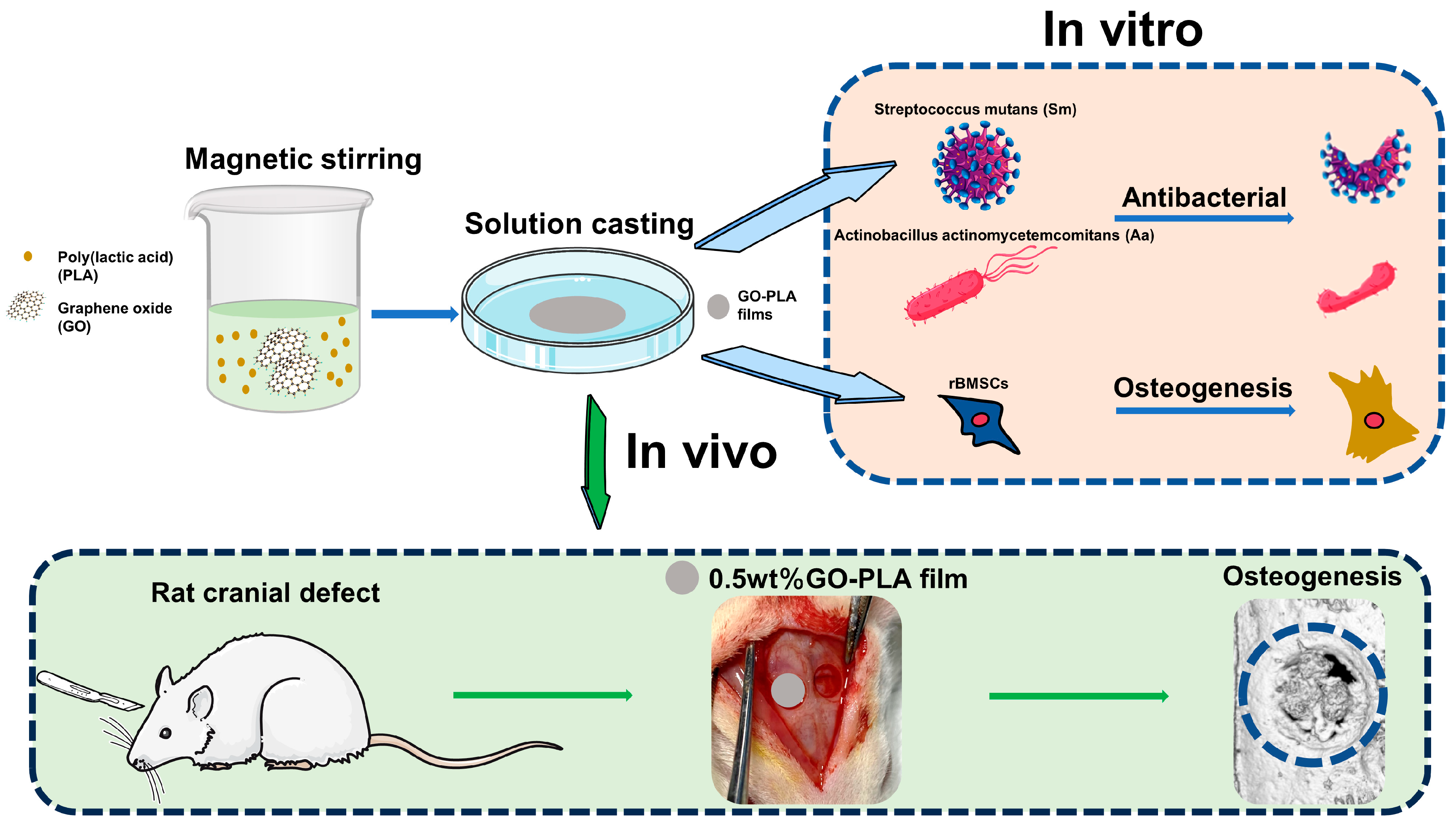
Enhanced Osteogenic Activity and Antibacterial Properties of Graphene Oxide-Poly(Lactic Acid) Films for the Repair of Cranial Defects in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterization of the GO-PLA Films
2.3. Cell Isolation and Culture
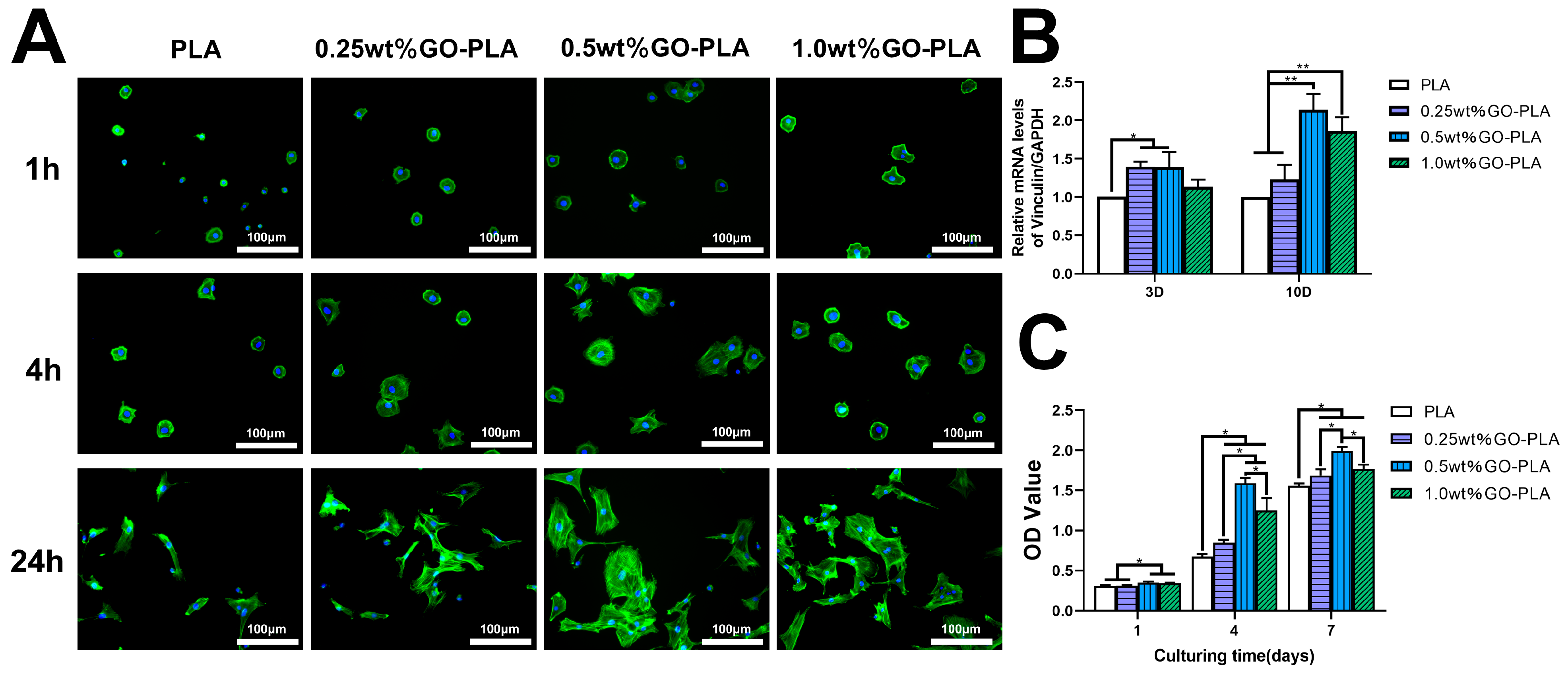
2.4. Cell Adhesion Activity Assay
2.5. Cell Proliferation Measurement
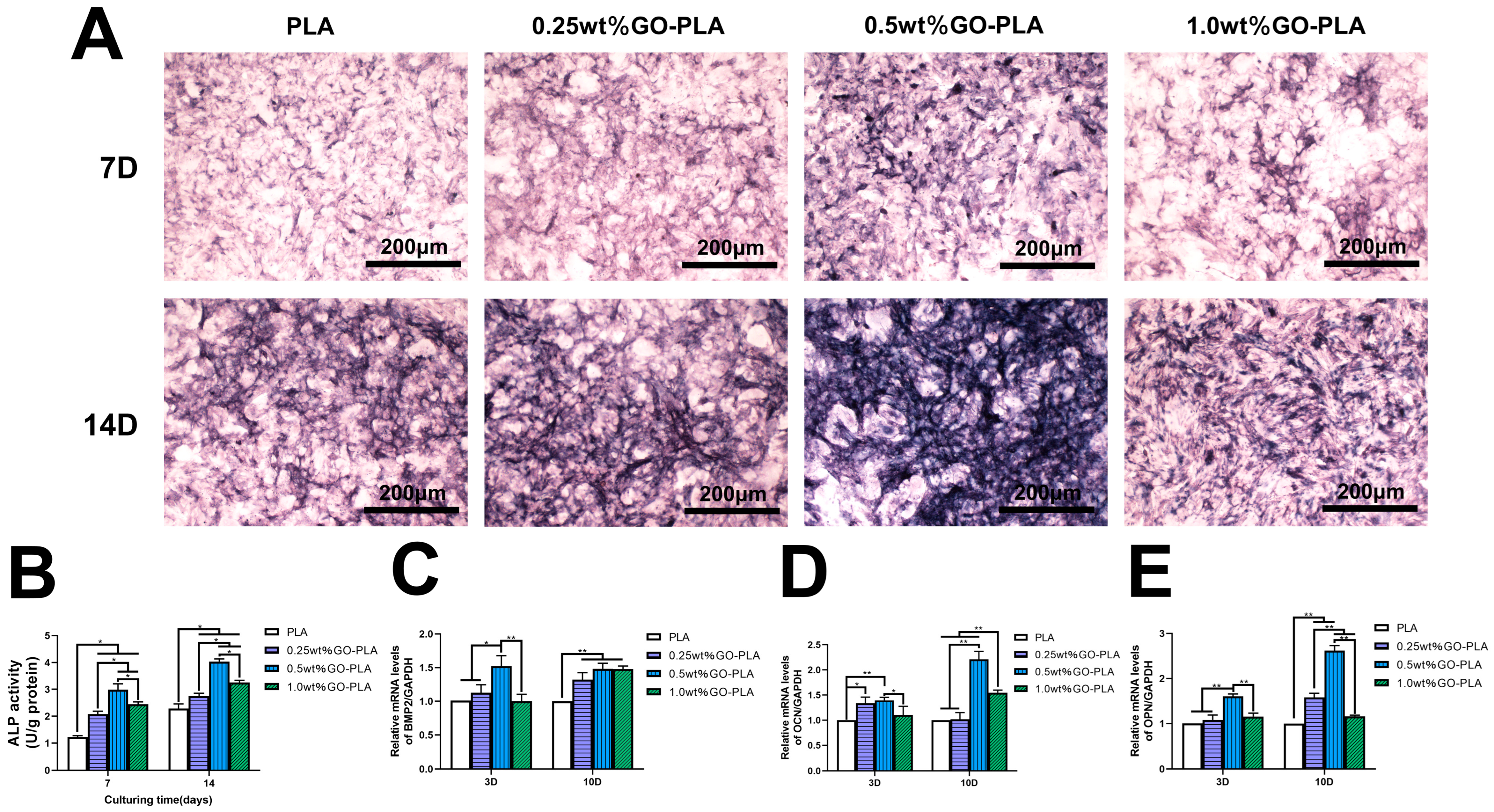
2.6. ALP Staining and ALP Activity Assay
2.7. Real-Time Quantitative Polymerase Chain Reaction Analysis
2.7.1. RNA Extraction
2.7.2. RT-PCR Analysis
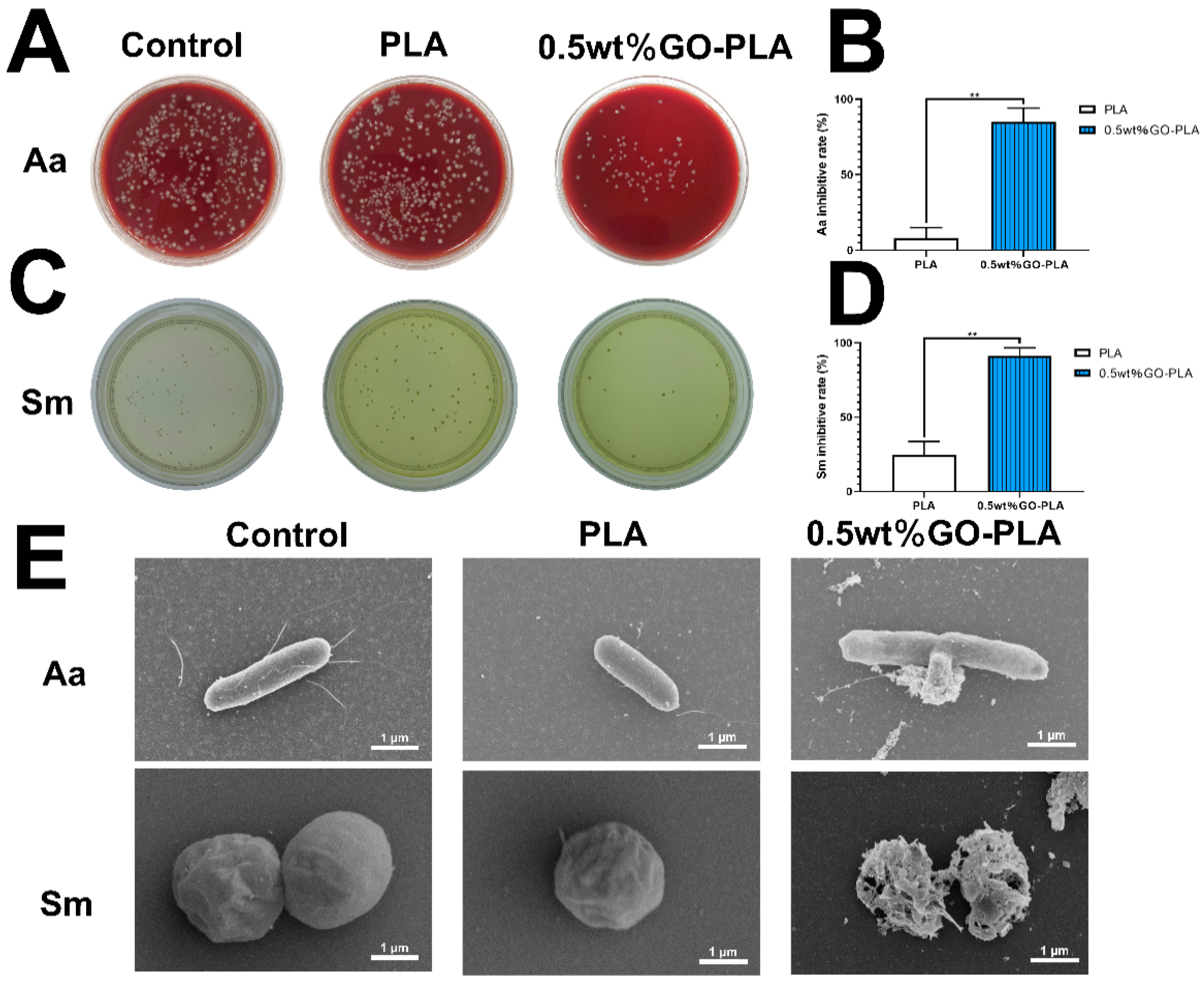
2.8. Antibacterial Assay In Vitro
2.9. In Vivo Osseointegration Assessment
2.9.1. Animals and Surgical Procedure
2.9.2. Micro-CT Assay
2.9.3. Histological Observation
2.10. Statistical Analysis
3. Results
3.1. Characterization of the Graphene Oxide-Incorporated-PLA Films
3.2. Effect of Graphene Oxide Incorporation on the Adhesion and Proliferation of rBMSCs on PLA Films
3.3. Effect of Graphene Oxide Incorporation on the ALP Activity and Osteogenic-Related Genes Expression of rBMSCs on PLA Films
3.4. Effect of Graphene Oxide Incorporation on the Antibacterial Activity on PLA Films
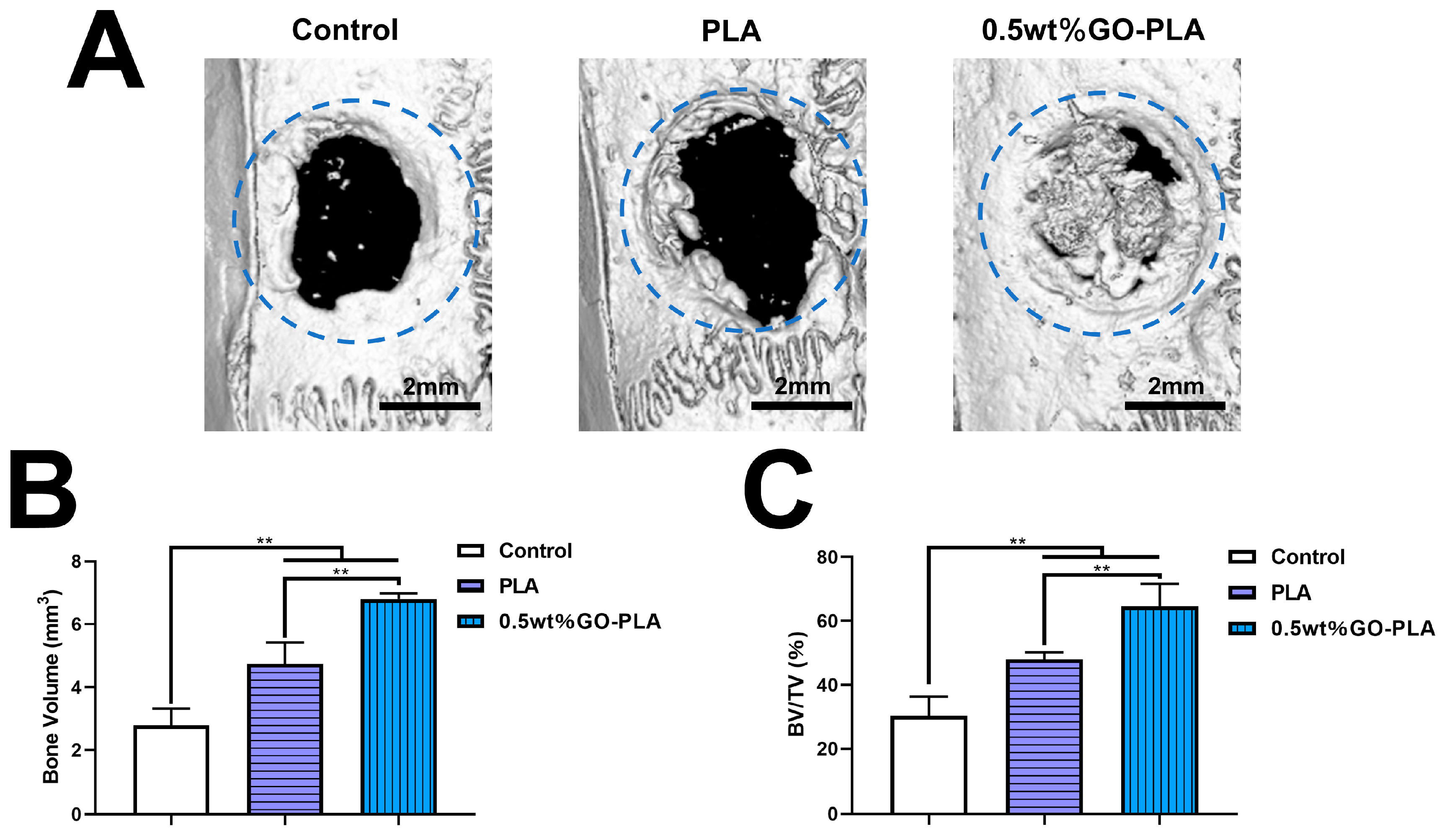
3.5. Guiding the Bone Tissue Regeneration of Graphene Oxide-Incorporated PLA Films In Vivo
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jia, Z.; Xiu, P.; Xiong, P.; Zhou, W.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; Liu, Z.; et al. Additively Manufactured Macroporous Titanium with Silver-Releasing Micro-/Nanoporous Surface for Multipurpose Infection Control and Bone Repair—A Proof of Concept. ACS Appl. Mater. Interfaces 2016, 8, 28495–28510. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; Tang, T. Dual-Functional 3D-Printed Composite Scaffold for Inhibiting Bacterial Infection and Promoting Bone Regeneration in Infected Bone Defect Models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef]
- Majidinia, M.; Sadeghpour, A.; Yousefi, B. The Roles of Signaling Pathways in Bone Repair and Regeneration. J. Cell. Physiol. 2018, 233, 2937–2948. [Google Scholar] [CrossRef]
- Ou, L.; Tan, X.; Qiao, S.; Wu, J.; Su, Y.; Xie, W.; Jin, N.; He, J.; Luo, R.; Lai, X.; et al. Graphene-Based Material-Mediated Immunomodulation in Tissue Engineering and Regeneration: Mechanism and Significance. ACS Nano 2023, 17, 18669–18687. [Google Scholar] [CrossRef]
- Koushik, T.M.; Miller, C.M.; Antunes, E. Bone Tissue Engineering Scaffolds: Function of Multi-Material Hierarchically Structured Scaffolds. Adv. Healthc. Mater. 2023, 12, 2202766. [Google Scholar] [CrossRef]
- Lu, Y.; Li, L.; Zhu, Y.; Wang, X.; Li, M.; Lin, Z.; Hu, X.; Zhang, Y.; Yin, Q.; Xia, H.; et al. Multifunctional Copper-Containing Carboxymethyl Chitosan/Alginate Scaffolds for Eradicating Clinical Bacterial Infection and Promoting Bone Formation. ACS Appl. Mater. Interfaces 2018, 10, 127–138. [Google Scholar] [CrossRef]
- Chen, M.; Li, Y.; Hou, W.-X.; Peng, D.-Y.; Li, J.-K.; Zhang, H.-X. The Antibacterial Effect, Biocompatibility, and Osteogenesis of Vancomycin-Nanodiamond Composite Scaffold for Infected Bone Defects. Int. J. Nanomed. 2023, 18, 1365–1380. [Google Scholar] [CrossRef]
- Yao, H.; Wang, J.; Deng, Y.; Li, Z.; Wei, J. Osteogenic and Antibacterial PLLA Membrane for Bone Tissue Engineering. Int. J. Biol. Macromol. 2023, 247, 125671. [Google Scholar] [CrossRef]
- Loh, K.P.; Bao, Q.; Eda, G.; Chhowalla, M. Graphene Oxide as a Chemically Tunable Platform for Optical Applications. Nat. Chem. 2010, 2, 1015–1024. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.; Sim, S.H.; Sung, M.G.; Kim, K.S.; Hong, B.H.; Hong, S. Enhanced Differentiation of Human Neural Stem Cells into Neurons on Graphene. Adv. Mater. 2011, 23, H263–H267. [Google Scholar] [CrossRef]
- Chawda, N.; Basu, M.; Majumdar, D.; Poddar, R.; Mahapatra, S.K.; Banerjee, I. Engineering of Gadolinium-Decorated Graphene Oxide Nanosheets for Multimodal Bioimaging and Drug Delivery. ACS Omega 2019, 4, 12470–12479. [Google Scholar] [CrossRef]
- Tian, X.; Hu, J.; Wei, T.; Ding, W.; Miao, Q.; Ning, Z.; Fan, S.; Wu, H.; Lu, J.; Lyu, M.; et al. Fast and Sensitive Graphene oxide-DNAzyme-based Biosensor for Vibrio Alginolyticus Detection. J. Fish. Dis. 2022, 45, 687–697. [Google Scholar] [CrossRef]
- Karki, N.; Tiwari, H.; Tewari, C.; Rana, A.; Pandey, N.; Basak, S.; Sahoo, N.G. Functionalized Graphene Oxide as a Vehicle for Targeted Drug Delivery and Bioimaging Applications. J. Mater. Chem. B 2020, 8, 8116–8148. [Google Scholar] [CrossRef]
- Holt, B.D.; Wright, Z.M.; Arnold, A.M.; Sydlik, S.A. Graphene Oxide as a Scaffold for Bone Regeneration. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1437. [Google Scholar] [CrossRef]
- Yang, J.-W.; Hsieh, K.Y.; Kumar, P.V.; Cheng, S.-J.; Lin, Y.-R.; Shen, Y.-C.; Chen, G.-Y. Enhanced Osteogenic Differentiation of Stem Cells on Phase-Engineered Graphene Oxide. ACS Appl. Mater. Interfaces 2018, 10, 12497–12503. [Google Scholar] [CrossRef]
- Li, Q.; Shen, A.; Wang, Z. Enhanced Osteogenic Differentiation of BMSCs and M2-Phenotype Polarization of Macrophages on a Titanium Surface Modified with Graphene Oxide for Potential Implant Applications. RSC Adv. 2020, 10, 16537–16550. [Google Scholar] [CrossRef]
- Wang, H.; Lai, Y.; Xie, Z.; Lin, Y.; Cai, Y.; Xu, Z.; Chen, J. Graphene Oxide-Modified Concentric Microgrooved Titanium Surfaces for the Dual Effects of Osteogenesis and Antiosteoclastogenesis. ACS Appl. Mater. Interfaces 2022, 14, 54500–54516. [Google Scholar] [CrossRef]
- Shao, W.; He, J.; Wang, Q.; Cui, S.; Ding, B. Biomineralized Poly(L-Lactic-Co-Glycolic Acid)/Graphene Oxide/Tussah Silk Fibroin Nanofiber Scaffolds with Multiple Orthogonal Layers Enhance Osteoblastic Differentiation of Mesenchymal Stem Cells. ACS Biomater. Sci. Eng. 2017, 3, 1370–1380. [Google Scholar] [CrossRef]
- Liu, S.; Zhou, C.; Mou, S.; Li, J.; Zhou, M.; Zeng, Y.; Luo, C.; Sun, J.; Wang, Z.; Xu, W. Biocompatible Graphene Oxide–Collagen Composite Aerogel for Enhanced Stiffness and in Situ Bone Regeneration. Mater. Sci. Eng. C 2019, 105, 110137. [Google Scholar] [CrossRef]
- Panda, S.; Rout, T.K.; Prusty, A.D.; Ajayan, P.M.; Nayak, S. Electron Transfer Directed Antibacterial Properties of Graphene Oxide on Metals. Adv. Mater. 2018, 30, 1702149. [Google Scholar] [CrossRef]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing Dental Pathogens Using Antibacterial Graphene Oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Zou, F.; Zhou, H.; Jeong, D.Y.; Kwon, J.; Eom, S.U.; Park, T.J.; Hong, S.W.; Lee, J. Wrinkled Surface-Mediated Antibacterial Activity of Graphene Oxide Nanosheets. ACS Appl. Mater. Interfaces 2017, 9, 1343–1351. [Google Scholar] [CrossRef]
- Liu, S.; Zeng, T.H.; Hofmann, M.; Burcombe, E.; Wei, J.; Jiang, R.; Kong, J.; Chen, Y. Antibacterial Activity of Graphite, Graphite Oxide, Graphene Oxide, and Reduced Graphene Oxide: Membrane and Oxidative Stress. ACS Nano 2011, 5, 6971–6980. [Google Scholar] [CrossRef]
- Pulingam, T.; Thong, K.L.; Ali, M.E.; Appaturi, J.N.; Dinshaw, I.J.; Ong, Z.Y.; Leo, B.F. Graphene Oxide Exhibits Differential Mechanistic Action towards Gram-Positive and Gram-Negative Bacteria. Colloids Surf. B Biointerfaces 2019, 181, 6–15. [Google Scholar] [CrossRef]
- Song, C.; Yang, C.-M.; Sun, X.-F.; Xia, P.-F.; Qin, J.; Guo, B.-B.; Wang, S.-G. Influences of Graphene Oxide on Biofilm Formation of Gram-Negative and Gram-Positive Bacteria. Environ. Sci. Pollut. Res. 2018, 25, 2853–2860. [Google Scholar] [CrossRef]
- Qiu, J.; Geng, H.; Wang, D.; Qian, S.; Zhu, H.; Qiao, Y.; Qian, W.; Liu, X. Layer-Number Dependent Antibacterial and Osteogenic Behaviors of Graphene Oxide Electrophoretic Deposited on Titanium. ACS Appl. Mater. Interfaces 2017, 9, 12253–12263. [Google Scholar] [CrossRef]
- Zhang, Y.; Ali, S.F.; Dervishi, E.; Xu, Y.; Li, Z.; Casciano, D.; Biris, A.S. Cytotoxicity Effects of Graphene and Single-Wall Carbon Nanotubes in Neural Phaeochromocytoma-Derived PC12 Cells. ACS Nano 2010, 4, 3181–3186. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Haroosh, H.J.; Dong, Y.; Jasim, S.; Ramakrishna, S. Improvement of Drug Release and Compatibility between Hydrophilic Drugs and Hydrophobic Nanofibrous Composites. Materials 2021, 14, 5344. [Google Scholar] [CrossRef]
- Deng, X.; Gould, M.; Ali, M.A. A Review of Current Advancements for Wound Healing: Biomaterial Applications and Medical Devices. J. Biomed. Mater. Res. 2022, 110, 2542–2573. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, Q.; Xu, H.; Zhang, S.; Wang, W.; Zhao, B. Effect of Polylactic Acid Membrane on Guided Bone Regeneration in Anterior Maxillary Implantation. Med. Sci. Monit. 2023, 29, e938566. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kang, M.S.; Kim, W.-H.; Jo, H.J.; Lee, S.-H.; Hahm, E.J.; Oh, J.H.; Hong, S.W.; Kim, B.; Han, D.-W. 3D Printed Membranes of Polylactic Acid and Graphene Oxide for Guided Bone Regeneration. Nanoscale Adv. 2023, 5, 3619–3628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhou, G.; Junka, R.; Chang, N.; Anwar, A.; Wang, H.; Yu, X. Fabrication of Polylactic Acid (PLA)-Based Porous Scaffold through the Combination of Traditional Bio-Fabrication and 3D Printing Technology for Bone Regeneration. Colloids Surf. B Biointerfaces 2021, 197, 111420. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Cheng, X.; Xiao, L.; Wang, Q.; Yan, K.; Su, Z.; Wang, L.; Ma, C.; Wang, Y. Inside-Outside Ag Nanoparticles-Loaded Polylactic Acid Electrospun Fiber for Long-Term Antibacterial and Bone Regeneration. Int. J. Biol. Macromol. 2021, 167, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, X.; Qiu, K.; Feng, W.; Mo, H.; Wang, M.; Wang, J.; He, C. Strontium-Incorporated Mineralized PLLA Nanofibrous Membranes for Promoting Bone Defect Repair. Colloids Surf. B Biointerfaces 2019, 179, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Chen, C.; Zhou, Q.; Wang, X.S.; Zhou, G.; Liu, W.; Zhang, Z.-Y.; Cao, Y.; Zhang, W.J. The Treatment Efficacy of Bone Tissue Engineering Strategy for Repairing Segmental Bone Defects Under Osteoporotic Conditions. Tissue Eng. Part A 2015, 21, 2346–2355. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, K.; Wen, Z.; Liu, W.; Zhang, L.; Su, J. Double-Edged Effects and Mechanisms of Zn2+ Microenvironments on Osteogenic Activity of BMSCs: Osteogenic Differentiation or Apoptosis. RSC Adv. 2020, 10, 14915–14927. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of Bone Regeneration Using the Rat Critical Size Calvarial Defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Dong, X.; Liang, X.; Zhou, Y.; Bao, K.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Liu, Y. Preparation of Polylactic Acid/TiO2/GO Nano-Fibrous Films and Their Preservation Effect on Green Peppers. Int. J. Biol. Macromol. 2021, 177, 135–148. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Gao, S.; Zhang, Y.-W.; Zhou, R.-B.; Zhou, F. Antibacterial Biomaterials in Bone Tissue Engineering. J. Mater. Chem. B 2021, 9, 2594–2612. [Google Scholar] [CrossRef]
- Ren, G.; Li, R.; Hu, Y.; Chen, Y.; Chen, C.; Yu, B. Treatment Options for Infected Bone Defects in the Lower Extremities: Free Vascularized Fibular Graft or Ilizarov Bone Transport? J. Orthop. Surg. Res. 2020, 15, 439. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-Centered Infection: Microbial Adhesion Versus Tissue Integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Hai, J.H.; Lee, C.; Kapila, Y.L.; Chaffee, B.W.; Armitage, G.C. Antibiotic Prescribing Practices in Periodontal Surgeries with and without Bone Grafting. J. Periodontol. 2020, 91, 508–515. [Google Scholar] [CrossRef]
- Hussain, S.; Joo, J.; Kang, J.; Kim, B.; Braun, G.B.; She, Z.-G.; Kim, D.; Mann, A.P.; Mölder, T.; Teesalu, T.; et al. Antibiotic-Loaded Nanoparticles Targeted to the Site of Infection Enhance Antibacterial Efficacy. Nat. Biomed. Eng. 2018, 2, 95–103. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Khodabukus, A.; Guyer, T.; Moore, A.C.; Stevens, M.M.; Guldberg, R.E.; Bursac, N. Translating Musculoskeletal Bioengineering into Tissue Regeneration Therapies. Sci. Transl. Med. 2022, 14, eabn9074. [Google Scholar] [CrossRef]
- Xiao, Y.; Pang, Y.X.; Yan, Y.; Qian, P.; Zhao, H.; Manickam, S.; Wu, T.; Pang, C.H. Synthesis and Functionalization of Graphene Materials for Biomedical Applications: Recent Advances, Challenges, and Perspectives. Adv. Sci. 2023, 10, 2205292. [Google Scholar] [CrossRef]
- Bhatt, S.; Punetha, V.D.; Pathak, R.; Punetha, M. Graphene in Nanomedicine: A Review on Nano-Bio Factors and Antibacterial Activity. Colloids Surf. B Biointerfaces 2023, 226, 113323. [Google Scholar] [CrossRef]
- Shi, D.; Zhuang, J.; Fan, Z.; Zhao, H.; Zhang, X.; Su, G.; Xie, L.; Ge, D.; Hou, Z. Self-Targeting Nanotherapy Based on Functionalized Graphene Oxide for Synergistic Thermochemotherapy. J. Colloid Interface Sci. 2021, 603, 70–84. [Google Scholar] [CrossRef]
- Itoo, A.M.; Vemula, S.L.; Gupta, M.T.; Giram, M.V.; Kumar, S.A.; Ghosh, B.; Biswas, S. Multifunctional Graphene Oxide Nanoparticles for Drug Delivery in Cancer. J. Control. Release 2022, 350, 26–59. [Google Scholar] [CrossRef]
- Lin, J.; Chen, X.; Huang, P. Graphene-Based Nanomaterials for Bioimaging. Adv. Drug Deliv. Rev. 2016, 105, 242–254. [Google Scholar] [CrossRef]
- Petrochenko, P.E.; Zhang, Q.; Bayati, R.; Skoog, S.A.; Phillips, K.S.; Kumar, G.; Narayan, R.J.; Goering, P.L. Cytotoxic Evaluation of Nanostructured Zinc Oxide (ZnO) Thin Films and Leachates. Toxicol. Vitr. 2014, 28, 1144–1152. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z. Involvement of FAK/P38 Signaling Pathways in Mediating the Enhanced Osteogenesis Induced by Nano-Graphene Oxide Modification on Titanium Implant Surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef]
- Cicuéndez, M.; Silva, V.S.; Hortigüela, M.J.; Matesanz, M.C.; Vila, M.; Portolés, M.T. MC3T3-E1 Pre-Osteoblast Response and Differentiation after Graphene Oxide Nanosheet Uptake. Colloids Surf. B Biointerfaces 2017, 158, 33–40. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Li, Z.; Lu, Z.; Li, Y.; Yin, J.; Zhou, Y.; Gao, X.; Fang, Y.; Nie, G.; et al. Unraveling Stress-Induced Toxicity Properties of Graphene Oxide and the Underlying Mechanism. Adv. Mater. 2012, 24, 5391–5397. [Google Scholar] [CrossRef]
- Wang, Q.; Li, M.; Cui, T.; Wu, R.; Guo, F.; Fu, M.; Zhu, Y.; Yang, C.; Chen, B.; Sun, G. A Novel Zwitterionic Hydrogel Incorporated with Graphene Oxide for Bone Tissue Engineering: Synthesis, Characterization, and Promotion of Osteogenic Differentiation of Bone Mesenchymal Stem Cells. Int. J. Mol. Sci. 2023, 24, 2691. [Google Scholar] [CrossRef]
- Ao, Q.; Wang, S.; He, Q.; Ten, H.; Oyama, K.; Ito, A.; He, J.; Javed, R.; Wang, A.; Matsuno, A. Fibrin Glue/Fibronectin/Heparin-Based Delivery System of BMP2 Induces Osteogenesis in MC3T3-E1 Cells and Bone Formation in Rat Calvarial Critical-Sized Defects. ACS Appl. Mater. Interfaces 2020, 12, 13400–13410. [Google Scholar] [CrossRef]
- Bailey, S.; Karsenty, G.; Gundberg, C.; Vashishth, D. Osteocalcin and Osteopontin Influence Bone Morphology and Mechanical Properties. Ann. N. Y. Acad. Sci. 2017, 1409, 79–84. [Google Scholar] [CrossRef]
- Marom, R.; Shur, I.; Solomon, R.; Benayahu, D. Characterization of Adhesion and Differentiation Markers of Osteogenic Marrow Stromal Cells. J. Cell. Physiol. 2005, 202, 41–48. [Google Scholar] [CrossRef]
- Hui, Y.; Yan, Z.; Yang, H.; Xu, X.; Yuan, W.-E.; Qian, Y. Graphene Family Nanomaterials for Stem Cell Neurogenic Differentiation and Peripheral Nerve Regeneration. ACS Appl. Bio Mater. 2022, 5, 4741–4759. [Google Scholar] [CrossRef]
- Bayaraa, O.; Dashnyam, K.; Singh, R.K.; Mandakhbayar, N.; Lee, J.H.; Park, J.-T.; Lee, J.-H.; Kim, H.-W. Nanoceria-GO-Intercalated Multicellular Spheroids Revascularize and Salvage Critical Ischemic Limbs through Anti-Apoptotic and pro-Angiogenic Functions. Biomaterials 2023, 292, 121914. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, S.; Ye, Y.; Wu, Y.; Lin, K.; Su, J. Enhanced Osteogenic Differentiation and Bone Regeneration of Poly(Lactic-Co-Glycolic Acid) by Graphene via Activation of PI3K/Akt/GSK-3β/β-Catenin Signal Circuit. Biomater. Sci. 2018, 6, 1147–1158. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, H.; Fang, Y.; Cao, Y.; Huang, J.; Zhang, M.; Dai, J.; Shi, X.; Zhang, Z. Enhanced Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells on Graphene Oxide-Incorporated Electrospun Poly(Lactic-Co-Glycolic Acid) Nanofibrous Mats. ACS Appl. Mater. Interfaces 2015, 7, 6331–6339. [Google Scholar] [CrossRef]
- Guo, C.; Lu, R.; Wang, X.; Chen, S. Antibacterial activity, bio-compatibility and osteogenic differentiation of graphene oxide coating on 3D-network poly-ether-ether-ketone for orthopaedic implants. J. Mater. Sci. Mater. Med. 2021, 32, 135. [Google Scholar] [CrossRef]
- Sun, N.; Yin, S.; Lu, Y.; Zhang, W.; Jiang, X. Graphene oxide-coated porous titanium for pulp sealing: An antibacterial and dentino-inductive restorative material. J. Mater. Chem. B 2020, 8, 5606–5619. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Tu, Y.; Lv, M.; Xiu, P.; Huynh, T.; Zhang, M.; Castelli, M.; Liu, Z.; Huang, Q.; Fan, C.; Fang, H.; et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nat. Nanotech. 2013, 8, 594–601. [Google Scholar] [CrossRef]
- Alava, T.; Mann, J.A.; Théodore, C.; Benitez, J.J.; Dichtel, W.R.; Parpia, J.M.; Craighead, H.G. Control of the Graphene–Protein Interface Is Required to Preserve Adsorbed Protein Function. Anal. Chem. 2013, 85, 2754–2759. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Pathmanapan, S.; Periyathambi, P.; Anandasadagopan, S.K. Fibrin hydrogel incorporated with graphene oxide functionalized nanocomposite scaffolds for bone repair—In vitro and in vivo study. Nanomed. Nanotechnol. Biol. Med. 2020, 29, 102251. [Google Scholar] [CrossRef] [PubMed]


| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| Vinculin | TGGACGGCAAAGCCATTCC | GCTGGTGGCATATCTCTCTTCAG |
| BMP2 | AACGAGAAAAGCGTCAAGCC | CCAGTCATTCCACCCCACA |
| OCN | CATCTATGGCACCACCGTTTA | CTGTGCCGTCCATACTTTCG |
| OPN | TGGATGAACCAAGCGTGGA | TCGCCTGACTGTCGATAGCA |
| GAPDH | GGCAAGTTCAACGGCACAGT | GCCAGTAGACTCCACGACAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Lai, W.; Wu, J.; Lu, Y. Enhanced Osteogenic Activity and Antibacterial Properties of Graphene Oxide-Poly(Lactic Acid) Films for the Repair of Cranial Defects in Rats. Coatings 2024, 14, 223. https://doi.org/10.3390/coatings14020223
Liu K, Lai W, Wu J, Lu Y. Enhanced Osteogenic Activity and Antibacterial Properties of Graphene Oxide-Poly(Lactic Acid) Films for the Repair of Cranial Defects in Rats. Coatings. 2024; 14(2):223. https://doi.org/10.3390/coatings14020223
Chicago/Turabian StyleLiu, Kai, Wen Lai, Jianyong Wu, and Yongjian Lu. 2024. "Enhanced Osteogenic Activity and Antibacterial Properties of Graphene Oxide-Poly(Lactic Acid) Films for the Repair of Cranial Defects in Rats" Coatings 14, no. 2: 223. https://doi.org/10.3390/coatings14020223
APA StyleLiu, K., Lai, W., Wu, J., & Lu, Y. (2024). Enhanced Osteogenic Activity and Antibacterial Properties of Graphene Oxide-Poly(Lactic Acid) Films for the Repair of Cranial Defects in Rats. Coatings, 14(2), 223. https://doi.org/10.3390/coatings14020223





