Metal Ions’ Dynamic Effect on Metal-Assisted Catalyzed Etching of Silicon in Acid Solution
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
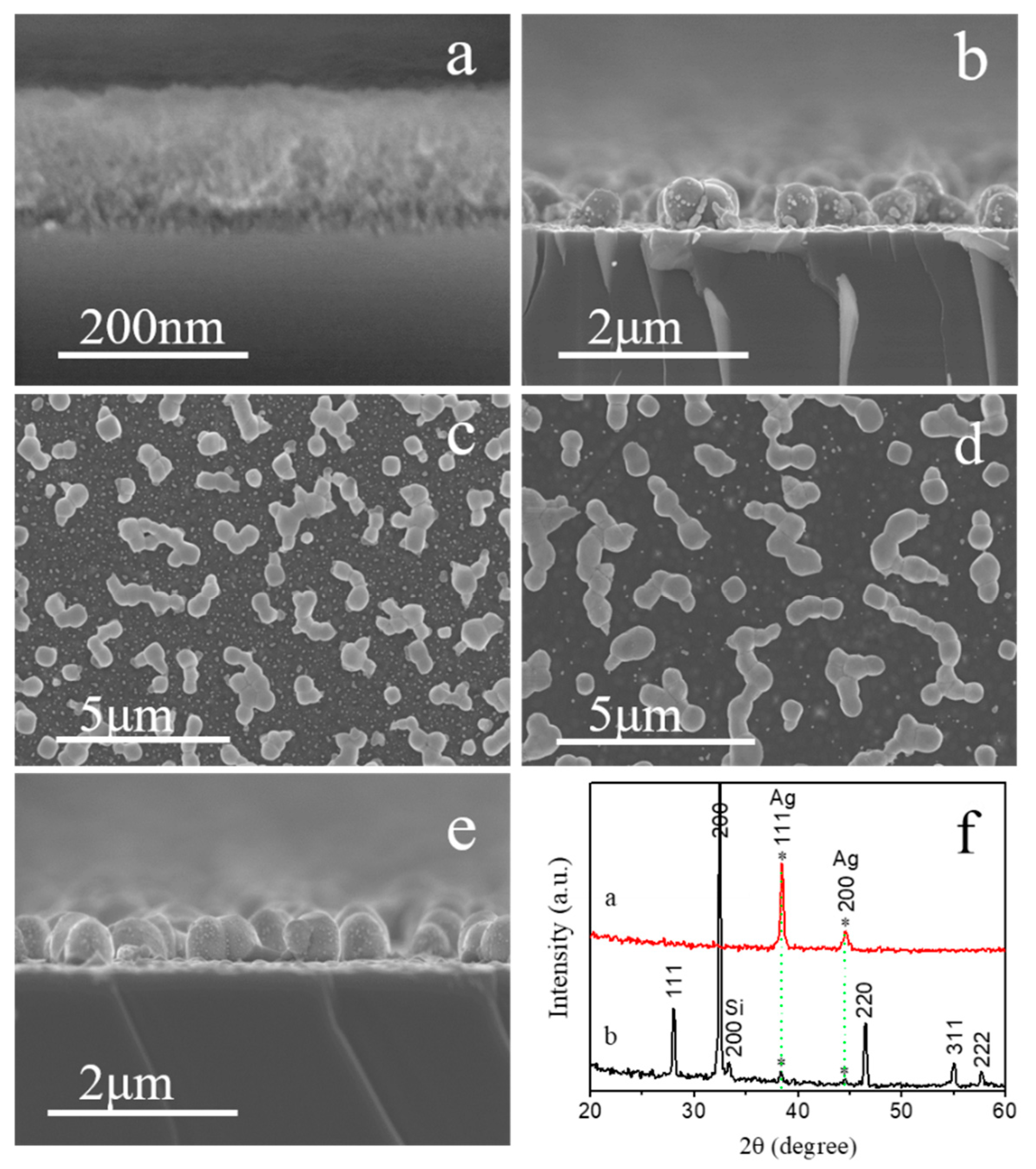
3.1. Oxidation Validation of Fe3+ and NO3−
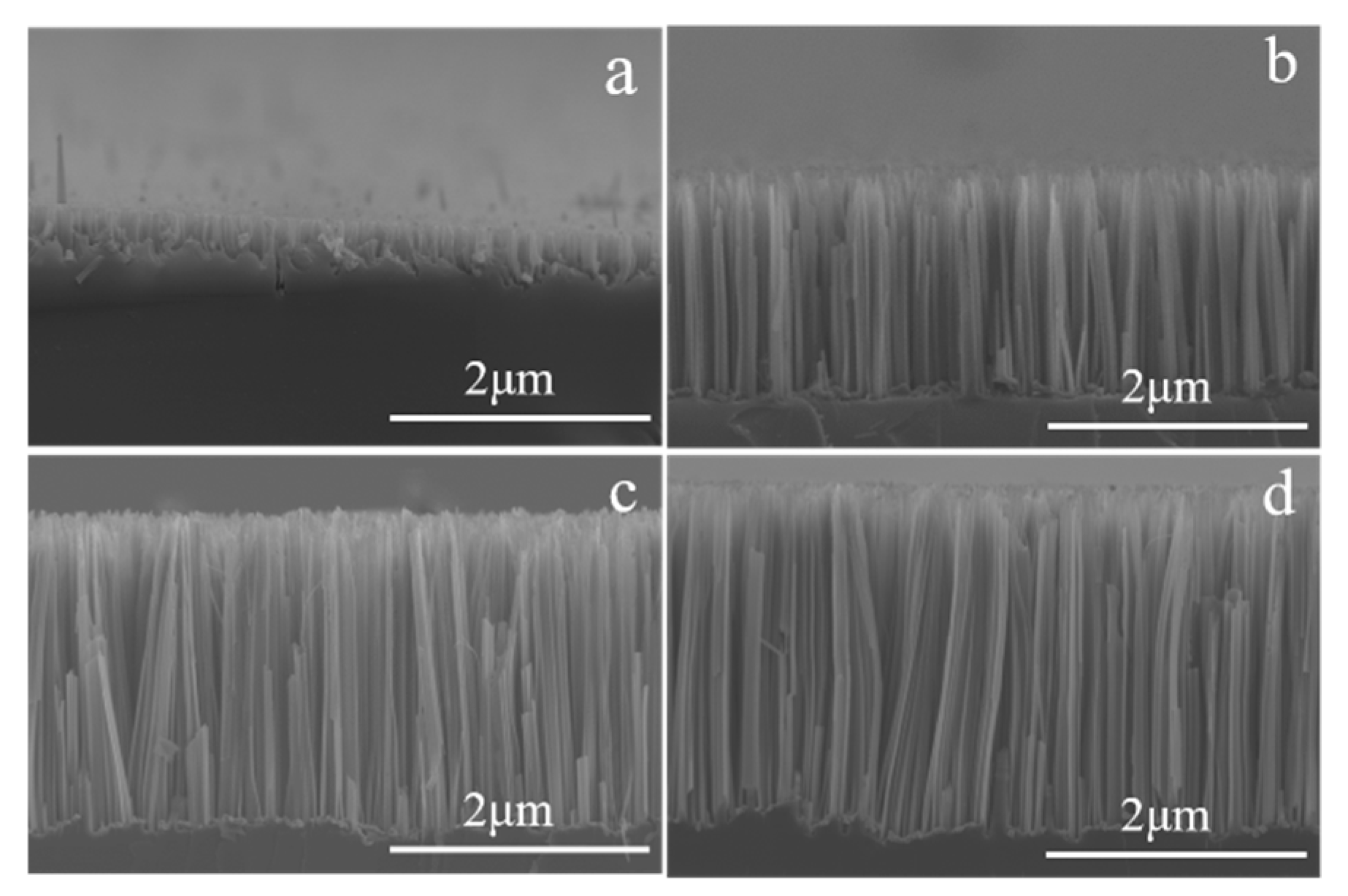
3.2. Metal Ions’ Dynamic Effect Analysis
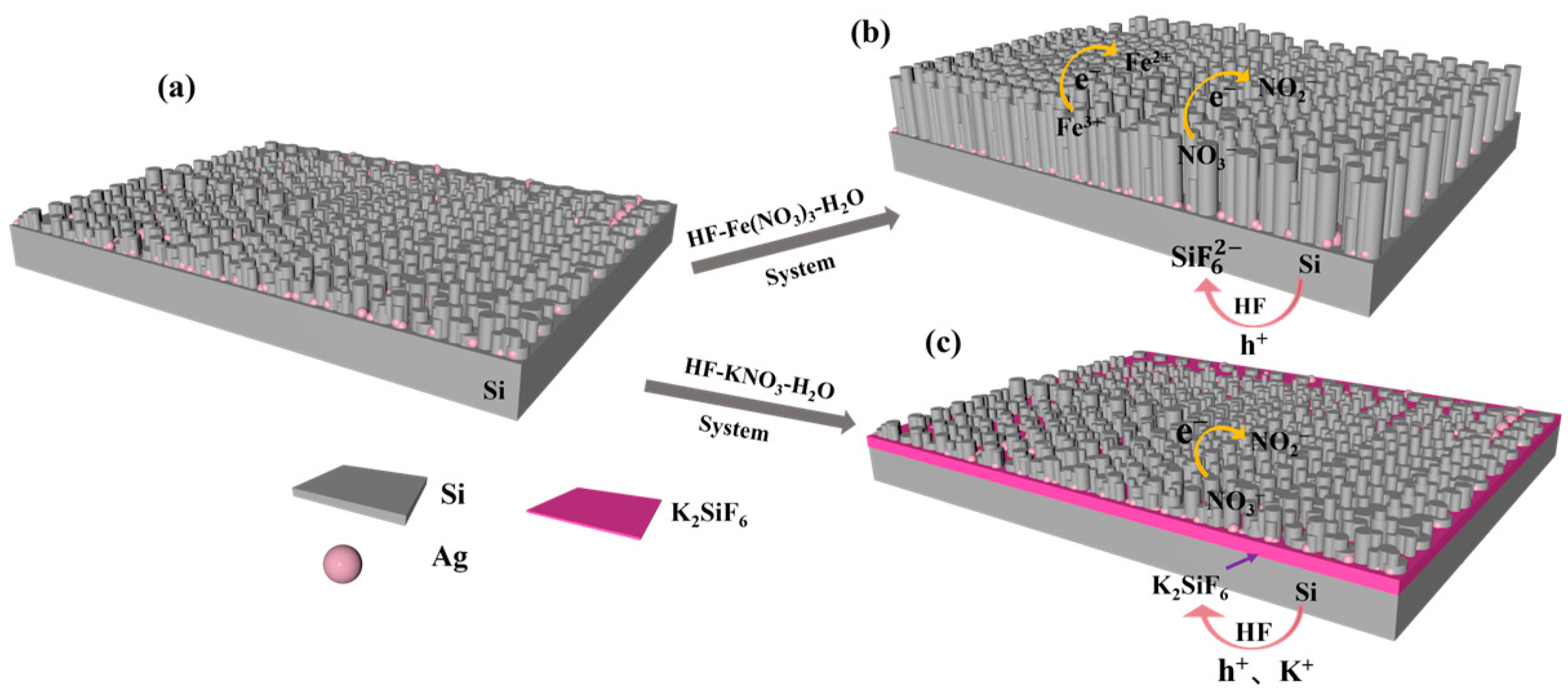
3.3. Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhanabalan, A.; Song, B.F.; Biswal, S.L. Extreme Rate Capability Cycling of Porous Silicon Composite Anodes for Lithium-Ion Batteries. ChemElectroChem 2021, 8, 3318–3325. [Google Scholar] [CrossRef]
- Nulu, A.; Nulu, V.; Sohn, K.Y. Si/SiOx Nanoparticles Embedded in a Conductive and Durable Carbon Nanoflake Matrix as an Efficient Anode for Lithium-Ion Batteries. ChemElectroChem 2020, 7, 4055–4065. [Google Scholar] [CrossRef]
- Ulvestad, A.; Reksten, A.H.; Andersen, H.F.; Carvalho, P.A.; Jensen, I.J.T.; Nagell, M.U.; Mæhlen, J.P.; Kirkengen, M.; Koposov, A.Y. Crystallinity of Silicon Nanoparticles: Direct Influence on the Electrochemical Performance of Lithium Ion Battery Anodes. ChemElectroChem 2020, 7, 4349–4353. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Han, J. Comprehensive Study of Au Nano-Mesh as a Catalyst in the Fabrication of Silicon Nanowires Arrays by Metal-Assisted Chemical Etching. Coatings 2019, 9, 149. [Google Scholar] [CrossRef]
- Prigoda, K.; Ermina, A.; Bolshakov, V.; Nazarov, D.; Ezhov, I.; Lutakov, O.; Maximov, M.; Tolmachev, V.; Zharova, Y. The Array of Si Nanowires Covered with Ag Nanoparticles by ALD: Fabrication Process and Optical Properties. Coatings 2022, 12, 1748. [Google Scholar] [CrossRef]
- Shanshan, W.; Yan, Z. Effect of High-Temperature Annealing on Raman Characteristics of Silicon Nanowire Arrays. Coatings 2023, 13, 793. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, G.; Zhu, J.; Fan, C.; Li, X.G.; Zhang, Y. Ultraviolet Light Emission from Porous Silicon Hydrothermally Prepared. Phys. Lett. A 1996, 224, 133–136. [Google Scholar] [CrossRef]
- Chen, Q.; Zhu, J.; Li, X.-G.; Fan, C.G.; Zhang, Y.H. Photoluminescence in Porous Silicon Obtained by Hydrothermal Etching. Phys. Lett. A 1996, 220, 293–296. [Google Scholar] [CrossRef]
- Peng, K.Q.; Hu, J.J.; Yan, Y.J.; Wu, Y.; Fang, H.; Xu, Y.; Lee, S.T.; Zhu, J. Fabrication of Single-Crystalline Silicon Nanowires by Scratching a Silicon Surface with Catalytic Metal Particles. Adv. Funct. Mater. 2005, 16, 387–394. [Google Scholar] [CrossRef]
- Hu, Y.; Peng, K.-Q.; Qiao, Z.; Huang, X.; Zhang, F.-Q.; Sun, R.-N.; Meng, X.-M.; Lee, S.-T. Metal-Catalyzed Electroless Etching of Silicon in Aerated HF/H2O Vapor for Facile Fabrication of Silicon Nanostructures. Nano Lett. 2014, 14, 4212–4219. [Google Scholar] [CrossRef]
- Jiang, W.; Ya, H.; Haichuan, Z.; Haoxin, F.; Yachun, W.; Chenliang, H.; Kui-Qing, P. Oxidant Concentration Modulated Metal/Silicon Interface Electrical Field Mediates Metal-Assisted Chemical Etching of Silicon. Adv. Mater. Interfaces 2018, 5, 1801132. [Google Scholar]
- Peng, K.; Lu, A.; Zhang, R.; Lee, S.-T. Motility of Metal Nanoparticles in Silicon and Induced Anisotropic Silicon Etching. Adv. Funct. Mater. 2008, 18, 3026–3035. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, C.; Liu, Y.; Yang, X.; Liao, Z.; Zhang, B.; Zhou, Y.; Chen, A.; Wu, L.; Liu, J.; et al. Metal Particle Evolution Behavior during Metal Assisted Chemical Etching of Silicon. ECS J. Solid State Sci. Technol. 2021, 10, 084002. [Google Scholar] [CrossRef]
- Peng, K.Q.; Yan, Y.J.; Gao, S.P.; Zhu, J. Synthesis of Large-Area Silicon Nanowire Arrays via Self-Assembling Nanoelectrochemistry. Adv. Mater. 2002, 14, 1164–1167. [Google Scholar] [CrossRef]
- To, W.-K.; Tsang, C.-H.; Li, H.-H.; Huang, Z. Fabrication of N-Type Mesoporous Silicon Nanowires by One-Step Etching. Nano Lett. 2011, 11, 5252–5258. [Google Scholar] [CrossRef]
- Leng, X.; Wang, C.; Yuan, Z. Progress in Metal-Assisted Chemical Etching of Silicon Nanostructures. Procedia CIRP 2020, 89, 26–32. [Google Scholar] [CrossRef]
- Venkatesan, R.; Arivalagan, M.K.; Venkatachalapathy, V.; Pearce, J.M.; Mayandi, J. Effects of Silver Catalyst Concentration in Metal Assisted Chemical Etching of Silicon. Mater. Lett. 2018, 221, 206–210. [Google Scholar] [CrossRef]
- Aca-López, V.; Quiroga-González, E.; Gómez-Barojas, E.; Światowska, J.; Luna-López, J.A. Effects of the Doping Level in the Production of Silicon Nanowalls by Metal Assisted Chemical Etching. Mater. Sci. Semicond. Process. 2020, 118, 105206. [Google Scholar] [CrossRef]
- Han, H.; Huang, Z.; Lee, W. Metal-Assisted Chemical Etching of Silicon and Nanotechnology Applications. Nano Today 2014, 9, 271–304. [Google Scholar] [CrossRef]
- Hu, Y.; Fu, H.; Wang, J.; Sun, R.; Wu, L.; Liu, Y.; Xu, J.; Liu, J.; Peng, K.-Q. Carbon Induced Galvanic Etching of Silicon in Aerated HF/H2O Vapor. Corros. Sci. 2019, 157, 268–273. [Google Scholar] [CrossRef]
- Hassan, H.H.; Chazalviel, J.N.; Neumann-Spallart, M.; Ozanam, F.; Etman, M. Chemical Limitations to the Anodic Dissolution of P-Si in Fluoride Media in the Presence of Alkali Metal Cations. J. Electroanal. Chem. 1995, 381, 211–214. [Google Scholar] [CrossRef]
- Hassan, H.H.; Fotouhi, B.; Sculfort, J.L.; Abdel-Rehiem, S.S.; Etman, M.; Ozanam, F.; Chazalviel, J.N. Effect of Alkali-Metal and Some Quaternary-Ammonium Cations on the Anodic Dissolution of p-Si in Fluoride Media. J. Electroanal. Chem. 1996, 407, 105–113. [Google Scholar] [CrossRef]
- Nahm, K.S.; Seo, Y.H.; Lee, H.J. Formation Mechanism of Stains during Si Etching Reaction in HF–Oxidizing Agent–H2O Solutions. J. Appl. Phys. 1997, 81, 2418–2424. [Google Scholar] [CrossRef]
- Hun, S.Y.; Suk, N.K.; Bang, L.K. Mechanistic Study of Silicon Etching in HF-KBrO3-H2O Solution. J. Electrochem. Soc. 1993, 140, 1453. [Google Scholar]
- Lin, H.; Wu, F.; Gao, P.; Shen, W. Shape-Controlled Silicon Microwire Arrays from Au–Ag-Catalyzed Metal-Assisted Chemical Etching for Radial Junction Solar Cells. ACS Appl. Energy Mater. 2019, 2, 5871–5876. [Google Scholar] [CrossRef]
- Booker, K.; Rahman, S.; Chong, T.-K.; Mankelow, R.; Weber, K.; Blakers, A. A Robust Metal-Assisted Etching Process for Ag-Catalyzed Texturing of Silicon. IEEE J. Photovolt. 2015, 5, 766–773. [Google Scholar] [CrossRef]
- Shaoyuan, L.; Wenhui, M.; Yang, Z.; Xiuhua, C.; Yongyin, X.; Mingyu, M.; Wenjie, Z.; Feng, W. Fabrication of Porous Silicon Nanowires by MACE Method in HF/H2O2/AgNO3 System at Room Temperature. Nanoscale Res. Lett. 2014, 9, 196. [Google Scholar] [CrossRef]
- Milazzo, R.G.; D’arrigo, G.; Spinella, C.; Grimaldi, M.G.; Rimini, E. Ag-Assisted Chemical Etching of (100) and (111) n-Type Silicon Substrates by Varying the Amount of Deposited Metal. J. Electrochem. Soc. 2012, 159, D521. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, J. Morphology-Controlled Synthesis and Applications of Silver Halide Photocatalytic Materials. Catal. Surv. Asia 2012, 16, 210–230. [Google Scholar] [CrossRef]
- Bi, Y.; Ye, J. In Situ Oxidation Synthesis of Ag/AgCl Core–Shell Nanowires and Their Photocatalytic Properties. Chem. Commun. 2009, 2009, 6551–6553. [Google Scholar] [CrossRef]
- Bi, Y.; Ye, J. Direct Conversion of Commercial Silver Foils into High Aspect Ratio AgBr Nanowires with Enhanced Photocatalytic Properties. Chem.—A Eur. J. 2010, 16, 10327–10331. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Ye, J. Heteroepitaxial Growth of Platinum Nanocrystals on AgCl Nanotubes via Galvanic Replacement Reaction. Chem. Commun. 2010, 46, 1532. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.; Koper, M.T. The Influence of Nitrate Concentration and Acidity on the Electrocatalytic Reduction of Nitrate on Platinum. J. Electroanal. Chem. 2004, 562, 81–94. [Google Scholar] [CrossRef]
- Dima, G.E.; Beltramo, G.L.; Koper, M.T.M. Nitrate Reduction on Single-Crystal Platinum Electrodes. Electrochim. Acta 2005, 50, 4318–4326. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Liu, Y.; Wu, L.; Liao, Z.; Zhang, B.; Tembo, T.; Wang, Y.; Hu, Y. Metal Ions’ Dynamic Effect on Metal-Assisted Catalyzed Etching of Silicon in Acid Solution. Coatings 2024, 14, 1405. https://doi.org/10.3390/coatings14111405
Yang X, Liu Y, Wu L, Liao Z, Zhang B, Tembo T, Wang Y, Hu Y. Metal Ions’ Dynamic Effect on Metal-Assisted Catalyzed Etching of Silicon in Acid Solution. Coatings. 2024; 14(11):1405. https://doi.org/10.3390/coatings14111405
Chicago/Turabian StyleYang, Xiaoyu, Ying Liu, Lin Wu, Zhiyuan Liao, Baoguo Zhang, Tinashe Tembo, Yichen Wang, and Ya Hu. 2024. "Metal Ions’ Dynamic Effect on Metal-Assisted Catalyzed Etching of Silicon in Acid Solution" Coatings 14, no. 11: 1405. https://doi.org/10.3390/coatings14111405
APA StyleYang, X., Liu, Y., Wu, L., Liao, Z., Zhang, B., Tembo, T., Wang, Y., & Hu, Y. (2024). Metal Ions’ Dynamic Effect on Metal-Assisted Catalyzed Etching of Silicon in Acid Solution. Coatings, 14(11), 1405. https://doi.org/10.3390/coatings14111405





