Coconut-Solid-Waste-Derived Hard-Carbon Anode Materials for Fast Potassium Ion Storage
Abstract
:1. Introduction
2. Materials, Methods
2.1. Synthesis of CHC Samples
2.2. Material Characterisations
3. Results
3.1. Morphology and Structural Characterisation
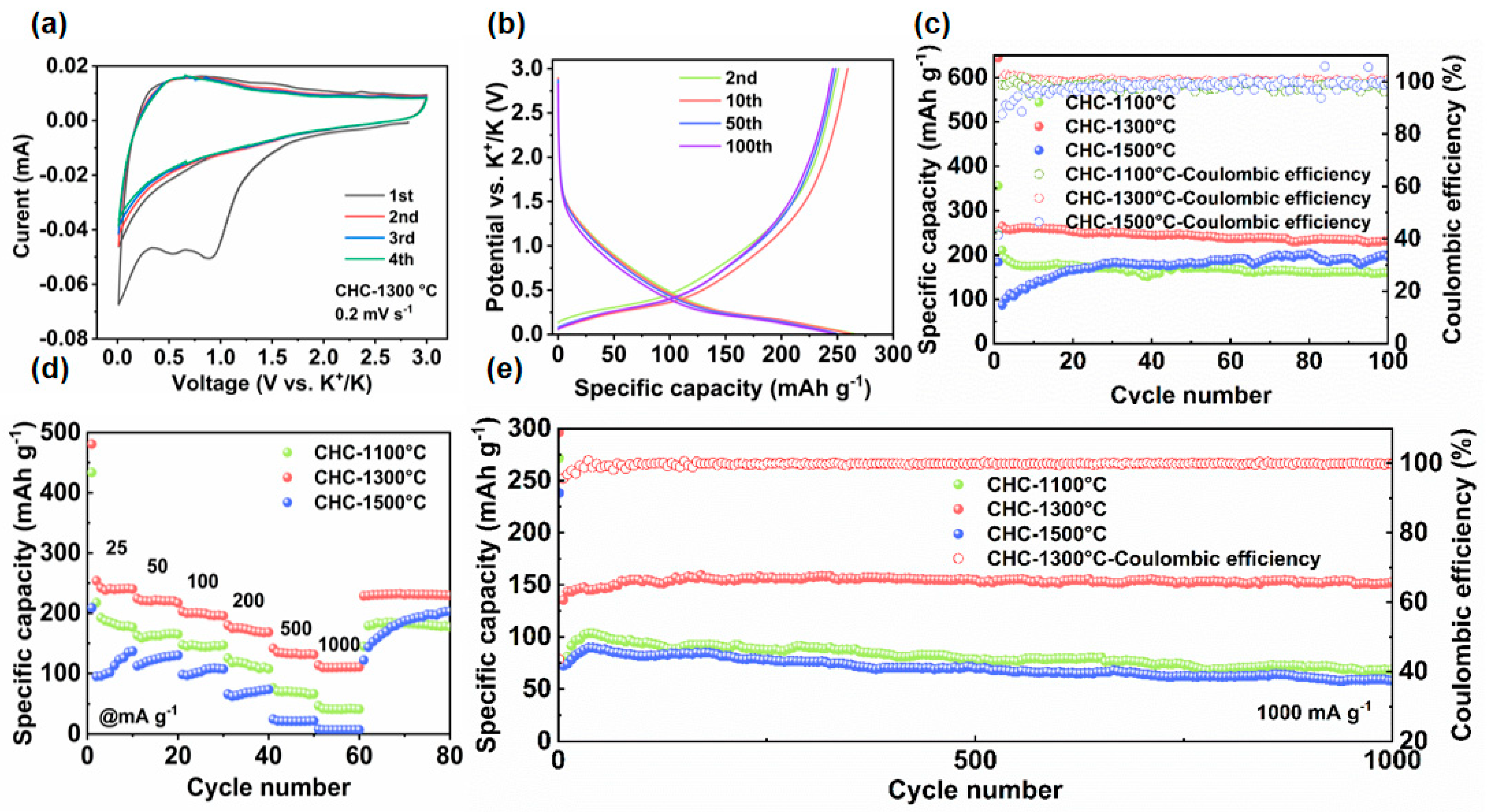
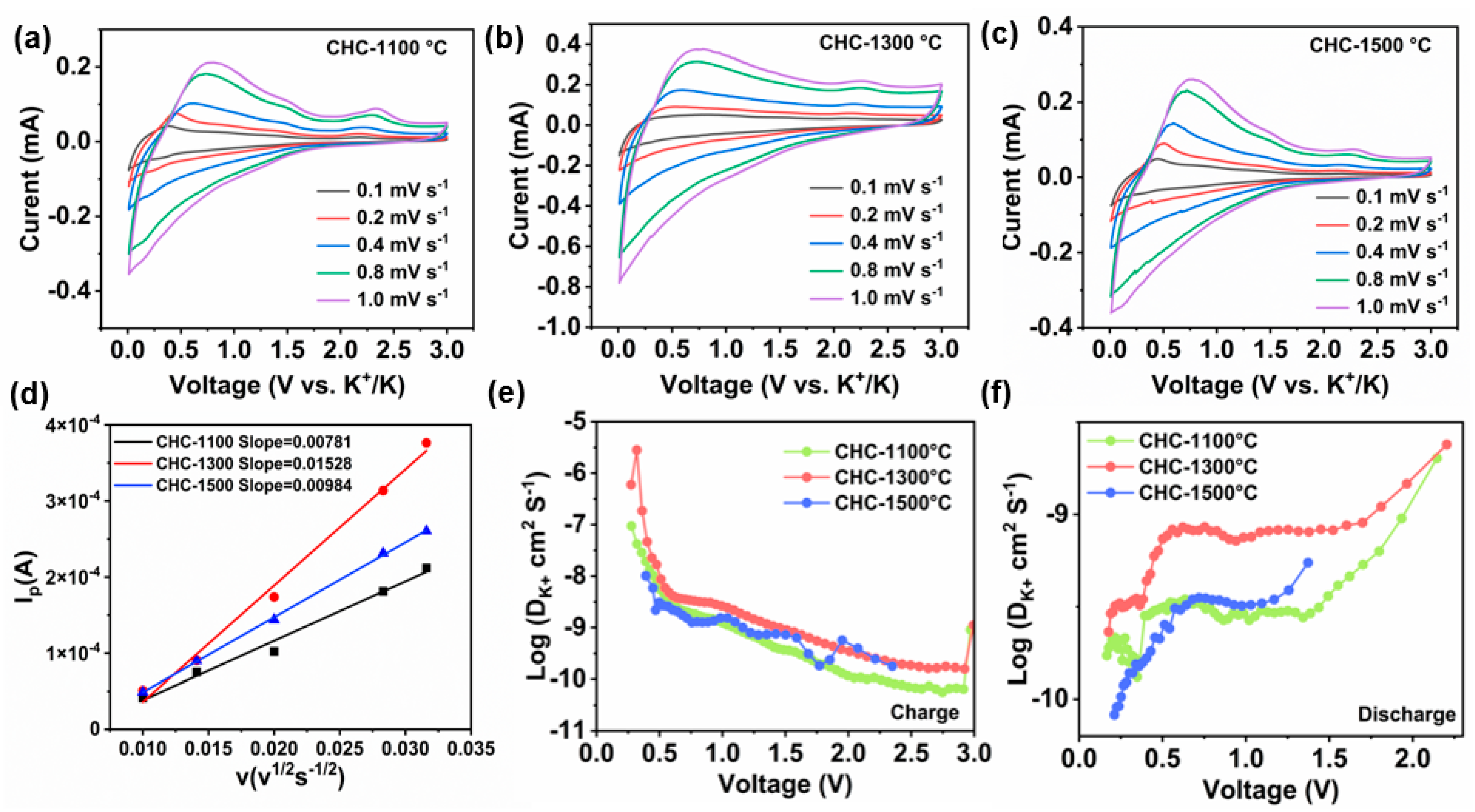
3.2. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| KIB | Potassium-ion battery |
| HC | Hard carbon |
| CHC | Hard carbon derived from coconut solid wastes |
| CMCNa | Sodium carboxymethyl cellulose |
| KFSI | Potassium difluorosulfonimide |
| DME | Dimethyl ether |
| TEM | Transmission electron microscope |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscope |
| SAED | Electron diffraction |
| XPS | X-ray photoelectron spectroscopy |
| BET | Brunauer–Emmett–Teller |
| CV | Cyclic voltammetry |
| SEI | Solid electrolyte interface |
| EIS | Electrochemical impedance spectroscopy |
| GITT | Galvanostatic intermittent titration technique |
References
- Chen, S.; Wang, J.; Fan, L.; Ma, R.; Zhang, E.; Liu, Q.; Lu, B. An Ultrafast Rechargeable Hybrid Sodium-Based Dual-Ion Capacitor Based on Hard Carbon Cathodes. Adv. Energy Mater. 2018, 8, 1800140. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Gong, D.; Xu, Z.; Lu, B. Graphene Nanoribbons on Highly Porous 3D Graphene for High-Capacity and Ultrastable Al-Ion Batteries. Adv. Mater. 2017, 29, 1604118. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Li, X.; Qiao, Y.; Jia, M.; Qiu, F.L.; He, Y.B.; He, P.; Zhou, H.S. Restraining Oxygen Loss and Suppressing Structural Distortion in a Newly Ti-Substituted Layered Oxide P2-Na0.66Li0.22Ti0.15Mn0.63O2. ACS Energy Lett. 2019, 4, 2409. [Google Scholar] [CrossRef]
- Kim, H.; Kim, J.C.; Bianchini, M.; Seo, D.H.; Rodriguez-Garcia, J.; Ceder, G. Recent Progress and Perspective in Electrode Materials for K-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702384. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, G.; Liu, Z.; Li, H.; Liu, Y.; Wang, Z.; Li, X.; Shih, K.; Mai, L. Li3V(MoO4)3 as a Novel Electrode Material with Good Lithium Storage Properties and Improved Initial Coulombic Efficiency. Nano Energy 2018, 44, 272–278. [Google Scholar] [CrossRef]
- Chen, S.; Fan, L.; Xu, L.; Liu, Q.; Qin, Y.; Lu, B. 100 K cycles: Core-shell H-FeS@C Based Lithium-Ion Battery Anode. Energy Storage Mater. 2017, 8, 20–27. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Zhang, Q. Nanostructured Energy Materials for Electrochemical Energy Conversion and Storage: A Review. J. Energy Chem. 2016, 25, 967–984. [Google Scholar] [CrossRef]
- Fan, L.; Lin, K.; Wang, J.; Ma, R.; Lu, B. A Nonaqueous Potassium-Based Battery-Supercapacitor Hybrid Device. Adv. Mater. 2018, 30, e1800804. [Google Scholar] [CrossRef]
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Recent Progress in Rechargeable Potassium Batteries. Adv. Funct. Mater. 2018, 28, 1802938. [Google Scholar] [CrossRef]
- Jian, Z.; Luo, W.; Ji, X. Carbon Electrodes for K-Ion Batteries. J. Am. Chem. Soc. 2015, 137, 11566–11569. [Google Scholar] [CrossRef]
- Luo, W.; Wan, J.; Ozdemir, B.; Bao, W.; Chen, Y.; Dai, J.; Lin, H.; Xu, Y.; Gu, F.; Barone, V.; et al. Potassium Ion Batteries with Graphited Materials. Nano Lett. 2015, 15, 7671–7677. [Google Scholar] [CrossRef]
- Pramudita, J.C.; Sehrawat, D.; Goonetilleke, D.; Sharma, N. An Initial Review of the Status of Electrode Materials for Potassium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602911. [Google Scholar] [CrossRef]
- Beltrop, K.; Beuker, S.; Heckmann, A.; Winter, M.; Placke, T. Alternative Electrochemical Energy Storage: Potassium-Based Dualgraphite Batteries. Energy Environ. Sci. 2017, 10, 2090–2094. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, W.; Carter, M.; Zhou, L.; Dai, J.; Fu, K.; Lacey, S.; Li, T.; Wan, J.; Han, X.; et al. Organic Electrode for Nonaqueous Potassium-Ion Batteries. Nano Energy 2015, 18, 205–211. [Google Scholar] [CrossRef]
- Fan, L.; Chen, S.; Ma, R.; Wang, J.; Wang, L.; Zhang, Q.; Zhang, E.; Liu, Z.; Lu, B. Ultrastable Potassium Storage Performance Realized by Highly Effective Solid Electrolyte Interphase Layer. Small 2018, 14, e1801806. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. Thermodynamic Functions of Transfer of Single Ions from Water to Nonaqueous and Mixed Solvents: Part 3—Standard Potentials of Selected Electrodes. Pure Appl. Chem. 1985, 57, 1129. [Google Scholar] [CrossRef]
- Ramasamy, H.V.; Senthilkumar, B.; Barpanda, P.; Lee, Y.-S. Superior Potassium-Ion Hybrid Capacitor Based on Novel P3-Type Layered K0.45Mn0.5Co0.5O2 as High Capacity Cathode. Chem. Eng. J. 2019, 368, 235–243. [Google Scholar] [CrossRef]
- Ming, F.; Liang, H.; Zhang, W.; Ming, J.; Lei, Y.; Emwas, A.-H.; Alshareef, H.N. Porous MXenes Enable High Performance Potassium Ion Capacitors. Nano Energy 2019, 62, 853–860. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Zhang, B.; Miao, L.; Cai, J.; Peng, L.; Huang, Y.; Jiang, J.; Huang, Y.; Zhang, L.; et al. Nitrogen-Rich Hard Carbon as a Highly Durable Anode for High-Power Potassium-Ion Batteries. Energy Storage Mater. 2017, 8, 161–168. [Google Scholar] [CrossRef]
- Chen, M.; Wang, W.; Liang, X.; Gong, S.; Liu, J.; Wang, Q.; Guo, S.J.; Yang, H. Sulfur/Oxygen Codoped Porous Hard Carbon Microspheres for High-Performance Potassium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1800171. [Google Scholar] [CrossRef]
- Jian, Z.L.; Xing, Z.Y.; Bommier, C.; Li, Z.F.; Ji, X.L. Hard Carbon Microspheres: Potassium-Ion Anode Versus Sodium-Ion Anode. Adv. Energy Mater. 2016, 6, 1501874. [Google Scholar] [CrossRef]
- Wu, F.; Liu, L.; Yuan, Y.; Li, Y.; Bai, Y.; Li, T.; Lu, J.; Wu, C. Expanding Interlayer Spacing of Hard Carbon by Natural K+ Doping to Boost Na-Ion Storage. ACS Appl. Mater. Interfaces 2018, 10, 27030–27038. [Google Scholar] [CrossRef] [PubMed]
- Dahbi, M.; Kiso, M.; Kubota, K.; Horiba, T.; Chafik, T.; Hida, K.; Matsuyama, T.; Komaba, S.J. Synthesis of hard carbon from argan shells for Na-ion batteries. Mater. Chem. A 2017, 5, 9917–9928. [Google Scholar] [CrossRef]
- Lotfabad, E.M.; Ding, J.; Cui, K.; Kohandehghan, A.; Kalisvaart, W.P.; Hazelton, M.; Mitlin, D. High-density sodium and lithium ion battery anodes from banana peels. ACS Nano 2014, 8, 7115–7129. [Google Scholar] [CrossRef] [PubMed]
- Rios, C.D.M.S.; Simone, V.; Simonin, L.; Martinet, S.; Dupont, C. Biochars from various biomass types as precursors for hard carbon anodes in sodium-ion batteries. Biomass Bioenergy 2018, 117, 32–37. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Z.H.; Yin, S.Y.; Guo, Z.P.; Wang, S.Q.; Feng, C.Q. Biomass carbon micro/nano-structures derived from ramie fibers and corncobs as anode materials for lithium-ion and sodium-ion batteries. Appl. Surface Sci. 2016, 379, 73–82. [Google Scholar] [CrossRef]
- Zhu, T.; Mai, B.; Hu, P.; Cai, C.; Xing, B.; Wei, Z.; Chen, C.; Fan, H.; Li, M.; Wang, X. Bagasse-Derived Hard Carbon Anode with an Adsorption–Intercalation Mechanism for High-Rate Potassium Storage. ACS Appl. Energy Mater. 2023, 6, 2370–2377. [Google Scholar] [CrossRef]
- Ghimbeu, C.M.; Górka, J.; Simone, V.; Simonin, L.; Martinet, S.; Vix-Guterl, C. Insights on the Na+ ion storage mechanism in hard carbon: Discrimination between the porosity, surface functional groups and defects. Nano Energy 2018, 44, 327–335. [Google Scholar] [CrossRef]
- Gomez-Martin, A.; Martinez-Fernandez, J.; Ruttert, M.; Winter, M.; Placke, T.; RamirezRico, J. Correlation of Structure and Performance of Hard Carbons as Anodes for Sodium Ion Batteries. Chem. Mater. 2019, 31, 7288–7299. [Google Scholar] [CrossRef]
- Sun, N.; Guan, Z.R.X.; Liu, Y.W.; Cao, Y.L.; Zhu, Q.Z.; Liu, H.; Wang, Z.X.; Zhang, P.; Xu, B. Extended “Adsorption–Insertion” Model: A New Insight into the Sodium Storage Mechanism of Hard Carbons. Adv. Energy Mater. 2019, 9, 1901351. [Google Scholar] [CrossRef]
- Buiel, E.R.; George, A.E.; Dahn, J.R. Model of micropore closure in hard carbon prepared from sucrose. Carbon 1999, 37, 1399–1407. [Google Scholar] [CrossRef]
- Yang, J.; Ju, Z.; Jiang, Y.; Xing, Z.; Xi, B.; Feng, J.; Xiong, S. Enhanced Capacity and Rate Capability of Nitrogen/Oxygen Dual-Doped Hard Carbon in Capacitive Potassium-Ion Storage. Adv. Mater. 2018, 30, 1700104. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Saurel, D.; Orayech, B.; Xiao, B.; Carriazo, D.; Li, X.; Rojo, T. From Charge Storage Mechanism to Performance: A Roadmap toward High Specific Energy Sodium-Ion Batteries through Carbon Anode Optimization. Adv. Energy Mater. 2018, 8, 1703268. [Google Scholar] [CrossRef]
- Hou, H.; Qiu, X.; Wei, W.; Zhang, Y.; Ji, X. Carbon Anode Materials for Advanced Sodium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1602898. [Google Scholar] [CrossRef]
- Bommier, C.; Mitlin, D.; Ji, X.L. Internal structure–Na storage mechanisms–Electrochemical performance relations in carbons. Mater. Sci. 2018, 97, 170. [Google Scholar] [CrossRef]
- Qiu, S.; Xiao, L.; Sushko, M.L.; Han, K.S.; Shao, Y.; Yan, M.; Liang, X.; Mai, L.; Feng, J.; Cao, Y.; et al. Manipulating Adsorption-Insertion Mechanisms in Nanostructured Carbon Materials for High-Efficiency Sodium Ion Storage. Adv. Energy Mater. 2017, 7, 1700403. [Google Scholar] [CrossRef]
- Dou, X.W.; Hasa, I.; Saurel, D.; Vaalma, C.; Wu, L.M.; Buchholz, D.; Bresser, D.; Komaba, S.; Passerini, S. Hard carbons for sodium-ion batteries: Structure, analysis, sustainability, and electrochemistry. Mater. Today 2019, 23, 87–104. [Google Scholar] [CrossRef]
- Shrestha, D.; Maensiri, S.; Wongpratat, U.; Lee, S.W.; Nyachhyon, A.R. Shorea Robusta Derived Activated Carbon Decorated with Manganese Dioxide Hybrid Composite for Improved Capacitive Behaviors. J. Environ. Chem. Eng. 2019, 7, 103227. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Reddeppa, M.; Nam, D.-J.; Bak, N.-H.; Peta, K.R.; Cho, H.D.; Kim, S.-K.; Kim, M.D. Boosting of NO2 Gas Sensing Performances Using GO-PEDOT: PSS Nanocomposite Chemical Interface Coated on Langasite-Based Surface Acoustic Wave Sensor. Sens. Actuators B Chem. 2021, 344, 130267. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gomez-Romero, P. All Nanocarbon Li-Ion Capacitor with High Energy and High Power Density. Mater. Today Energy 2018, 8, 109–117. [Google Scholar] [CrossRef]
- Fondard, J.; Irisarri, E.; Courreges, C.; Palacin, M.R.; Ponrouch, A.; Dedryvere, R. SEI Composition on Hard Carbon in Na-Ion Batteries After Long Cycling: Influence of Salts (NaPF6, NaTFSI) and Additives (FEC, DMCF). J. Electrochem. Soc. 2020, 167, 070526. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C. Galvanostatic Intermittent Titration Technique for Phase-Transformation Electrodes. Adv. Energy Mater. 2010, 114, 2830–2841. [Google Scholar] [CrossRef]
- Xing, Z.; Qi, Y.; Jian, Z.; Ji, X. Polynanocrystalline graphite: A new carbon anode with superior cycling performance for K-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 4343–4351. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.-J.; Hou, B.-H.; Li, W.-H.; Ning, Q.-L.; Yang, X.; Gu, Z.-Y.; Nie, X.-J.; Wang, G.; Wu, X.-L. Staging Na/K-ion de-/intercalation of graphite retrieved from spent Li-ion batteries: In operando X-ray diffraction studies and an advanced anode material for Na/K-ion batteries. Energy Environ. Sci. 2019, 12, 3575–3584. [Google Scholar] [CrossRef]
- Hao, R.; Lan, H.; Kuang, C.; Wang, H.; Guo, L. Superior potassium storage in chitin-derived natural nitrogen-doped carbon nanofibers. Carbon 2018, 128, 224–230. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, L.; Huang, J.; Zou, J.; Chen, S.; Cheng, H.; Jiang, C.; Gao, P.; Niu, X. Loofah-derived carbon as an anode material for potassium ion and lithium ion batteries. Electrochim. Acta 2019, 306, 446–453. [Google Scholar] [CrossRef]
- Shan, J.; Wang, J.-J.; Kiekens, P.; Zhao, Y.; Huang, J.-j. Effect of co-activation of petroleum coke and Artemisia Hedinii on potassium loss during activation and its promising application in anode material of potassium-ion batteries. Solid State Sci. 2019, 92, 96–105. [Google Scholar] [CrossRef]
- Li, W.; Li, Z.; Zhang, C.; Liu, W.; Han, C.; Yan, B.; An, S.; Qiu, X. Hard carbon derived from rice husk as anode material for high performance potassium-ion batteries. Solid State Ionics 2020, 351, 115319. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Liu, W.; Liu, W.; Zhang, G.; Wang, Y.; Wang, H.; Chen, W.; Huang, M.; Wang, X. Coconut-Solid-Waste-Derived Hard-Carbon Anode Materials for Fast Potassium Ion Storage. Coatings 2024, 14, 208. https://doi.org/10.3390/coatings14020208
Ma Y, Liu W, Liu W, Zhang G, Wang Y, Wang H, Chen W, Huang M, Wang X. Coconut-Solid-Waste-Derived Hard-Carbon Anode Materials for Fast Potassium Ion Storage. Coatings. 2024; 14(2):208. https://doi.org/10.3390/coatings14020208
Chicago/Turabian StyleMa, Yi, Wenhao Liu, Wenhan Liu, Guangwan Zhang, Yu Wang, Haokai Wang, Wei Chen, Meng Huang, and Xuanpeng Wang. 2024. "Coconut-Solid-Waste-Derived Hard-Carbon Anode Materials for Fast Potassium Ion Storage" Coatings 14, no. 2: 208. https://doi.org/10.3390/coatings14020208




