Preparation of Environmentally Friendly Anticorrosive Coatings with Aniline Trimer-Modified Waterborne Polyurethane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methodologies
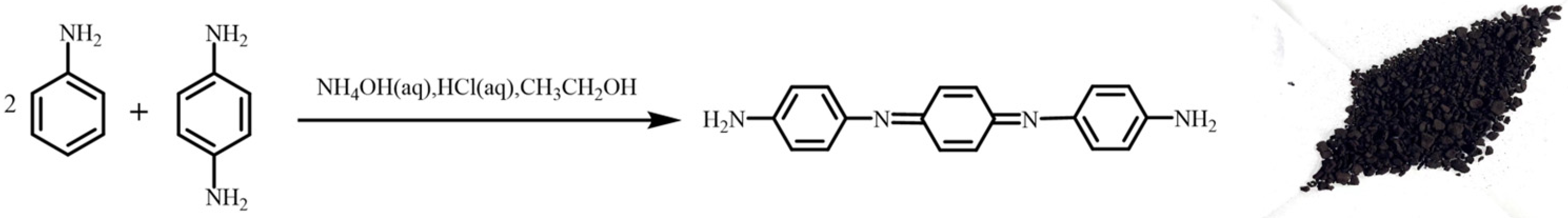
2.2.1. Synthesis of Amine-Capped Aniline Trimer (ACAT)
2.2.2. Preparation of Waterborne Polyurethane Modified with Aniline Trimer
2.2.3. Preparation of Waterborne Polyurethane Modified with Aniline Trimer Coatings
3. Characterization
3.1. 1H NMR
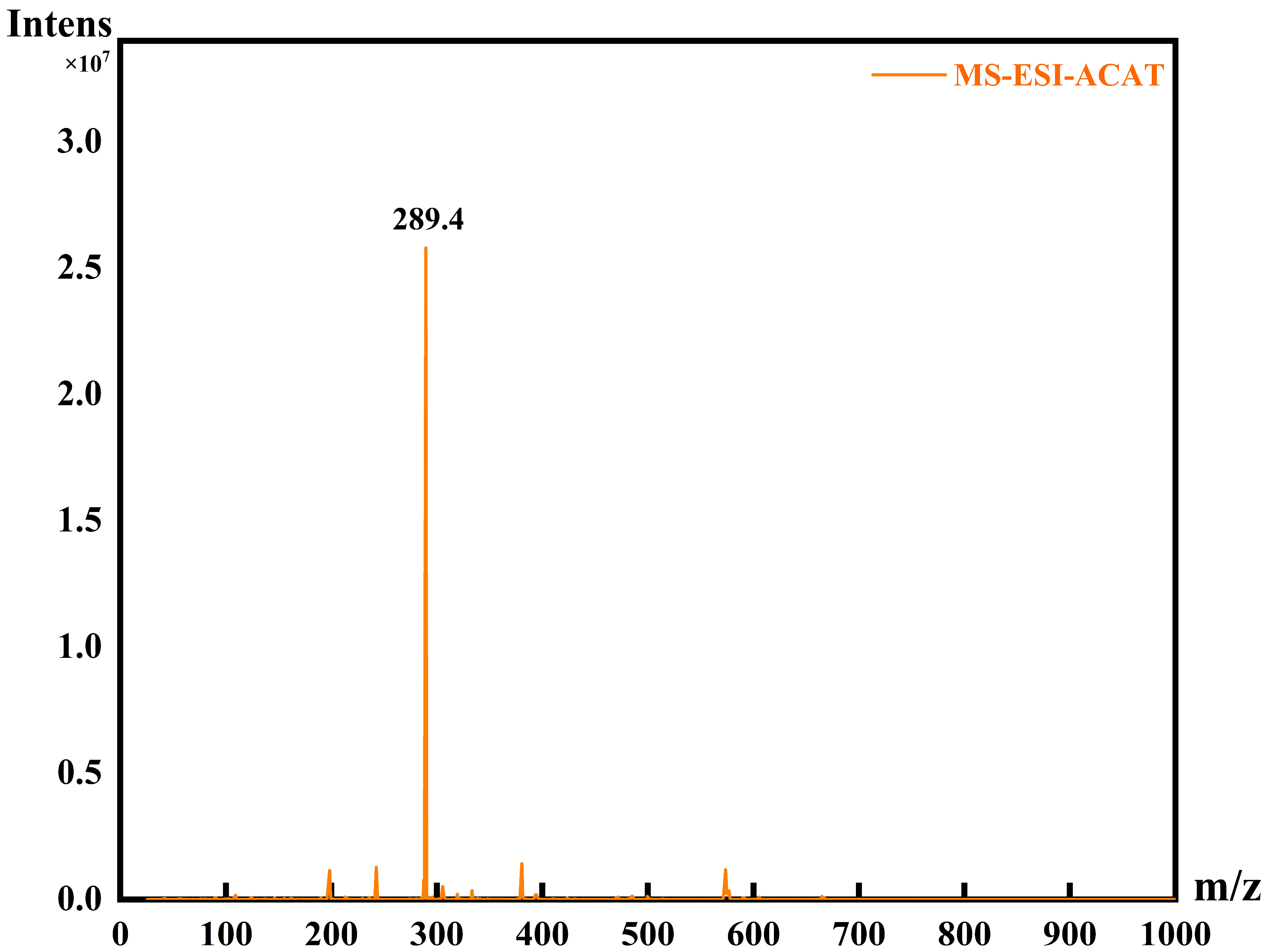
3.2. ESI-MS
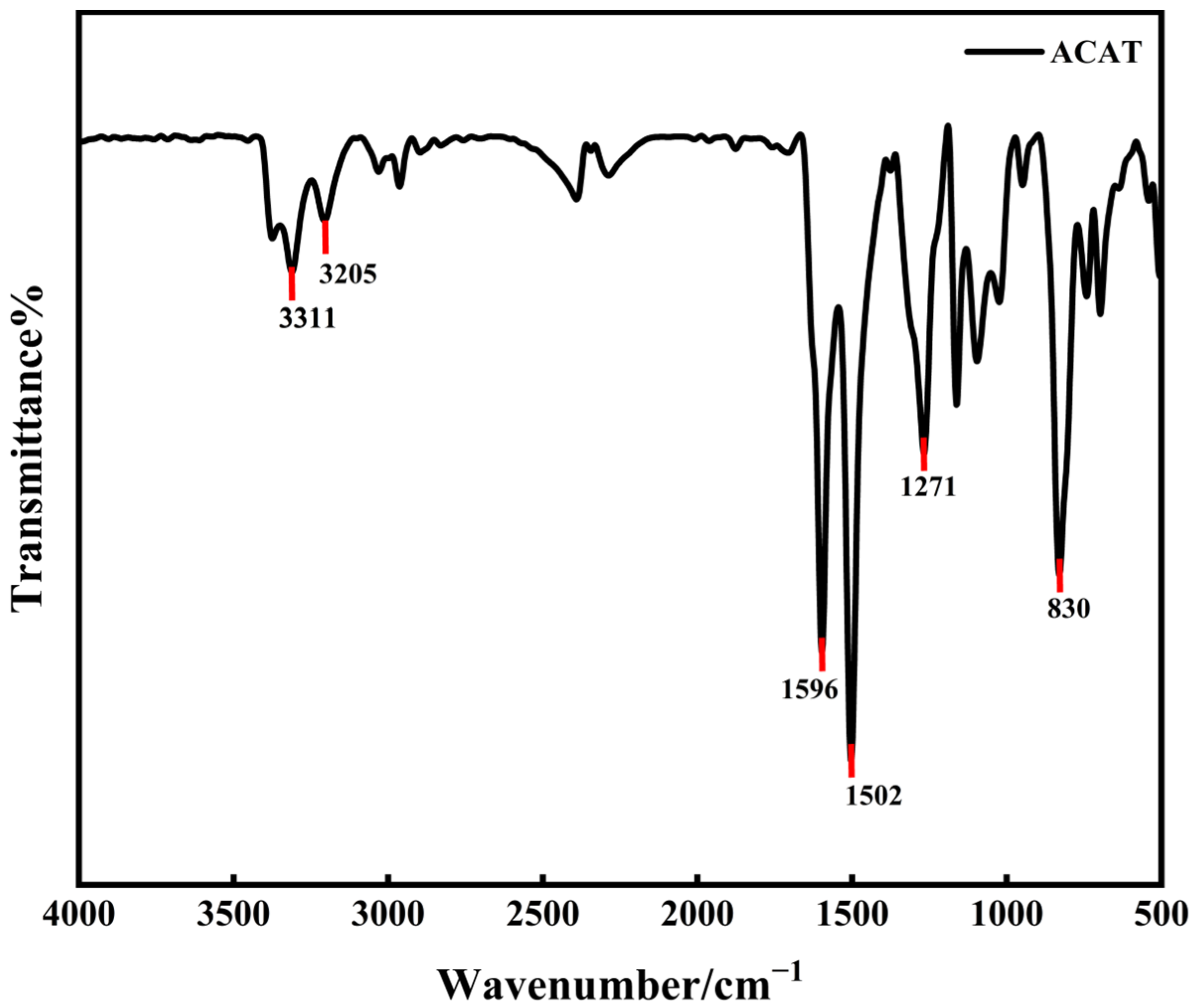
3.3. FTIR Spectroscopy
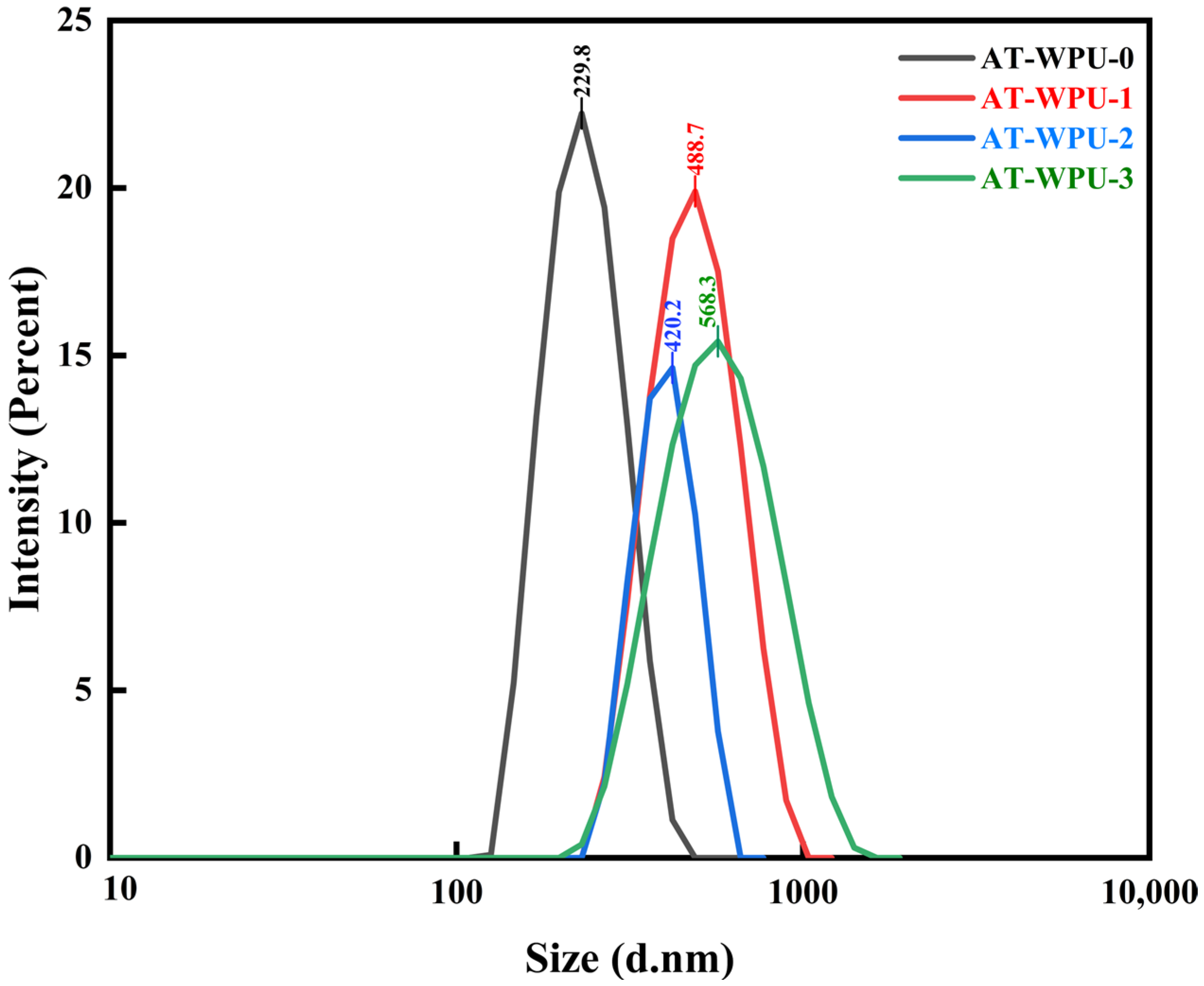
3.4. Particle Size Testing
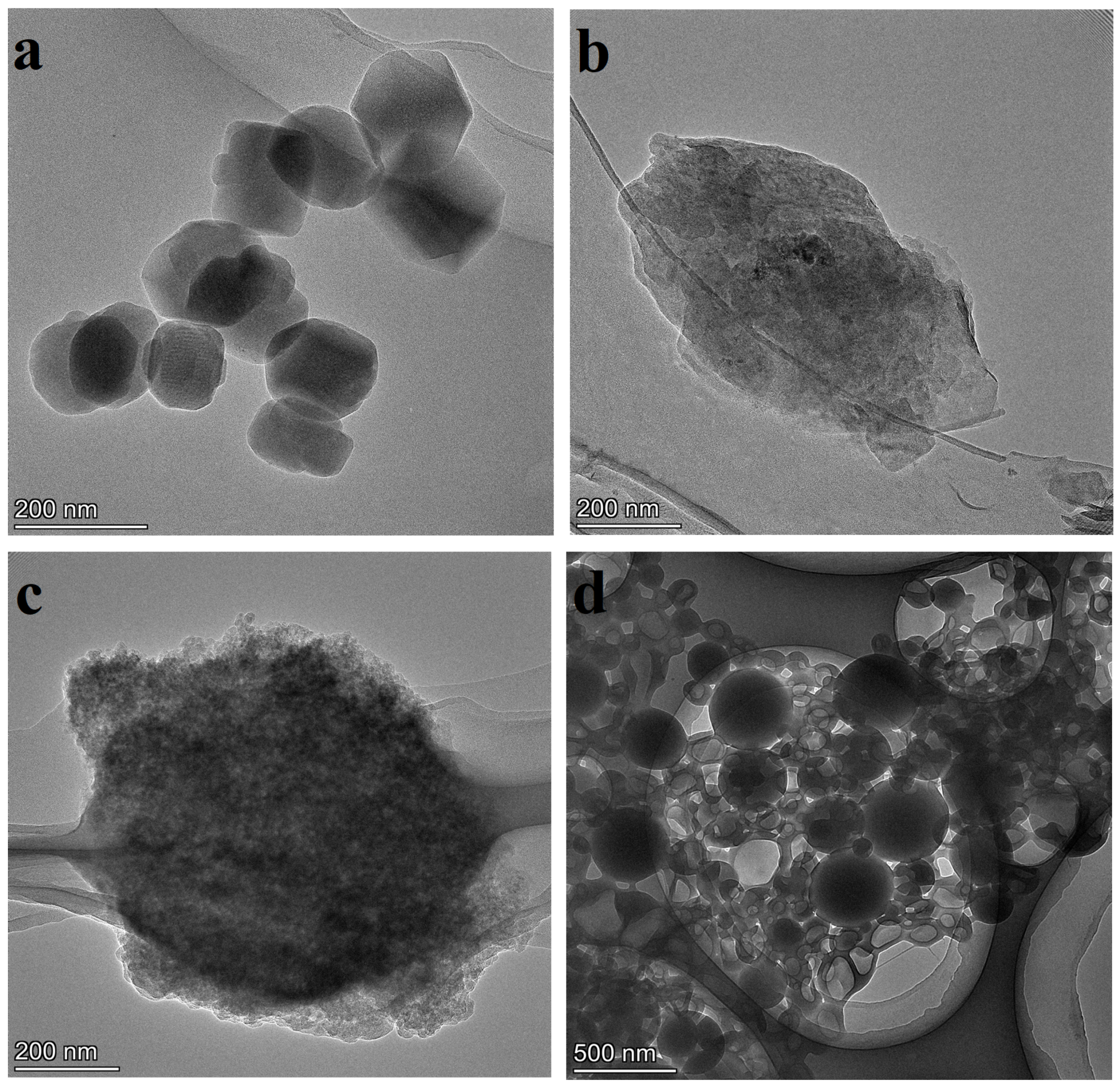
3.5. TEM
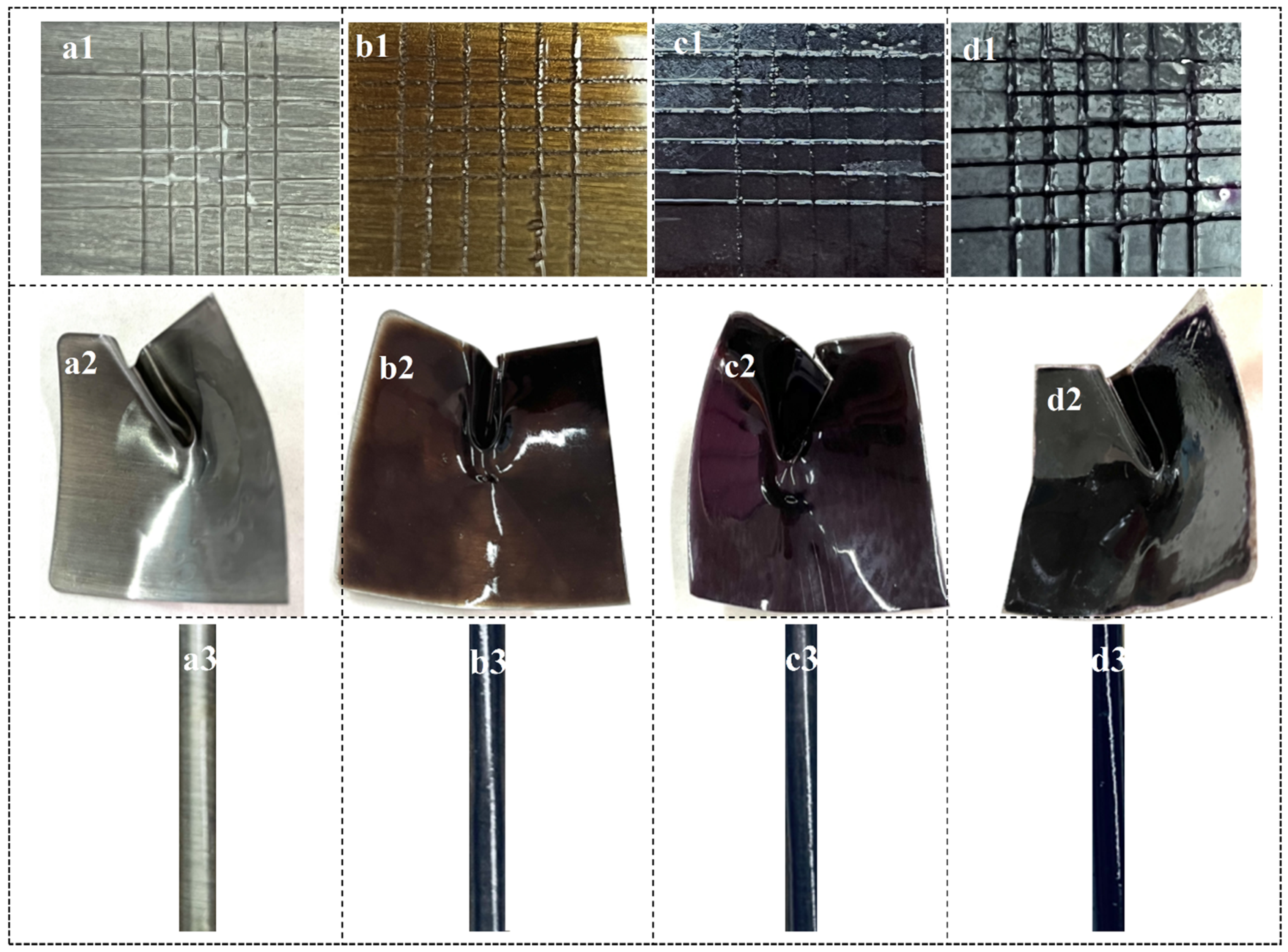
3.6. Fundamental Property Testing of the Coatings
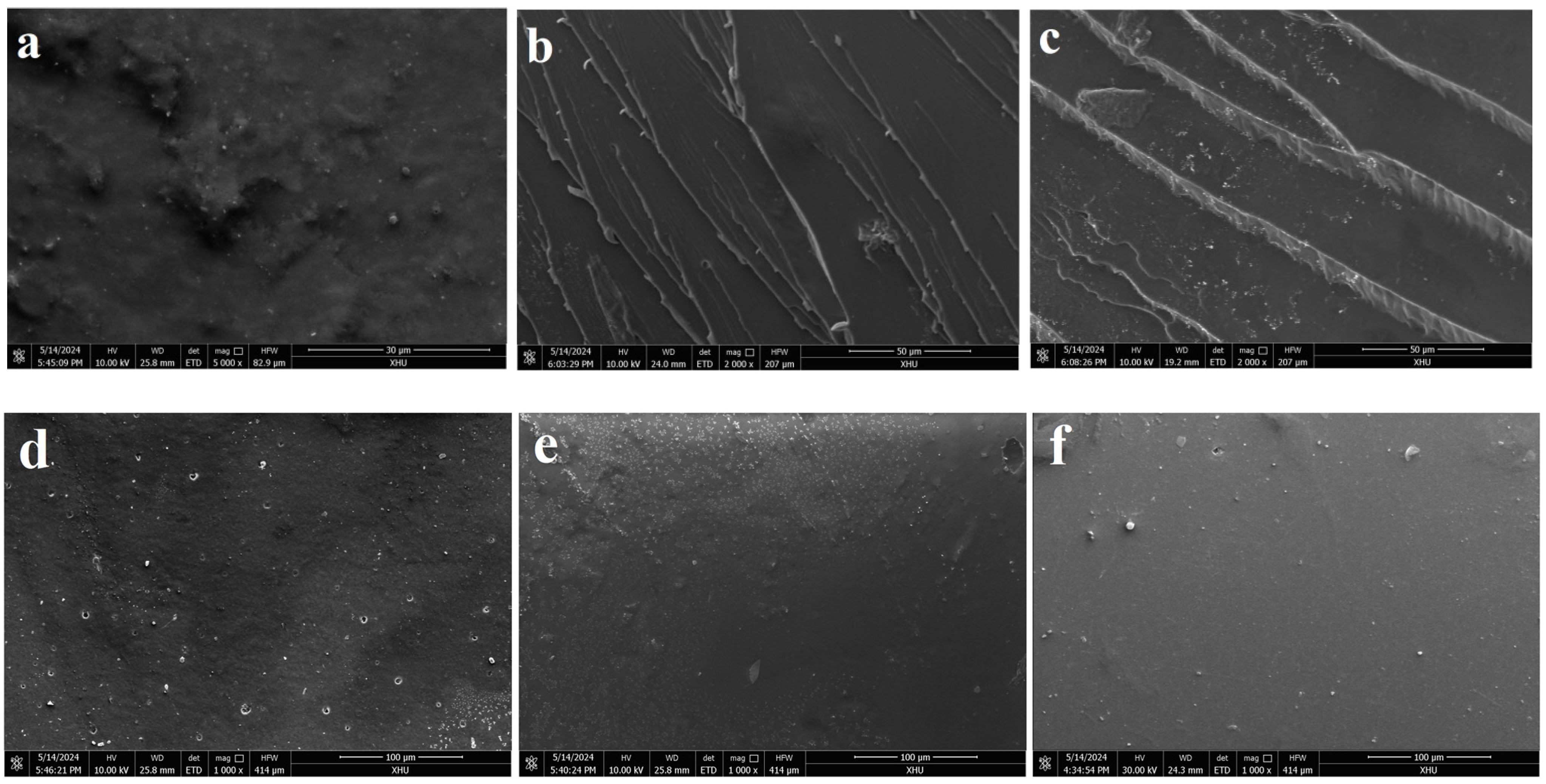
3.7. SEM
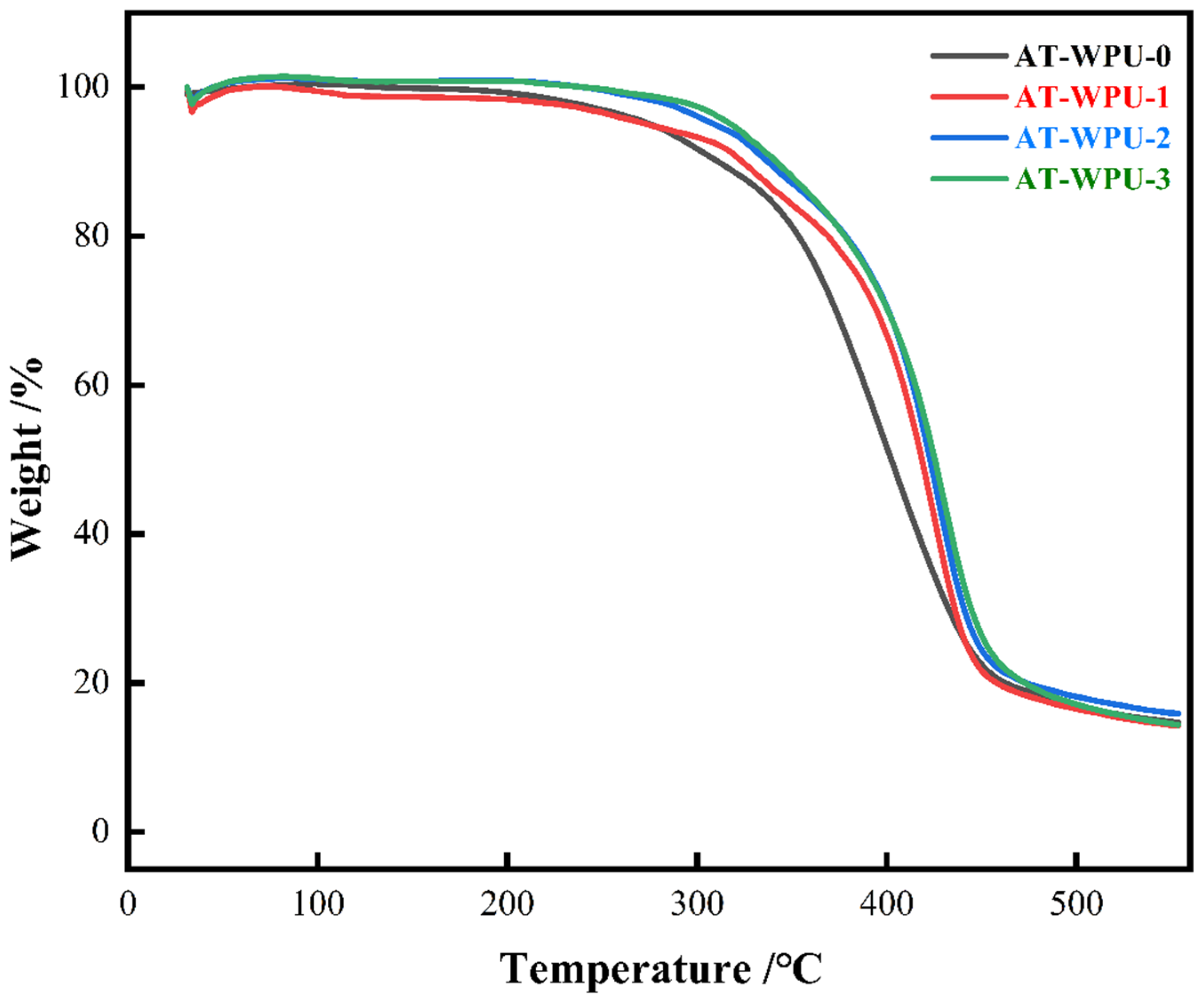
3.8. Thermogravimetric Analysis Test
3.9. Differential Scanning Calorimetry
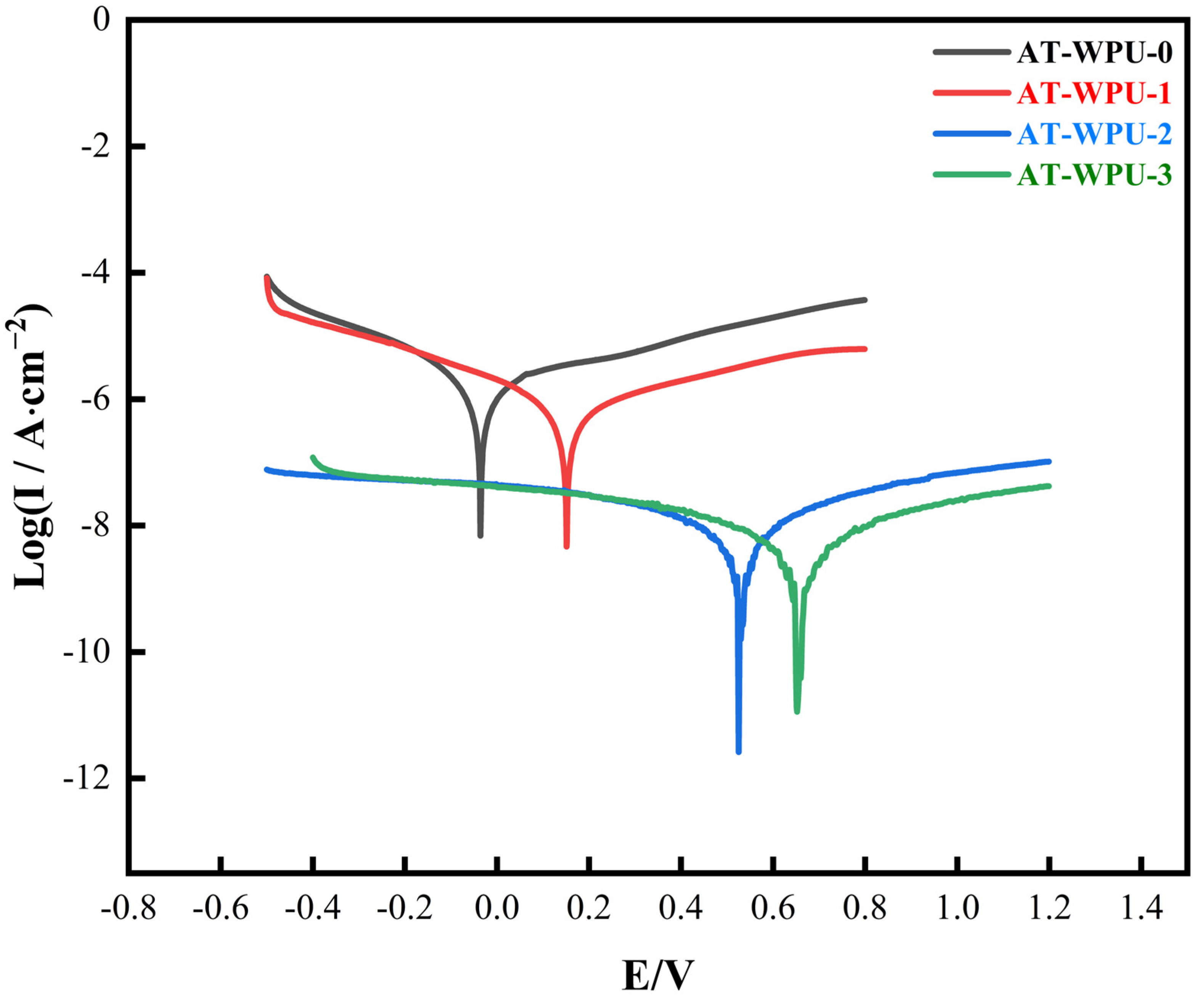
3.10. Electrochemical Corrosion Studies
4. Results and Discussion
4.1. Structural Characterisation of Amine-Capped Aniline Trimer (ACAT)
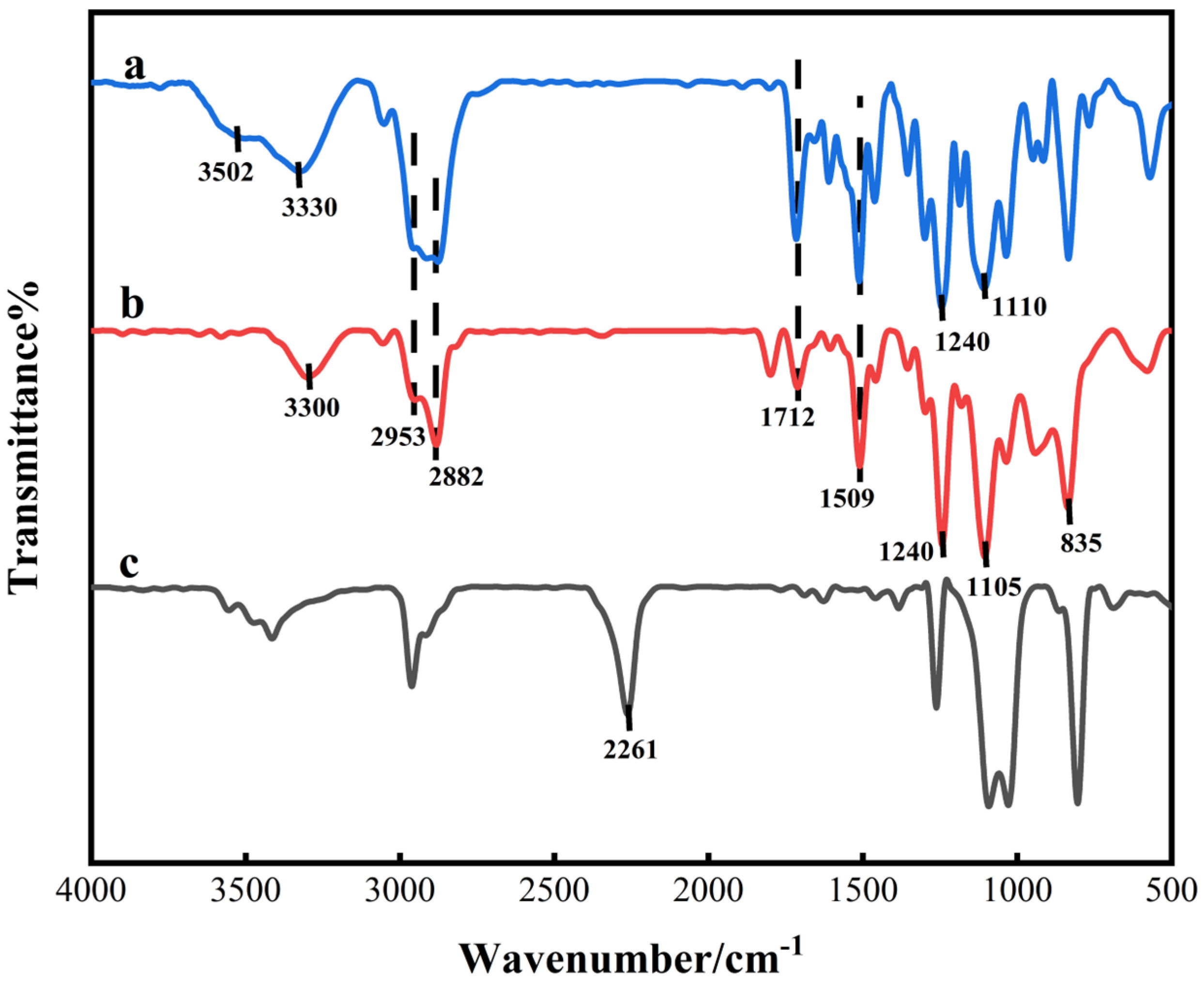
4.2. FTIR Analysis of AT-WPU
4.3. Particle Size Analysis
4.4. TEM Images
4.5. Fundamental Properties of Coatings
4.6. Investigation of Film Surface
4.7. Thermogravimetric Analysis
4.8. Potentiodynamic Measurements
4.9. Corrosion Protection Mechanism of Coating
5. Conclusions
- (1)
- The DSC analysis results and thermogravimetric graphs indicate that the introduction of E-44 epoxy resin and the use of the amine-type curing agent for high-temperature curing facilitated a ring-opening reaction, which effectively increased thermal stability and the crosslinking density of the waterborne polyurethane coating. Additionally, the coating exhibits superior fundamental properties compared to the conventional waterborne polyurethane coating AT-WPU-0, such as improved hardness and adhesion.
- (2)
- Electrochemical evaluation of the aniline trimer-modified waterborne polyurethane coatings reveals that when the addition amount of aniline trimer was increased from 3% to 15%, the coatings exhibited better corrosion resistance. The corrosion potential of AT-WPU-3 reaches 0.67 V, and the corrosion current density is 7.245 × 10−9 A·cm−2, which is three orders of magnitude lower than that of the unmodified conventional waterborne polyurethane coating. This improvement is primarily ascribed to the ability of the aniline trimer to induce the formation of a passivation layer on the substrate metal through its redox properties.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yellishetty, M.; Ranjith, P.G.; Tharumarajah, A. Iron ore and steel production trends and material flows in the world: Is this really sustainable? Resour. Conserv. Recycl. 2010, 54, 1084–1094. [Google Scholar] [CrossRef]
- Barbara, S.; Robert, K. What is corrosion. Electrochem. Soc. Interface 2006, 6, 24–26. [Google Scholar]
- Nazari, M.H.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite organic coatings for corrosion protection of metals: A review of recent advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, Y.; Yuan, Y.; Zhou, X.; Liu, Y.; Meng, X. Recent advances of metal–organic frameworks in corrosion protection: From synthesis to applications. Chem. Eng. J. 2022, 430, 132823. [Google Scholar] [CrossRef]
- Adibzadeh, E.; Mirabedini, S.M.; Behzadnasab, M.; Farnood, R.R. A novel two-component self-healing coating comprising vinyl ester resin-filled microcapsules with prolonged anticorrosion performance. Prog. Org. Coat. 2021, 154, 106220. [Google Scholar] [CrossRef]
- Huang, S.; Kong, G.; Yang, B.; Zhang, S.; Che, C. Effects of graphene on the corrosion evolution of zinc particles in waterborne epoxy zinc-containing coatings. Prog. Org. Coat. 2020, 140, 105531. [Google Scholar] [CrossRef]
- Ren, S.; Zhou, W.; Song, K.; Gao, X.; Zhang, X.; Fang, H.; Li, X.; Ding, Y. Robust, self-healing, anti-corrosive waterborne polyurethane urea composite coatings enabled by dynamic hindered urea bonds. Prog. Org. Coat. 2023, 180, 107571. [Google Scholar] [CrossRef]
- Ai, D.; Mo, R.; Wang, H.; Lai, Y.; Jiang, X.; Zhang, X. Preparation of waterborne epoxy dispersion and its application in 2K waterborne epoxy coatings. Prog. Org. Coat. 2019, 136, 105258. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Z.; Shi, J.; Chen, G.; Zhang, Q.; Weng, Z.; Wu, K.; Lu, M. Green synthesis of graphene with the assistance of modified lignin and its application in anticorrosive waterborne epoxy coatings. Appl. Surf. Sci. 2019, 484, 759–770. [Google Scholar] [CrossRef]
- Nan, D.; Li, X.; Li, D.; Liu, Q.; Wang, B.; Gao, X.; Ma, T.; He, N.; Xu, Y.; Dong, J. Preparation and Anticorrosive Performance of Waterborne Epoxy Resin Composite Coating with Amino-Modified Graphene Oxide. Polymers 2022, 15, 27. [Google Scholar] [CrossRef]
- Wang, X.; Sun, W.; Li, W.; Zuo, C.; Jiang, Y.; Wang, S. Development of waterborne heavy-duty anticorrosive coatings with modified nanoscale titania. Coatings 2022, 12, 1651. [Google Scholar] [CrossRef]
- Sili, H.; Yuntao, L.; Chunxia, Z.; Jiaojiao, W.; Hui, L.; Dong, X. Advanced anticorrosion coatings prepared from polybenzoxazine/siloxane-containing epoxy resin. Polym. Eng. Sci. 2020, 60, 1812–1821. [Google Scholar] [CrossRef]
- Sun, D.; Bai, Y.; He, Y.; Li, Z.; Li, C.; Zhao, Y.; Yin, X. Polydopamine coated Co2(OH)2BDC nanosheets for anticorrosive reinforcement of water-borne epoxy coating. Prog. Org. Coat. 2023, 175, 107368. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Gao, Z.; He, H.; Chen, Y.; He, F.; Lin, Y.; Yan, B.; Chen, S. Preparation of polyaniline/cellulose nanofiber composites with enhanced anticorrosion performance for waterborne epoxy resin coatings. Polym. Eng. Sci. 2023, 63, 1613–1622. [Google Scholar] [CrossRef]
- Liao, G.; Li, Q.; Xu, Z. The chemical modification of polyaniline with enhanced properties: A review. Prog. Org. Coat. 2019, 126, 35–43. [Google Scholar] [CrossRef]
- Gao, X.Z.; Liu, H.J.; Cheng, F.; Chen, Y. Thermoresponsive polyaniline nanoparticles: Preparation, characterization, and their potential application in waterborne anticorrosion coatings. Chem. Eng. J. 2016, 283, 682–691. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, X.H. Eco-friendly water-borne conducting polyaniline. Chin. J. Polym. Sci. 2013, 31, 853–869. [Google Scholar] [CrossRef]
- Tian, Z.; Yu, H.; Wang, L.; Saleem, M.; Ren, F.; Ren, P.; Chen, Y.; Sun, R.; Sun, Y.; Huang, L. Recent progress in the preparation of polyaniline nanostructures and their applications in anticorrosive coatings. RSC Adv. 2014, 4, 28195–28208. [Google Scholar] [CrossRef]
- Zhu, Z.; Wen, Z.; Chen, S.; Liu, Y.; Zhang, Y.; Wang, W. Enhanced anticorrosion properties of composite coatings containing polyvinyl butyral and polyaniline-carbonized polyaniline. Prog. Org. Coat. 2023, 180, 107559. [Google Scholar] [CrossRef]
- Gao, F.; Mu, J.; Bi, Z.; Wang, S.; Li, Z. Recent advances of polyaniline composites in anticorrosive coatings: A review. Prog. Org. Coat. 2021, 151, 106071. [Google Scholar] [CrossRef]
- Peng, C.W.; Hsu, C.H.; Lin, K.H.; Li, P.L.; Hsieh, M.F.; Wei, Y.; Yeh, J.M.; Yu, Y.H. Electrochemical corrosion protection studies of aniline-capped aniline trimer-based electroactive polyurethane coatings. Electrochim. Acta 2011, 58, 614–620. [Google Scholar] [CrossRef]
- Chen, K.Y.; Lai, Y.S.; You, J.K.; Santiago, K.S.; Yeh, J.M. Effective anticorrosion coatings prepared from sulfonated electroactive polyurea. Polymer 2019, 166, 98–107. [Google Scholar] [CrossRef]
- Huang, H.Y.; Huang, T.C.; Lin, J.C.; Chang, J.H.; Lee, Y.T.; Yeh, J.M. Advanced environmentally friendly coatings prepared from amine-capped aniline trimer-based waterborne electroactive polyurethane. Mater. Chem. Phys. 2013, 137, 772–780. [Google Scholar] [CrossRef]
- Zeng, Q.; Xue, S.; Li, J.; Jiang, W.; Ding, Y.; Shen, L. Preparation of bio-based air-drying water-borne polyurea coatings with excellent coating properties and anticorrosive performance. Prog. Org. Coat. 2022, 171, 107040. [Google Scholar] [CrossRef]
- Guo, H.; Chao, B.; Zhao, Z.; Nan, D. Preparation of aniline trimer modified graphene oxide new composite coating and study on anticorrosion performance. Mater. Res. Express 2020, 7, 125601. [Google Scholar] [CrossRef]
- Gu, L.; Liu, S.; Zhao, H.; Yu, H. Facile preparation of water-dispersible graphene sheets stabilized by carboxylated oligoanilines and their anticorrosion coatings. ACS Appl. Mater. Interfaces 2015, 7, 17641–17688. [Google Scholar] [CrossRef] [PubMed]
- David, D.J.; Staley, H.B. Analytical Chemistry of the Polyurethanes; Wiley-Interscience: New York, NY, USA, 1969. [Google Scholar]
- GB/T 13452.2-2008; Paints and Varnishes—Determination of Film Thickness. Standards Press of China: Beijing, China, 2008.
- GB/T 6739-2022; Paints and Varnishes—Determination of Film Hardness by Pencil Test. Standards Press of China: Beijing, China, 2022.
- GB/T 9286-2021; Paints and Varnishes—Cross-Cut Test. Standards Press of China: Beijing, China, 2021.
- GB/T 1731-2020; Determination of Flexibility of Coating and Putty Films. Standards Press of China: Beijing, China, 2020.
- Weng, C.J.; Huang, J.Y.; Huang, K.Y.; Jhuo, Y.S.; Tsai, M.H.; Yeh, J.M. Advanced anticorrosive coatings prepared from electroactive polyimide–TiO2 hybrid nanocomposite materials. Electrochim. Acta 2010, 55, 8430–8438. [Google Scholar] [CrossRef]
- Jhon, Y.K.; Cheong, I.W.; Kim, J.H. Chain extension study of aqueous polyurethane dispersions. Colloids Surf. A Physicochem. Eng. Asp. 2001, 179, 71–78. [Google Scholar] [CrossRef]
- Assanvo, E.F.; Baruah, S.D. Synthesis and properties of Ricinodendron heudelotii oil based hybrid alkyd–acrylate latexes via miniemulsion polymerization. Prog. Org. Coat. 2015, 86, 25–32. [Google Scholar] [CrossRef]
- Fang, Y.; Du, X.; Jiang, Y.; Du, Z.; Pan, P.; Cheng, X.; Wang, H. Thermal-driven self-healing and recyclable waterborne polyurethane films based on reversible covalent interaction. ACS Sustain. Chem. Eng. 2018, 6, 14490–14500. [Google Scholar] [CrossRef]
- Li, Q.; Liao, G.; Zhang, S.; Pang, L.; Tong, H.; Zhao, W.; Xu, Z. Effect of adjustable molecular chain structure and pure silica zeolite nanoparticles on thermal, mechanical, dielectric, UV-shielding and hydrophobic properties of fluorinated copolyimide composites. Appl. Surf. Sci. 2018, 427, 437–450. [Google Scholar] [CrossRef]
- Liang, H.; Liu, L.; Lu, J.; Chen, M.; Zhang, C. Castor oil-based cationic waterborne polyurethane dispersions: Storage stability, thermo-physical properties and antibacterial properties. Ind. Crops Prod. 2018, 117, 169–178. [Google Scholar] [CrossRef]
- Patel, C.J.; Mannari, V. Air-drying bio-based polyurethane dispersion from cardanol: Synthesis and characterization of coatings. Prog. Org. Coat. 2014, 77, 997–1006. [Google Scholar] [CrossRef]
- Yu, C.; de Luna, M.S.; Russo, A.; Adamiano, I.; Scherillo, F.; Wang, Z.; Zhang, X.; Xia, H.; Lavorgna, M. Role of diisocyanate structure on self-healing and anticorrosion properties of waterborne polyurethane coatings. Adv. Mater. Interfaces 2021, 8, 2100117. [Google Scholar] [CrossRef]
- Lu, W.K.; Elsenbaumer, R.L.; Wessling, B. Corrosion protection of mild steel by coatings containing polyaniline. Synth. Met. 1995, 71, 2163–2166. [Google Scholar] [CrossRef]
- Wessling, B. Corrosion prevention with an organic metal (polyaniline): Surface ennobling, passivation. Corrosion Test Results. Mater. Corros. 1996, 47, 439–445. [Google Scholar] [CrossRef]
- Wessling, B. Passivation of metals by coating with polyaniline: Corrosion potential shift and morphological changes. Adv. Mater. 1994, 6, 226–228. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Jia, X.; Yeh, J.M.; Spellane, P. Polyaniline as corrosion protection coatings on cold rolled steel. Polymer 1995, 36, 4535–4537. [Google Scholar] [CrossRef]
- Yağan, A.; Pekmez, N.Ö.; Yıldız, A. Electrochemical synthesis of poly (N-methylaniline) on an iron electrode and its corrosion performance. Electrochim. Acta 2008, 53, 5242–5251. [Google Scholar] [CrossRef]
- Wang, X.; Lu, J.; Li, J.; Jing, X.; Wang, F. Solvent-free polyaniline coating for corrosion prevention of metal. In Electroactive Polymers for Corrosion Control; American Chemical Society: Washington, DC, USA, 2003. [Google Scholar]
- Gamboa, S.A.; Gonzalez-Rodriguez, J.G.; Valenzuela, E.; Campillo, B.; Sebastian, P.J.; Reyes-Rojas, A. Evaluation of the corrosion resistance of Ni–Co–B coatings in simulated PEMFC environment. Electrochim. Acta 2006, 51, 4045–4051. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Muthkrishnan, S.; Venkatachari, G. Corrosion protection of steel by polyaniline blended coating. Electrochim. Acta 2006, 51, 6313–6319. [Google Scholar] [CrossRef]





| Types | AT Content (1) | Component/g | |||||||
|---|---|---|---|---|---|---|---|---|---|
| IPDI | E-44 | DBTDL | PEG200 | PEG2000 | Acetone | DMF | AT | ||
| AT-WPU-0 | 0% | 6.67 | 22.7 | 0.3 | 2.3 | 22 | 20 | 0 | 0 |
| AT-WPU-1 | 3% | 7.33 | 22.7 | 0.3 | 2.3 | 22 | 30 | 20 | 0.8 |
| AT-WPU-2 | 9% | 8.23 | 22.7 | 0.3 | 2.3 | 22 | 50 | 30 | 2.21 |
| AT-WPU-3 | 15% | 9.34 | 22.7 | 0.3 | 2.3 | 22 | 70 | 40 | 3.72 |
| Sample | Thickness (μm) | Adhesion | Pencil Hardness | Flexibility (mm) | Impact Resistance (cm) |
|---|---|---|---|---|---|
| AT-WPU-0 | 41.2–43.7 | 1 | 2H | 2 | 120 |
| AT-WPU-1 | 52.6–58.4 | 1 | 3H | 2 | 80 |
| AT-WPU-2 | 54.2–61.6 | 0 | 4H | 2 | 60 |
| AT-WPU-3 | 55.6–63.3 | 0 | 4H | 2 | 60 |
| Sample | T5%/°C | T10%/°C | T50%/°C | Tg |
|---|---|---|---|---|
| AT-WPU-0 | 274 | 310 | 402 | 79.53 |
| AT-WPU-1 | 276 | 323 | 418 | 88.27 |
| AT-WPU-2 | 309 | 337 | 422 | 93.53 |
| AT-WPU-3 | 318 | 341 | 425 | 101.71 |
| Sample | Ecorr (v) | Icorr (A·cm−2) | ba (mv) | bc (mv) | Rp (MΩ·cm−2) | Rcorr (μm·Y−1) |
|---|---|---|---|---|---|---|
| AT-WPU-0 | −0.045 | 1.731 × 10−6 | 350 | 150 | 26.32 | 20.12 |
| AT-WPU-1 | 0.18 | 7.524 × 10−7 | 250 | 150 | 56.15 | 8.75 |
| AT-WPU-2 | 0.52 | 1.947 × 10−8 | 74.5 | 125 | 1036.9 | 0.23 |
| AT-WPU-3 | 0.67 | 7.245 × 10−9 | 137 | 187 | 4735.5 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, S.; Dong, X.; Zhao, Y.; Han, J.; Ji, Y.; Kuang, R.; Zhang, S.; Ma, S. Preparation of Environmentally Friendly Anticorrosive Coatings with Aniline Trimer-Modified Waterborne Polyurethane. Coatings 2024, 14, 1380. https://doi.org/10.3390/coatings14111380
Xu S, Dong X, Zhao Y, Han J, Ji Y, Kuang R, Zhang S, Ma S. Preparation of Environmentally Friendly Anticorrosive Coatings with Aniline Trimer-Modified Waterborne Polyurethane. Coatings. 2024; 14(11):1380. https://doi.org/10.3390/coatings14111380
Chicago/Turabian StyleXu, Shaoxiong, Xiaoying Dong, Yufei Zhao, Jinhui Han, Yanbing Ji, Run Kuang, Suhang Zhang, and Sude Ma. 2024. "Preparation of Environmentally Friendly Anticorrosive Coatings with Aniline Trimer-Modified Waterborne Polyurethane" Coatings 14, no. 11: 1380. https://doi.org/10.3390/coatings14111380
APA StyleXu, S., Dong, X., Zhao, Y., Han, J., Ji, Y., Kuang, R., Zhang, S., & Ma, S. (2024). Preparation of Environmentally Friendly Anticorrosive Coatings with Aniline Trimer-Modified Waterborne Polyurethane. Coatings, 14(11), 1380. https://doi.org/10.3390/coatings14111380





