Advances in Bacterial Cellulose Production: A Scoping Review
Abstract
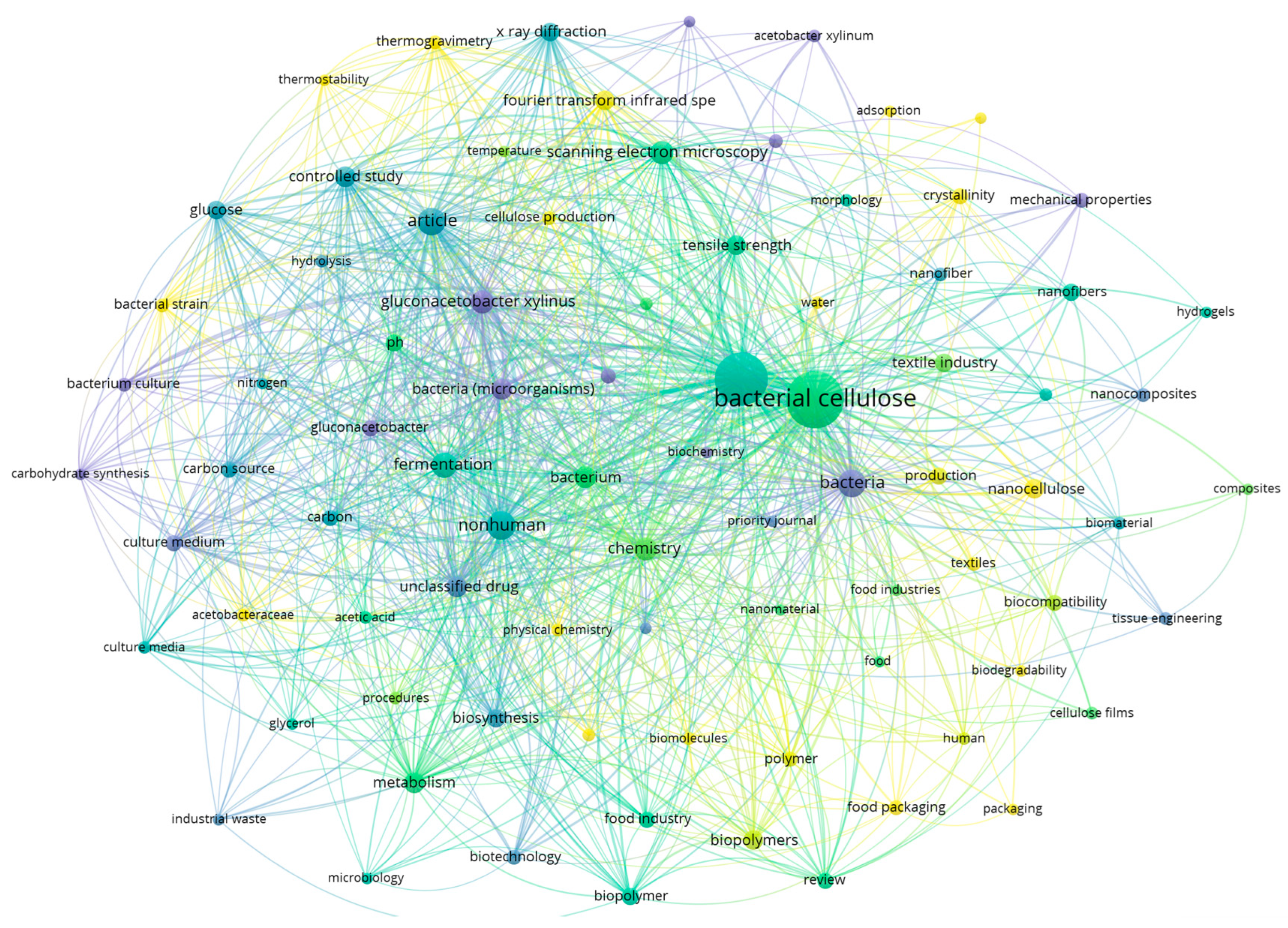
:1. Introduction
2. Methodology
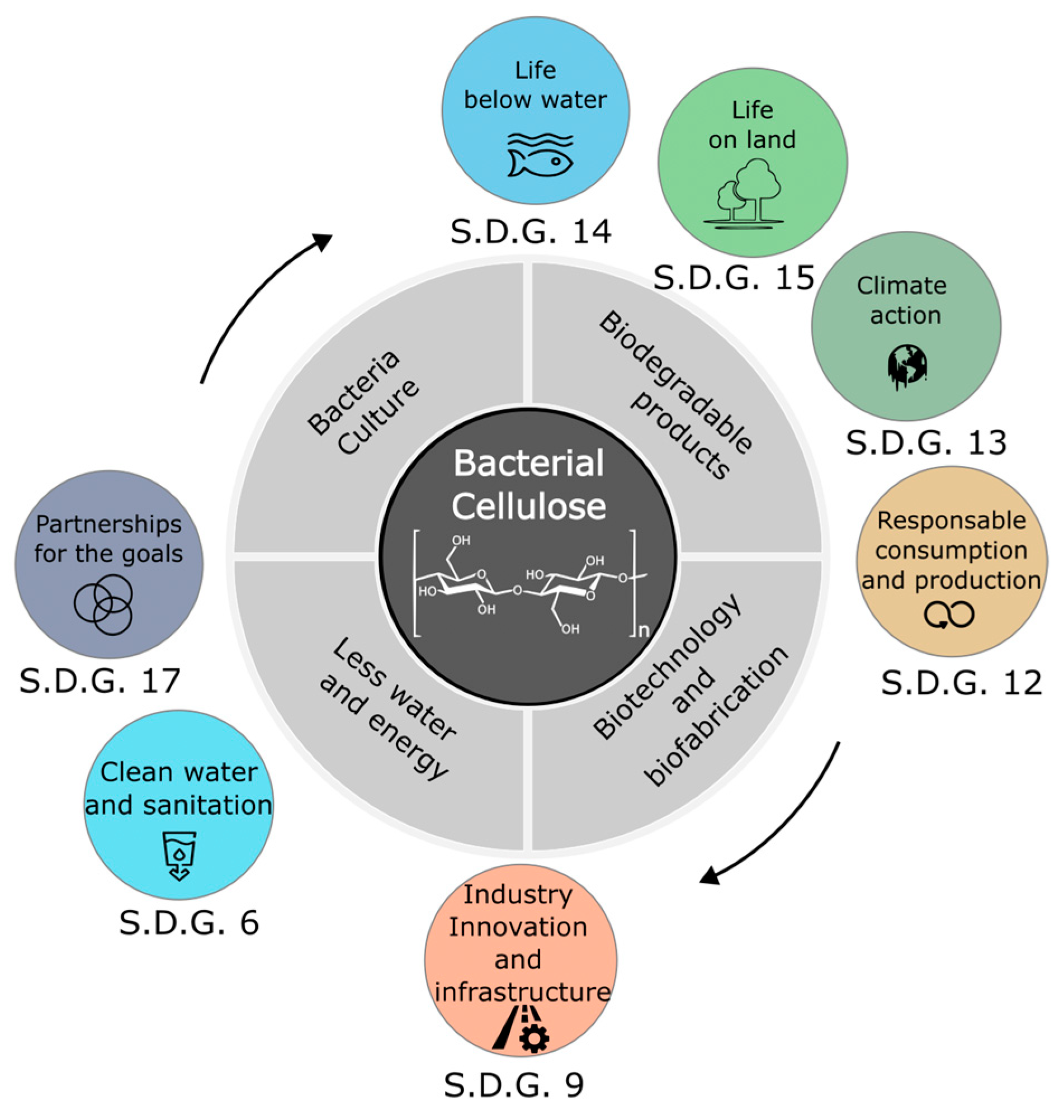
3. Potential Applications of Bacterial Cellulose
4. Key Technologies for Bacterial Cellulose Production
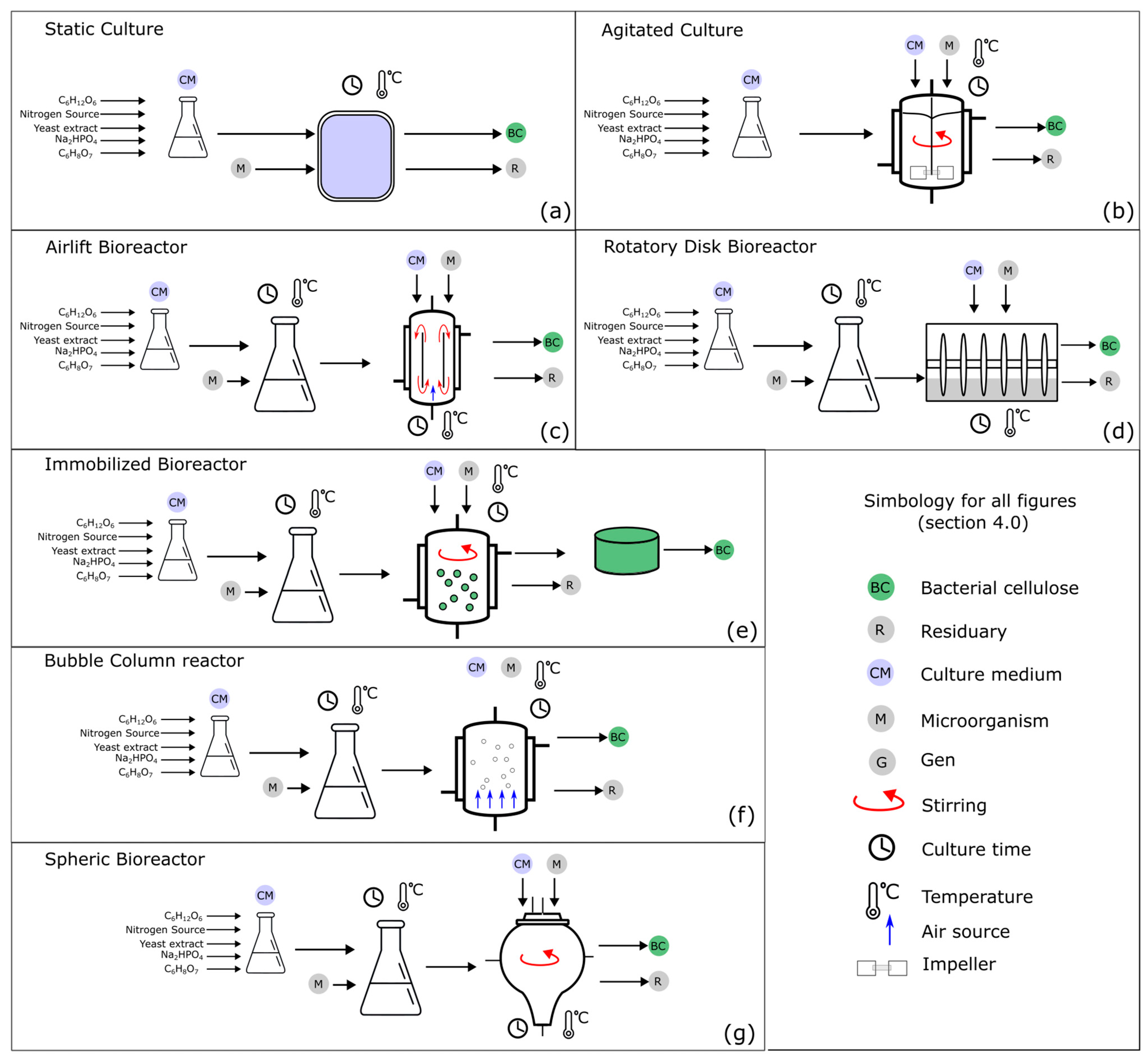
4.1. Culture Methods
4.1.1. Culture Conditions
4.1.2. Bioreactors
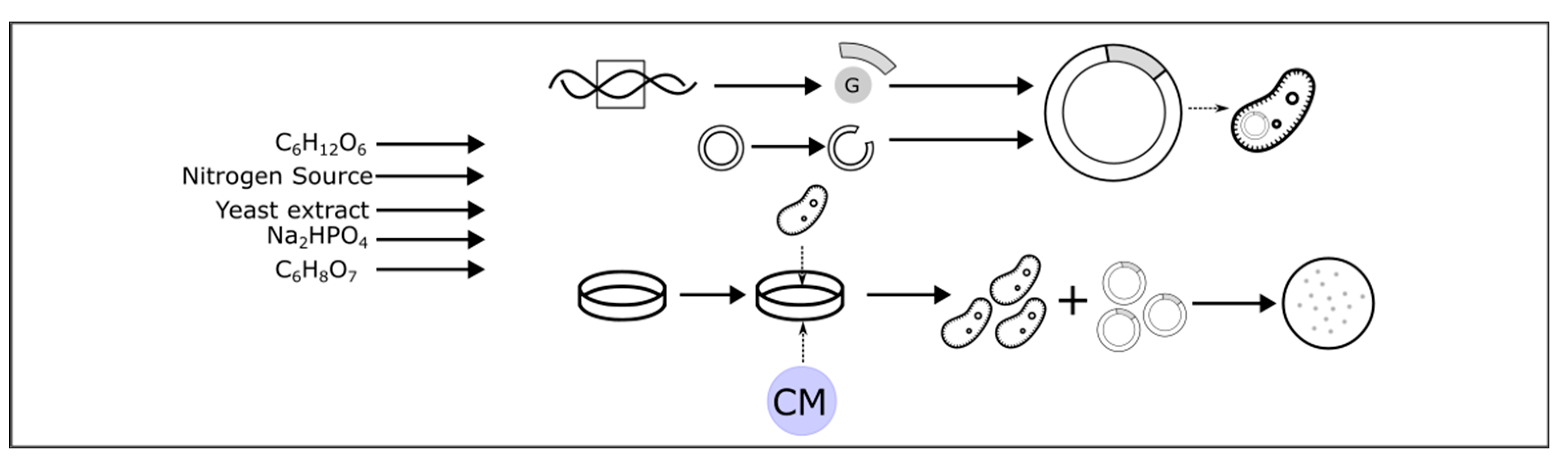
4.2. Strain Selection and Genetic Modification
4.2.1. Bacterial Strains
4.2.2. Genetic Modification
4.3. Optimization and Improvement Technologies
4.4. Sustainable Production and Waste Use Technologies
4.5. Production of Cellulose-Based Biopolymers
4.6. Innovations and Specific Applications
4.7. Strategies to Improve Bacterial Cellulose Production
4.8. Limitations and Difficulties to Producing Bacterial Cellulose
4.9. Evaluation of the Economic Aspects of the Preparation or Production of Bacterial Cellulose
5. Physical and Mechanical Properties of Bacterial Cellulose
6. The Future of Bacterial Cellulose Technology
7. Patents
8. Discussion
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brugnoli, M.; Robotti, F.; La China, S.; Anguluri, K.; Haghighi, H.; Bottan, S.; Ferrari, A.; Gullo, M. Assessing Effectiveness of Komagataeibacter Strains for Producing Surface-Microstructured Cellulose via Guided Assembly-Based Biolithography. Sci. Rep. 2021, 11, 19311. [Google Scholar] [CrossRef] [PubMed]
- Molina-Ramírez, C.; Álvarez, J.; Zuluaga, R.; Castro, C.; Gañán, P. A Novel Approach Using Conventional Methodologies to Scale up BNC Production Using Komagataeibacter Medellinensis and Rotten Banana Waste as Alternative. Processes 2020, 8, 1469. [Google Scholar] [CrossRef]
- Blanco Parte, F.G.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current Progress on the Production, Modification, and Applications of Bacterial Cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed]
- Picheth, G.F.; Pirich, C.L.; Sierakowski, M.R.; Woehl, M.A.; Sakakibara, C.N.; De Souza, C.F.; Martin, A.A.; Da Silva, R.; De Freitas, R.A. Bacterial Cellulose in Biomedical Applications: A Review. Int. J. Biol. Macromol. 2017, 104, 97–106. [Google Scholar] [CrossRef]
- Gedarawatte, S.T.G.; Ravensdale, J.T.; Johns, M.L.; Li, M.; Al-Salami, H.; Dykes, G.A.; Coorey, R. Evaluation of the Water-Holding and Anti-Spoilage Effect of a Bacterial Cellulose Nanocrystal Coating for the Storage of Vacuum-Packaged Beef. Food Packag. Shelf Life 2022, 31, 100818. [Google Scholar] [CrossRef]
- Ahmed, M.; Saini, P.; Iqbal, U.; Sahu, K. Edible Microbial Cellulose-Based Antimicrobial Coatings and Films Containing Clove Extract. Food Prod. Process. Nutr. 2024, 6, 65. [Google Scholar] [CrossRef]
- Prilepskii, A.; Nikolaev, V.; Klaving, A. Conductive Bacterial Cellulose: From Drug Delivery to Flexible Electronics. Carbohydr. Polym. 2023, 313, 120850. [Google Scholar] [CrossRef]
- Lourenço, A.F.; Martins, D.; Dourado, F.; Sarmento, P.; Ferreira, P.J.T.; Gamelas, J.A.F. Impact of Bacterial Cellulose on the Physical Properties and Printing Quality of Fine Papers. Carbohydr. Polym. 2023, 314, 120915. [Google Scholar] [CrossRef]
- Wang, J.; Tavakoli, J.; Tang, Y. Bacterial Cellulose Production, Properties and Applications with Different Culture Methods—A Review. Carbohydr. Polym. 2019, 219, 63–76. [Google Scholar] [CrossRef]
- Yanti, N.A. Characteristics of Biocellulose from Sago Liquid Waste with Different Ammonium Sulfate Concentration. Int. J. Ecophysiol. 2019, 1, 56–64. [Google Scholar] [CrossRef]
- Molina-Ramírez, C.; Castro, M.; Osorio, M.; Torres-Taborda, M.; Gómez, B.; Zuluaga, R.; Gómez, C.; Gañán, P.; Rojas, O.; Castro, C. Effect of Different Carbon Sources on Bacterial Nanocellulose Production and Structure Using the Low pH Resistant Strain Komagataeibacter Medellinensis. Materials 2017, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized Culture Conditions for Bacterial Cellulose Production by Acetobacter Senegalensis MA1. BMC Biotechnol 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in Bacterial Cellulose Matrices for Biotechnological Applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef]
- Ghozali, M.; Meliana, Y.; Chalid, M. Synthesis and Characterization of Bacterial Cellulose by Acetobacter Xylinum Using Liquid Tapioca Waste. Mater. Today: Proc. 2021, 44, 2131–2134. [Google Scholar] [CrossRef]
- Reimer, L.C.; Sarda Carbasse, J.; Schober, I.; Koblitz, J.; Podstawka, A.; Overmann, J. Komagataeibacter Xylinus (Brown 1886) Yamada et al. 2013. 2024. Available online: https://bacdive.dsmz.de/pdf-view/89?site=pdf_view&id=89&doi=doi%3A10.13145%2Fbacdive89.20240916.9.1 (accessed on 16 September 2024).
- Pesaran, M.; Amoabediny, G.; Yazdian, F. Effect of Cultivation Time and Medium Condition in Production of Bacterial Cellulose Nanofiber for Urease Immobilization. Int. J. Polym. Sci. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Provin, A.P.; Cubas, A.L.V.; Dutra, A.R.D.A.; Schulte, N.K. Textile Industry and Environment: Can the Use of Bacterial Cellulose in the Manufacture of Biotextiles Contribute to the Sector? Clean Techn Env. Policy 2021, 23, 2813–2825. [Google Scholar] [CrossRef]
- Moreno-Díaz, C.; Maresca, P.; Barajas, C.; Menéndez, P. Implementation of Bacterial Cellulose in Production Plants for Waste Disposal. KEM 2023, 961, 181–190. [Google Scholar] [CrossRef]
- Páez, M.A.; Casa-Villegas, M.; Aldas, M.; Luna, M.; Cabrera-Valle, D.; López, O.; Fernández, D.; Cruz, M.A.; Flor-Unda, O.; García, M.D.; et al. Insights into Agitated Bacterial Cellulose Production with Microbial Consortia and Agro-Industrial Wastes. Fermentation 2024, 10, 425. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Gardner, D.J.; Shaler, S.M.; Cai, Z. Towards a Cellulose-Based Society: Opportunities and Challenges. Cellulose 2021, 28, 4511–4543. [Google Scholar] [CrossRef]
- El-Gendi, H.; Taha, T.H.; Ray, J.B.; Saleh, A.K. Recent Advances in Bacterial Cellulose: A Low-Cost Effective Production Media, Optimization Strategies and Applications. Cellulose 2022, 29, 7495–7533. [Google Scholar] [CrossRef]
- Żywicka, A.; Ciecholewska-Juśko, D.; Drozd, R.; Rakoczy, R.; Konopacki, M.; Kordas, M.; Junka, A.; Migdał, P.; Fijałkowski, K. Preparation of Komagataeibacter Xylinus Inoculum for Bacterial Cellulose Biosynthesis Using Magnetically Assisted External-Loop Airlift Bioreactor. Polymers 2021, 13, 3950. [Google Scholar] [CrossRef] [PubMed]
- Flor, O.; Cruz, M.-A.; Avila, A. Technologies for the Production of Bacterial Cellulose 2024. Available online: https://data.mendeley.com/datasets/rvvmcdnf8p/1 (accessed on 16 September 2024).
- Zuo, K.; Cheng, H.-P.; Wu, S.-C.; Wu, W.-T. A Hybrid Model Combining Hydrodynamic and Biological Effects for Production of Bacterial Cellulose with a Pilot Scale Airlift Reactor. Biochem. Eng. J. 2006, 29, 81–90. [Google Scholar] [CrossRef]
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial Cellulose: Molecular Regulation of Biosynthesis, Supramolecular Assembly, and Tailored Structural and Functional Properties. Prog. Mater. Sci. 2022, 129, 100972. [Google Scholar] [CrossRef]
- Tsouko, E.; Maina, S.; Ladakis, D.; Kookos, I.K.; Koutinas, A. Integrated Biorefinery Development for the Extraction of Value-Added Components and Bacterial Cellulose Production from Orange Peel Waste Streams. Renew. Energy 2020, 160, 944–954. [Google Scholar] [CrossRef]
- Efthymiou, M.-N.; Tsouko, E.; Papagiannopoulos, A.; Athanasoulia, I.-G.; Georgiadou, M.; Pispas, S.; Briassoulis, D.; Tsironi, T.; Koutinas, A. Development of Biodegradable Films Using Sunflower Protein Isolates and Bacterial Nanocellulose as Innovative Food Packaging Materials for Fresh Fruit Preservation. Sci. Rep. 2022, 12, 6935. [Google Scholar] [CrossRef]
- Girard, V.; Chaussé, J.; Vermette, P. Bacterial Cellulose: A Comprehensive Review. J. Appl. Polym. Sci. 2024, 141, e55163. [Google Scholar] [CrossRef]
- Medeiros, A.D.M.; Amorim, J.D.P.D.; Silva Junior, C.J.G.; Durval, I.J.B.; Costa, A.F.S.; Sarubbo, L.A. Environmental, Medical and Textile Applications of Bacterial Nanocellulose: A Patent Review. Lett. Od. Appl. NanoBioSci. 2024, 13, 13. [Google Scholar] [CrossRef]
- Liu, K.; Catchmark, J.M. Bacterial Cellulose/Hyaluronic Acid Nanocomposites Production through Co-Culturing Gluconacetobacter Hansenii and Lactococcus Lactis in a Two-Vessel Circulating System. Bioresour. Technol. 2019, 290, 121715. [Google Scholar] [CrossRef]
- Li, X.; Tang, J.; Bao, L.; Chen, L.; Hong, F.F. Performance Improvements of the BNC Tubes from Unique Double-Silicone-Tube Bioreactors by Introducing Chitosan and Heparin for Application as Small-Diameter Artificial Blood Vessels. Carbohydr. Polym. 2017, 178, 394–405. [Google Scholar] [CrossRef]
- Wacker, M.; Kießwetter, V.; Slottosch, I.; Awad, G.; Paunel-Görgülü, A.; Varghese, S.; Klopfleisch, M.; Kupitz, D.; Klemm, D.; Nietzsche, S.; et al. In Vitro Hemo- and Cytocompatibility of Bacterial Nanocelluose Small Diameter Vascular Grafts: Impact of Fabrication and Surface Characteristics. PLoS ONE 2020, 15, e0235168. [Google Scholar] [CrossRef]
- Konopacki, M.; Grygorcewicz, B.; Kordas, M.; Ossowicz-Rupniewska, P.; Nowak, A.; Perużyńska, M.; Rakoczy, R. Intensification of Bacterial Cellulose Production Process with Sequential Electromagnetic Field Exposure Aided by Dynamic Modelling. Biochem. Eng. J. 2022, 182, 108432. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Risberg, B.; Gatenholm, P. Observations on Bacterial Cellulose Tube Formation for Application as Vascular Graft. Mater. Sci. Eng. C 2011, 31, 14–21. [Google Scholar] [CrossRef]
- Jonas, R.; Farah, L.F. Production and Application of Microbial Cellulose. Polym. Degrad. Stab. 1998, 59, 101–106. [Google Scholar] [CrossRef]
- Reshmy, R.; Eapen, P.; Deepa, T.; Aravind, M.; Raveendran, S.; Binod, P.; Varjani, S.; Awasthi, M.K.; Pandey, A. Bacterial Nanocellulose: Engineering, Production, and Applications. Bioengineered 2021, 12, 11463–11483. [Google Scholar] [CrossRef]
- Tan, H.-F.; Ooi, B.S.; Leo, C.P. Future Perspectives of Nanocellulose-Based Membrane for Water Treatment. J. Water Process Eng. 2020, 37, 101502. [Google Scholar] [CrossRef]
- Paleczny, J.; Junka, A.F.; Krzyżek, P.; Czajkowska, J.; Kramer, A.; Benkhai, H.; Żyfka-Zagrodzińska, E.; Bartoszewicz, M. Comparison of Antibiofilm Activity of Low-Concentrated Hypochlorites vs Polyhexanide-Containing Antiseptic. Front. Cell. Infect. Microbiol. 2023, 13, 1119188. [Google Scholar] [CrossRef]
- Ramírez-Carmona, M.; Gálvez-Gómez, M.P.; González-Perez, L.; Pinedo-Rangel, V.; Pineda-Vasquez, T.; Hotza, D. Production of Bacterial Cellulose Hydrogel and Its Evaluation as a Proton Exchange Membrane. J. Polym. Env. 2023, 31, 2462–2472. [Google Scholar] [CrossRef]
- Rahman, S.S.A.; Vaishnavi, T.; Vidyasri, G.S.; Sathya, K.; Priyanka, P.; Venkatachalam, P.; Karuppiah, S. Production of Bacterial Cellulose Using Gluconacetobacter Kombuchae Immobilized on Luffa Aegyptiaca Support. Sci. Rep. 2021, 11, 2912. [Google Scholar] [CrossRef]
- Kouda, T.; Yano, H.; Yoshinaga, F.; Kaminoyama, M.; Kamiwano, M. Characterization of Non-Newtonian Behavior during Mixing of Bacterial Cellulose in a Bioreactor. J. Ferment. Bioeng. 1996, 82, 382–386. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Buldum, G.; Mantalaris, A.; Bismarck, A. More Than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing, and Applications in Advanced Fiber Composites: More Than Meets the Eye in Bacterial Cellulose: Biosynthesis, Bioprocessing. Macromol. Biosci. 2014, 14, 10–32. [Google Scholar] [CrossRef]
- Lahiri, D.; Nag, M.; Dutta, B.; Dey, A.; Sarkar, T.; Pati, S.; Edinur, H.A.; Abdul Kari, Z.; Mohd Noor, N.H.; Ray, R.R. Bacterial Cellulose: Production, Characterization, and Application as Antimicrobial Agent. IJMS 2021, 22, 12984. [Google Scholar] [CrossRef] [PubMed]
- Shavyrkina, N.A.; Skiba, E.A.; Kazantseva, A.E.; Gladysheva, E.K.; Budaeva, V.V.; Bychin, N.V.; Gismatulina, Y.A.; Kashcheyeva, E.I.; Mironova, G.F.; Korchagina, A.A.; et al. Static Culture Combined with Aeration in Biosynthesis of Bacterial Cellulose. Polymers 2021, 13, 4241. [Google Scholar] [CrossRef] [PubMed]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Kinetics of Cell Growth and Crystalline Nanocellulose Production by Komagataeibacter Xylinus. Biochem. Eng. J. 2017, 127, 21–31. [Google Scholar] [CrossRef]
- Öz, Y.E.; Kalender, M. A Novel Static Cultivation of Bacterial Cellulose Production from Sugar Beet Molasses: Series Static Culture (SSC) System. Int. J. Biol. Macromol. 2023, 225, 1306–1314. [Google Scholar] [CrossRef]
- Wu, S.-C.; Li, M.-H. Production of Bacterial Cellulose Membranes in a Modified Airlift Bioreactor by Gluconacetobacter Xylinus. J. Biosci. Bioeng. 2015, 120, 444–449. [Google Scholar] [CrossRef]
- Doran, P.M. Bioprocess Engineering Principles; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-220851-5. [Google Scholar]
- Soleimani, A.; Hamedi, S.; Babaeipour, V.; Rouhi, M. Design, Construction and Optimization a Flexible Bench-Scale Rotating Biological Contactor (RBC) for Enhanced Production of Bacterial Cellulose by Acetobacter Xylinium. Bioprocess Biosyst. Eng. 2021, 44, 1071–1080. [Google Scholar] [CrossRef]
- Sharma, C.; Bhardwaj, N.K.; Pathak, P. Rotary Disc Bioreactor-Based Approach for Bacterial Nanocellulose Production Using Gluconacetobacter Xylinus NCIM 2526 Strain. Cellulose 2022, 29, 7177–7191. [Google Scholar] [CrossRef]
- Song, H.-J.; Li, H.; Seo, J.-H.; Kim, M.-J.; Kim, S.-J. Pilot-Scale Production of Bacterial Cellulose by a Spherical Type Bubble Column Bioreactor Using Saccharified Food Wastes. Korean J. Chem. Eng. 2009, 26, 141–146. [Google Scholar] [CrossRef]
- Cielecka, I.; Ryngajłło, M.; Bielecki, S. BNC Biosynthesis with Increased Productivity in a Newly Designed Surface Air-Flow Bioreactor. Appl. Sci. 2020, 10, 3850. [Google Scholar] [CrossRef]
- Chen, G.; Chen, L.; Wang, W.; Chen, S.; Wang, H.; Wei, Y.; Hong, F.F. Improved Bacterial Nanocellulose Production from Glucose without the Loss of Quality by Evaluating Thirteen Agitator Configurations at Low Speed. Microb. Biotechnol. 2019, 12, 1387–1402. [Google Scholar] [CrossRef]
- Zhou, J.; Gu, F.; Yang, H.; Cao, G.; Xu, W.; Sun, S.; Zhang, Y. Sequential Fermentation Strategy Improves Microbial Conversion of Waste Jasmine Flower to Bacterial Cellulose with Antibacterial Properties. Ind. Crops Prod. 2022, 185, 115147. [Google Scholar] [CrossRef]
- Ramírez Tapias, Y.A.; Di Monte, M.V.; Peltzer, M.A.; Salvay, A.G. Bacterial Cellulose Films Production by Kombucha Symbiotic Community Cultured on Different Herbal Infusions. Food Chem. 2022, 372, 131346. [Google Scholar] [CrossRef] [PubMed]
- Toyosaki, H.; Naritomi, T.; Seto, A.; Matsuoka, M.; Tsuchida, T.; Yoshinaga, F. Screening of Bacterial Cellulose-Producing Acetobacter Strains Suitable for Agitated Culture. Biosci. Biotechnol. Biochem. 1995, 59, 1498–1502. [Google Scholar] [CrossRef]
- Gorgieva. Trček Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, C.; Yang, J.; Nie, Y.; Chen, C.; Sun, D. Recent Advances in Bacterial Cellulose. Cellulose 2014, 21, 1–30. [Google Scholar] [CrossRef]
- Avcioglu, N.H. Bacterial Cellulose: Recent Progress in Production and Industrial Applications. World J Microbiol. Biotechnol. 2022, 38, 86. [Google Scholar] [CrossRef]
- Senthilnathan, S.; Rahman, S.S.A.; Pasupathi, S.; Venkatachalam, P.; Karuppiah, S. Stoichiometric Analysis and Production of Bacterial Cellulose by Gluconacetobacter Liquefaciens Using Borassus Flabellifer L. Jaggery. Appl. Biochem. Biotechnol. 2022, 194, 3645–3667. [Google Scholar] [CrossRef]
- Potočnik, V.; Gorgieva, S.; Trček, J. From Nature to Lab: Sustainable Bacterial Cellulose Production and Modification with Synthetic Biology. Polymers 2023, 15, 3466. [Google Scholar] [CrossRef]
- Carreira, P.; Mendes, J.A.S.; Trovatti, E.; Serafim, L.S.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Utilization of Residues from Agro-Forest Industries in the Production of High Value Bacterial Cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef]
- Hu, H.; Catchmark, J.M.; Demirci, A. Co-Culture Fermentation on the Production of Bacterial Cellulose Nanocomposite Produced by Komagataeibacter Hansenii. Carbohydr. Polym. Technol. Appl. 2021, 2, 100028. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Tseng, Y.-S.; Kumar, V.; Chen, C.-W.; Haldar, D.; Saini, J.K.; Dong, C.-D. Developments in Bioprocess for Bacterial Cellulose Production. Bioresour. Technol. 2022, 344, 126343. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Cao, Z.; Li, C.; Chen, L.; Wu, G.; Zhou, X.; Hong, F.F. A Recombinant Strain of Komagataeibacter Xylinus ATCC 23770 for Production of Bacterial Cellulose from Mannose-Rich Resources. New Biotechnol. 2023, 76, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, N.N.; Abd Rahman, N.; Esa, F. Monitoring Production of Bacterial Cellulose by Acetobacter Xylinum 0416 with Fuzzy Logic via Simulation. J. Kejuruter. 2018, SI, 21–26. [Google Scholar] [CrossRef]
- Jung, J.Y.; Khan, T.; Park, J.K.; Chang, H.N. Production of Bacterial Cellulose by Gluconacetobacter Hansenii Using a Novel Bioreactor Equipped with a Spin Filter. Korean J. Chem. Eng. 2007, 24, 265–271. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Dsouza, V.; Yaraguppi, D.A.; Mulla, S.I.; Deshpande, S.H.; Shettar, S.S. Low Cost Production of Bacterial Cellulose through Statistical Optimization and Developing Its Composites for Multipurpose Applications. Process Biochem. 2023, 125, 47–60. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter Xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Rivas, B.; Moldes, A.B.; Domı́nguez, J.M.; Parajó, J.C. Development of Culture Media Containing Spent Yeast Cells of Debaryomyces Hansenii and Corn Steep Liquor for Lactic Acid Production with Lactobacillus Rhamnosus. Int. J. Food Microbiol. 2004, 97, 93–98. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from Agricultural Residues by Two Komagataeibacter sp. Strains. Bioeng. 2022, 13, 10010–10025. [Google Scholar] [CrossRef]
- Revin, V.; Liyaskina, E.; Nazarkina, M.; Bogatyreva, A.; Shchankin, M. Cost-Effective Production of Bacterial Cellulose Using Acidic Food Industry by-Products. Braz. J. Microbiol. 2018, 49, 151–159. [Google Scholar] [CrossRef]
- Algar, I.; Fernandes, S.C.M.; Mondragon, G.; Castro, C.; Garcia-Astrain, C.; Gabilondo, N.; Retegi, A.; Eceiza, A. Pineapple Agroindustrial Residues for the Production of High Value Bacterial Cellulose with Different Morphologies. J. Appl. Polym. Sci. 2015, 132, 41237. [Google Scholar] [CrossRef]
- Andritsou, V.; De Melo, E.M.; Tsouko, E.; Ladakis, D.; Maragkoudaki, S.; Koutinas, A.A.; Matharu, A.S. Synthesis and Characterization of Bacterial Cellulose from Citrus-Based Sustainable Resources. ACS Omega 2018, 3, 10365–10373. [Google Scholar] [CrossRef]
- Saavedra-Sanabria, O.L.; Durán, D.; Cabezas, J.; Hernández, I.; Blanco-Tirado, C.; Combariza, M.Y. Cellulose Biosynthesis Using Simple Sugars Available in Residual Cacao Mucilage Exudate. Carbohydr. Polym. 2021, 274, 118645. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xia, J.; Wang, J.; Yan, X.; Wang, C.; Lei, T.; Xian, M.; Zhang, H. Production of Bacterial Cellulose Using Polysaccharide Fermentation Wastewater as Inexpensive Nutrient Sources. Biotechnol. Biotechnol. Equip. 2018, 32, 350–356. [Google Scholar] [CrossRef]
- Çakar, F.; Özer, I.; Aytekin, A.Ö.; Şahin, F. Improvement Production of Bacterial Cellulose by Semi-Continuous Process in Molasses Medium. Carbohydr. Polym. 2014, 106, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Hasanin, M.S.; Abdelraof, M.; Hashem, A.H.; El Saied, H. Sustainable Bacterial Cellulose Production by Achromobacter Using Mango Peel Waste. Microb. Cell Fact. 2023, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter Xylinus. J. Polym. Env. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Lu, T.; Gao, H.; Liao, B.; Wu, J.; Zhang, W.; Huang, J.; Liu, M.; Huang, J.; Chang, Z.; Jin, M.; et al. Characterization and Optimization of Production of Bacterial Cellulose from Strain CGMCC 17276 Based on Whole-Genome Analysis. Carbohydr. Polym. 2020, 232, 115788. [Google Scholar] [CrossRef]
- Heydorn, R.L.; Lammers, D.; Gottschling, M.; Dohnt, K. Effect of Food Industry By-Products on Bacterial Cellulose Production and Its Structural Properties. Cellulose 2023, 30, 4159–4179. [Google Scholar] [CrossRef]
- Souza, K.C.D.; Trindade, N.M.; Amorim, J.D.P.D.; Nascimento, H.A.D.; Costa, A.F.S.; Henrique, M.A.; Caetano, V.F.; Sarubbo, L.A.; Vinhas, G.M. Kinetic Study of a Bacterial Cellulose Production by Komagataeibacter Rhaeticus Using Coffee Grounds and Sugarcane Molasses. Mat. Res. 2021, 24, e20200454. [Google Scholar] [CrossRef]
- Tan, H.; Xiao, D.; Navik, R.; Zhao, Y. Facile Fabrication of Polyaniline/Pristine Graphene–Bacterial Cellulose Composites as High-Performance Electrodes for Constructing Flexible All-Solid-State Supercapacitors. ACS Omega 2021, 6, 11427–11435. [Google Scholar] [CrossRef]
- Salari, M.; Sowti Khiabani, M.; Rezaei Mokarram, R.; Ghanbarzadeh, B.; Samadi Kafil, H. Preparation and Characterization of Cellulose Nanocrystals from Bacterial Cellulose Produced in Sugar Beet Molasses and Cheese Whey Media. Int. J. Biol. Macromol. 2019, 122, 280–288. [Google Scholar] [CrossRef]
- Gao, G.; Cao, Y.; Zhang, Y.; Wu, M.; Ma, T.; Li, G. In Situ Production of Bacterial Cellulose/Xanthan Gum Nanocomposites with Enhanced Productivity and Properties Using Enterobacter Sp. FY-07. Carbohydr. Polym. 2020, 248, 116788. [Google Scholar] [CrossRef]
- Toscano Ávila, J.A.; Terán, D.A.; Debut, A.; Vizuete, K.; Martínez, J.; Cerda-Mejía, L.A. Shelf Life Estimation of Blackberry ( Rubus Glaucus Benth) with Bacterial Cellulose Film Coating from Komagataeibacter Xylinus. Food Sci. Nutr. 2020, 8, 2173–2179. [Google Scholar] [CrossRef] [PubMed]
- Khanpanuek, S.; Lunprom, S.; Reungsang, A.; Salakkam, A. Repeated-Batch Simultaneous Saccharification and Fermentation of Cassava Pulp for Ethanol Production Using Amylases and Saccharomyces Cerevisiae Immobilized on Bacterial Cellulose. Biochem. Eng. J. 2022, 177, 108258. [Google Scholar] [CrossRef]
- Saejung, C.; Phonaiam, S.; Kotthale, P.; Chaiyarat, A. Bacterial Cellulose as a Reinforcement Material of Alginate Beads Improves Effectiveness and Recycling Potential of Immobilized Photosynthetic Bacteria for Cooking Oil Waste Removal. Carbohydr. Polym. 2024, 324, 121532. [Google Scholar] [CrossRef]
- Fusco, D.; Meissner, F.; Podesser, B.K.; Marsano, A.; Grapow, M.; Eckstein, F.; Winkler, B. Small-Diameter Bacterial Cellulose-Based Vascular Grafts for Coronary Artery Bypass Grafting in a Pig Model. Front. Cardiovasc. Med. 2022, 9, 881557. [Google Scholar] [CrossRef]
- Taokaew, S.; Nunkaew, N.; Siripong, P.; Phisalaphong, M. Characteristics and Anticancer Properties of Bacterial Cellulose Films Containing Ethanolic Extract of Mangosteen Peel. J. Biomater. Sci. Polym. Ed. 2014, 25, 907–922. [Google Scholar] [CrossRef]
- Sajjad, W.; He, F.; Ullah, M.W.; Ikram, M.; Shah, S.M.; Khan, R.; Khan, T.; Khalid, A.; Yang, G.; Wahid, F. Fabrication of Bacterial Cellulose-Curcumin Nanocomposite as a Novel Dressing for Partial Thickness Skin Burn. Front. Bioeng. Biotechnol. 2020, 8, 553037. [Google Scholar] [CrossRef]
- Hur, D.H.; Rhee, H.-S.; Lee, J.H.; Shim, W.Y.; Kim, T.Y.; Lee, S.Y.; Park, J.H.; Jeong, K.J. Enhanced Production of Cellulose in Komagataeibacter Xylinus by Preventing Insertion of IS Element into Cellulose Synthesis Gene. Biochem. Eng. J. 2020, 156, 107527. [Google Scholar] [CrossRef]
- Shigematsu, T.; Takamine, K.; Kitazato, M.; Morita, T.; Naritomi, T.; Morimura, S.; Kida, K. Cellulose Production from Glucose Using a Glucose Dehydrogenase Gene (Gdh)-Deficient Mutant of Gluconacetobacter Xylinus and Its Use for Bioconversion of Sweet Potato Pulp. J. Biosci. Bioeng. 2005, 99, 415–422. [Google Scholar] [CrossRef]
- Fang, J.; Kawano, S.; Tajima, K.; Kondo, T. In Vivo Curdlan/Cellulose Bionanocomposite Synthesis by Genetically Modified Gluconacetobacter Xylinus. Biomacromolecules 2015, 16, 3154–3160. [Google Scholar] [CrossRef]
- Jang, W.D.; Kim, T.Y.; Kim, H.U.; Shim, W.Y.; Ryu, J.Y.; Park, J.H.; Lee, S.Y. Genomic and Metabolic Analysis of Komagataeibacter Xylinus DSM 2325 Producing Bacterial Cellulose Nanofiber. Biotech Bioeng. 2019, 116, 3372–3381. [Google Scholar] [CrossRef] [PubMed]
- Business Research Insights Microbial and Bacterial Cellulose Market Size, Share, Global Industry Analysis, by Type (Plant Cellulose and Bacterial Cellulose), by Application, (Paper and Packaging, Composites Materials, Hygiene and Absorbent Products, Paints and Coatings, Food, Biomedical and Pharmaceutical, and Others), and COVID-19 Impact, Latest Trends, Driving Factors, Restraining Factors, Regional Insights, and Forecast from 2024 to 2032. Available online: https://www.businessresearchinsights.com/market-reports/microbial-and-bacterial-cellulose-market-100001 (accessed on 16 October 2024).
- Ul-Islam, M.; Ullah, M.W.; Khan, S.; Park, J.K. Production of Bacterial Cellulose from Alternative Cheap and Waste Resources: A Step for Cost Reduction with Positive Environmental Aspects. Korean J. Chem. Eng. 2020, 37, 925–937. [Google Scholar] [CrossRef]
- Quijano, L.; Rodrigues, R.; Fischer, D.; Tovar-Castro, J.D.; Payne, A.; Navone, L.; Hu, Y.; Yan, H.; Pinmanee, P.; Poon, E.; et al. Bacterial Cellulose Cookbook: A Systematic Review on Sustainable and Cost-Effective Substrates. J. Bioresour. Bioprod. 2024, 9, 379–409. [Google Scholar] [CrossRef]
- Qi, G.; Luo, M.; Huang, C.; Guo, H.; Chen, X.; Xiong, L.; Wang, B.; Lin, X.; Peng, F.; Chen, X. Comparison of Bacterial Cellulose Production by Gluconacetobacter xylinus on Bagasse Acid and Enzymatic Hydrolysates. J. Appl. Polym. Sci. 2017, 134, 45066. [Google Scholar] [CrossRef]
- Goelzer, F.D.E.; Faria-Tischer, P.C.S.; Vitorino, J.C.; Sierakowski, M.-R.; Tischer, C.A. Production and Characterization of Nanospheres of Bacterial Cellulose from Acetobacter Xylinum from Processed Rice Bark. Mater. Sci. Eng. C 2009, 29, 546–551. [Google Scholar] [CrossRef]
- Dima, S.-O.; Panaitescu, D.-M.; Orban, C.; Ghiurea, M.; Doncea, S.-M.; Fierascu, R.; Nistor, C.; Alexandrescu, E.; Nicolae, C.-A.; Trică, B.; et al. Bacterial Nanocellulose from Side-Streams of Kombucha Beverages Production: Preparation and Physical-Chemical Properties. Polymers 2017, 9, 374. [Google Scholar] [CrossRef]
- McKenna, B.A.; Mikkelsen, D.; Wehr, J.B.; Gidley, M.J.; Menzies, N.W. Mechanical and Structural Properties of Native and Alkali-Treated Bacterial Cellulose Produced by Gluconacetobacter Xylinus Strain ATCC 53524. Cellulose 2009, 16, 1047–1055. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Hamid, S.B.A. Preparation and Characterization of Nanocrystalline Cellulose Using Ultrasonication Combined with a Microwave-Assisted Pretreatment Process. BioResources 2016, 11, 3397–3415. [Google Scholar] [CrossRef]
- Lu, H.; Jiang, X. Structure and Properties of Bacterial Cellulose Produced Using a Trickling Bed Reactor. Appl. Biochem. Biotechnol. 2014, 172, 3844–3861. [Google Scholar] [CrossRef]
- Gatenholm, P.; Klemm, D. Bacterial Nanocellulose as a Renewable Material for Biomedical Applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Gao, X.; Sozumert, E.; Shi, Z.; Yang, G.; Silberschmidt, V.V. Assessing Stiffness of Nanofibres in Bacterial Cellulose Hydrogels: Numerical-Experimental Framework. Mater. Sci. Eng. C 2017, 77, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Gorgieva, S.; Jančič, U.; Cepec, E.; Trček, J. Production Efficiency and Properties of Bacterial Cellulose Membranes in a Novel Grape Pomace Hydrolysate by Komagataeibacter Melomenusus AV436T and Komagataeibacter Xylinus LMG 1518. Int. J. Biol. Macromol. 2023, 244, 125368. [Google Scholar] [CrossRef] [PubMed]
- Dayal, M.S.; Catchmark, J.M. Mechanical and Structural Property Analysis of Bacterial Cellulose Composites. Carbohydr. Polym. 2016, 144, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, G.; Alriksson, B.; Wang, W.; Hong, F.F.; Jonsson, L.J. Bioconversion of Waste Fiber Sludge to Bacterial Nanocellulose and Use for Reinforcement of CTMP Paper Sheets. Polymers 2017, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.; Lv, P.; Zhou, H.; Naveed, T.; Wei, Q. A Novel In Situ Self-Assembling Fabrication Method for Bacterial Cellulose-Electrospun Nanofiber Hybrid Structures. Polymers 2018, 10, 712. [Google Scholar] [CrossRef]
- Sederavičiūtė, F.; Bekampienė, P.; Domskienė, J. Effect of Pretreatment Procedure on Properties of Kombucha Fermented Bacterial Cellulose Membrane. Polym. Test. 2019, 78, 105941. [Google Scholar] [CrossRef]
- Abral, H.; Lawrensius, V.; Handayani, D.; Sugiarti, E. Preparation of Nano-Sized Particles from Bacterial Cellulose Using Ultrasonication and Their Characterization. Carbohydr. Polym. 2018, 191, 161–167. [Google Scholar] [CrossRef]
- Kawee, N.; Lam, N.T.; Sukyai, P. Homogenous Isolation of Individualized Bacterial Nanofibrillated Cellulose by High Pressure Homogenization. Carbohydr. Polym. 2018, 179, 394–401. [Google Scholar] [CrossRef]
- Andrade, F.K.; Morais, J.P.S.; Muniz, C.R.; Nascimento, J.H.O.; Vieira, R.S.; Gama, F.M.P.; Rosa, M.F. Stable Microfluidized Bacterial Cellulose Suspension. Cellulose 2019, 26, 5851–5864. [Google Scholar] [CrossRef]
- Ullah, M.W.; Ul-Islam, M.; Wahid, F.; Yang, G. Editorial: Nanocellulose: A Multipurpose Advanced Functional Material, Volume II. Front. Bioeng. Biotechnol. 2022, 10, 931256. [Google Scholar] [CrossRef]
- Ghosh, T.; Dhar, P.; Katiyar, V. 2. Nanocellulose: Extraction and Fabrication Methodologies. In Cellul. Nanocrystals; De Gruyter: Berlin, Germany, 2020; pp. 23–48. ISBN 978-3-11-064801-0. [Google Scholar]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural Features and Properties of Bacterial Cellulose Produced in Agitated Culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Carmen Bañó, M. Nanocomposites of Bacterial Cellulose/Hydroxyapatite for Biomedical Applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar] [CrossRef]
- Chen, G.; Koon-Yang, L.; Bismarck, A.; Li, R. Cellulose Materials And Methods of Making And Using The Same. International Patent Application No. PCT/CN2017/074728, 24 February 2017. [Google Scholar]


| Quality Assessment Questions | Answer |
|---|---|
| Does the paper describe technologies used for bacterial cellulose production? | (+1) Yes/(+0) No |
| Does the paper describe the technical aspects of and methodologies for bacterial cellulose production? | (+1) Yes/(+0) No |
| Does the paper describe the limitations and disadvantages of bacterial cellulose production? | (+1) Yes/(+0) No |
| Is the journal or conference in which the paper was published indexed by the SJR? | (+1) if it is ranked Q1, (+0.75) if it is ranked Q2, (+0.50) if it is ranked Q3, (+0.25) if it is ranked Q4, (+0.0) if it is not ranked |
| Microorganism | Description | References |
|---|---|---|
| Gluconacetobacter/Komagataeibacter | Gluconacetobacter/Komagataeibacter xylinus is one of the most efficient producers of B.C, often using various carbon sources like agro-industrial residues for large-scale production. B.C. from this bacterium is used in biomedical applications and nanocomposites. | [58,59,60] |
| Acetobacter | Similar to Gluconacetobacter, Acetobacter species are frequently studied in regard to fermentation processes for B.C. production, using bioprocess optimization to increase yields. | [58] |
| Achromobacter | Less frequently studied, but has potential in regard to B.C. production, especially when used in conjunction with other bacteria to enhance efficiency and adjust B.C. properties for industrial use. | [59] |
| Rhizobium | Shows promise in B.C. production and has been studied alongside other microorganisms to optimize B.C. yield and tailor it for specific industrial applications. | [61] |
| Sarcina | Known for producing B.C., though not as extensively researched as Gluconacetobacter or Acetobacter, it has potential for use in specific applications in industrial cellulose production. | [62] |
| Property | Value | Reference |
|---|---|---|
| Fibril size (bundle of nanofibrils) | <100 nm wide, 2 µm length | [57,101] |
| Elementary nanofibril size | 7-8 nm wide | [57] |
| Crystal size Static culture Agitated culture Trickling bed reactor | 11. 95 nm 7.91 nm 10.07 nm | [104] |
| Crystallinity Static culture Agitated culture Trickling bed reactor | 84–91% 64% 69% | [57,104] |
| Purity | 96–98% | [104] |
| Thermal properties Onset degradation temperature (Ton) Temperature at the maximum degradation rate (Td) | 253.9 °C 273.9/328.3 °C | [101] |
| Porosity | 83–86% | [104] |
| Initial water-holding capacity | 98–99% | [57] |
| Rehydration ratio | 72–83% | [104] |
| Flexibility (Young’s modulus) | 15–35 GPa | [57,102,105] |
| Stiffness | 53.7–64.9 GPa | [106] |
| Tensile strength | 200–300 MPa | [105,107] |
| Elongation | 1.5–5.6% | [105,107] |
| Compressive modulus | 7–8 kPa | [108] |
| Intrinsic viscosity | 411 mL/g a | [8] |
| Degree of polymerization | 1765 b | [8] |
| Hydrophilicity | 1 g B.C.:100 mL water | [101] |
| Patent Title | Summary | Filed Year | Applications | Ref. |
|---|---|---|---|---|
| 20170191100 | To provide a B.C. that is highly dispersible in liquid, shows excellent molding properties, and high miscibility with other materials when applied to materials and, therefore, has high applicability as a practical material and a bacterium which produces the B.C. | 2017 | Better B.C. production | [119] |
| 20220315760 | B.C.–polyurethane composite material, preparation method, and use are described. The preparation method comprises performing organic solvent exchange on B.C. microfibers and obtaining a B.C. microfiber composite. | 2022 | B.C.–polyurethane | [119] |
| 20130211308 | Nanosilver-coated B.C. nanofiber and a method of producing the nanosilver-coated B.C. nanofiber. The nanosilver-coated B.C. nanofiber is produced by preparing a suspension of B.C. fibers and oxidizing the B.C. fibers. | 2013 | Medical textiles, tissue engineering, wound healing applications | [119] |
| EP2331699 | Describes a production method for cellulose synthesized by bacteria, enabling the large-scale production of homogeneous materials. | 2019 | Textiles, biopolymer production | [28] |
| CN103481720A | Outlines a method for manufacturing decorative designs using B.C., involving dyeing and molding processes. | 2013 | Textiles, decorative products | [28] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, M.A.; Flor-Unda, O.; Avila, A.; Garcia, M.D.; Cerda-Mejía, L. Advances in Bacterial Cellulose Production: A Scoping Review. Coatings 2024, 14, 1401. https://doi.org/10.3390/coatings14111401
Cruz MA, Flor-Unda O, Avila A, Garcia MD, Cerda-Mejía L. Advances in Bacterial Cellulose Production: A Scoping Review. Coatings. 2024; 14(11):1401. https://doi.org/10.3390/coatings14111401
Chicago/Turabian StyleCruz, María Alejandra, Omar Flor-Unda, Alec Avila, Mario D. Garcia, and Liliana Cerda-Mejía. 2024. "Advances in Bacterial Cellulose Production: A Scoping Review" Coatings 14, no. 11: 1401. https://doi.org/10.3390/coatings14111401
APA StyleCruz, M. A., Flor-Unda, O., Avila, A., Garcia, M. D., & Cerda-Mejía, L. (2024). Advances in Bacterial Cellulose Production: A Scoping Review. Coatings, 14(11), 1401. https://doi.org/10.3390/coatings14111401









