in Aerospace Applications. In Thermosets; Guo, Q., Ed.; Woodhead Publishing: Cambridge, CA, USA, 2012; pp. 189–227. [Google Scholar]
- Moghtadernejad, S.; Barjasteh, E.; Johnson, Z.; Stolpe, T.; Banuelos, J. Effect of Thermo-Oxidative Aging on Surface Characteristics of Benzoxazine and Epoxy Copolymer. J. Appl. Polym. Sci. 2021, 138, 50211. [Google Scholar] [CrossRef]
- Huang, Z.; Zhao, W.; Zhao, W.; Ci, X.; Li, W. Tribological and anti-corrosion performance of epoxy resin composite coatings reinforced with differently sized cubic boron nitride (CBN) particles. Friction 2021, 9, 104–118. [Google Scholar] [CrossRef]
- Li, Y. The development of carbon fiber epoxy resin composite material and its applications in aerospace. Appl. Comput. Mech. 2023, 23, 75–80. [Google Scholar] [CrossRef]
- Zhou, C.; Fu, M.; Xie, H.; Gong, Y.; Chen, J.; Liu, J.; Xin, Z. Polybenzoxazine/epoxy composite coatings: Effect of crosslinking on corrosion resistance. Ind. Eng. Chem. Res. 2021, 60, 1675–1683. [Google Scholar] [CrossRef]
- Soliman, A.M.; Aly, K.I.; Mohamed, M.G.; Amer, A.A.; Belal, M.R.; Abdel-Hakim, M. Synthesis, characterization and protective efficiency of novel polybenzoxazine precursor as an anticorrosive coating for mild steel. Sci. Rep. 2023, 13, 5581. [Google Scholar] [CrossRef]
- Barjasteh, E.; Gouni, S.; Sutanto, C.; Narongdej, P. Bisphenol-A benzoxazine and cycloaliphatic epoxy copolymer for composite processing by resin infusion. J. Compos. Mater. 2019, 53, 1777–1790. [Google Scholar] [CrossRef]
- Narongdej, P.; Hanson, J.; Barjasteh, E.; Moghtadernejad, S. Liquid-Solid Interaction to Evaluate Thermal Aging Effects on Carbon Fiber-Reinforced Composites. Fluids 2024, 9, 100. [Google Scholar] [CrossRef]
- Eral, H.B.; ’t Mannetje, D.J.C.M.; Oh, J.M. Contact angle hysteresis: A review of fundamentals and applications. Colloid Polym. Sci. 2013, 291, 247–260. [Google Scholar] [CrossRef]
- Kotnarowska, D. Epoxy coating destruction as a result of sulphuric acid aqueous solution action. Prog. Org. Coat. 2010, 67, 324–328. [Google Scholar] [CrossRef]
- Thomas, R.R.; Brusic, V.A.; Rush, B.M. Correlation of Surface Wettability and Corrosion Rate for Benzotriazole-Treated Copper. J. Electrochem. Soc. 1992, 139, 678. [Google Scholar] [CrossRef]
- Kotnarowska, D. Influence of ultraviolet radiation and aggressive media on epoxy coating degradation. Prog. Org. Coat. 1999, 37, 149–159. [Google Scholar] [CrossRef]
- Maurya, S.K.; Chechi, P.; Prasad, R.; Susheel, C.K.; Manna, A. Effect of surface finish on corrosion behavior of polished Al6061 alloy in simulated acid rain environment. Mater. Today Proc. 2022, 64, 755–759. [Google Scholar] [CrossRef]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Zheng, M.; Sakairi, M.; Jha, H. Influence of desiccation procedures on the surface wettability and corrosion resistance of porous aluminium anodic oxide films. Corros. Sci. 2012, 55, 332–338. [Google Scholar] [CrossRef]
- Payus, C.M.; Jikilim, C.; Sentian, J. Rainwater chemistry of acid precipitation occurrences due to long-range transboundary haze pollution and prolonged drought events during southwest monsoon season: Climate change driven. Heliyon 2020, 6, e04997. [Google Scholar] [CrossRef]
- Casas, G.; Martinez-Varela, A.; Vila-Costa, M.; Jiménez, B.; Dachs, J. Rain amplification of persistent organic pollutants. Environ. Sci. Tech. 2021, 55, 12961–12972. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, R.; Godinez, V.; Amin, M. Performance of thermoplastic elastomeric and thermoset insulators under accelerated acid rain multistress conditions. In Proceedings of the 15th National Power Systems Conference, Bombay, India, 16–18 December 2008; pp. 356–360. [Google Scholar]
- Likens, G.E. Acid Rain. In Fundamentals of Ecosystem Science; Weathers, K.C., Strayer, D.L., Likens, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 259–264. [Google Scholar]
- Barjasteh, E.; Narongdej, P.; Shipley, W.; Denk, J. Development and characterization of graphite nanoplatelets filled copolymer of benzoxazine and epoxy. Polym. Compos. 2020, 41, 3528–3540. [Google Scholar] [CrossRef]
- Lin, H.C.; Chang, H.L.; Wang, C.F.; Huang, C.F.; Chang, F.C. Polybenzoxazine-silica hybrid surface with environmentally responsive wettability behavior. J. Adhes. Sci. Technol. 2009, 23, 503–511. [Google Scholar]
- Kim, G.; Lee, H.; Kim, M.; Kim, D.U. Investigating the effects of nitric acid treatments on the properties of recycled carbon fiber. Polymers 2023, 15, 824. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tao, M.; Liu, J.; Liu, T.; Lu, X.; Xin, Z. Effects of interfacial interaction on corrosion resistance of polybenzoxazine/SiO2 nanocomposite coatings. ACS Appl. Polym. Mater. 2019, 1, 381–391. [Google Scholar] [CrossRef]
- Kadhim, A.; Al-Okbi, A.K.; Jamil, D.M.; Qussay, A.; Al-Amiery, A.A.; Gaaz, T.S.; Kadhum, A.A.H.; Mohamad, A.B.; Nassir, M.H. Experimental and theoretical studies of benzoxazines corrosion inhibitors. Results Phys. 2017, 7, 4013–4019. [Google Scholar] [CrossRef]
- Renaud, A.; Bonnaud, L.; Dumas, L.; Zhang, T.; Paint, Y.; Fasano, F.; Kulyk, O.; Pospisilova, E.; Nysten, B.; Delcorte, A.; et al. A benzoxazine/substituted borazine composite coating: A new resin for improving the corrosion resistance of the pristine benzoxazine coating applied on aluminum. Eur. Polym. J. 2018, 109, 460–472. [Google Scholar] [CrossRef]
- ASTM D790-17; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D2344-22; Standard Test Methods for Short-Beam Strength of Polymer Matrix Composite Materials and Their Laminates. ASTM International: West Conshohocken, PA, USA, 2022.
- Butt, H.J.; Liu, J.; Koynov, K.; Straub, B.; Hinduja, C.; Roismann, I.; Berger, R.; Li, X.; Vollmer, D.; Steffen, W.; et al. Contact angle hysteresis. Curr. Opin. Colloid Interface Sci. 2022, 59, 101574. [Google Scholar] [CrossRef]
- Law, K.Y. Contact angle hysteresis on smooth/flat and rough surfaces. Interpretation, mechanism, and origin. Acc. Mater. Res. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An approach to chemical recycling of epoxy resin cured with amine using nitric acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Sembokuya, H.; Tsuda, K. Chemical recycling of glass fiber reinforced epoxy resin cured with amine using nitric acid. Polymer 2005, 46, 1905–1912. [Google Scholar] [CrossRef]
- Lo, J.N.; Nutt, S.R.; Williams, T.J. Recycling benzoxazine–epoxy composites via catalytic oxidation. ACS Sustain. Chem. Eng. 2018, 6, 7227–7231. [Google Scholar] [CrossRef]
- Afzal, A.; Bangash, M.K.; Hafeez, A.; Shaker, K. Aging Effects on the Mechanical Performance of Carbon Fiber-Reinforced Composites. Int. J. Polym. Sci. 2023, 1, 4379307. [Google Scholar] [CrossRef]
- Yang, T.; Lu, S.; Song, D.; Zhu, X.; Almira, I.; Liu, J.; Zhu, Y. Effect of Nanofiller on the mechanical properties of carbon fiber/epoxy composites under different aging conditions. Materials 2021, 14, 7810. [Google Scholar] [CrossRef]
- Roseno, S.; Ammarullah, M.I.; Rohman, S.; Kurniawati, F.; Wahyudi, T.; Wargadipura, A.H.S.; Masmui, M.; Budiyanto, D.; Effendi, M.D.; Wahyudin, W.; et al. The effects of carbon fiber surface treatment by oxidation process for enhanced mechanical properties of carbon fiber/epoxy composites for biomedical application. AIP Adv. 2024, 14, 015044. [Google Scholar] [CrossRef]
- Soucek, M.D.; Teng, G.; Wu, S. Cycloaliphatic epoxide crosslinkable core-shell latexes: A new strategy for waterborne epoxide coatings. J. Coat. Technol. 2001, 73, 117–125. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Z.; Huang, J.; Han, D.; Qiao, Z.; Liu, H.; Du, J.; Yan, H.; Ma, Y.; Zhang, C.; et al. Synthesis, curing mechanism, thermal stability, and surface properties of fluorinated polybenzoxazines for coating applications. Adv. Compos. Mater. 2022, 5, 322–334. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narongdej, P.; Gomez, R.; Tseng, D.; Barjasteh, E.; Moghtadernejad, S. Characterization of Mechanical Properties and Surface Wettability of Epoxy Resin/Benzoxazine Composites in a Simulated Acid Rain Environment. Coatings 2024, 14, 1279. https://doi.org/10.3390/coatings14101279
Narongdej P, Gomez R, Tseng D, Barjasteh E, Moghtadernejad S. Characterization of Mechanical Properties and Surface Wettability of Epoxy Resin/Benzoxazine Composites in a Simulated Acid Rain Environment. Coatings. 2024; 14(10):1279. https://doi.org/10.3390/coatings14101279
Chicago/Turabian StyleNarongdej, Poom, Riley Gomez, Daniel Tseng, Ehsan Barjasteh, and Sara Moghtadernejad. 2024. "Characterization of Mechanical Properties and Surface Wettability of Epoxy Resin/Benzoxazine Composites in a Simulated Acid Rain Environment" Coatings 14, no. 10: 1279. https://doi.org/10.3390/coatings14101279








