Investigating the Concurrent Effect of Cerium/Hydroxyapatite Coatings on Mg-Based Implant for Enhancing Corrosion Performance and In-Vitro Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Preparation
2.3. Coatings Preparation
2.3.1. Direct Addition Method
2.3.2. Pre-Treatment Method
2.4. Coatings Surface Characterization
2.5. Anticorrosion Performance
2.6. In Vitro Study
3. Results and Discussion
3.1. Ce Conversion Coating and HA Coating Formation
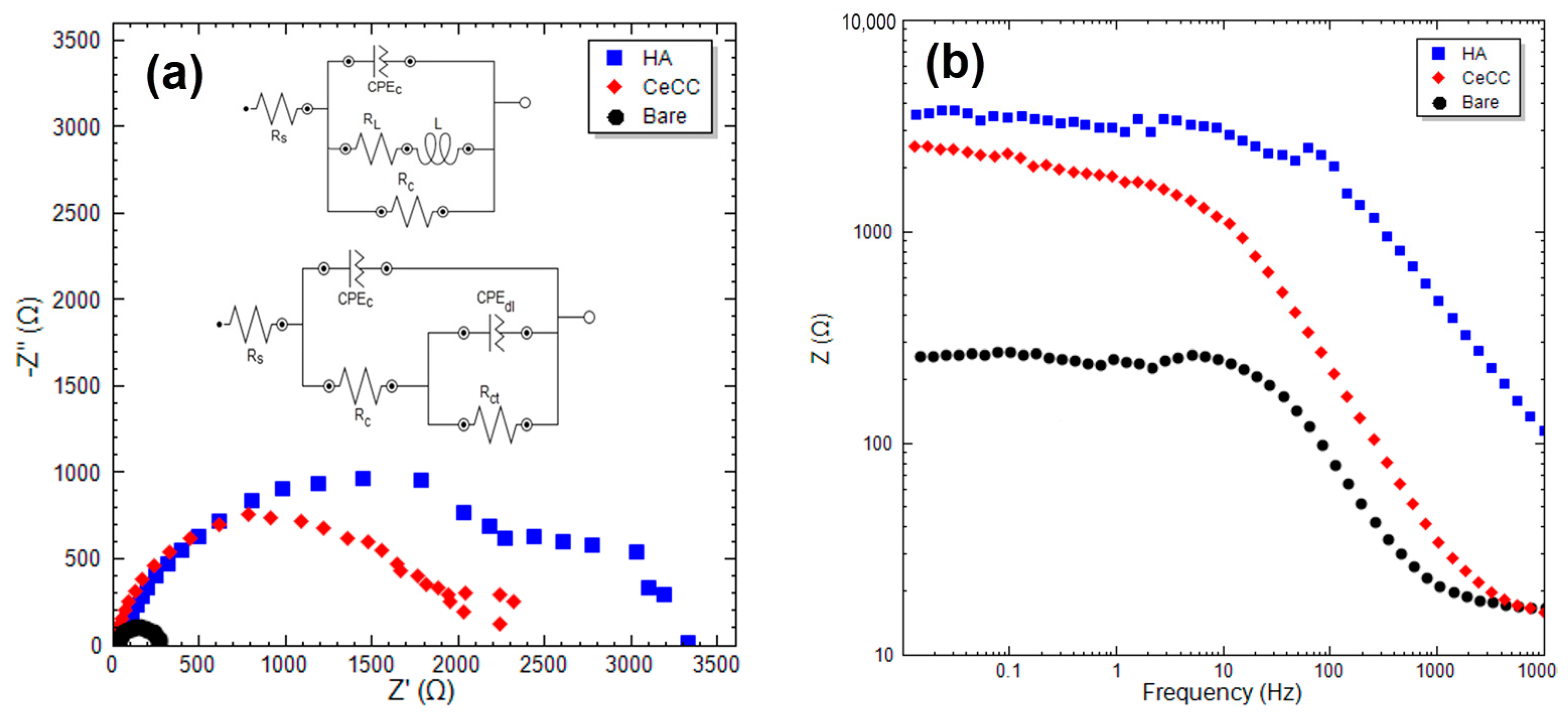
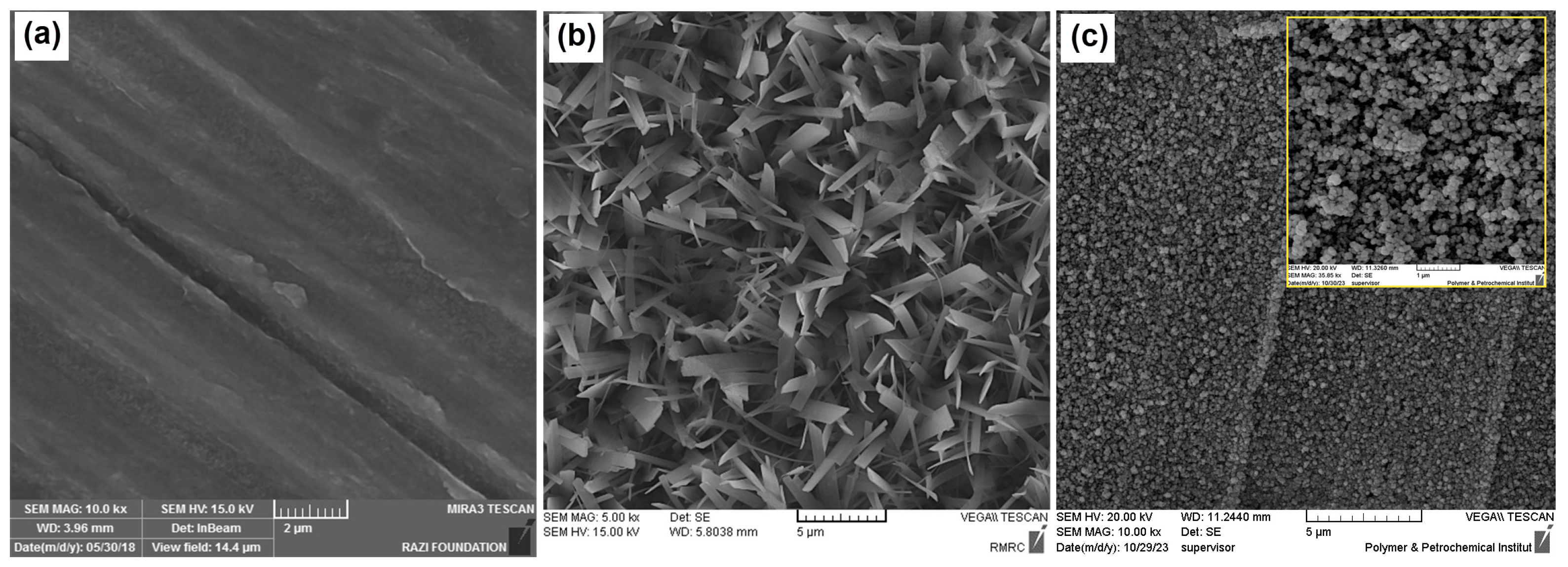
3.1.1. EIS and FE-SEM Study
3.1.2. Formation Mechanism
3.2. Modification of HA Coating with Ce
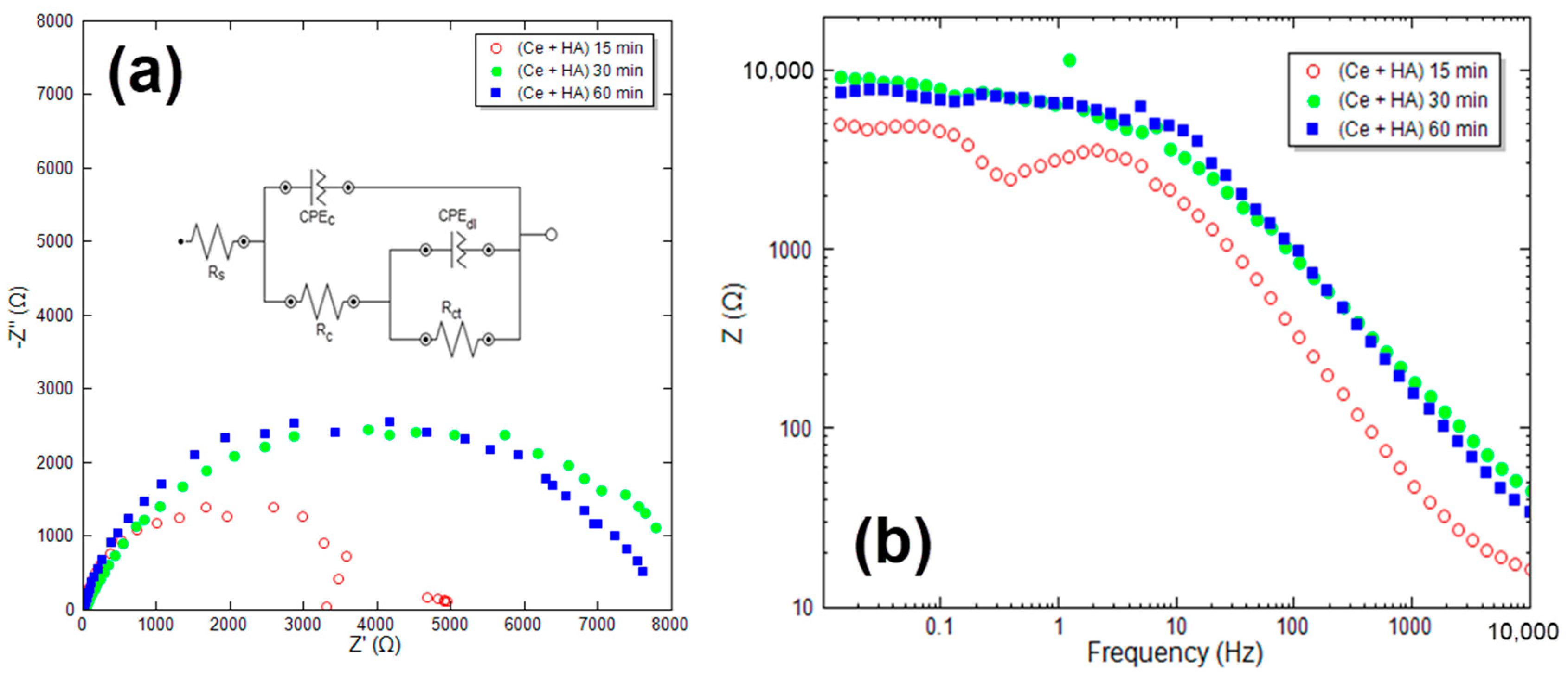
3.2.1. HA + Ce Coating on CeCC
3.2.2. DC Polarization and OCP Measurements
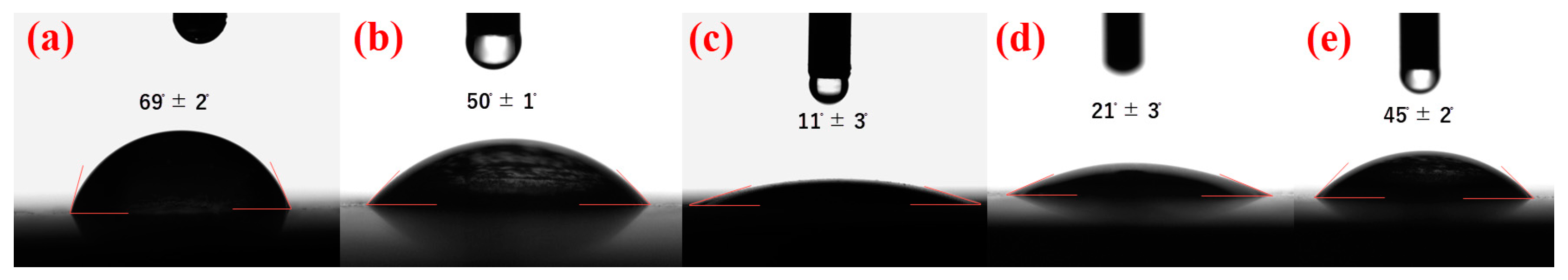
3.3. Contact Angle Study
3.4. XRD Study
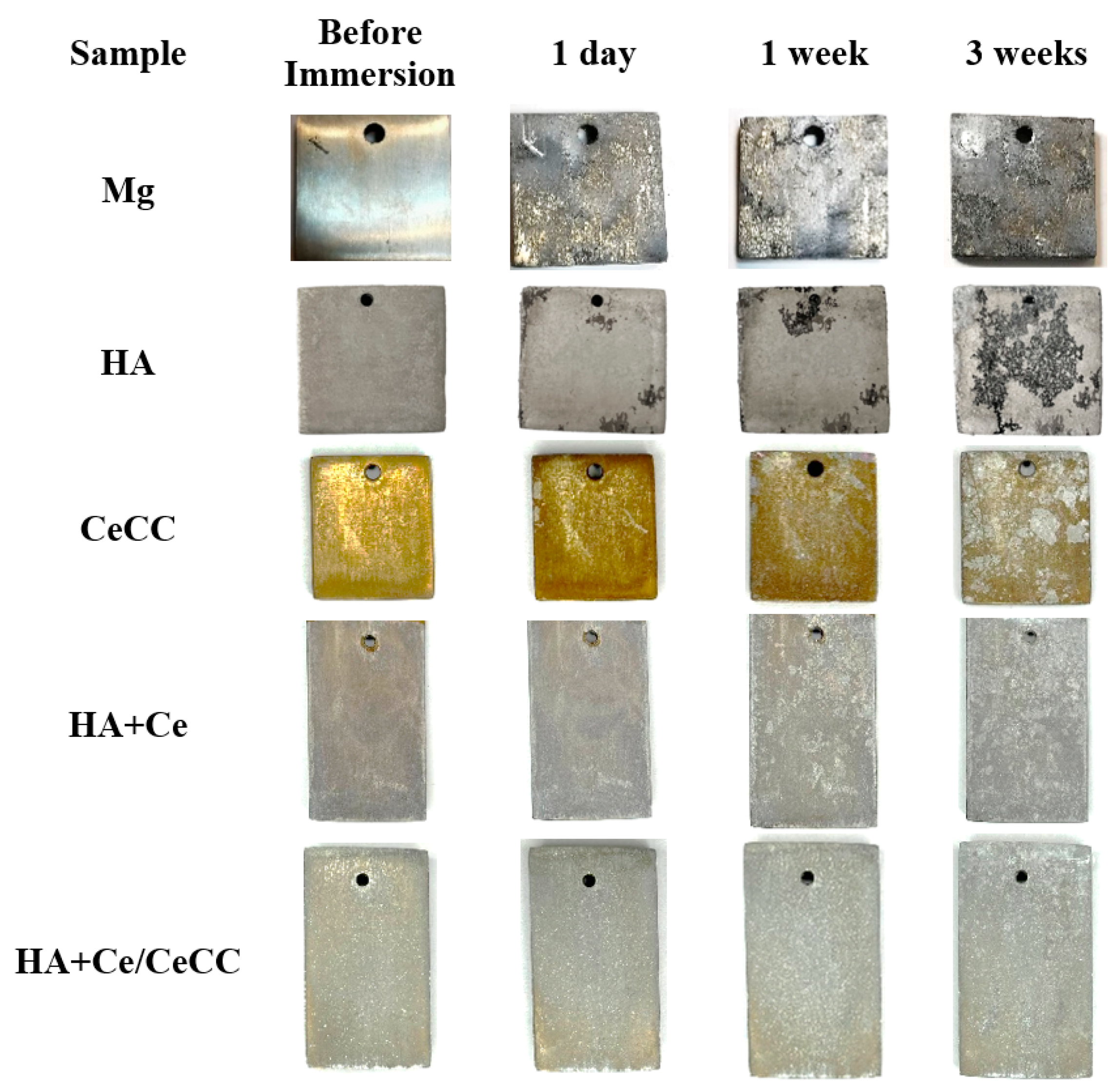
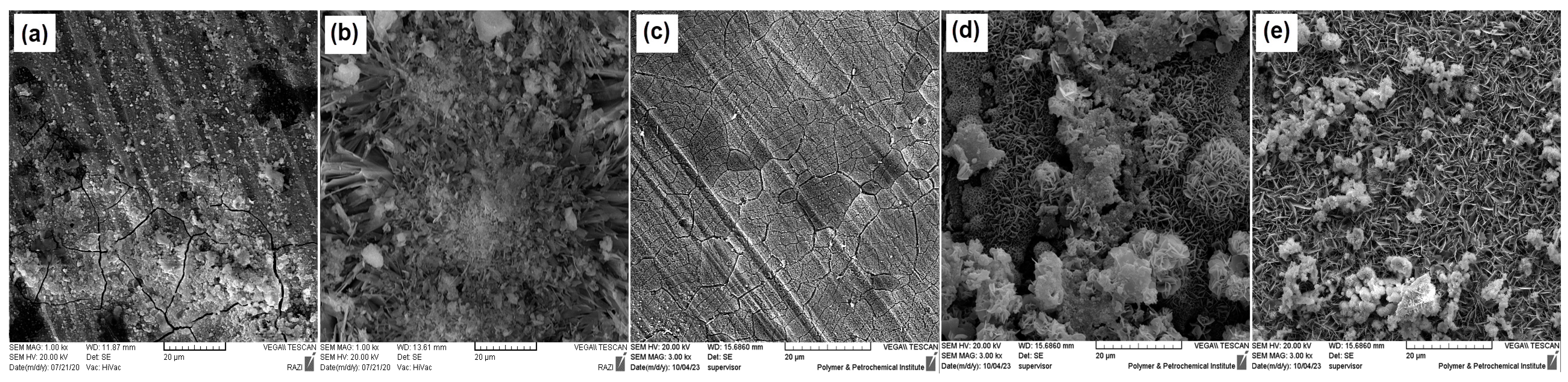
3.5. Immersion in SBF
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niu, L.Y.; Jiang, Z.H.; Li, G.Y.; Gu, C.D.; Lian, J.S. A study and application of zinc phosphate coating on AZ91D magnesium alloy. Surf. Coat. Technol. 2006, 200, 3021–3026. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef] [PubMed]
- Hollstein, F.; Wiedemann, R.; Scholz, J. Characteristics of PVD-coatings on AZ31hp magnesium alloys. Surf. Coat. Technol. 2003, 162, 261–268. [Google Scholar] [CrossRef]
- Jayasathyakawin, S.; Ravichandran, M.; Baskar, N.; Chairman, C.A.; Balasundaram, R. Mechanical properties and applications of Magnesium alloy—Review. Mater. Today Proc. 2020, 27, 909–913. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, F.; Wu, Y.; Le, J.; Liu, L.; He, M.; Zhu, J.; Hu, W. Protective diffusion coatings on magnesium alloys: A review of recent developments. J. Alloys Compd. 2012, 520, 11–21. [Google Scholar] [CrossRef]
- Chen, X.B.; Birbilis, N.; Abbott, T.B. A simple route towards a hydroxyapatite-Mg(OH)2 conversion coating for magnesium. Corros. Sci. 2011, 53, 2263–2268. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Kim, W.J. Enhancement of mechanical properties and corrosion resistance of Mg-Ca alloys through microstructural refinement by indirect extrusion. Corros. Sci. 2014, 82, 392–403. [Google Scholar] [CrossRef]
- Li, Q.; Ye, W.; Gao, H.; Gao, L. Improving the corrosion resistance of ZEK100 magnesium alloy by combining high-pressure torsion technology with hydroxyapatite coating. Mater. Des. 2019, 181, 107933. [Google Scholar] [CrossRef]
- Ronc, I.S.; Grubac, Z.; Metikos, M. Electrodeposition of hydroxyapatite coating on AZ91D alloy for biodegradable implant application. Int. J. Electrochem. Sci. 2014, 9, 5907–5923. [Google Scholar] [CrossRef]
- Ho, Y.H.; Man, K.; Joshi, S.S.; Pantawane, M.V.; Wu, T.C.; Yang, Y.; Dahotre, N.B. In-vitro biomineralization and biocompatibility of friction stir additively manufactured AZ31B magnesium alloy-hydroxyapatite composites. Bioact. Mater. 2020, 5, 891–901. [Google Scholar] [CrossRef]
- Ren, Y.; Babaie, E.; Bhaduri, S.B. Nanostructured amorphous magnesium phosphate/poly (lactic acid) composite coating for enhanced corrosion resistance and bioactivity of biodegradable AZ31 magnesium alloy. Prog. Org. Coat. 2018, 118, 1–8. [Google Scholar] [CrossRef]
- Durdu, S.; Bayramoglu, S.; Demirtaş, A.; Usta, M.; Üçşk, A.H. Characterization of AZ31 Mg Alloy coated by plasma electrolytic oxidation. Vacuum 2013, 88, 130–133. [Google Scholar] [CrossRef]
- Nabizadeh, M.; Sarabi, A.A.; Mohammadloo, H.E. Comparative investigation of Cu ion and adipic acid addition on electrochemical and microstructure characteristics of vanadium conversion coating on AZ31 Mg alloy. Surf. Coat. Technol. 2019, 357, 1–11. [Google Scholar] [CrossRef]
- Yue, J.; Lou, G.; Zhou, G.; Leng, J.; Feng, Y.; Teng, X. Corrosion resistance of zinc phosphate conversion coatings on az91d surface. Mater. Sci. Forum Trans. Tech. Publ. 2020, 993, 1110–1117. [Google Scholar] [CrossRef]
- Van Phuong, N.; Lee, K.; Chang, D.; Kim, M.; Lee, S.; Moon, S. Zinc phosphate conversion coatings on magnesium alloys: A review. Met. Mater. Int. 2013, 19, 273–281. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S. Surface pretreatment by phosphate conversion coatings—A review. Rev. Adv. Mater. Sci. 2005, 9, 130–177. [Google Scholar]
- Cui, L.Y.; Cheng, S.C.; Liang, L.X.; Zhang, J.C.; Li, S.Q.; Wang, Z.L.; Zeng, R.C. In vitro corrosion resistance of layer-by-layer assembled polyacrylic acid multilayers induced Ca–P coating on magnesium alloy AZ31. Bioact. Mater. 2020, 5, 153–163. [Google Scholar] [CrossRef]
- Yadav, V.S.; Kumar, A.; Das, A.; Pamu, D.; Pandey, L.M.; Sankar, M.R. Degradation kinetics and surface properties of bioceramic hydroxyapatite coated AZ31 magnesium alloys for biomedical applications. Mater. Lett. 2020, 270, 127732. [Google Scholar] [CrossRef]
- Saei, E.; Ramezanzadeh, B.; Amini, R.; Kalajahi, M.S. Effects of combined organic and inorganic corrosion inhibitors on the nanostructure cerium based conversion coating performance on AZ31 magnesium alloy: Morphological and corrosion studies. Corros. Sci. 2017, 127, 186–200. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, F.; Yi, A.; Liao, Z.; Li, W.; Li, K.; Chen, K.; Liang, G.; Guo, J.T.; Wu, Q. Titanium, zirconium and vanadium conversion coatings (TZVCCs) on AZ91D magnesium alloy sheets. Mater. Res. Express. 2020, 7, 86402. [Google Scholar] [CrossRef]
- Guo, Y.; Su, Y.; Gu, R.; Zhang, Z.; Li, G.; Lian, J.; Ren, L. Enhanced corrosion resistance and biocompatibility of biodegradable magnesium alloy modified by calcium phosphate/collagen coating. Surf. Coat. Technol. 2020, 401, 127322. [Google Scholar] [CrossRef]
- Seitz, J.M.; Collier, K.; Wulf, E.; Bormann, D.; Bach, F.W. Comparison of the Corrosion Behavior of Coated and Uncoated Magnesium Alloys in an In Vitro Corrosion Environment. Adv. Eng. Mater. 2011, 13, B313–B323. [Google Scholar] [CrossRef]
- Hahn, B.-D.; Park, D.-S.; Choi, J.-J.; Ryu, J.; Yoon, W.-H.; Choi, J.-H.; Kim, H.-E.; Kim, S.-G. Aerosol deposition of hydroxyapatite–chitosan composite coatings on biodegradable magnesium alloy. Surf. Coat. Technol. 2011, 205, 3112–3118. [Google Scholar] [CrossRef]
- Rahimi, M.; Aghdam, R.M.; Sohi, M.H.; Rezayan, A.H.; Ettelaei, M. Improving biocompatibility and corrosion resistance of anodized AZ31 Mg alloy by electrospun chitosan/mineralized bone allograft (MBA) nanocoatings. Surf. Coat. Technol. 2021, 405, 126627. [Google Scholar] [CrossRef]
- Chen, X.B.; Nisbet, D.R.; Li, R.W.; Smith, P.N.; Abbott, T.B.; Easton, M.A.; Zhang, D.H.; Birbilis, N. Controlling initial biodegradation of magnesium by a biocompatible strontium phosphate conversion coating. Acta Biomater. 2014, 10, 1463–1474. [Google Scholar] [CrossRef]
- Astala, R.; Stott, M.J. First principles investigation of mineral component of bone: CO3 substitutions in hydroxyapatite. Chem. Mater. 2005, 17, 4125–4133. [Google Scholar] [CrossRef]
- Ahmadi, H.; Ghamsarizade, R.; Haddadi-Asl, V.; Mohammadloo, H.E.; Ramezanzadeh, B. Designing a novel bio-compatible hydroxyapatite (HA)/hydroxyquinoline (8-HQ)-inbuilt polyvinylalcohol (PVA) composite coatings on Mg AZ31 implants via electrospinning and immersion protocols: Smart anti-corrosion and anti-bacterial properties reinforcements. J. Ind. Eng. Chem. 2022, 116, 556–571. [Google Scholar] [CrossRef]
- Negroiu, G.; Piticescu, R.M.; Chitanu, G.C.; Mihailescu, I.N.; Zdrentu, L.; Miroiu, M. Biocompatibility evaluation of a novel hydroxyapatite-polymer coating for medical implants (in vitro tests). J. Mater. Sci. Mater. Med. 2008, 19, 1537–1544. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.P.; Silva, T.M.E.; Fernandes, J.C.S.; Fernandes, M.H.; Montemor, M.D.F. Biofunctional composite coating architectures based on polycaprolactone and nanohydroxyapatite for controlled corrosion activity and enhanced biocompatibility of magnesium AZ31 alloy. Mater. Sci. Eng. C 2015, 48, 434–443. [Google Scholar] [CrossRef]
- Jamesh, M.; Kumar, S.; Narayanan, T.S.N.S. Electrodeposition of hydroxyapatite coating on magnesium for biomedical applications. J. Coat. Technol. Res. 2012, 9, 495–502. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N. Development and characterization of MgO/hydroxyapatite composite coating on AZ31 magnesium alloy by plasma electrolytic oxidation coupled with electrophoretic deposition. Mater. Lett. 2012, 68, 439–442. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate coatings on magnesium and its biodegradable alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef]
- Zhou, Z.; Zheng, B.; Gu, Y.; Shen, C.; Wen, J.; Meng, Z.; Chen, S.; Ou, J.; Qin, A. New approach for improving anticorrosion and biocompatibility of magnesium alloys via polydopamine intermediate layer-induced hydroxyapatite coating. Surf. Interfaces 2020, 19, 100501. [Google Scholar] [CrossRef]
- Jian, S.-Y.; Yang, C.-Y.; Chang, J.-K. Robust corrosion resistance and self-healing characteristics of a novel Ce/Mn conversion coatings on EV31 magnesium alloys. Appl. Surf. Sci. 2020, 510, 145385. [Google Scholar] [CrossRef]
- Hsieh, C.-Y.; Huang, S.-Y.; Chu, Y.-R.; Yen, H.-W.; Lin, H.-C.; Shih, D.S.; Kawamura, Y.; Lee, Y.-L. Role of second phases in the corrosion resistance and cerium conversion coating treatment of as-extruded Mg–8Al–4Ca magnesium alloy. J. Mater. Res. Technol. 2023, 22, 2343–2359. [Google Scholar] [CrossRef]
- Khast, F.; Saybani, M.; Dariani, A.A.S. Effects of copper and manganese cations on cerium-based conversion coating on galvanized steel: Corrosion resistance and microstructure characterizations. J. Rare Earths. 2022, 40, 1002–1006. [Google Scholar] [CrossRef]
- Wu, G.; Wang, C.; Zhang, Q.; Kang, P. Characterization of Ce conversion coating on Gr-f/6061Al composite surface for corrosion protection. J. Alloys Compd. 2008, 461, 389–394. [Google Scholar] [CrossRef]
- Lin, C.S.; Fang, S.K. Formation of cerium conversion coatings on AZ31 magnesium alloys. J. Electrochem. Soc. 2005, 152, B54–B59. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chu, Y.R.; Lin, C.S. Mechanism of the formation of stannate and cerium conversion coatings on AZ91D magnesium alloys. Appl. Surf. Sci. 2013, 276, 578–585. [Google Scholar] [CrossRef]
- Lee, Y.L.; Chen, F.J.; Lin, C.S. Corrosion resistance studies of cerium conversion coating with a fluoride-free pretreatment on AZ91D magnesium alloy. J. Electrochem. Soc. 2013, 160, C28–C35. [Google Scholar] [CrossRef]
- Kazemi, S.; Ghamsarizade, R.; Yazdani, S.; Mohammadloo, H.E.; Yeganeh, H. The combined effect of hydroxyapatite and zirconia coatings on magnesium-based-implant to improve corrosion resistance and induce antibacterial activity. J. Alloys Compd. 2024, 971, 172790. [Google Scholar] [CrossRef]
- AhadiParsa, M.; Mohammadloo, H.E.; Mirabedini, S.M.; Roshan, S. Bio-corrosion assessment and surface study of hydroxyapatite-coated AZ31 Mg alloy pre-treated with vinyl tri-ethoxy silane. Mater. Chem. Phys. 2022, 287, 126147. [Google Scholar] [CrossRef]
- Afshari, M.; Mohammadloo, H.E.; Sarabi, A.A.; Roshan, S. Modification of hydroxyapatite-based coating in the presence of polyvinylalcohol (PVA) for implant application: Corrosion, structure and surface study. Corros. Sci. 2021, 192, 109859. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A.A.; Hosseini, R.M.; Sarayloo, M.; Sameie, H.; Salimi, R. A comprehensive study of the green hexafluorozirconic acid-based conversion coating. Prog. Org. Coat. 2014, 77, 322–330. [Google Scholar] [CrossRef]
- Mohammadloo, H.E.; Sarabi, A.A.; Alvani, A.A.S.; Salimi, R.; Sameie, H. The effect of solution temperature and pH on corrosion performance and morphology of nanoceramic-based conversion thin film. Mater. Corros. 2013, 64, 535–543. [Google Scholar] [CrossRef]
- Ramezanzadeh, B.; Attar, M.M.; Farzam, M. A study on the anticorrosion performance of the epoxy-polyamide nanocomposites containing ZnO nanoparticles. Prog. Org. Coat. 2011, 72, 410–422. [Google Scholar] [CrossRef]
- Lostak, T.; Krebs, S.; Maljusch, A.; Gothe, T.; Giza, M.; Kimpel, M.; Flock, J.; Schulz, S. Formation and characterization of Fe3+-/Cu2+-modified zirconium oxide conversion layers on zinc alloy coated steel sheets. Electrochim. Acta. 2013, 112, 14–23. [Google Scholar] [CrossRef]
- Asemabadi, Z.; Korrani, A.M.; Dolatabadi, M.M.; Mohammadloo, H.E.; Sarabi, A.A.; Roshan, S. Modification of hydroxyapatite coating in the presence of adipic acid for Mg-based implant application. Prog. Org. Coat. 2022, 172, 107088. [Google Scholar] [CrossRef]
- Ji, X.-J.; Cheng, Q.; Wang, J.; Zhao, Y.-B.; Han, Z.-Z.; Zhang, F.; Li, S.-Q.; Zeng, R.-C.; Wang, Z.-L. Corrosion resistance and antibacterial effects of hydroxyapatite coating induced by polyacrylic acid and gentamicin sulfate on magnesium alloy. Front. Mater. Sci. 2019, 13, 87–98. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Shuang, C.P.; Berto, F. Preparation of poly(ε-caprolactone)-hydroxyapatite composite coating for improvement of corrosion performance of biode-gradable magnesium. Mater. Des. Process. Commun. 2020, 2, e170. [Google Scholar] [CrossRef]
- Kiahosseini, S.R.; Afshar, A.; Larijani, M.M.; Yousefpour, M. Structural and corrosion characterization of hydroxyapatite/zirconium nitride-coated AZ91 magnesium alloy by ion beam sputtering. Appl. Surf. Sci. 2017, 401, 172–180. [Google Scholar] [CrossRef]
- Roshan, S.; Mohammadloo, H.E.; Sarabi, A.A.; Afshari, M. Biocompatible hybrid chitosan/hydroxyapatite coating applied on the AZ31 Mg alloy substrate: In-vitro corrosion, surface and structure studies. Mater. Today Commun. 2022, 30, 103153. [Google Scholar] [CrossRef]
- Lu, C.; Mu, S.; Du, J.; Zhang, K.; Guo, M.; Chen, L. Investigation on the composition and corrosion resistance of cerium-based conversion treatment by alkaline methods on aluminum alloy 6063. RSC Adv. 2020, 10, 36654–36666. [Google Scholar] [CrossRef]
- Ardelean, H.; Frateur, I.; Marcus, P. Corrosion protection of magnesium alloys by cerium, zirconium and niobium-based conversion coatings. Corros. Sci. 2008, 50, 1907–1918. [Google Scholar] [CrossRef]
- Scholes, F.; Soste, C.; Hughes, A.; Hardin, S.; Curtis, P. The role of hydrogen peroxide in the deposition of cerium-based conversion coatings. Appl. Surf. Sci. 2006, 253, 1770–1780. [Google Scholar] [CrossRef]
- Wang, C.; Jiang, F.; Wang, F. Cerium chemical conversion coating for aluminum alloy 2024-T3 and its corrosion resistance. Corrosion 2004, 60, 237–243. [Google Scholar] [CrossRef]
- Wheeler, D.W. Kinetics and mechanism of the oxidation of cerium in air at ambient temperature. Corros. Sci. 2016, 111, 52–60. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Localized corrosion of the Mg alloys with inhibitor-containing coatings: SVET and SIET studies. Corros. Sci. 2016, 102, 269–278. [Google Scholar] [CrossRef]
- Aldykiewicz, A.; Davenport, A.; Isaacs, H. Studies of the formation of cerium-rich protective films using X-ray absorption near-edge spectroscopy and rotating disk electrode methods. J. Electrochem. Soc. 2016, 143, 147–154. [Google Scholar] [CrossRef]
- Decroly, A.; Petitjean, J.-P. Study of the deposition of cerium oxide by conversion on to aluminium alloys. Surf. Coat. Technol. 2005, 194, 1–9. [Google Scholar] [CrossRef]
- Jayaraj, J.; Raj, S.A.; Srinivasan, A. Composite magnesium phosphate coatings for improved corrosion resistance of magnesium AZ31 alloy. Corros. Sci. 2016, 113, 104–115. [Google Scholar] [CrossRef]
- Qiu, D.; Wang, A.; Yin, Y. Characterization and corrosion behavior of hydroxyapatite/zirconia composite coating on NiTi fabricated by electrochemical deposition. Appl. Surf. Sci. 2010, 257, 1774–1778. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, K.H.; Ong, J.L. A review on calcium phosphate coatings produced using a sputtering process—An alternative to plasma spraying. Biomaterials 2005, 26, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Van Phuong, N.; Moon, S. Comparative corrosion study of zinc phosphate and magnesium phosphate conversion coatings on AZ31 Mg alloy. Mater. Lett. 2014, 122, 341–344. [Google Scholar] [CrossRef]
- Kim, M.S.; Ryu, J.J.; Sung, Y.M. One-step approach for nano-crystalline hydroxyapatite coating on titanium via micro-arc oxidation. Electrochem. Commun. 2007, 9, 1886–1891. [Google Scholar] [CrossRef]
- Prevéy, P.S. X-ray diffraction characterization of crystallinity and phase composition in plasma-sprayed hydroxyapatite coatings. J. Therm. Spray. Technol. 2000, 9, 369–376. [Google Scholar] [CrossRef]
- Sroka-Bartnicka, A.; Borkowski, L.; Ginalska, G.; Ślósarczyk, A.; Kazarian, S.G. Structural transformation of synthetic hydroxyapatite under simulated in vivo conditions studied with ATR-FTIR spectroscopic imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 171, 155–161. [Google Scholar] [CrossRef]
- Gheisari, H.; Karamian, E.; Abdellahi, M. A novel hydroxyapatite–Hardystonite nanocomposite ceramic. Ceram. Int. 2015, 41, 5967–5975. [Google Scholar] [CrossRef]
- Ahmed, Y.; Rehman, M.A.U. Improvement in the surface properties of stainless steel via zein/hydroxyapatite composite coatings for biomedical applications. Surf. Interfaces 2020, 20, 100589. [Google Scholar] [CrossRef]
- Corradini, E.; Curti, P.S.; Meniqueti, A.B.; Martins, A.F.; Rubira, A.F.; Muniz, E.C. Recent advances in food-packing, pharmaceutical and biomedical applications of zein and zein-based materials. Int. J. Mol. Sci. 2014, 15, 22438–22470. [Google Scholar] [CrossRef]
- Yan, T.T.; Tan, L.L.; Xiong, D.S.; Liu, X.J.; Zhang, B.C.; Yang, K. Fluoride treatment and in vitro corrosion behavior of an AZ31B magnesium alloy. Mater. Sci. Eng. 2010, 30, 740–748. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Kasiri-Asgarani, M.; Medraj, M. Synthesis and biodegradation evaluation of nano-Si and nano-Si/TiO2 coatings on biodegradable Mg–Ca alloy in simulated body fluid. Ceram. Int. 2014, 40, 14009–14018. [Google Scholar] [CrossRef]
- Kotharu, V.; Nagumothu, R.; Arumugam, C.B.; Veerappan, M.; Sankaran, S.; Davoodbasha, M.; Nooruddin, T. Fabrication of corrosion resistant, bioactive and antibacterial silver substituted hydroxyapatite/titania composite coating on Cp Ti. Ceram. Int. 2012, 38, 731–740. [Google Scholar] [CrossRef]






| Reagents | Amount in 1000 mL |
|---|---|
| NaCl | 8.035 g |
| NaHCO3 | 0.355 g |
| KCl | 0.225 g |
| K2HPO4·3H2O | 0.231 g |
| MgCl2·6H2O | 0.311 g |
| 1.0 M HCl | 39.0 mL |
| CaCl2 | 0.292 g |
| Na2SO4 | 0.072 g |
| ((HOCH2)3CNH2) | 6.118 g |
| 1.0 M HCl | for adjusting the pH~7.4 |
| Sample | RS (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | Rp (Ω·cm2) | n1 | Y0 (1) (sn/Ω/cm2) | n2 | Y0 (2) (sn/Ω/cm2) |
|---|---|---|---|---|---|---|---|---|
| Bare | 13.5 | - | 253 | 253 | 0.92 | 55.60 | - | - |
| HA | 6.1 | 2602 | 1028 | 3630 | 0.88 | 18.11 | 0.90 | 8.90 |
| CeCC | 11.2 | - | 2430 | 2430 | 0.90 | 22.70 | - | - |
| Sample | RS (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | Rp (Ω·cm2) | n1 | Y0 (1) (sn/Ω/cm2) | n2 | Y0 (2) (sn/Ω/cm2) |
|---|---|---|---|---|---|---|---|---|
| Ce + HA 15 min | 18.50 | 1498 | 3522 | 5020 | 0.78 | 8.05 | 0.83 | 11.43 |
| Ce + HA 30 min | 25.77 | 3472 | 5680 | 9152 | 0.89 | 12.77 | 0.81 | 4.72 |
| Ce + HA 60 min | 26.12 | 3145 | 4665 | 7810 | 0.90 | 15.20 | 0.85 | 7.86 |
| Immersion Time | Rs (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | Rp (Ω·cm2) |
|---|---|---|---|---|
| Bare AZ31 | ||||
| 1 h | 24.80 | 370 | - | 370 |
| 1 day | 49.55 | 506 | - | 506 |
| 1 week | 38.20 | 727 | - | 727 |
| 2 weeks | 13.85 | 510 | - | 510 |
| 3 weeks | 12.59 | 312 | - | 312 |
| HA | ||||
| 1 h | 9.21 | 8110 | 4119 | 12,229 |
| 1 day | 13.35 | 3552 | 713 | 4265 |
| 1 week | 10.50 | 2891 | 1209 | 4100 |
| 2 weeks | 14.12 | 991 | 2224 | 3215 |
| 3 weeks | 12.62 | 36 | 2501 | 2537 |
| Immersion Time | Rs (Ω·cm2) | Rc (Ω·cm2) | Rct (Ω·cm2) | Rp (Ω·cm2) |
|---|---|---|---|---|
| HA + Ce | ||||
| 1 h | 54.12 | 11,877 | 2173 | 14,050 |
| 24 h | 45.98 | 9376 | 3244 | 12,620 |
| 1 week | 59.01 | 7494 | 1938 | 9432 |
| 2 weeks | 28.90 | 5545 | 2285 | 7830 |
| 3 weeks | 62.55 | 4216 | 974 | 5190 |
| HA + Ce/CeCC | ||||
| 1 h | 36.58 | 21,850 | 18,700 | 40,550 |
| 24 h | 59.77 | 16,538 | 4662 | 21,200 |
| 1 week | 42.51 | 15,088 | 3812 | 18,900 |
| 2 weeks | 67.15 | 14,140 | 3105 | 17,245 |
| 3 weeks | 51.14 | 12,071 | 2491 | 14,562 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, Z.; Afshar Taromi, F.; Pourmahdian, S.; Eivaz Mohammadloo, H. Investigating the Concurrent Effect of Cerium/Hydroxyapatite Coatings on Mg-Based Implant for Enhancing Corrosion Performance and In-Vitro Activity. Coatings 2024, 14, 179. https://doi.org/10.3390/coatings14020179
Rajabi Z, Afshar Taromi F, Pourmahdian S, Eivaz Mohammadloo H. Investigating the Concurrent Effect of Cerium/Hydroxyapatite Coatings on Mg-Based Implant for Enhancing Corrosion Performance and In-Vitro Activity. Coatings. 2024; 14(2):179. https://doi.org/10.3390/coatings14020179
Chicago/Turabian StyleRajabi, Zeinab, Faramarz Afshar Taromi, Saeed Pourmahdian, and Hossein Eivaz Mohammadloo. 2024. "Investigating the Concurrent Effect of Cerium/Hydroxyapatite Coatings on Mg-Based Implant for Enhancing Corrosion Performance and In-Vitro Activity" Coatings 14, no. 2: 179. https://doi.org/10.3390/coatings14020179






