Hybrid Activity of P–Si–N Moieties for Improved Fire Retardancy of Cotton Fabric Coated Using Sol-Gel Process
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of Sol
2.3. Cotton Treatment
2.4. Characterization Techniques
3. Results and Discussion
3.1. Microstructural and Physical Properties
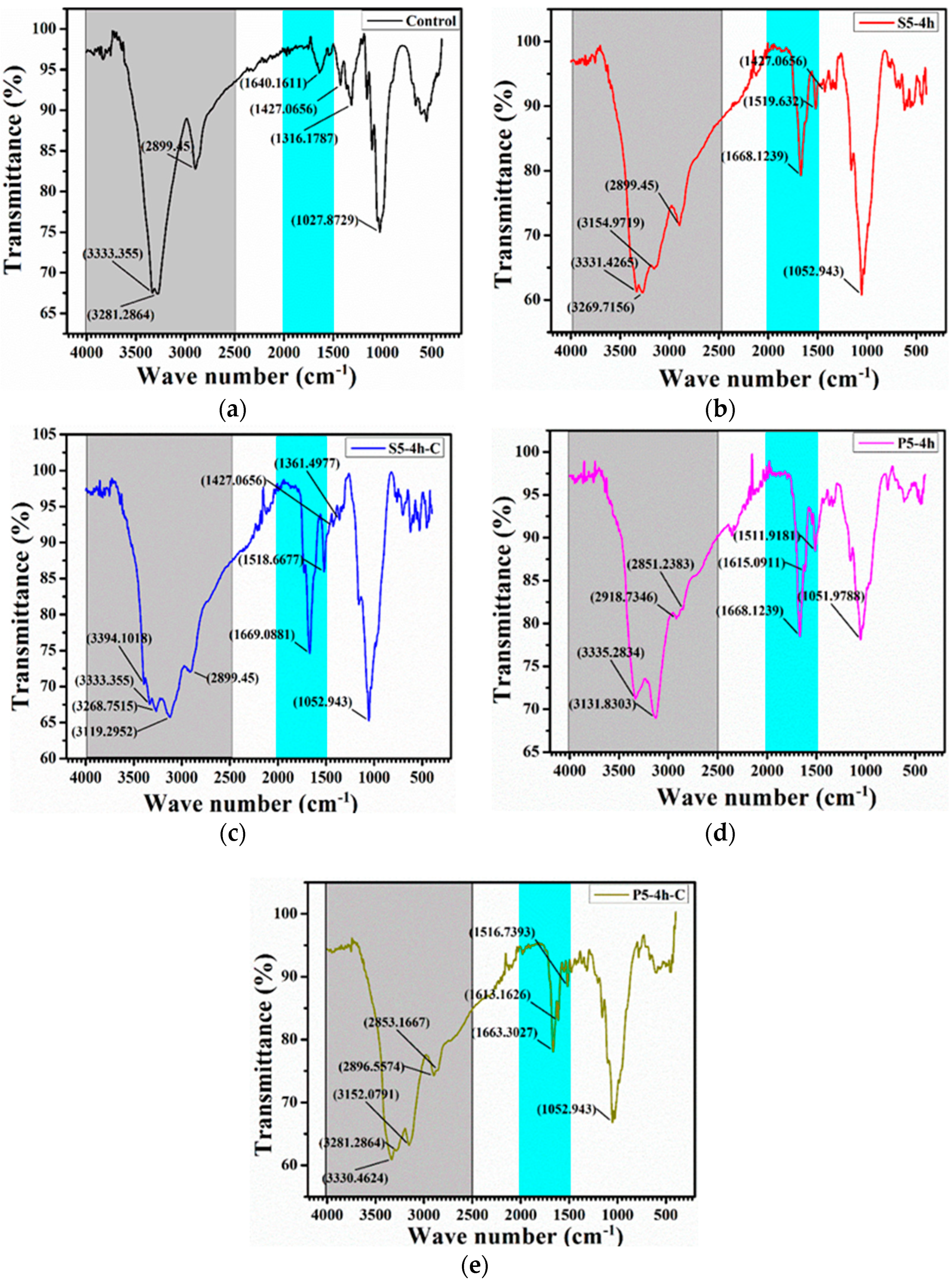
3.2. Compositional Analysis
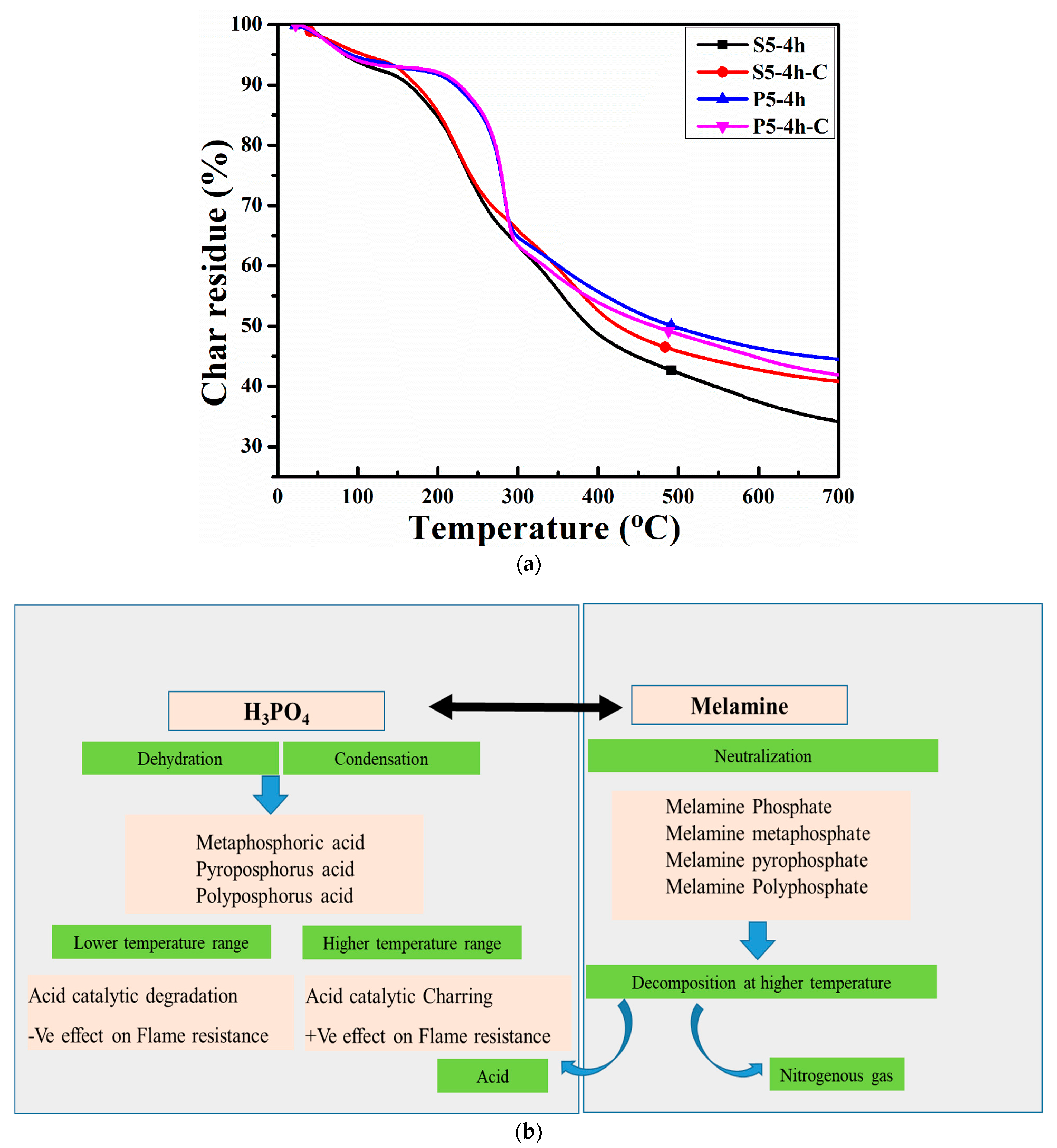
3.3. Thermal Analysis




3.4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, S.-Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Horrocks, A.R. Flame-retardant Finishing of Textiles. Rev. Prog. Color. Relat. Top. 1986, 16, 62–101. [Google Scholar] [CrossRef]
- Nabil, B.; Ahmida, E.A.; Christine, C.; Julien, V.; Abdelkrim, A. Polyfunctional cotton fabrics with catalytic activity and antibacterial capacity. Chem. Eng. J. 2018, 351, 328–339. [Google Scholar] [CrossRef]
- Wang, W.; Wang, J.; Wang, X.; Wang, S.; Liu, X.; Qi, P.; Li, H.; Sun, J.; Tang, W.; Zhang, S.; et al. Improving flame retardancy and self-cleaning performance of cotton fabric via a coating of in-situ growing layered double hydroxides (LDHs) on polydopamine. Prog. Org. Coat. 2020, 149, 105930. [Google Scholar] [CrossRef]
- Horrocks, A.R. Flame retardant challenges for textiles and fibres: New chemistry versus innovatory solutions. Polym. Degrad. Stab. 2011, 96, 377–392. [Google Scholar] [CrossRef]
- Birnbaum, L.S.; Staskal, D.F. Brominated flame retardants: Cause for concern? Environ. Health Perspect. 2004, 112, 9–17. [Google Scholar] [CrossRef]
- de Wit, C.A. An overview of brominated flame retardants in the environment. Chemosphere 2002, 46, 583–624. [Google Scholar] [CrossRef]
- Horrocks, A.R. Flame retardant finishes and finishing. Text. Finish. 2003, 2, 214–250. [Google Scholar]
- Li, Y.-C.; Schulz, J.; Mannen, S.; Delhom, C.; Condon, B.; Chang, S.; Zammarano, M.; Grunlan, J.C. Flame Retardant Behavior of Polyelectrolyte−Clay Thin Film Assemblies on Cotton Fabric. ACS Nano 2010, 4, 3325–3337. [Google Scholar] [CrossRef]
- Li, Y.-C.; Mannen, S.; Morgan, A.B.; Chang, S.; Yang, Y.-H.; Condon, B.; Grunlan, J.C. Intumescent All-Polymer Multilayer Nanocoating Capable of Extinguishing Flame on Fabric. Adv. Mater. 2011, 23, 3926–3931. [Google Scholar] [CrossRef]
- Chang, S.; Slopek, R.P.; Condon, B.; Grunlan, J.C. Surface Coating for Flame-Retardant Behavior of Cotton Fabric Using a Continuous Layer-by-Layer Process. Ind. Eng. Chem. Res. 2014, 53, 3805–3812. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Alongi, J.; Malucelli, G. Green DNA-based flame retardant coatings assembled through Layer by Layer. Polymer 2013, 54, 5148–5153. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Niaz, A.K.; Song, J.-I.; Koo, B.H. Excellent Fire Retardant Properties of CNF/VMT Based LBL Coatings Deposited on Polypropylene and Wood-Ply. Polymers 2021, 13, 303. [Google Scholar] [CrossRef]
- Song, K.; Hou, B.; Ur Rehman, Z.; Pan, Y.-T.; He, J.; Wang, D.-Y.; Yang, R. “Sloughing” of metal-organic framework retaining nanodots via step-by-step carving and its flame-retardant effect in epoxy resin. Chem. Eng. J. 2022, 448, 137666. [Google Scholar] [CrossRef]
- Hou, B.; Song, K.; Ur Rehman, Z.; Song, T.; Lin, T.; Zhang, W.; Pan, Y.-T.; Yang, R. Precise Control of a Yolk-Double Shell Metal–Organic Framework-Based Nanostructure Provides Enhanced Fire Safety for Epoxy Nanocomposites. ACS Appl. Mater. Interfaces 2022, 14, 14805–14816. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Kaseem, M.; Churchill, D.G.; Pan, Y.-T.; Heun Koo, B. Macro and micro thermal investigation of nanoarchitectonics-based coatings on cotton fabric using new quaternized starch. RSC Adv. 2022, 12, 2888–2900. [Google Scholar] [CrossRef]
- Ur Rehman, Z.; Huh, S.-H.; Ullah, Z.; Pan, Y.-T.; Churchill, D.G.; Koo, B.H. LBL generated fire retardant nanocomposites on cotton fabric using cationized starch-clay-nanoparticles matrix. Carbohydr. Polym. 2021, 274, 118626. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Q.; Wu, W. Combination of a hydroxy-functional organophosphorus oligomer and a multifunctional carboxylic acid as a flame retardant finishing system for cotton: Part I. The chemical reactions. Fire Mater. 2003, 27, 223–237. [Google Scholar] [CrossRef]
- Wu, W.; Yang, C.Q. Comparison of different reactive organophosphorus flame retardant agents for cotton: Part I. The bonding of the flame retardant agents to cotton. Polym. Degrad. Stab. 2006, 91, 2541–2548. [Google Scholar] [CrossRef]
- Yang, C.Q.; He, Q. Applications of micro-scale combustion calorimetry to the studies of cotton and nylon fabrics treated with organophosphorus flame retardants. J. Anal. Appl. Pyrolysis 2011, 91, 125–133. [Google Scholar] [CrossRef]
- Chang, S.; Sachinvala, N.D.; Sawhney, P.; Parikh, D.V.; Jarrett, W.; Grimm, C. Epoxy phosphonate crosslinkers for providing flame resistance to cotton textiles. Polym. Adv. Technol. 2007, 18, 611–619. [Google Scholar] [CrossRef]
- Shi, Y.; Nie, C.; Jiang, S.; Wang, H.; Feng, Y.; Gao, J.; Tang, L.; Song, P. Tunable construction of fire safe and mechanically strong hierarchical composites towards electromagnetic interference shielding. J. Colloid Interface Sci. 2023, 652, 1554–1567. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, C.; Duan, Z.; Yu, B.; Liu, M.; Song, P. Interface engineering of MXene towards super-tough and strong polymer nanocomposites with high ductility and excellent fire safety. Chem. Eng. J. 2020, 399, 125829. [Google Scholar] [CrossRef]
- Alongi, J.; Milnes, J.; Malucelli, G.; Bourbigot, S.; Kandola, B. Thermal degradation of DNA-treated cotton fabrics under different heating conditions. J. Anal. Appl. Pyrolysis 2014, 108, 212–221. [Google Scholar] [CrossRef]
- Carosio, F.; Di Blasio, A.; Cuttica, F.; Alongi, J.; Malucelli, G. Flame Retardancy of Polyester and Polyester–Cotton Blends Treated with Caseins. Ind. Eng. Chem. Res. 2014, 53, 3917–3923. [Google Scholar] [CrossRef]
- Xing, W.; Jie, G.; Song, L.; Hu, S.; Lv, X.; Wang, X.; Hu, Y. Flame retardancy and thermal degradation of cotton textiles based on UV-curable flame retardant coatings. Thermochim. Acta 2011, 513, 75–82. [Google Scholar] [CrossRef]
- Opwis, K.; Wego, A.; Bahners, T.; Schollmeyer, E. Permanent flame retardant finishing of textile materials by a photochemical immobilization of vinyl phosphonic acid. Polym. Degrad. Stab. 2011, 96, 393–395. [Google Scholar] [CrossRef]
- Tsafack, M.J.; Levalois-Grützmacher, J. Flame retardancy of cotton textiles by plasma-induced graft-polymerization (PIGP). Surf. Coat. Technol. 2006, 201, 2599–2610. [Google Scholar] [CrossRef]
- Totolin, V.; Sarmadi, M.; Manolache, S.O.; Denes, F.S. Atmospheric pressure plasma enhanced synthesis of flame retardant cellulosic materials. J. Appl. Polym. Sci. 2010, 117, 281–289. [Google Scholar] [CrossRef]
- Brancatelli, G.; Colleoni, C.; Massafra, M.R.; Rosace, G. Effect of hybrid phosphorus-doped silica thin films produced by sol-gel method on the thermal behavior of cotton fabrics. Polym. Degrad. Stab. 2011, 96, 483–490. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments on cotton fabrics for improving thermal and flame stability: Effect of the structure of the alkoxysilane precursor. Carbohydr. Polym. 2012, 87, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Thermal stability, flame retardancy and mechanical properties of cotton fabrics treated with inorganic coatings synthesized through sol–gel processes. Carbohydr. Polym. 2012, 87, 2093–2099. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Malucelli, G.; Rosace, G. Hybrid phosphorus-doped silica architectures derived from a multistep sol–gel process for improving thermal stability and flame retardancy of cotton fabrics. Polym. Degrad. Stab. 2012, 97, 1334–1344. [Google Scholar] [CrossRef]
- Zhou, T.; He, X.; Guo, C.; Yu, J.; Lu, D.; Yang, Q. Synthesis of a novel flame retardant phosphorus/nitrogen/siloxane and its application on cotton fabrics. Text. Res. J. 2015, 85, 701–708. [Google Scholar] [CrossRef]
- Mahltig, B.; Haufe, H.; Böttcher, H. Functionalisation of textiles by inorganic sol–gel coatings. J. Mater. Chem. 2005, 15, 4385–4398. [Google Scholar] [CrossRef]
- Mahltig, B.; Böttcher, H.; Rauch, K.; Dieckmann, U.; Nitsche, R.; Fritz, T. Optimized UV protecting coatings by combination of organic and inorganic UV absorbers. Thin Solid Film. 2005, 485, 108–114. [Google Scholar] [CrossRef]
- Mahltig, B.; Fiedler, D.; Böttcher, H. Antimicrobial Sol–Gel Coatings. J. Sol-Gel Sci. Technol. 2004, 32, 219–222. [Google Scholar] [CrossRef]
- Xing, Y.; Ding, X. UV photo-stabilization of tetrabutyl titanate for aramid fibers via sol–gel surface modification. J. Appl. Polym. Sci. 2007, 103, 3113–3119. [Google Scholar] [CrossRef]
- Xing, Y.; Yang, X.; Dai, J. Antimicrobial finishing of cotton textile based on water glass by sol–gel method. J. Sol-Gel Sci. Technol. 2007, 43, 187–192. [Google Scholar] [CrossRef]
- Abidi, N.; Hequet, E.; Tarimala, S.; Dai, L.L. Cotton fabric surface modification for improved UV radiation protection using sol–gel process. J. Appl. Polym. Sci. 2007, 104, 111–117. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Sol–gel derived architectures for enhancing cotton flame retardancy: Effect of pure and phosphorus-doped silica phases. Polym. Degrad. Stab. 2014, 99, 92–98. [Google Scholar] [CrossRef]
- Alongi, J.; Colleoni, C.; Rosace, G.; Malucelli, G. Phosphorus- and nitrogen-doped silica coatings for enhancing the flame retardancy of cotton: Synergisms or additive effects? Polym. Degrad. Stab. 2013, 98, 579–589. [Google Scholar] [CrossRef]
- Fidalgo, A.; Ilharco, L.M. The defect structure of sol–gel-derived silica/polytetrahydrofuran hybrid films by FTIR. J. Non-Cryst. Solids 2001, 283, 144–154. [Google Scholar] [CrossRef]
- Vasiliu, I.; Gartner, M.; Anastasescu, M.; Todan, L.; Predoana, L.; Elisa, M.; Negrila, C.; Ungureanu, F.; Logofatu, C.; Moldovan, A.; et al. Structural and optical properties of the SiO2–P2O5 films obtained by sol–gel method. Thin Solid Film. 2007, 515, 6601–6605. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R Rep. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Dobele, G.; Rossinskaja, G.; Telysheva, G.; Meier, D.; Faix, O. Cellulose dehydration and depolymerization reactions during pyrolysis in the presence of phosphoric acid. J. Anal. Appl. Pyrolysis 1999, 49, 307–317. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Woodbridge, C.R.; Jones, J.M. Phosphorus catalysis in the pyrolysis behaviour of biomass. J. Anal. Appl. Pyrolysis 2008, 83, 197–204. [Google Scholar] [CrossRef]
- Babushok, V.; Tsang, W. Inhibitor rankings for alkane combustion. Combust. Flame 2000, 123, 488–506. [Google Scholar] [CrossRef]
- Alongi, J.; Ciobanu, M.; Malucelli, G. Sol–gel treatments for enhancing flame retardancy and thermal stability of cotton fabrics: Optimisation of the process and evaluation of the durability. Cellulose 2011, 18, 167–177. [Google Scholar] [CrossRef]
- Cireli, A.; Onar, N.; Ebeoglugil, M.F.; Kayatekin, I.; Kutlu, B.; Culha, O.; Celik, E. Development of flame retardancy properties of new halogen-free phosphorous doped SiO2 thin films on fabrics. J. Appl. Polym. Sci. 2007, 105, 3748–3756. [Google Scholar] [CrossRef]
- Hribernik, S.; Smole, M.S.; Kleinschek, K.S.; Bele, M.; Jamnik, J.; Gaberscek, M. Flame retardant activity of SiO2-coated regenerated cellulose fibres. Polym. Degrad. Stab. 2007, 92, 1957–1965. [Google Scholar] [CrossRef]
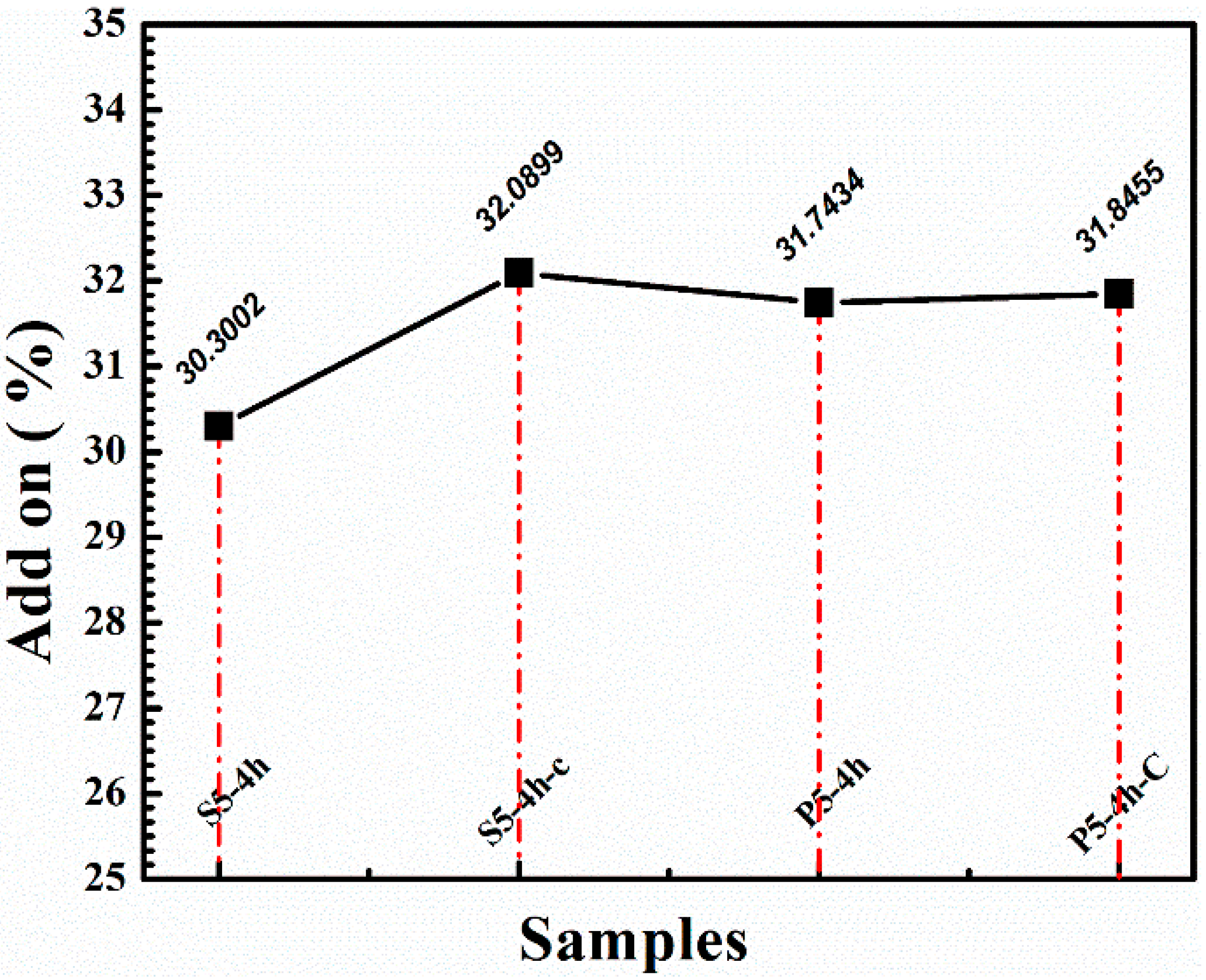



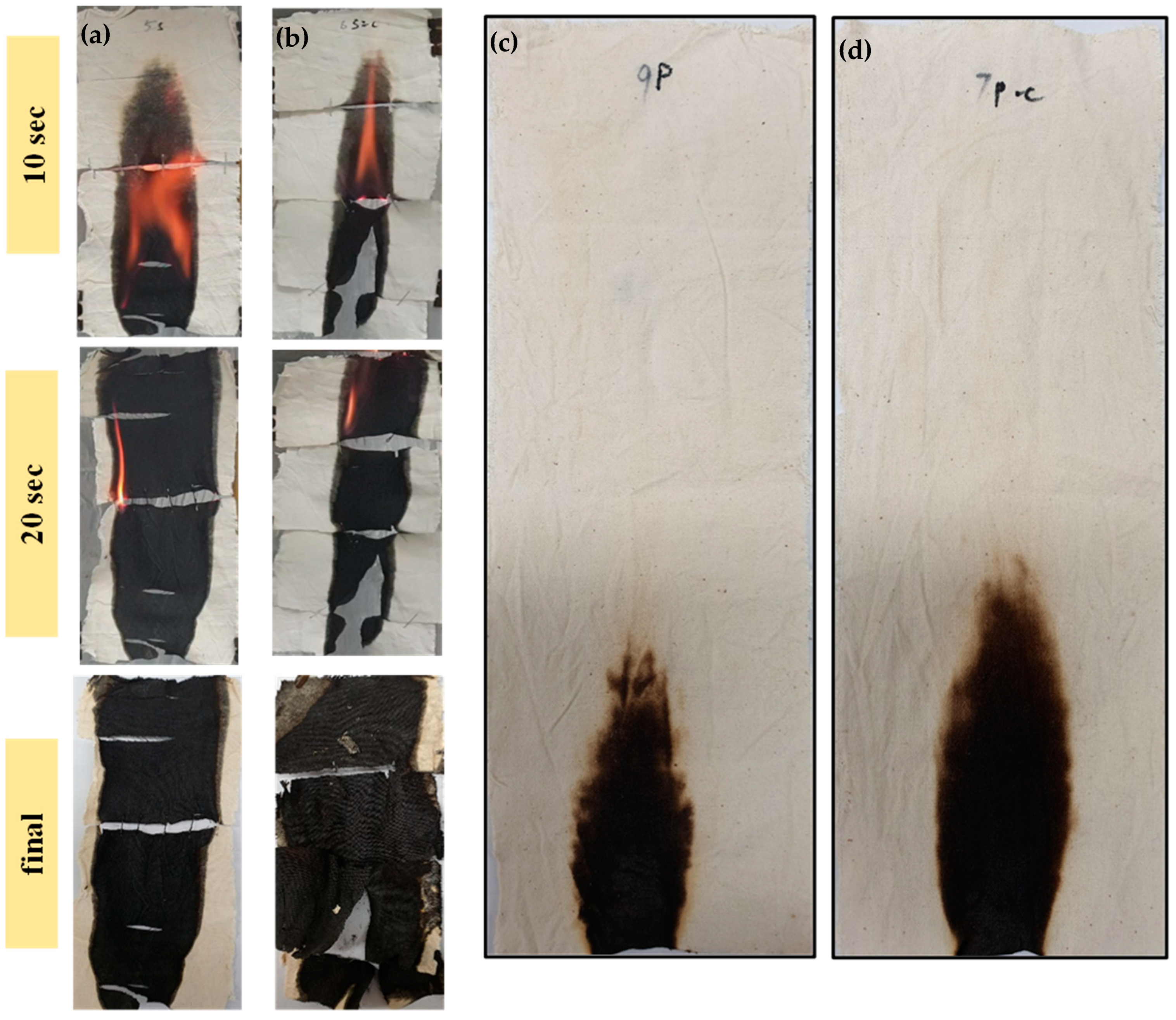
| Samples | Dipping Time (hrs) | Weight before Coating (g) | Weight after Coating (g) | Adds on (%) | Heating Time/T | Curing Time/T |
|---|---|---|---|---|---|---|
| S5-4h | 4 | 3.46 | 4.97 | 30.30 | 12 h/65 °C | 60 s/130 °C |
| S5-4h-C | 4 | 3.49 | 5.14 | 32.11 | 12 h/65 °C | 60 s/130 °C |
| P5-4h | 4 | 3.50 | 5.13 | 31.74 | 12 h/65 °C | 60 s/130 °C |
| P5-4h-C | 4 | 3.39 | 4.97 | 31.84 | 12 h/65 °C | 60 s/130 °C |
| Elements# | S5-4h | S5-4h-C | P5-4h | P5-4h-C |
|---|---|---|---|---|
| C | 44.63 | 41.79 | 46.72 | 46.78 |
| P | 1.38 | 2.11 | ||
| O | 46.62 | 46.37 | 45.91 | 46.67 |
| Si | 6.25 | 8.37 | 5.90 | 4.23 |
| S | 2.36 | 3.26 | ----- | ----- |
| N | 0.14 | 0.21 | 0.09 | 0.21 |
| Total | 100.00 | 100.00 | 100.00 | 100.00 |
| # | Ton | Ron | Toff | Roff | Tpeak1 | Tpeak2 | Char Residue (%) |
|---|---|---|---|---|---|---|---|
| S5-4h | 155.27 | 92.44 | 340.53 | 53.1 | 232.61 | 297.18 | 34.0 |
| S5-4h-C | 146.77 | 95.82 | 355.45 | 56.24 | 226.26 | 281.75 | 40.81 |
| P5-4h | 227.60 | 92.37 | 281.99 | 68.03 | 145.5 | 44.5 | |
| P5-4h-C | 229.87 | 92.59 | 284.07 | 65.84 | 283.87 | 41.99 |
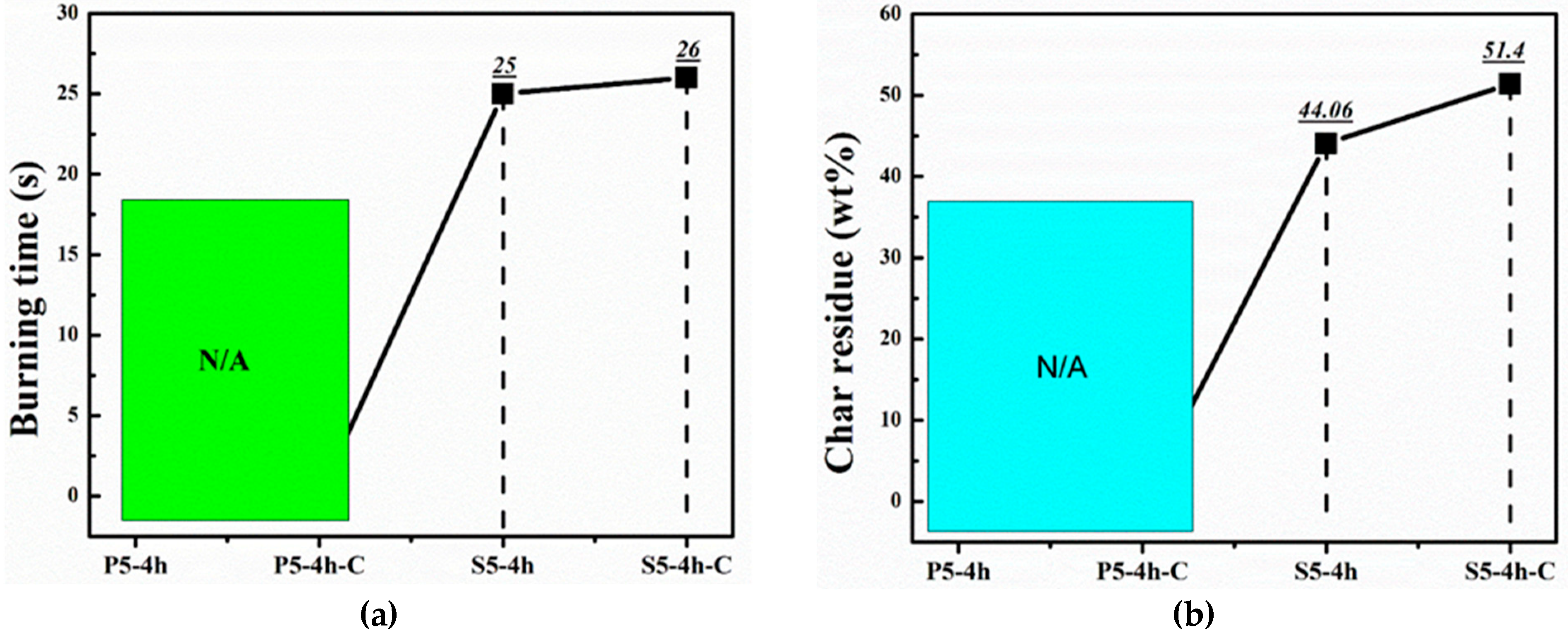
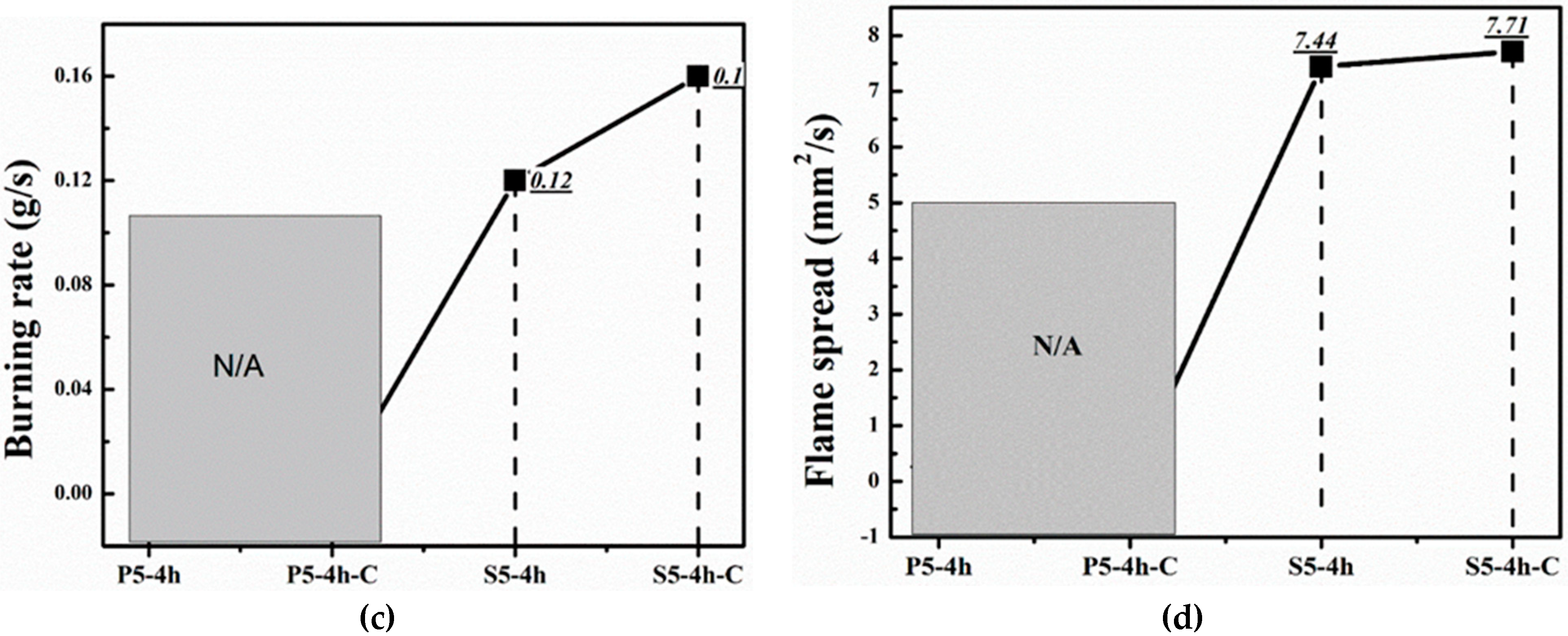
| Samples | Flame Spread (mm2/s) | Char Residue (%) | Burning Rate (g/s) | Burning Time (s) |
|---|---|---|---|---|
| S5-4h | 7.44 | 44.06 | 0.12 | 25 |
| S5-4h-C | 7.71 | 51.40 | 0.16 | 26 |
| P5-4h | Fire Extinguishes | |||
| P5-4h-C | Fire Extinguishes | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, Z.U.; Hassan, H.; Khan, L.; Hwain, L.; Chiho, Y.; Koo, B.H. Hybrid Activity of P–Si–N Moieties for Improved Fire Retardancy of Cotton Fabric Coated Using Sol-Gel Process. Coatings 2024, 14, 1283. https://doi.org/10.3390/coatings14101283
Rehman ZU, Hassan H, Khan L, Hwain L, Chiho Y, Koo BH. Hybrid Activity of P–Si–N Moieties for Improved Fire Retardancy of Cotton Fabric Coated Using Sol-Gel Process. Coatings. 2024; 14(10):1283. https://doi.org/10.3390/coatings14101283
Chicago/Turabian StyleRehman, Zeeshan Ur, Hamid Hassan, Laila Khan, Lee Hwain, Yun Chiho, and Bon Heun Koo. 2024. "Hybrid Activity of P–Si–N Moieties for Improved Fire Retardancy of Cotton Fabric Coated Using Sol-Gel Process" Coatings 14, no. 10: 1283. https://doi.org/10.3390/coatings14101283
APA StyleRehman, Z. U., Hassan, H., Khan, L., Hwain, L., Chiho, Y., & Koo, B. H. (2024). Hybrid Activity of P–Si–N Moieties for Improved Fire Retardancy of Cotton Fabric Coated Using Sol-Gel Process. Coatings, 14(10), 1283. https://doi.org/10.3390/coatings14101283






