Performance of Cobalt-Doped C3N5 Electrocatalysis Nitrate in Ammonia Production
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Apparatus and Reagents
2.2. Synthesis of Catalysts
2.2.1. Preparation of C3N5 Precursor
2.2.2. Preparation of Co-C3N5 Catalysts
2.2.3. Pretreatment of Carbon Paper
2.2.4. Preparation of Working Electrode
2.3. Catalyst Characterization
2.4. Electrochemical Performance Test
2.4.1. Cyclic Voltammetry, CV
2.4.2. Linear Sweep Voltammetry, LSV
2.5. Product Detection Methods
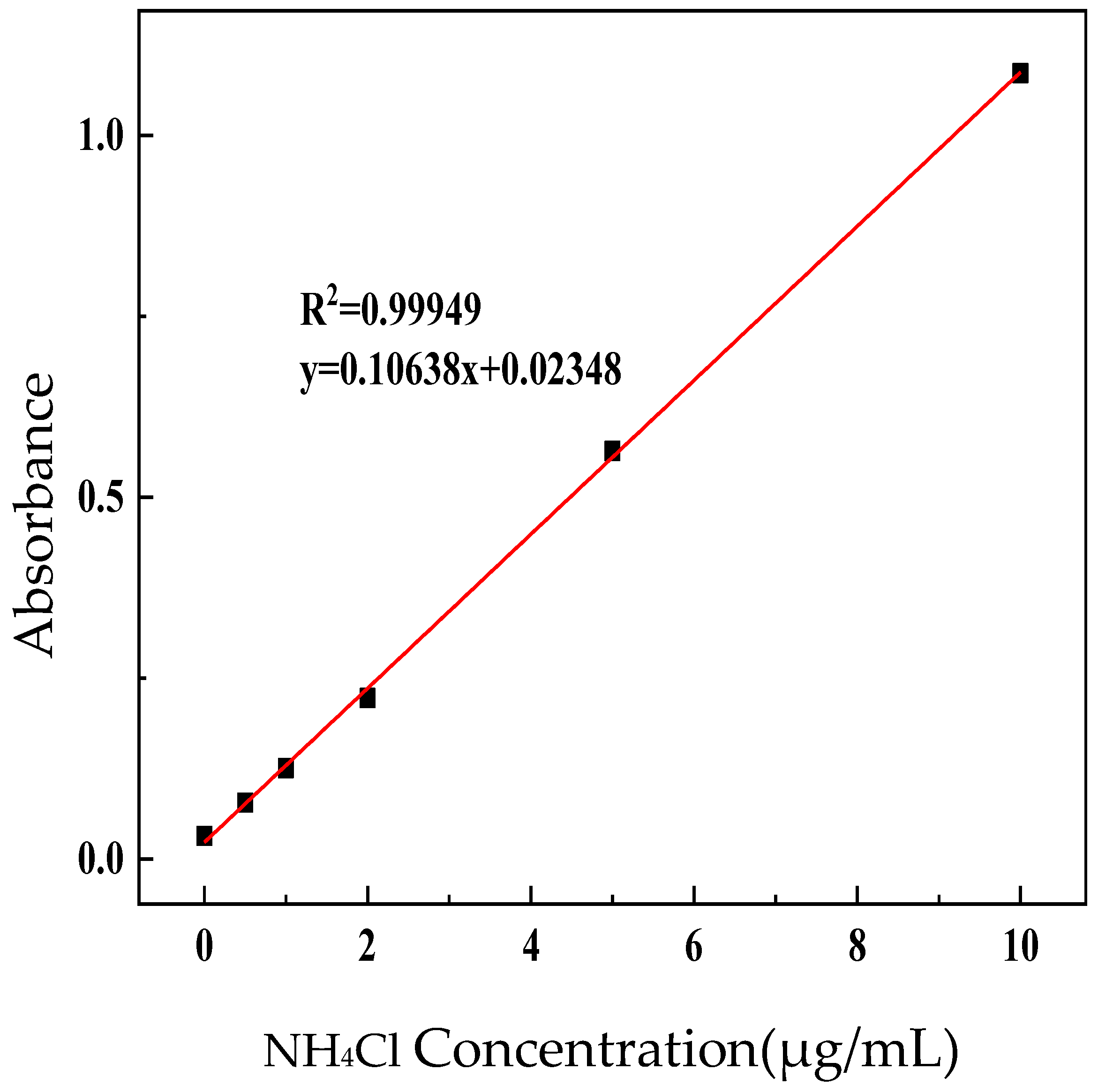
2.5.1. NH4+ Standard Curve
2.5.2. Calculation of Ammonia Yield (Mmol·H−1·Mgcat−1)
2.5.3. Faraday Efficiency Calculation (%)
3. Results and Discussion
3.1. Catalyst Characterization Experiments
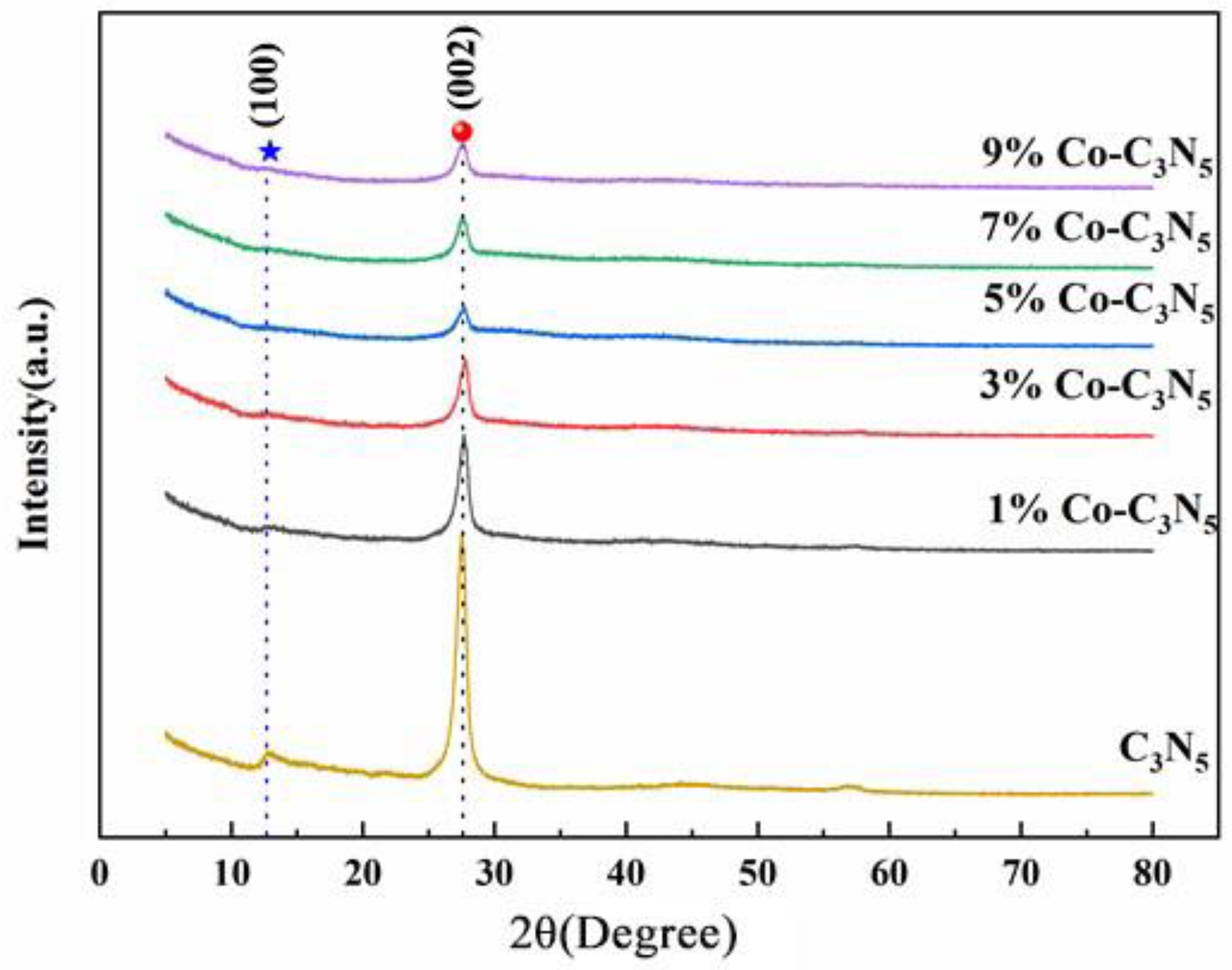
3.1.1. X-ray Diffraction, XRD
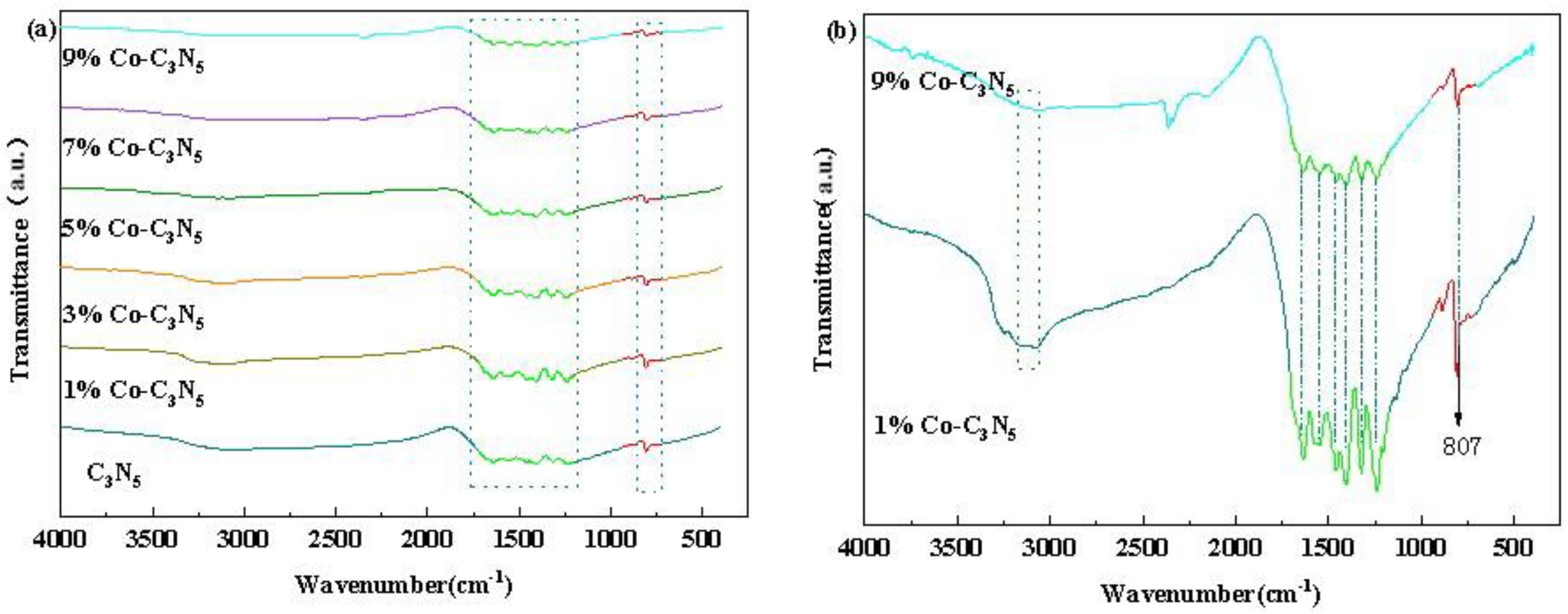
3.1.2. Fourier Transform Infrared, FTIR
3.1.3. X-ray Photoelectron Spectroscopy, XPS
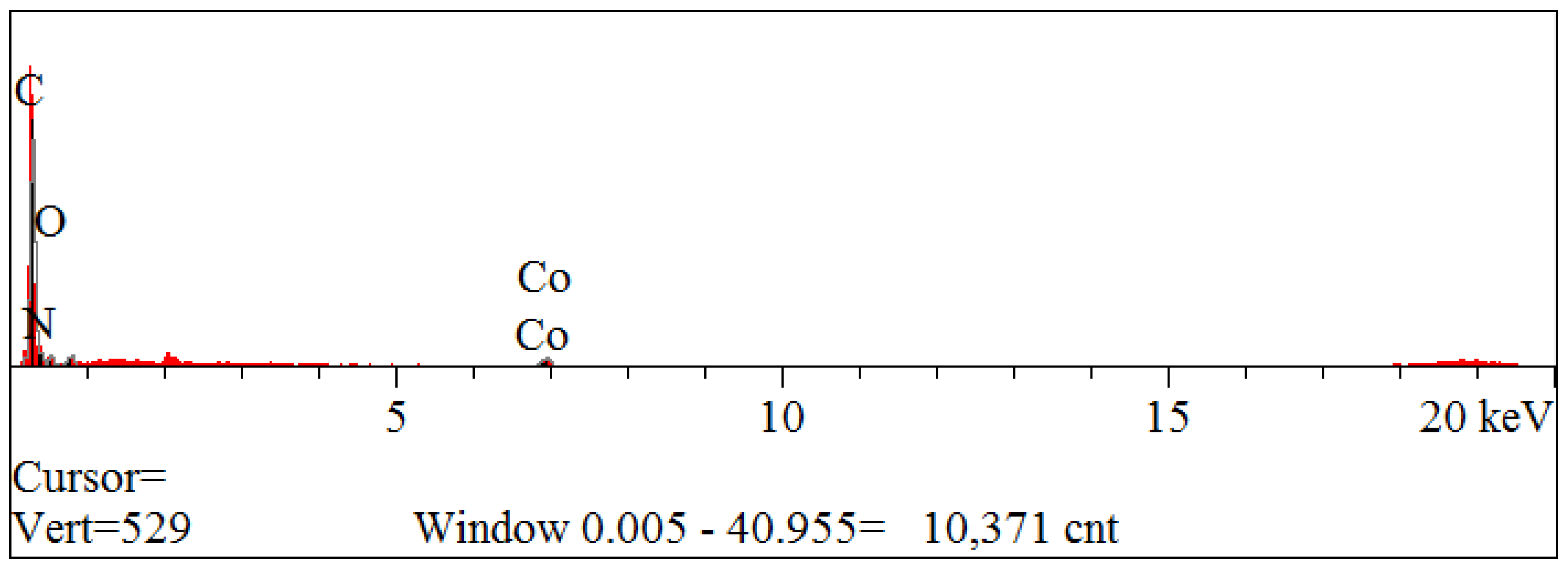
3.1.4. Scanning Electron Microscope and Energy-Dispersive X-ray Spectroscopy, SEM and EDS

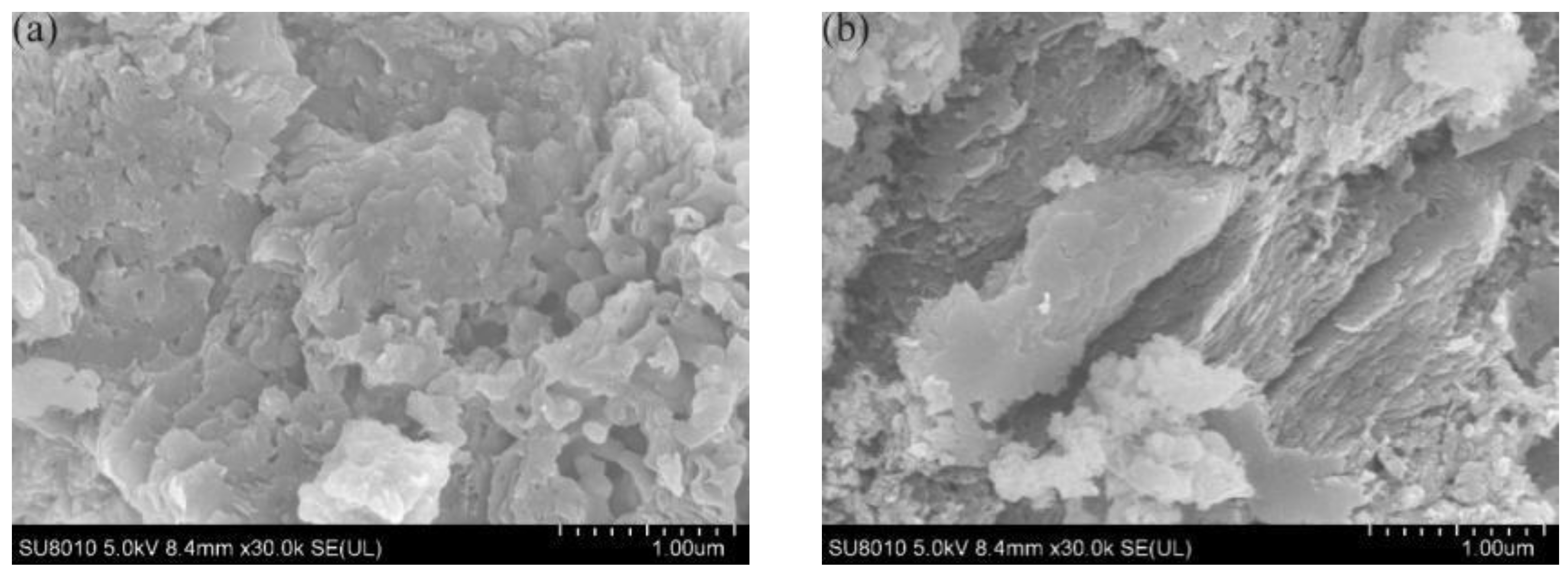

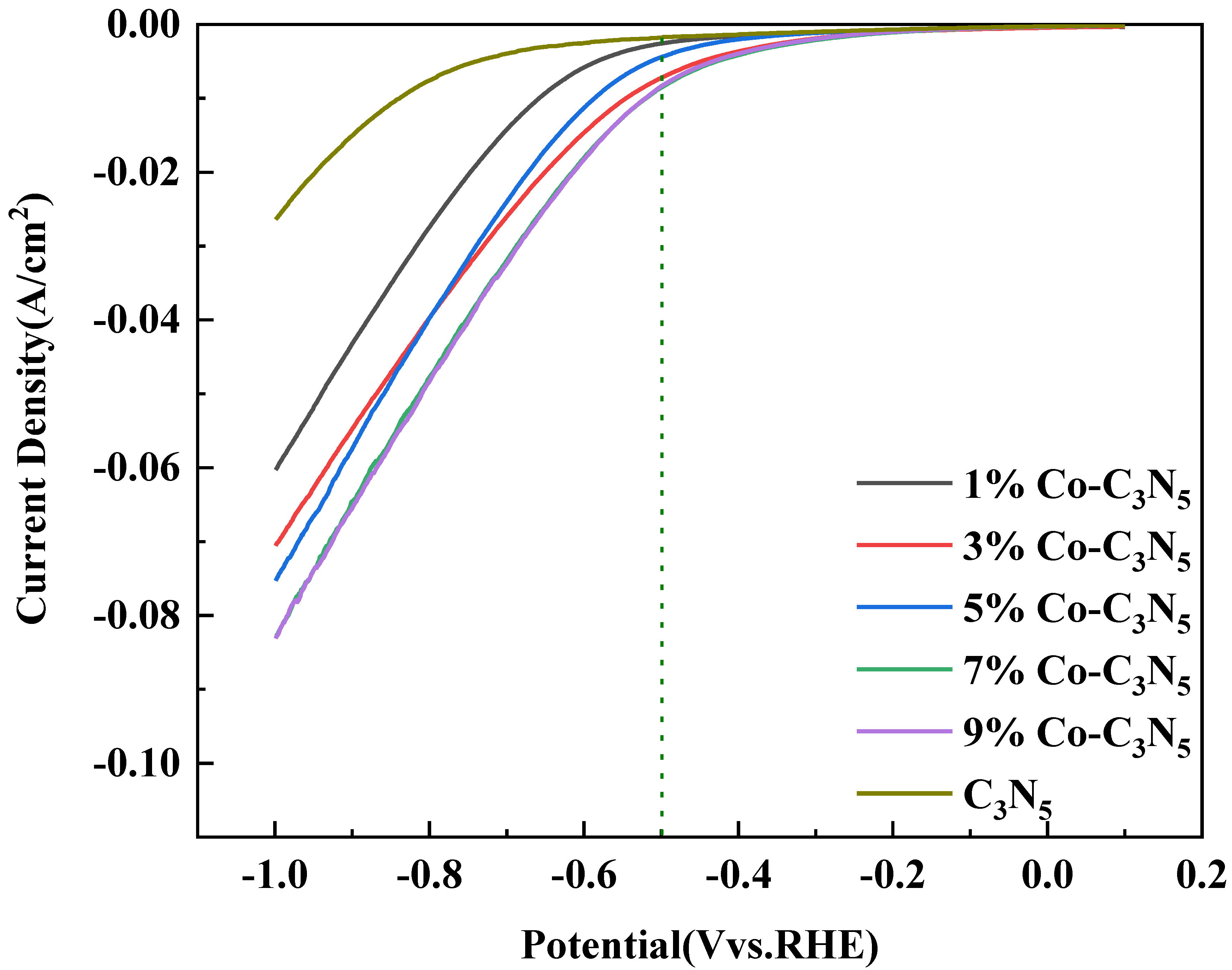
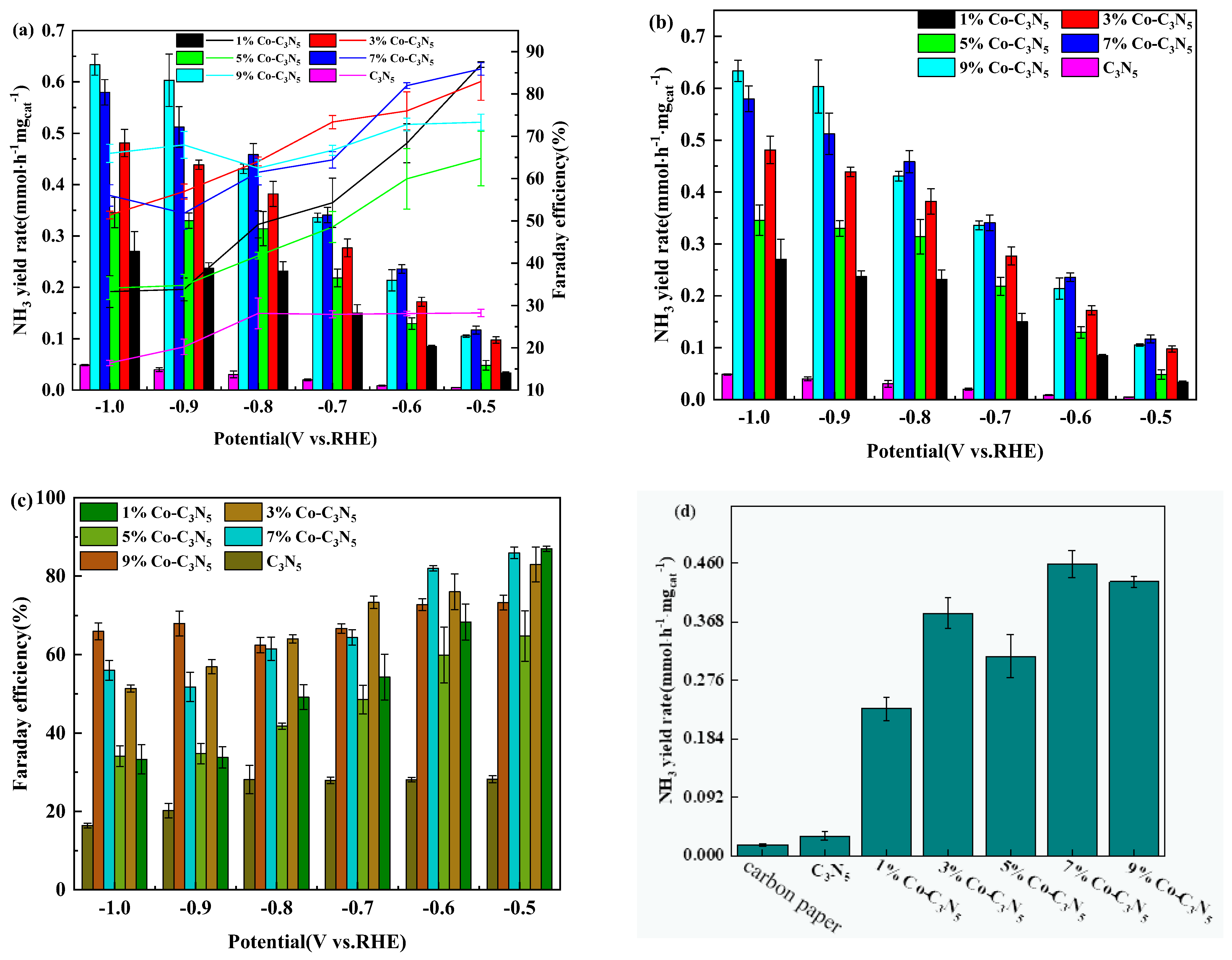
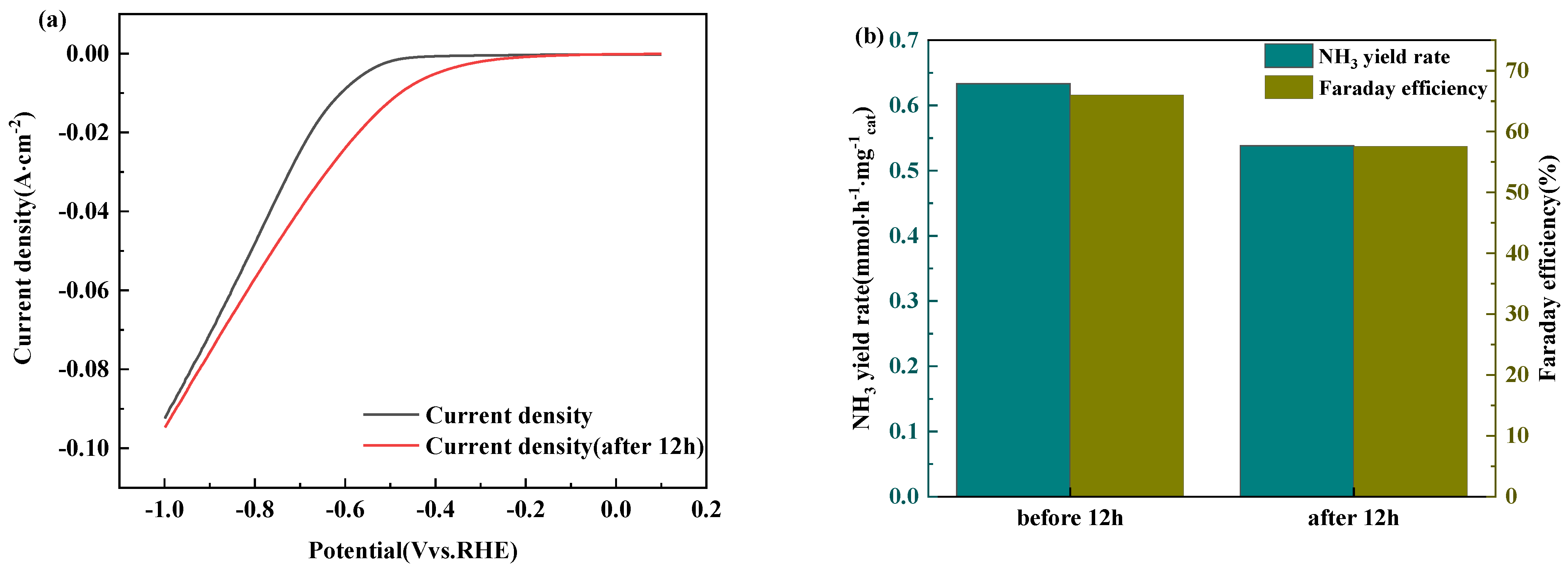
3.2. Co-C3N5 Catalyst Electrochemical Performance Testing Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiong, Y.; Wang, Y.; Zhou, J.; Liu, F.; Hao, F.; Fan, Z. Electrochemical nitrate reduction: Ammonia synthesis and the beyond. Adv. Mater. 2024, 36, 2304021. [Google Scholar] [CrossRef] [PubMed]
- Yu, J. Surface Modification of Porous Graphitic Carbon Nitride and Its Electrocatalytic Ammonia Synthesis Performance. Master’s Thesis, Yanshan University, Qinhuangdao, China, 2022. [Google Scholar]
- Gao, J.; Jiang, B.; Ni, C.; Qi, Y.; Zhang, Y.; Oturan, N.; Oturan, M.A. Non-precious Co3O4-TiO2/Ti cathode based electrocatalytic nitrate reduction: Preparation, performance and mechanism. Appl. Catal. B Environ. 2019, 254, 391–402. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Pan, L.; Pei, P.; Song, X.; Ren, P.; Zhang, L. Cobalt-based oxygen electrocatalysts for zinc-air batteries: Recent progress, challenges, and perspectives. Nano Res. 2022, 15, 5038–5063. [Google Scholar] [CrossRef]
- Cheng, S.H.; Su, Y.O. Electrocatalysis of nitric oxide reduction by water-soluble cobalt porphyrin. Spectral and electrochemical studies. Inorg. Chem. 1994, 33, 5847–5854. [Google Scholar] [CrossRef]
- Guo, Y.; Stroka, J.R.; Kandemir, B.; Dickerson, C.E.; Bren, K.L. Cobalt metallopeptide electrocatalyst for the selective reduction of nitrite to ammonium. J. Am. Chem. Soc. 2018, 140, 16888–16892. [Google Scholar] [CrossRef]
- Wang, J.; Cai, C.; Wang, Y.; Yang, X.; Wu, D.; Zhu, Y.; Li, M.; Gu, M.; Shao, M. Electrocatalytic reduction of nitrate to ammonia on low-cost ultrathin CoOx nanosheets. ACS Catal. 2021, 11, 15135–15140. [Google Scholar] [CrossRef]
- Hong, Q.L.; Zhou, J.; Zhai, Q.G.; Jiang, Y.C.; Hu, M.C.; Xiao, X.; Li, S.N.; Chen, Y. Cobalt phosphide nanorings towards efficient electrocatalytic nitrate reduction to ammonia. Chem. Commun. 2021, 57, 11621–11624. [Google Scholar] [CrossRef]
- Deng, X.; Yang, Y.; Wang, L.; Fu, X.Z.; Luo, J.L. Metallic Co nanoarray catalyzes selective NH3 production from electrochemical nitrate reduction at current densities exceeding 2 A cm−2. Adv. Sci. 2021, 8, 2004523. [Google Scholar] [CrossRef]
- Wang, J.; Feng, T.; Chen, J.; Ramalingam, V.; Li, Z.; Kabtamu, D.M.; He, J.H.; Fang, X. Electrocatalytic nitrate/nitrite reduction to ammonia synthesis using metal nanocatalysts and bio-inspired metalloenzymes. Nano Energy 2021, 86, 106088. [Google Scholar] [CrossRef]
- Xu, H.; Ci, S.; Ding, Y.; Wang, G.; Wen, Z. Recent advances in precious metal-free bifunctional catalysts for electrochemical conversion systems. J. Mater. Chem. A 2019, 7, 8006–8029. [Google Scholar] [CrossRef]
- Sun, X.W.; Bai, J.; Li, C.P.; Liang, H.O.; Xu, T.; Sun, W.Y. Modification strategy and application of cobalt-based electrode materials. Acta Mater. Compos. Sin. 2023, 40, 2550–2565. [Google Scholar] [CrossRef]
- Agayev, F.G.; Trukhanov, S.V.; Trukhanov, A.V.; Jabarov, S.H.; Ayyubova, G.S.; Mirzayev, M.N.; Trukhanova, E.L.; Vinnik, D.A.; Kozlovskiy, A.L.; Zdorovets, M.V.; et al. Study of structural features and thermal properties of barium hexaferrite upon indium doping. J. Therm. Anal. Calor. 2022, 147, 14107–14114. [Google Scholar] [CrossRef]
- Wang, S.; Chen, S.; Ma, L.; Zapien, J.A. Recent progress in cobalt-based carbon materials as oxygen electrocatalysts for zinc-air battery applications. Mater. Today Energy 2021, 20, 100659. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Panina, L.V.; Trukhanov, S.V.; Kostishyn, V.G.; Turchenko, V.A.; Vinnik, D.A.; Zubar, T.I.; Yakovenko, E.S.; Macuy, L.Y.; Trukhanova, E.L. Critical influence of different diamagnetic ions on electromagnetic properties of BaFe12O19. Ceram Int. 2018, 44, 13520–13529. [Google Scholar] [CrossRef]
- He, P.; Gu, C.; Tang, B.; Zhou, Y.; Gan, M.; Zhu, J. Expeditious degradation of SMX by high-valent cobalt-oxo species derived from cobalt-doped C3N5-activated peroxymonosulfate with the assistance of visible light. Sep. Purif. Technol. 2022, 301, 122009. [Google Scholar] [CrossRef]
- Li, M.; Lu, Q.; Liu, M.; Yin, P.; Wu, C.; Li, H.; Zhang, Y.; Yao, S. Photoinduced charge separation via the double-electron transfer mechanism in nitrogen vacancies g-C3N5/BiOBr for the photoelectrochemical nitrogen reduction. ACS Appl. Mater. Interfaces 2020, 12, 38266–38274. [Google Scholar] [CrossRef]
- Liu, T.; Yang, G.; Wang, W.; Wang, C.; Wang, M.; Sun, X.; Xu, P.; Zhang, J. Preparation of C3N5 nanosheets with enhanced performance in photocatalytic methylene blue (MB) degradation and H2-evolution from water splitting. Environ. Res. 2020, 188, 109741. [Google Scholar] [CrossRef]
- Hu, X.; Zeng, X.; Liu, Y.; Lu, J.; Yuan, S.; Yin, Y.; Hu, J.; McCarthy, D.T.; Zhang, X. Nano-layer based 1T-rich MoS2/g-C3N4 co-catalyst system for enhanced photocatalytic and photoelectrochemical activity. Appl. Catal. B Environ. 2020, 268, 118466. [Google Scholar] [CrossRef]
- Wei, W.; Gong, H.; Sheng, L.; Wu, H.; Zhu, S.; Feng, L.; Li, X.; You, W. Highly efficient photocatalytic activity and mechanism of novel Er3+ and Tb3+ co-doped BiOBr/g-C3N5 towards sulfamethoxazole degradation. Ceram. Int. 2021, 47, 24062–24072. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.; Jin, X.; Premkumar, S.; Chandra, G.; Lee, N.S.; Mane, G.P.; Hwang, S.J.; Umapathy, S.; Vinu, A. Ordered Mesoporous C3N5 with a Combined Triazole and Triazine Framework and Its Graphene Hybrids for the Oxygen Reduction Reaction (ORR). Angew. Chem. 2018, 130, 17381–17386. [Google Scholar] [CrossRef]
- Sathish, C.I.; Premkumar, S.; Chu, X.; Yu, X.; Breese, M.B.; Al-Abri, M.; Al-Muhtaseb, A.A.; Karakoti, A.; Yi, J.; Vinu, A. Microporous Carbon Nitride (C3N5.4) with Tetrazine based Molecular Structure for Efficient Adsorption of CO2 and Water. Angew. Chem. 2021, 133, 21412–21419. [Google Scholar] [CrossRef]
- Chen, W.; He, Z.C.; Huang, G.B.; Wu, C.L.; Chen, W.F.; Liu, X.H. Direct Z-scheme 2D/2D MnIn2S4/g-C3N4 architectures with highly efficient photocatalytic activities towards treatment of pharmaceutical wastewater and hydrogen evolution. Chem. Eng. J. 2019, 359, 244–253. [Google Scholar] [CrossRef]
- Wang, B. The Study on the Photocatalytic Activity of ZnO/C3N5 and B-C3N5. Master’s Thesis, Shanxi University, Taiyuan, China, 2021. [Google Scholar]
- Gong, Y.N.; Jiao, L.; Qian, Y.Y.; Pan, C.Y.; Zheng, L.R.; Cai, X.C.; Liu, B.; Yu, S.H.; Jiang, H.L. Regulating the coordination environment of MOF-templated single-atom nickel electrocatalysts for boosting CO2 reduction. Angew. Chem. Int. Ed. 2020, 59, 2705–2709. [Google Scholar] [CrossRef]
- Mane, G.P.; Talapaneni, S.N.; Lakhi, K.S.; Ilbeygi, H.; Ravon, U.; Al-Bahily, K.; Mori, T.; Park, D.H.; Vinu, A. Highly ordered nitrogen-rich mesoporous carbon nitrides and their superior performance for sensing and photocatalytic hydrogen generation. Angew. Chem. Int. Ed. 2017, 56, 8481–8485. [Google Scholar] [CrossRef]
- Song, T.L.; Zou, M.S.; Lu, D.F. Textbook of X-ray Photoelectron Spectroscopy Data Analysis; Beijing Institute of Technology Press: Beijing, China, 2022. [Google Scholar]
- Yang, M.; Wu, D.; Cheng, D. Biomass-derived porous carbon supported CoCoO yolk-shell nanoparticles as enhanced multifunctional electrocatalysts. Int. J. Hydrogen Energy 2019, 44, 6525–6534. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Vasiliev, A.N.; Szymczak, H. Frustrated exchange interactions formation at low temperatures and high hydrostatic pressures in La0.70Sr0.30MnO2.85. J. Exp. Theor. Phys. 2010, 111, 209–214. [Google Scholar] [CrossRef]
- Huang, P.; Huang, J.; Pantovich, S.A.; Carl, A.D.; Fenton, T.G.; Caputo, C.A.; Grimm, R.L.; Frenkel, A.I.; Li, G. Selective CO2 reduction catalyzed by single cobalt sites on carbon nitride under visible-light irradiation. J. Am. Chem. Soc. 2018, 140, 16042–16047. [Google Scholar] [CrossRef]
- Shi, Y.B. Preparation of Cobalt and Iron-Based Electrocatalysts and Their Application in Electrochemical Ammonia Synthesis. Master’s Thesis, Dalian University of Technology, Dalian, China, 2022. [Google Scholar]
- Kozhukh, J.; Lippard, S.J. Influence of tetraazamacrocyclic ligands on the nitric oxide reactivity of their cobalt (II) complexes. J. Am. Chem. Soc. 2012, 134, 11120–11123. [Google Scholar] [CrossRef]
- Rhine, M.A.; Rodrigues, A.V.; Urbauer, R.J.; Urbauer, J.L.; Stemmler, T.L.; Harrop, T.C. Proton-induced reactivity of NO− from a {CoNO}8 complex. J. Am. Chem. Soc. 2014, 136, 12560–12563. [Google Scholar] [CrossRef]
- Walter, M.R.; Dzul, S.P.; Rodrigues, A.V.; Stemmler, T.L.; Telser, J.; Conradie, J.; Ghosh, A.; Harrop, T.C. Synthesis of CoII–NO−complexes and their reactivity as a source of nitroxyl. J. Am. Chem. Soc. 2016, 138, 12459–12471. [Google Scholar] [CrossRef]

| Elt. | Intensity (c/s) | Atomic % | Conc. | Units | |
|---|---|---|---|---|---|
| C | 326.18 | 60.363 | 53.094 | wt.% | |
| N | 15.20 | 25.145 | 25.792 | wt.% | |
| O | 17.89 | 13.177 | 15.440 | wt.% | |
| Co | 23.31 | 1.315 | 5.674 | wt.% | |
| 100.000 | 100.000 | wt.% | Total |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, B.; Wu, Y.; Han, J.; Deng, W.; Zhang, X.; Li, R.; Hong, Y.; Du, J.; Fu, L.; Liao, R. Performance of Cobalt-Doped C3N5 Electrocatalysis Nitrate in Ammonia Production. Coatings 2024, 14, 1327. https://doi.org/10.3390/coatings14101327
Liang B, Wu Y, Han J, Deng W, Zhang X, Li R, Hong Y, Du J, Fu L, Liao R. Performance of Cobalt-Doped C3N5 Electrocatalysis Nitrate in Ammonia Production. Coatings. 2024; 14(10):1327. https://doi.org/10.3390/coatings14101327
Chicago/Turabian StyleLiang, Boyu, Yueqi Wu, Jing Han, Wenqiang Deng, Xinyao Zhang, Runrun Li, Yan Hong, Jie Du, Lichun Fu, and Runhua Liao. 2024. "Performance of Cobalt-Doped C3N5 Electrocatalysis Nitrate in Ammonia Production" Coatings 14, no. 10: 1327. https://doi.org/10.3390/coatings14101327
APA StyleLiang, B., Wu, Y., Han, J., Deng, W., Zhang, X., Li, R., Hong, Y., Du, J., Fu, L., & Liao, R. (2024). Performance of Cobalt-Doped C3N5 Electrocatalysis Nitrate in Ammonia Production. Coatings, 14(10), 1327. https://doi.org/10.3390/coatings14101327





