In Situ Evaluation of New Silica Nanosystems as Long-Lasting Methods to Prevent Stone Monument Biodeterioration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biocide, Lithotypes, and Coatings
2.1.1. Biocide and Their Nanocontainers
2.1.2. Lithotypes Selection and Aging
2.1.3. Coating Development and Aging
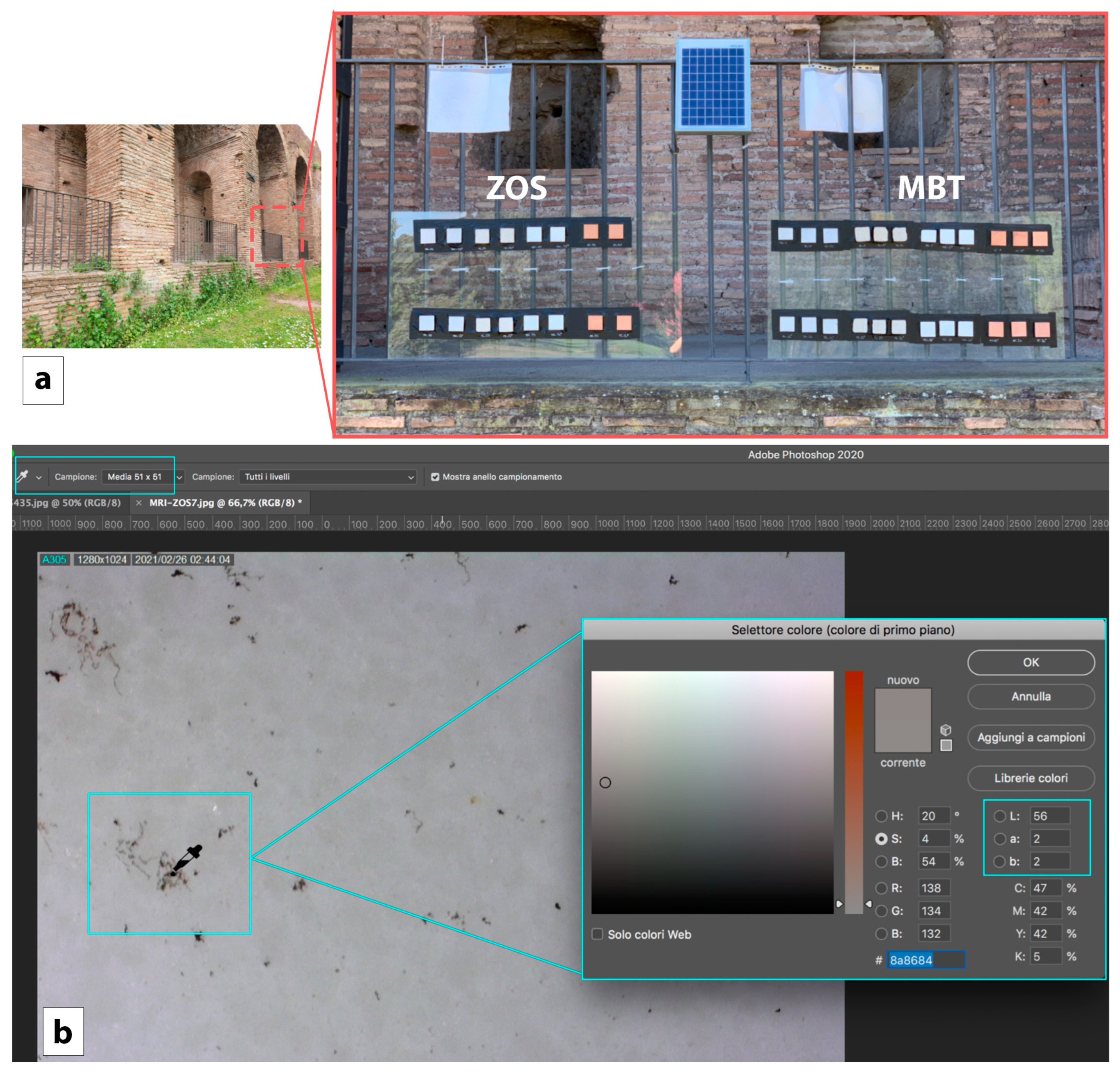
2.2. In Situ Tests
2.2.1. Microscopic and Colorimetric Analysis
2.2.2. Microclimate Evaluation
3. Results
3.1. Coating Tests
3.1.1. Comparison between Pretreating and After-Treating Samples
3.1.2. Comparison between Pre-Aging and After-Aging Samples
3.2. In Situ Tests
3.2.1. Microclimate
3.2.2. Biocidal Efficiency
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Herrera, L.K.; Videla, H.A. The importance of atmospheric effects on biodeterioration of cultural heritage constructional materials. Int. Biodeterior. Biodegrad. 2004, 54, 125–134. [Google Scholar] [CrossRef]
- Nuhoglu, Y.; Oguz, E.; Uslu, H.; Ozbek, A.; Ipekoglu, B.; Ocak, I.; Hasenekoglu, I. The accelerating effects of the microorganisms on biodeterioration of stone monuments under air pollution and continental-cold climatic conditions in Erzurum, Turkey. Sci. Total Environ. 2006, 364, 272–283. [Google Scholar] [CrossRef]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Microbial deterioration of stone monuments—An updated overview. Adv. Appl. Microbiol. 2009, 66, 97–139. [Google Scholar] [CrossRef] [PubMed]
- Austigard, M.S.; Mattsson, J. Monitoring climate change related biodeterioration of protected historic buildings. Int. J. Build. Pathol. Adapt. 2019, 38, 529–538. [Google Scholar] [CrossRef]
- Caneva, G.; Nugari, M.P.; Salvadori, O. Plant Biology for Cultural Heritage: Biodeterioration and Conservation; The Getty Conservation Institute: Los Angeles, CA, USA, 2008. [Google Scholar]
- Liu, X.; Koestler, R.J.; Warscheid, T.; Katayama, Y.; Gu, J.D. Microbial deterioration and sustainable conservation of stone monuments and buildings. Nat. Sustain. 2020, 3, 991–1004. [Google Scholar] [CrossRef]
- Negi, A.; Sarethy, I.P. Microbial biodeterioration of cultural heritage: Events, colonization, and analyses. Microb. Ecol. 2019, 78, 1014–1029. [Google Scholar] [CrossRef] [PubMed]
- Valentin, N. Trends on biological deterioration of rocks and monumental stones. In Congrés International sur la Conservation de la Pierre et Autres Matériaux; UNESCO: Paris, France, 1993; pp. 30–34. [Google Scholar]
- Warscheid, T.; Braams, J. Biodeterioration of stone: A review. Int. Biodeterior. Biodegrad. 2000, 46, 343–368. [Google Scholar] [CrossRef]
- Ciferri, O. The role of microorganisms in the degradation of cultural heritage. Stud. Conserv. 2002, 47, 35–45. [Google Scholar] [CrossRef]
- Crispim, C.; Gaylarde, C. Cyanobacteria and Biodeterioration of Cultural Heritage: A Review. Microb. Ecol. 2005, 49, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Gong, C.; Gu, J.; Katayama, Y.; Someya, T.; Gu, J.D. Biochemical reactions and mechanisms involved in the biodeterioration of stone world cultural heritage under the tropical climate conditions. Int. Biodeterior. Biodegrad. 2019, 143, 104723. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Vannini, A.; Benesperi, R.; Bianchi, E.; Fačkovcová, Z.; Giordani, P.; Malaspina, P.; Martire, L.; Matteucci, E.; Paoli, L.; et al. The application protocol impacts the effectiveness of biocides against lichens. Int. Biodeterior. Biodegrad. 2020, 155, 105105. [Google Scholar] [CrossRef]
- Demoulin, T.; Girardet, F.; Flatt, R.J. Reprofiling of altered building sandstones: On-site measurement of the environmental conditions and their evolution in the stone. In 32èmes Rencontres de l’AUGC; ETH: Zürich, Switzerland, 2014. [Google Scholar] [CrossRef]
- Juhász, P.; Kopecskó, K.; Suhajda, Á. Analysis of capillary absorption properties of porous limestone material and its relation to the migration depth of bacteria in the absorbed biomineralizing compound. Period. Polytech. Civ. Eng. 2014, 58, 113–120. [Google Scholar] [CrossRef]
- Monte, M. The influence of environmental conditions on the reproduction and distribution of epilithic lichens. Aerobiologia 1993, 9, 169–179. [Google Scholar] [CrossRef]
- Ortega-Calvo, J.J.; Ariño, X.; Hernandez-Marine, M.; Saiz-Jimenez, C. Factors affecting the weathering and colonization of monuments by phototrophic microorganisms. Sci. Total Environ. 1995, 167, 329–341. [Google Scholar] [CrossRef]
- Pinna, D. Microbial growth and its effects on inorganic heritage materials. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 3–36. [Google Scholar]
- Darlington, A. Ecology of Walls; Heinemann Educational Publishers: Portsmouth, NH, USA, 1981. [Google Scholar]
- Chung, Y.J.; Seo, M.S.; Lee, K.S.; Han, S.H. The biodeterioration and conservation of stone historical monuments. Conserv. Sci. Res. 2003, 5–28. [Google Scholar]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration; Cambridge University Press: Cambridge, UK, 2004. [Google Scholar]
- Jeong, S.H.; Lee, H.J.; Kim, D.W.; Chung, Y.J. New biocide for eco-friendly biofilm removal on outdoor stone monuments. Int. Biodeterior. Biodegrad. 2018, 131, 19–28. [Google Scholar] [CrossRef]
- Kakakhel, M.A.; Wu, F.; Gu, J.D.; Feng, H.; Shah, K.; Wang, W. Controlling biodeterioration of cultural heritage objects with biocides: A review. Int. Biodeterior. Biodegrad. 2019, 143, 104721. [Google Scholar] [CrossRef]
- Scheerer, S. Microbial Biodeterioration of Outdoor Stone Monuments: Assessment Methods and Control Strategies; Cardiff University: Cardiff, UK, 2008. [Google Scholar]
- Cappitelli, F.; Cattò, C.; Villa, F. The control of cultural heritage microbial deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Nugari, M.P.; Pinna, D.; Salvadori, O. Il Controllo del Degrado Biologico: I Biocidi nel Restauro dei Materiali Lapidei; Nardini Editore: Fiesole, Italy, 1996; p. 151. [Google Scholar]
- Nugari, M.P.; Salvadori, O. Biodeterioration control of cultural heritage: Methods and products. In Molecular Biology and Cultural Heritage; Saiz-Jimenez, C., Ed.; Routledge: London, UK, 2017; pp. 233–242. [Google Scholar]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: Waretown, NJ, USA, 2017. [Google Scholar]
- Russell, A.D.; Chopra, I. Understanding Antibacterial Action and Resistance; Ellis Horwood: London, UK, 1996; Volume 107. [Google Scholar]
- Ghosh, S.K. Functional coatings and microencapsulation: A general perspective. In Functional Coatings: By Polymer Microencapsulation; Wiley: Hoboken, NJ, USA, 2006; pp. 1–28. [Google Scholar] [CrossRef]
- Tiano, P. Biodeterioration of monumental rocks: Decay mechanisms and control methods. Sci. Technol. Cult. Herit. 1998, 7, 19–38. [Google Scholar]
- Casanova Municchia, A.; Fidanza, M.R.; Caneva, G. Advances in testing the interference of biocides on stone materials: A comparative analysis and guidelines for a standardized approach. J. Cult. Herit. 2023, 64, 23–41. [Google Scholar] [CrossRef]
- Liu, X.; Qian, Y.; Wang, Y.; Wu, F.; Wang, W.; Gu, J.D. Innovative approaches for the processes involved in microbial biodeterioration of cultural heritage materials. Curr. Opin. Biotechnol. 2022, 75, 102716. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural biocides for the conservation of stone cultural heritage: A review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Villar-dePablo, M.; Ascaso, C.; Rodríguez-Pérez, E.; Urizal, M.; Wierzchos, J.; Pérez-Ortega, S.; de Los Ríos, A. Innovative approaches to accurately assess the effectiveness of biocide-based treatments to fight biodeterioration of Cultural Heritage monuments. Sci. Total Environ. 2023, 897, 165318. [Google Scholar] [CrossRef]
- Varona, S.; Martín, Á.; Cocero, M.J. Formulation of a natural biocide based on lavandin essential oil by emulsification using modified starches. Chem. Eng. Process. Process Intensif. 2009, 48, 1121–1128. [Google Scholar] [CrossRef]
- Fonseca, A.J.; Pina, F.; Macedo, M.F.; Leal, N.; Romanowska-Deskins, A.; Laiz, L.; Gómez-Bolea, A.; Saiz-Jimenez, C. Anatase as an alternative application for preventing biodeterioration of mortars: Evaluation and comparison with other biocides. Int. Biodeterior. Biodegrad. 2010, 64, 388–396. [Google Scholar] [CrossRef]
- Palla, F.; Bruno, M.; Mercurio, F.; Tantillo, A.; Rotolo, V. Essential oils as natural biocides in the conservation of cultural heritage. Molecules 2020, 25, 730. [Google Scholar] [CrossRef] [PubMed]
- Kaur, V.; Kaur, R.; Bhardwaj, U. A review on dill essential oil and its chief compounds as a natural biocide. Flavour Fragr. J. 2021, 36, 412–431. [Google Scholar] [CrossRef]
- Pawłowska, A.; Stepczyńska, M. Natural biocidal compounds of plant origin as biodegradable materials modifiers. J. Polym. Environ. 2021, 30, 1683–1708. [Google Scholar] [CrossRef]
- Gabriele, F.; Ranaldi, R.; Bruno, L.; Casieri, C.; Rugnini, L.; Spreti, N. Biodeterioration of stone monuments: Studies on the influence of bioreceptivity on cyanobacterial biofilm growth and on the biocidal efficacy of essential oils in natural hydrogel. Sci. Total Environ. 2023, 870, 161901. [Google Scholar] [CrossRef]
- Spada, M.; Cuzman, O.A.; Tosini, I.; Galeotti, M.; Sorella, F. Essential oils mixtures as an eco-friendly biocidal solution for a marble statue restoration. Int. Biodeterior. Biodegrad. 2021, 163, 105280. [Google Scholar] [CrossRef]
- Sasso, S.; Scrano, L.; Ventrella, E.; Bonomo, M.G.; Crescenzi, A.; Salzano, G.; Bufo, S.A. Natural biocides to prevent the microbial growth on cultural heritage. Built Herit. 2013, 1, 1035–1042. [Google Scholar]
- Rotolo, V.; Barresi, G.; Di Carlo, E.; Giordano, A.; Lombardo, G.; Crimi, E.; Costa, E.; Bruno, M.; Palla, F. Plant extracts as green potential strategies to control the biodeterioration of cultural heritage. Int. J. Conserv. Sci. 2016, 7, 839–846. [Google Scholar]
- Veneranda, M.; Blanco-Zubiaguirre, L.; Roselli, G.; Di Girolami, G.; Castro, K.; Madariaga, J.M. Evaluating the exploitability of several essential oils constituents as a novel biological treatment against cultural heritage biocolonization. Microchem. J. 2018, 138, 1–6. [Google Scholar] [CrossRef]
- Argyri, A.A.; Doulgeraki, A.I.; Varla, E.G.; Bikouli, V.C.; Natskoulis, P.I.; Haroutounian, S.A.; Moulas, G.A.; Tassou, C.C.; Chorianopoulos, N.G. Evaluation of plant origin essential oils as herbal biocides for the protection of caves belonging to natural and cultural heritage sites. Microorganisms 2021, 9, 1836. [Google Scholar] [CrossRef]
- Cattò, C.; Dell’Orto, S.; Villa, F.; Villa, S.; Gelain, A.; Vitali, A.; Marzano, V.; Baroni, S.; Forlani, F.; Cappitelli, F. Unravelling the structural and molecular basis responsible for the anti-biofilm activity of zosteric acid. PLoS ONE 2015, 10, e0131519. [Google Scholar] [CrossRef]
- Md Zain, W.S.; Hairul Salleh, N.I.; Abdullah, A. Natural Biocides for Mitigation of Sulphate Reducing Bacteria. Int. J. Corros. 2018, 2018, 3567569. [Google Scholar] [CrossRef]
- Borrego, S.; Valdés, O.; Vivar, I.; Lavin, P.; Guiamet, P.; Battistoni, P.; Gómez de Saravia, S.; Borges, P. Essential Oils of Plants as Biocides against Microorganisms Isolated from Cuban and Argentine Documentary Heritage. ISRN Microbiol. 2012, 826786. [Google Scholar] [CrossRef]
- Stupar, M.; Grbic, M.; Džamić, A.M.; Unković, N.; Ristić, M.S.; Jelikić, A.; Vukojevic, J. Antifungal activity of selected essential oils and biocide benzalkonium chloride against the fungi isolated from cultural heritage objects. S. Afr. J. Bot. 2014, 93, 118–124. [Google Scholar] [CrossRef]
- Elsayed, Y.; Shabana, Y. The effect of some essential oils on aspergillus niger and alternaria alternata infestation in archaeological oil paintings. Mediterr. Archaeol. Archaeom. 2018, 18, 71–87. [Google Scholar] [CrossRef]
- Macedo-Arantes, S.; Piçarra, A.; Caldeira, A.T.; Candeias, A.E.; Martins, M.R. Essential oils of Portuguese flavouring plants: Potential as green biocides in cultural heritage. Eur. Phys. J. Plus 2021, 136, 1106. [Google Scholar] [CrossRef]
- Maia, F.; Silva, A.P.; Fernandes, S.; Cunha, A.; Almeida, A.; Tedim, J.; Zheludkevich, M.L.; Ferreira, M.G.S. Incorporation of biocides in nanocapsules for protective coatings used in maritime applications. Chem. Eng. J. 2015, 270, 150–157. [Google Scholar] [CrossRef]
- Dresler, C.; Saladino, M.L.; Demirbag, C.; Caponetti, E.; Martino, D.F.C.; Alduina, R. Development of controlled release systems of biocides for the conservation of cultural heritage. Int. Biodeterior. Biodegrad. 2017, 125, 150–156. [Google Scholar] [CrossRef]
- Ruggiero, L.; Di Bartolomeo, E.; Gasperi, T.; Luisetto, I.; Talone, A.; Zurlo, F.; Peddis, D.; Ricci, M.A.; Sodo, A. Silica nanosystems for active antifouling protection: Nanocapsules and mesoporous nanoparticles in controlled release applications. J. Alloys Compd. 2019, 798, 144–148. [Google Scholar] [CrossRef]
- Ruggiero, L.; Bartoli, F.; Fidanza, M.R.; Zurlo, F.; Marconi, E.; Gasperi, T.; Tuti, S.; Crociani, L.; Di Bartolomeo, E.; Caneva, G.; et al. Encapsulation of environmentally-friendly biocides in silica nanosystems for multifunctional coatings. Appl. Surf. Sci. 2020, 514, 145908. [Google Scholar] [CrossRef]
- Marconi, E.; Luisetto, I.; Di Carlo, G. 3-APTES on Dendritic Fibrous Mesoporous Silica Nanoparticles for the pH-Controlled Release of Corrosion Inhibitors. Nanomaterials 2023, 13, 2543. [Google Scholar] [CrossRef] [PubMed]
- Jämsä, S.; Mahlberg, R.; Holopainen, U.; Ropponen, J.; Savolainen, A.; Ritschkoff, A.C. Slow release of a biocidal agent from polymeric microcapsules for preventing biodeterioration. Prog. Org. Coat. 2013, 76, 269–276. [Google Scholar] [CrossRef]
- Presentato, A.; Armetta, F.; Spinella, A.; Chillura Martino, D.F.; Alduina, R.; Saladino, M.L. Formulation of Mesoporous Silica Nanoparticles for Controlled Release of Antimicrobials for Stone Preventive Conservation. Front. Chem. 2020, 8, 699. [Google Scholar] [CrossRef]
- Bartoli, F.; Zuena, M.; Sodo, A.; Caneva, G. The Efficiency of Biocidal Silica Nanosystems for the Conservation of Stone Monuments: Comparative In Vitro Tests against Epilithic Green Algae. Appl. Sci. 2021, 11, 6804. [Google Scholar] [CrossRef]
- Heiden, P.; Matuana, L.; Dawson-Andoh, B. Nanotechnology: A Novel Approach to Prevent Biocide Leaching; US EPA Archive Document. 2016. Available online: https://archive.epa.gov/ncer/publications/web/html/heiden.html (accessed on 20 October 2023).
- Caldeira, A.T. Green mitigation strategy for cultural heritage using bacterial biocides. In Microorganisms in the Deterioration and Pre Servation of Cultural Heritage; Springer: Berlin/Heidelberg, Germany, 2021; p. 137. [Google Scholar]
- Franco-Castillo, I.; Hierro, L.; de la Fuente, J.M.; Seral-Ascaso, A.; Mitchell, S.G. Perspectives for antimicrobial nanomaterials in cultural heritage conservation. Chem 2021, 7, 629–669. [Google Scholar] [CrossRef]
- Gomez-Villalba, L.S.; Salcines, C.; Fort, R. Application of Inorganic Nanomaterials in Cultural Heritage Conservation, Risk of Toxicity, and Preventive Measures. Nanomaterials 2023, 13, 1454. [Google Scholar] [CrossRef] [PubMed]
- Geiger, T.; Delavy, P.; Hany, R.; Schleuniger, J.; Zinn, M. Encapsulated zosteric acid embedded in poly [3-hydroxyalkanoate] coatings—Protection against biofouling. Polym. Bull. 2004, 52, 65–72. [Google Scholar] [CrossRef]
- Villa, F.; Albanese, D.; Giussani, B.; Stewart, P.S.; Daffonchio, D.; Cappitelli, F. Hindering biofilm formation with zosteric acid. Biofouling 2010, 26, 739–752. [Google Scholar] [CrossRef]
- Villa, F.; Pitts, B.; Stewart, P.S.; Giussani, B.; Roncoroni, S.; Albanese, D.; Giordano, C.; Tunesi, M.; Cappitelli, F. Efficacy of zosteric acid sodium salt on the yeast biofilm model Candida albicans. Microb. Ecol. 2011, 62, 584–598. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, C.; Sousa, E.; Pinto, M.; Correia-da-Silva, M. An antifouling model from the sea: A review of 25 years of zosteric acid studies. Biofouling 2017, 33, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Barrios, C.A.; Cutright, T.; Zhang Newby, B.M. Evaluation of toxicity of capsaicin and zosteric acid and their potential application as antifoulants. Environ. Toxicol. Int. J. 2005, 20, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Franchini, C.; Muraglia, M.; Corbo, F.; Florio, M.A.; Di Mola, A.; Rosato, A.; Matucci, R.; Nesi, M.; Van Bambeke, F.; Vitali, C. Synthesis and biological evaluation of 2-mercapto-1, 3-benzothiazole derivatives with potential antimicrobial activity. Arch. Pharm. Int. J. Pharm. Med. Chem. 2009, 342, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Zuena, M.; Ruggiero, L.; Della Ventura, G.; Bemporad, E.; Ricci, M.A.; Sodo, A. Effectiveness and compatibility of nanoparticle based multifunctional coatings on natural and man-made stones. Coatings 2021, 11, 480. [Google Scholar] [CrossRef]
- Haake, S.; Simon, S.; Favaro, M. The bologna cocktail-evaluation of consolidation treatments on monuments in France and Italy after 20 years on natural aging. In Proceedings of the 10th International Congress on Deterioration and Conservation of Stone, Stockholm, Sweden, 27 June–2 July 2004; pp. 423–430. [Google Scholar]
- Commissione Beni Culturali Uni Normal. Normal 43/93 Misure Colorimetriche di Superfici Opache (Italian Normative on Stone Material-Colorimetric Measurement of Opaque Surfaces); Commissione Beni Culturali Uni Normal: Roma, Italy, 1993. [Google Scholar]
- Zuena, M.; Ruggiero, L.; Caneva, G.; Bartoli, F.; Ventura, G.D.; Ricci, M.A.; Sodo, A. Assessment of Stone Protective Coatings with a Novel Eco-Friendly Encapsulated Biocide. Coatings 2021, 11, 1109. [Google Scholar] [CrossRef]
- ASTM D4303-10; Standard Test Methods for Lightfastness of Colorants Used In Artists’ Materials. ASTM International: West Conshohocken, PA, USA, 2010.
- UNI-EN-15886; Misura del Colore Delle Superfici ICS: 97.195. Conservazione dei Beni Culturali 2010. 2010.
- Bartoli, F.; Municchia, A.C.; Leotta, M.; Luciano, S.; Caneva, G. Biological recolonization dynamics: Kentridge’s artwork disappearing along the Tiber embankments (Rome, Italy). Int. Biodeterior. Biodegrad. 2021, 160, 105214. [Google Scholar] [CrossRef]
- Rubio, F.; Rubio, J.; Oteo, J.L. FT-IR Study of the Hydrolysis of Tetraethylorthosilicate (TEOS), Spectroscopy Letters. Int. J. Rapid Commun. 1998, 31, 199–219. [Google Scholar] [CrossRef]
- Ruggiero, L.; Fidanza, M.R.; Iorio, M.; Tortora, L.; Caneva, G.; Ricci, M.A.; Sodo, A. Synthesis and characterization of TEOS coating added with innovative antifouling silica nanocontainers and TiO2 nanoparticles. Front. Mater. 2020, 7, 185. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Licciulli, A.; Munafò, P. Smart surfaces for architectural heritage: Preliminary results about the application of TiO2-based coatings on travertine. J. Cult. Herit. 2012, 13, 204–209. [Google Scholar] [CrossRef]
- Wibowo, D.; Hue, Y.; Middelberg, A.P.J.; Zhao, C.-X. Interfacial engineering for silica nanocapsules. Adv. Colloid Interface Sci. 2016, 236, 83–100. [Google Scholar] [CrossRef]
- Haynie, F.H. Deterioration of marble. Durab. Build. Mater. 1983, 1, 241–254. [Google Scholar]
- Wang, H.-P.; Fei, W.; Ngothai, Y.; O’Neill, B. A kinetic model of the ‘Fe2+ oxidisation’ process for colour enhancement in natural marble. Mater. Chem. Phys. 2004, 86, 51–58. [Google Scholar] [CrossRef]
- Wolfaardt, G.M.; Cloete, T.E. The effect of some environmental parameters on surface colonization by microorganisms. Water Res. 1992, 26, 527–537. [Google Scholar] [CrossRef]
- Tomaselli, L.; Lamenti, G.; Bosco, M.; Tiano, P. Biodiversity of photosynthetic micro-organisms dwelling on stone monuments. Int. Biodeterior. Biodegrad. 2000, 46, 251–258. [Google Scholar] [CrossRef]
- Macedo, M.F.; Miller, A.Z.; Dionísio, A.; Saiz-Jimenez, C. Biodiversity of cyanobacteria and green algae on monuments in the Mediterranean Basin: An overview. Microbiology 2009, 11, 3476–3490. [Google Scholar] [CrossRef] [PubMed]
- Aung, K.; Jiang, Y.; He, Y.S. The role of water in plant–microbe interactions. Plant J. 2018, 93, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Louati, M.; Ennis, N.J.; Ghodhbane-Gtari, F.; Hezbri, K.; Sevigny, J.L.; Fahnestock, M.F.; Cherif-Silini, H.; Bryce, J.G.; Tisa, L.S.; Gtari, M. Elucidating the ecological networks in stone-dwelling microbiomes. Environ. Microbiol. 2020, 22, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Yarwood, R.; Rockhold, M.; Niemet, M.R.; Selker, J.S.; Bottomley, P.J. Impact of microbial growth on water flow and solute transport in unsaturated porous media. Water Resour. Res. 2006, 42, 1–11. [Google Scholar] [CrossRef]
- Kapridaki, C.; Maravelaki-Kalaitzaki, P. TiO2–SiO2–PDMS nano-composite hydrophobic coating with self-cleaning properties for marble protection. Prog. Org. Coat. 2013, 76, 400–410. [Google Scholar] [CrossRef]
- Adamopoulos, F.G.; Vouvoudi, E.C.; Pavlidou, E.; Achilias, D.S.; Karapanagiotis, I. TEOS-Based Superhydrophobic Coating for the Protection of Stone-Built Cultural Heritage. Coatings 2021, 11, 135. [Google Scholar] [CrossRef]
- Bellinzoni, A.M.; Caneva, G.; Ricci, S. Ecological trends in travertine colonisation by pioneer algae and plant communities. Int. Biodeterior. Biodegrad. 2003, 51, 203–210. [Google Scholar] [CrossRef]
- Gaylarde, C. Influence of environment on microbial colonization of historic stone buildings with emphasis on cyanobacteria. Heritage 2020, 3, 1469–1482. [Google Scholar] [CrossRef]
- Bartoli, F.; Isola, D.; Casanova Municchia, A.; Kumbaric, A.; Caneva, G. Science for art: Multi-years’ evaluations of biocidal efficacy in support of artwork conservation. Front. Microbiol. 2023, 14, 1178900. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Bartoli, F.; Savo, V.; Futagami, Y.; Strona, G. Combining Statistical Tools and Ecological Assessments in the Study of Biodeterioration Patterns of Stone Temples in Angkor (Cambodia). Sci. Rep. 2016, 6, 32601. [Google Scholar] [CrossRef] [PubMed]
- Caneva, G.; Bartoli, F.; Ceschin, S.; Salvadori, O.; Futagami, Y.; Salvati, L. Exploring ecological relationships in the biodeterioration patterns of Angkor temples (Cambodia) along a forest canopy gradient. J. Cult. Herit. 2015, 16, 728–735. [Google Scholar] [CrossRef]
- Odum, E.P.; Barrett, G.W. Fundamentals of Ecology; Saunders: Philadelphia, PA, USA, 1971; Volume 3, p. 5. [Google Scholar]
- Achamlale, S.; Rezzonico, B.; Grignon-Dubois, M. Evaluation of Zostera detritus as a potential new source of zosteric acid. J. Appl. Phycol. 2009, 21, 347–352. [Google Scholar] [CrossRef]
- Newby, B.M.Z.; Cutright, T.; Barrios, C.A.; Xu, Q. Zosteric acid—An effective antifoulant for reducing freshwater bacterial attachment on coatings. JCT Res. 2006, 3, 69–76. [Google Scholar] [CrossRef]





| Coating Composition | |||
|---|---|---|---|
| No Inoculum | Inoculum * | ||
| Biocide | Samples | Biocide | Samples * |
| Untreated | A | Untreated | B * |
| MBT | NC | MBT | NC * |
| ZOS | NC | ZOS | NC * |
| MBT | MNP | MBT | MNP * |
| ZOS | MNP | ZOS | MNP * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartoli, F.; Hosseini, Z.; Graziani, V.; Zuena, M.; Venettacci, C.; Della Ventura, G.; Tortora, L.; Sodo, A.; Caneva, G. In Situ Evaluation of New Silica Nanosystems as Long-Lasting Methods to Prevent Stone Monument Biodeterioration. Coatings 2024, 14, 163. https://doi.org/10.3390/coatings14020163
Bartoli F, Hosseini Z, Graziani V, Zuena M, Venettacci C, Della Ventura G, Tortora L, Sodo A, Caneva G. In Situ Evaluation of New Silica Nanosystems as Long-Lasting Methods to Prevent Stone Monument Biodeterioration. Coatings. 2024; 14(2):163. https://doi.org/10.3390/coatings14020163
Chicago/Turabian StyleBartoli, Flavia, Zohreh Hosseini, Valerio Graziani, Martina Zuena, Carlo Venettacci, Giancarlo Della Ventura, Luca Tortora, Armida Sodo, and Giulia Caneva. 2024. "In Situ Evaluation of New Silica Nanosystems as Long-Lasting Methods to Prevent Stone Monument Biodeterioration" Coatings 14, no. 2: 163. https://doi.org/10.3390/coatings14020163
APA StyleBartoli, F., Hosseini, Z., Graziani, V., Zuena, M., Venettacci, C., Della Ventura, G., Tortora, L., Sodo, A., & Caneva, G. (2024). In Situ Evaluation of New Silica Nanosystems as Long-Lasting Methods to Prevent Stone Monument Biodeterioration. Coatings, 14(2), 163. https://doi.org/10.3390/coatings14020163









