Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices
Abstract
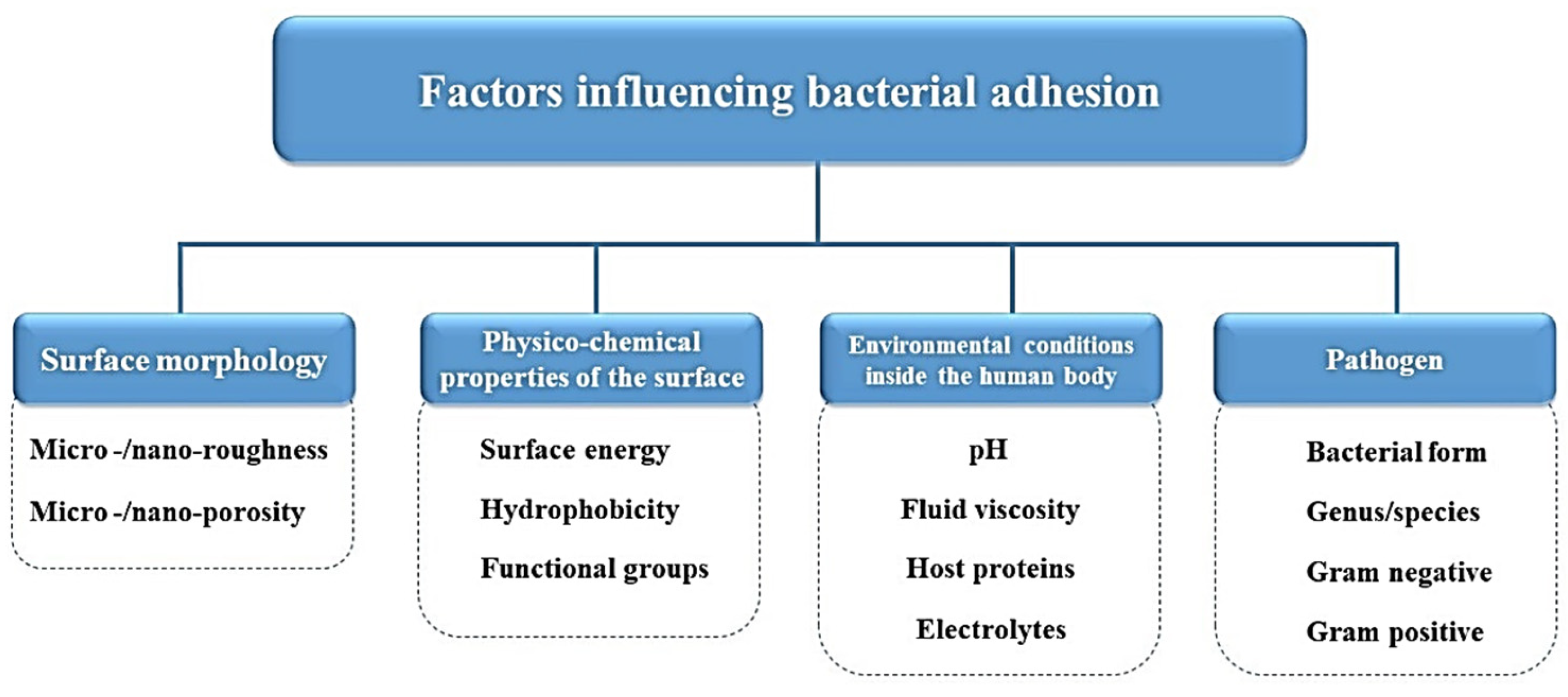
:1. Introduction
2. Infection on Implanted Medical Devices
3. Techniques for the Modification of Medical Device Surfaces
- (a)
- The plasma spraying technique is commercially viable and extensively used as a surface coating method for the orthopedic implants [28,29]. The plasma spraying technique permits the facile control of the coating thickness, and the sample size to be coated is not limited. However, by being a linear process, it limits the uniformity of the coating on a complex shape, and the high functioning temperature can change the structure and performance of the metal substrate [28,29]. Moreover, the bonding strength is limited by the stress concentration at the coating–substrate interface.
- (b)
- Chemical vapor deposition (CVD): The CVD process can deposit thick coatings but necessitates a relatively high temperature. The outcomes of CVD are conformal coatings. This technique does not require solvent use, which results in obtaining a complex coating on solvent-sensitive substrates [30].
- (c)
- Pulsed laser deposition (PLD) and matrix-assisted pulsed laser evaporation (MAPLE): The main advantages of using laser-assisted techniques in the fabrication of thin-film coatings for implants and other medical devices is their ability to control composition and topography, even at the nanoscale [14].
- (d)
- Ion implantation involves the acceleration of ions in an electrical field and impacting them within a solid. Electrons are stripped from the target atoms in order to form ions, which are directed using a region with opposite charge. The energy of the ions must be selected so that they are injected into the near-surface region of the solid [14].
- (e)
- The sol–gel process is as a well-known and reliable coating process. The advantages of this method include composition selection, the fact that it is easy to coat complex geometries, the homogeneity of the coating layer, and the easiness of the process. Also, the range of different compositions that can be produced by the sol–gel method comprises single oxides, mixed oxides, and non-oxides, such as nitrides, borides, and chlorides. The high purity of the compounds and coatings can be maintained, as grinding and high temperatures can be avoided [31].
- (f)
- Deep coating involves three successive stages: dipping, withdrawing, and drying. The substrate is dipped into the solution of interest and then withdrawn at a constant speed, resulting in a good control of the coating thickness and producing no waste. The coatings produced via this technique have a low adhesion strength to the substrate and tend to crack [32].
- (g)
- Electrophoretic deposition is attractive due to the fact that it can be used to fabricate uniform coatings with controlled assets on complex-shaped and porous substrates at ambient temperature and without the requirement for expensive equipment [33].
4. Drug Release from Coatings
Short Overview of Coating Materials Used to Deliver Anti-Infectious Compounds
5. Coatings on Common Medical Devices of the Head
5.1. Coatings on Dental Implants
5.2. Metals and Their Ions in Coatings on Dental Implants
5.3. Antibiotic-Based Coatings
5.4. Coatings on Ocular Prostheses
5.5. Coatings on Contact Lenses
5.6. Coatings on Sinus Stents
5.7. Coatings on Cochlear Implants
6. Challenges in Coating Implantable Devices of the Head
7. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamprou, D.A.; Scoutaris, N.; Ross, S.A.; Douroumis, D. Polymeric Coatings and Their Fabrication for Medical Devices. In Encyclopedia of Biomedical Engineering; Narayan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Liu, Z.; Liu, X.; Ramakrishna, S. Surface engineering of biomaterials in orthopedic and dental implants: Strategies to improve osteointegration, bacteriostatic and bactericidal activities. Biotechnol. J. 2021, 16, 2000116. [Google Scholar] [CrossRef]
- Gherasim, O.; Grumezescu, A.M.; Grumezescu, V.; Iordache, F.; Vasile, B.S.; Holban, A.M. Bioactive Surfaces of Polylactide and Silver Nanoparticles for the Prevention of Microbial Contamination. Materials 2020, 13, 768. [Google Scholar] [CrossRef]
- Polívková, M.; Hubáček, T.; Staszek, M.; Švorčík, V.; Siegel, J. Antimicrobial Treatment of Polymeric Medical Devices by Silver Nanomaterials and Related Technology. Int. J. Mol. Sci. 2017, 18, 419. [Google Scholar] [CrossRef]
- Costerton, J.; Montanaro, L.; Arciola, C. Biofilm in Implant Infections: Its Production and Regulation. Int. J. Artif. Organs 2005, 28, 1062–1068. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yu, Z.; Jin, S.; Liu, C.; Li, Y.; Guo, D.; Hu, M.; Ruan, R.; Liu, Y. Role of surface roughness in the algal short-term cell adhesion and long-term biofilm cultivation under dynamic flow condition. Algal Res. 2020, 46, 101787. [Google Scholar] [CrossRef]
- Manilal, A.; Sabu, K.R.; Shewangizaw, M.; Aklilu, A.; Seid, M.; Merdekios, B.; Tsegaye, B. In vitro antibacterial activity of medicinal plants against biofilm-forming methicillin-resistant Staphylococcus aureus: Efficacy of Moringa stenopetala and Rosmarinus officinalis extracts. Heliyon 2020, 6, e03303. [Google Scholar] [CrossRef] [PubMed]
- Subbiahdoss, G.; Reimhult, E. Biofilm formation at oil-water interfaces is not a simple function of bacterial hydrophobicity. Colloids Surf. B Biointerfaces 2020, 194, 111163. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, A.; Brambilla, E.; Sighinolfi, M.; Mattina, R. A new urinary catheter design reduces in-vitro biofilm formation by influencing hydrodynamics. J. Hosp. Infect. 2021, 114, 153–162. [Google Scholar] [CrossRef]
- Eick, S. Oral Biofilms; Karger Medical and Scientific Publishers: Basel, Switzerland, 2020. [Google Scholar]
- Yuan, Z.; Lin, C.; He, Y.; Tao, B.; Chen, M.; Zhang, J.; Liu, P.; Cai, K. Near-Infrared Light-Triggered Nitric-Oxide-Enhanced Photodynamic Therapy and Low-Temperature Photothermal Therapy for Biofilm Elimination. ACS Nano 2020, 14, 3546–3562. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.-C.; Fufa, O.; Apostol, A.I.; Popescu, D.; Grumezescu, A.M.; Andronescu, E. Chapter 9—Antimicrobial Thin Coatings Prepared by Laser Processing. In Nanostructures for Antimicrobial Therapy; Ficai, A., Grumezescu, A.M., Eds.; In Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2017; pp. 223–236. [Google Scholar] [CrossRef]
- Bisht, K.; Moore, J.L.; Caprioli, R.M.; Skaar, E.P.; Wakeman, C.A. Impact of temperature-dependent phage expression on Pseudomonas aeruginosa biofilm formation. Npj Biofilms Microbiomes 2021, 7, 22. [Google Scholar] [CrossRef]
- Dhar, Y.; Han, Y. Current developments in biofilm treatments: Wound and implant infections. Eng. Regen. 2020, 1, 64–75. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Fu, C.; Wang, Z.; Zhou, X.; Hu, B.; Li, C.; Yang, P. Protein-based bioactive coatings: From nanoarchitectonics to applications. Chem. Soc. Rev. 2024, 53, 1514–1551. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Cordeiro, J.M.; Nagay, B.E.; de Almeida, A.B.; Retamal-Valdes, B.; Nociti, F.H.; Feres, M.; Rangel, E.C.; et al. Targeting Pathogenic Biofilms: Newly Developed Superhydrophobic Coating Favors a Host-Compatible Microbial Profile on the Titanium Surface. ACS Appl. Mater. Interfaces 2020, 12, 10118–10129. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.C.; Nagay, B.E.; Bertolini, M.M.; Costa-Oliveira, B.E.; Sampaio, A.A.; Retamal-Valdes, B.; Shibli, J.A.; Feres, M.; Barão, V.A.; Souza, J.G.S. Fitting pieces into the puzzle: The impact of titanium-based dental implant surface modifications on bacterial accumulation and polymicrobial infections. Adv. Colloid Interface Sci. 2021, 298, 102551. [Google Scholar] [CrossRef]
- Garaicoa, J.L.; Bates, A.M.; Avila-Ortiz, G.; Brogden, K.A. Antimicrobial Prosthetic Surfaces in the Oral Cavity—A Perspective on Creative Approaches. Microorganisms 2020, 8, 1247. [Google Scholar] [CrossRef]
- van Oirschot, B.A.; Zhang, Y.; Alghamdi, H.S.; Cordeiro, J.M.; Nagay, B.E.; Barao, V.A.; de Avila, E.D.; Beucken, J.J.v.D. Surface Engineering for Dental Implantology: Favoring Tissue Responses Along the Implant. Tissue Eng. Part A 2022, 28, 555–572. [Google Scholar] [CrossRef]
- Alipal, J.; Pu’Ad, N.M.; Nayan, N.; Sahari, N.; Abdullah, H.; Idris, M.; Lee, T. An updated review on surface functionalisation of titanium and its alloys for implants applications. Mater. Today Proc. 2021, 42, 270–282. [Google Scholar] [CrossRef]
- Souza, J.C.M.; Sordi, M.B.; Kanazawa, M.; Ravindran, S.; Henriques, B.; Silva, F.S.; Aparicio, C.; Cooper, L.F. Nano-scale modification of titanium implant surfaces to enhance osseointegration. Acta Biomater. 2019, 94, 112–131. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.-D.; Liu, T.-T.; Wang, Q.-Q.; Zhang, J.; Cao, M.-S. Surface Modification and Functionalities for Titanium Dental Implants. ACS Biomater. Sci. Eng. 2023, 9, 4442–4461. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Saruta, J.; Hirota, M.; Taniyama, T.; Sugita, Y.; Kubo, K.; Ishijima, M.; Ikeda, T.; Maeda, H.; Ogawa, T. A Newly Created Meso-, Micro-, and Nano-Scale Rough Titanium Surface Promotes Bone-Implant Integration. Int. J. Mol. Sci. 2020, 21, 783. [Google Scholar] [CrossRef]
- Ke, D.; Robertson, S.F.; Dernell, W.S.; Bandyopadhyay, A.; Bose, S. Effects of MgO and SiO2 on Plasma-Sprayed Hydroxyapatite Coating: An in Vivo Study in Rat Distal Femoral Defects. ACS Appl. Mater. Interfaces 2017, 9, 25731–25737. [Google Scholar] [CrossRef]
- Sun, L.; Berndt, C.C.; Grey, C.P. Phase, structural and microstructural investigations of plasma sprayed hydroxyapatite coatings. Mater. Sci. Eng. A 2003, 360, 70–84. [Google Scholar] [CrossRef]
- Martin, T.; Kooi, S.; Chang, S.; Sedransk, K.; Gleason, K. Initiated chemical vapor deposition of antimicrobial polymer coatings. Biomaterials 2006, 28, 909–915. [Google Scholar] [CrossRef]
- Ben-Nissan, B.; Choi, A.H. Sol-gel production of bioactive nanocoatings for medical applications. Part 1: An introduction. Nanomedicine 2006, 1, 311–319. [Google Scholar] [CrossRef]
- Asri, R.I.M.; Harun, W.S.W.; Hassan, M.A.; Ghani, S.A.C.; Buyong, Z. A review of hydroxyapatite-based coating techniques: Sol-gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. Mater. 2016, 57, 95–108. [Google Scholar] [CrossRef]
- Single-Step Electrochemical Deposition of Antimicrobial Orthopaedic Coatings Based on a Bioactive glass/chitosan/nano-silver Composite System—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1742706113001244?via%3Dihub (accessed on 13 October 2021).
- Ratha, I.; Datta, P.; Balla, V.K.; Nandi, S.K.; Kundu, B. Effect of doping in hydroxyapatite as coating material on biomedical implants by plasma spraying method: A review. Ceram. Int. 2020, 47, 4426–4445. [Google Scholar] [CrossRef]
- Oukach, S.; Hamdi, H.; El Ganaoui, M.; Pateyron, B. Protective Plasma Sprayed Coating forThermo-Sensitive Substrates. MATEC Web Conf. 2020, 307, 01039. [Google Scholar] [CrossRef]
- Walsh, F.C.; Wang, S.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar] [CrossRef]
- Tallawi, M.; Rosellini, E.; Barbani, N.; Cascone, M.G.; Rai, R.; Saint-Pierre, G.; Boccaccini, A.R. Strategies for the chemical and biological functionalization of scaffolds for cardiac tissue engineering: A review. J. R. Soc. Interface 2015, 12, 20150254. Available online: https://royalsocietypublishing.org/doi/full/10.1098/rsif.2015.0254#d3e1886 (accessed on 13 October 2021). [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable Polymeric Drug Delivery Devices: Classification, Manufacture, Materials, and Clinical Applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef]
- Pan, C.; Zhou, Z.; Yu, X. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef]
- Rao, N.V.; Ko, H.; Lee, J.; Park, J.H. Recent Progress and Advances in Stimuli-Responsive Polymers for Cancer Therapy. Front. Bioeng. Biotechnol. 2018, 6, 110. [Google Scholar] [CrossRef]
- Belu, A.; Mahoney, C.; Wormuth, K. Chemical imaging of drug eluting coatings: Combining surface analysis and confocal Raman microscopy. J. Control. Release 2008, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Jahangirian, H.; Lemraski, E.G.; Webster, T.J.; Rafiee-Moghaddam, R.; Abdollahi, Y. A review of drug delivery systems based on nanotechnology and green chemistry: Green nanomedicine. Int. J. Nanomed. 2017, 12, 2957–2978. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. Available online: https://www.mdpi.com/1420-3049/24/6/1117 (accessed on 13 February 2024). [CrossRef]
- Gultepe, E.; Nagesha, D.; Sridhar, S.; Amiji, M. Nanoporous inorganic membranes or coatings for sustained drug delivery in implantable devices. Adv. Drug Deliv. Rev. 2010, 62, 305–315. [Google Scholar] [CrossRef]
- Langer, R. New Methods of Drug Delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Shahid, A.; Aslam, B.; Muzammil, S.; Aslam, N.; Shahid, M.; Almatroudi, A.; Allemailem, K.S.; Saqalein, M.; Nisar, M.A.; Rasool, M.H.; et al. The prospects of antimicrobial coated medical implants. J. Appl. Biomater. Funct. Mater. 2021, 19, 22808000211040304. [Google Scholar] [CrossRef]
- Soares, Í.; Faria, J.; Marques, A.; Ribeiro, I.A.C.; Baleizão, C.; Bettencourt, A.; Ferreira, I.M.M.; Baptista, A.C. Drug Delivery from PCL/Chitosan Multilayer Coatings for Metallic Implants. ACS Omega 2022, 7, 23096–23106. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, S.; Quadir, S.S.; Joshi, G.; Chouhan, M.; Puri, D.; Choudhary, D. Chapter 13—Polymeric (PLGA-based) nanocomposites for application in drug delivery: Current state of the art and forthcoming perspectives. In Bioresorbable Polymers and Their Composites; Verma, D., Okhawilai, M., Goh, K.L., Ramakrishna, S., Pasbakhsh, P., Sharma, M., Eds.; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Cambridge, UK, 2024; pp. 277–324. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Bhandari, P.; Singh, T.; Sharma, S.; Singh, J.; Singh, S. Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications. Molecules 2023, 28, 485. [Google Scholar] [CrossRef]
- del Olmo, J.A.; Pérez-Álvarez, L.; Martínez, V.S.; Cid, S.B.; Ruiz-Rubio, L.; González, R.P.; Vilas-Vilela, J.L.; Alonso, J.M. Multifunctional antibacterial chitosan-based hydrogel coatings on Ti6Al4V biomaterial for biomedical implant applications. Int. J. Biol. Macromol. 2023, 231, 123328. [Google Scholar] [CrossRef]
- Chen, H.; Feng, R.; Xia, T.; Wen, Z.; Li, Q.; Qiu, X.; Huang, B.; Li, Y. Progress in Surface Modification of Titanium Implants by Hydrogel Coatings. Gels 2023, 9, 423. [Google Scholar] [CrossRef]
- Pawar, R.; Pathan, A.; Nagaraj, S.; Kapare, H.; Giram, P.; Wavhale, R. Polycaprolactone and its derivatives for drug delivery. Polym. Adv. Technol. 2023, 34, 3296–3316. [Google Scholar] [CrossRef]
- Borges, M.H.; Nagay, B.E.; Costa, R.C.; Souza, J.G.S.; Mathew, M.T.; Barão, V.A. Recent advances of polypyrrole conducting polymer film for biomedical application: Toward a viable platform for cell-microbial interactions. Adv. Colloid Interface Sci. 2023, 314, 102860. [Google Scholar] [CrossRef]
- Rodríguez-Cendal, A.I.; Gómez-Seoane, I.; de Toro-Santos, F.J.; Fuentes-Boquete, I.M.; Señarís-Rodríguez, J.; Díaz-Prado, S.M. Biomedical Applications of the Biopolymer Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV): Drug Encapsulation and Scaffold Fabrication. Int. J. Mol. Sci. 2023, 24, 11674. [Google Scholar] [CrossRef]
- Brzeziński, M.; Basko, M. Polylactide-Based Materials: Synthesis and Biomedical Applications. Molecules 2023, 28, 1386. [Google Scholar] [CrossRef] [PubMed]
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palumbo, G.; Piccininni, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic Materials for Biomedical Applications: An Overview on Properties and Fabrication Processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Katić, J.; Krivačić, S.; Petrović, Ž.; Mikić, D.; Marciuš, M. Titanium Implant Alloy Modified by Electrochemically Deposited Functional Bioactive Calcium Phosphate Coatings. Coatings 2023, 13, 640. [Google Scholar] [CrossRef]
- Farjam, P.; Luckabauer, M.; de Vries, E.; Rangel, V.; Hekman, E.; Verkerke, G.; Rouwkema, J. Bioactive calcium phosphate coatings applied to flexible poly(carbonate urethane) foils. Surf. Coat. Technol. 2023, 470, 129838. [Google Scholar] [CrossRef]
- Heimann, R.B. Osseoconductive and Corrosion-Inhibiting Plasma-Sprayed Calcium Phosphate Coatings for Metallic Medical Implants. Metals 2017, 7, 468. [Google Scholar] [CrossRef]
- Jirofti, N.; Nakhaei, M.; Ebrahimzadeh, M.H.; Moradi, A. Review on Hydroxyapatite-Based Coatings as Antibiotic Delivery System on Bone Graft Substitution for Controlling Infection in Orthopedic Surgery. J. Polym. Environ. 2023. [Google Scholar] [CrossRef]
- Okereke, C.O.; Onaifo, J.O.; Omorogbe, S.O.; Ogbu, A.I.; Ifijen, I.H. Bioactive Glasses for Bone Repair Application: A Review of Osteointegration and Controlled Ion Release Capabilities. In TMS 2024 153rd Annual Meeting & Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Series; Springer Nature: Cham, Switzerland, 2024; pp. 311–326. [Google Scholar] [CrossRef]
- Jerdioui, S.; Zahraoui, K.; Elansari, L.L.; Bouammali, H.; Zelmimi, N.; Taibi, M.; Moumnassi, S.; Nandiyanto, A.B.D.; Bellaouchi, R.; Sabbahi, R.; et al. Synthesis and Characterization of Phosphocalcic Apatites with Phosphite Ions for Biomedical Applications. Moroc. J. Chem. 2023, 12, 233–248. [Google Scholar] [CrossRef]
- Körtvélyessy, G.; Tarjányi, T.; Baráth, Z.L.; Minarovits, J.; Tóth, Z. Bioactive coatings for dental implants: A review of alternative strategies to prevent peri-implantitis induced by anaerobic bacteria. Anaerobe 2021, 70, 102404. [Google Scholar] [CrossRef]
- Toribio, A.; Mart, H.; Rodr, L.; Ferrero, M.A.; Marrod, T.; Fern, I. In vitro adherence of conjunctival bacteria to different oculoplastic materials. Int. J. Ophthalmol. 2018, 11, 1895–1901. [Google Scholar] [CrossRef] [PubMed]
- Astley, R.A.; Mursalin, H.; Coburn, P.S.; Livingston, E.T.; Nightengale, J.W.; Bagaruka, E.; Hunt, J.J.; Callegan, M.C. Ocular Bacterial Infections: A Ten-Year Survey and Review of Causative Organisms Based on the Oklahoma Experience. Microorganisms 2023, 11, 1802. [Google Scholar] [CrossRef]
- Khan, S.A.; Lee, C.-S. Recent progress and strategies to develop antimicrobial contact lenses and lens cases for different types of microbial keratitis. Acta Biomater. 2020, 113, 101–118. [Google Scholar] [CrossRef]
- Tadj, Z.; Taleb, S. Infectious Complications of Contact Lenses: A Review of the Literature. Med. Technol. J. 2023, 5, 587–593. [Google Scholar] [CrossRef]
- Shariati, A.; Vesal, S.; Khoshbayan, A.; Goudarzi, P.; Darban-Sarokhalil, D.; Razavi, S.; Didehdar, M.; Chegini, Z. Novel strategies for inhibition of bacterial biofilm in chronic rhinosinusitis. J. Appl. Microbiol. 2022, 132, 2531–2546. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Asensio, G.; Vázquez-Lasa, B.; Rojo, L. Achievements in the Topographic Design of Commercial Titanium Dental Implants: Towards Anti-Peri-Implantitis Surfaces. J. Clin. Med. 2019, 8, 1982. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Macedo-De-Sousa, B.; López-Valverde, A.; Ramírez, J.M. Effectiveness of Antibacterial Surfaces in Osseointegration of Titanium Dental Implants: A Systematic Review. Antibiotics 2021, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.-X.; Li, W.; Gu, Y.-Y.; Zhao, D.; Qi, M.-C. Classification and research progress of implant surface antimicrobial techniques. J. Dent. Sci. 2021, 17, 1–7. [Google Scholar] [CrossRef]
- Camargo, S.E.A.; Mohiuddeen, A.S.; Fares, C.; Partain, J.L.; Carey, P.H.; Ren, F.; Hsu, S.-M.; Clark, A.E.; Esquivel-Upshaw, J.F. Anti-Bacterial Properties and Biocompatibility of Novel SiC Coating for Dental Ceramic. J. Funct. Biomater. 2020, 11, 33. [Google Scholar] [CrossRef]
- Birkett, M.; Dover, L.; Lukose, C.C.; Zia, A.W.; Tambuwala, M.M.; Serrano-Aroca, Á. Recent Advances in Metal-Based Antimicrobial Coatings for High-Touch Surfaces. Int. J. Mol. Sci. 2022, 23, 1162. [Google Scholar] [CrossRef]
- Mammoli, F.; Castiglioni, S.; Parenti, S.; Cappadone, C.; Farruggia, G.; Iotti, S.; Davalli, P.; Maier, J.A.; Grande, A.; Frassineti, C. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 2019, 20, 385. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]
- Su, Y.; Wang, K.; Gao, J.; Yang, Y.; Qin, Y.-X.; Zheng, Y.; Zhu, D. Enhanced cytocompatibility and antibacterial property of zinc phosphate coating on biodegradable zinc materials. Acta Biomater. 2019, 98, 174–185. [Google Scholar] [CrossRef]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100122. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Rutkowski, S.; Frueh, J.; Gogolev, A.S.; Chistyakov, S.G.; Gnedenkov, S.V.; Sinebryukhov, S.L.; Frueh, A.; Egorkin, V.S.; Choynzonov, E.L.; et al. Surface Modification of Additively Fabricated Titanium-Based Implants by Means of Bioactive Micro-Arc Oxidation Coatings for Bone Replacement. J. Funct. Biomater. 2022, 13, 285. [Google Scholar] [CrossRef]
- Kozelskaya, A.I.; Rutkowski, S.; Gogolev, A.S.; Chistyakov, S.G.; Krasovsky, I.B.; A Zheravin, A.; Tverdokhlebov, S.I. Bioactive coatings on 3D printed titanium implants with a complex internal structure for bone replacement. J. Phys. Conf. Ser. 2021, 2144, 012015. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A.T. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive Oxygen Species Formed by Metal and Metal Oxide Nanoparticles in Physiological Media—A Review of Reactions of Importance to Nanotoxicity and Proposal for Categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef]
- Ameh, T.; Gibb, M.; Stevens, D.; Pradhan, S.H.; Braswell, E.; Sayes, C.M. Silver and Copper Nanoparticles Induce Oxidative Stress in Bacteria and Mammalian Cells. Nanomaterials 2022, 12, 2402. [Google Scholar] [CrossRef] [PubMed]
- Fasnacht, M.; Polacek, N. Oxidative Stress in Bacteria and the Central Dogma of Molecular Biology. Front. Mol. Biosci. 2021, 8, 671037. Available online: https://www.frontiersin.org/articles/10.3389/fmolb.2021.671037 (accessed on 13 February 2024). [CrossRef]
- Salah, I.; Parkin, I.P.; Allan, E. Copper as an antimicrobial agent: Recent advances. RSC Adv. 2021, 11, 18179–18186. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.K.; Koo, H.C.; Kim, K.W.; Shin, S.; Kim, S.H.; Park, Y.H. Antibacterial Activity and Mechanism of Action of the Silver Ion in Staphylococcus aureus and Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 2171–2178. [Google Scholar] [CrossRef] [PubMed]
- Nešporová, K.; Pavlík, V.; Šafránková, B.; Vágnerová, H.; Odráška, P.; Žídek, O.; Císařová, N.; Skoroplyas, S.; Kubala, L.; Velebný, V. Effects of wound dressings containing silver on skin and immune cells. Sci. Rep. 2020, 10, 15216. [Google Scholar] [CrossRef] [PubMed]
- Pavlík, V.; Sobotka, L.; Pejchal, J.; Čepa, M.; Nešporová, K.; Arenbergerová, M.; Mrózková, A.; Velebný, V. Silver distribution in chronic wounds and the healing dynamics of chronic wounds treated with dressings containing silver and octenidine. FASEB J. 2021, 35, e21580. [Google Scholar] [CrossRef] [PubMed]
- Di, H.; Qiaoxia, L.; Yujie, Z.; Jingxuan, L.; Yan, W.; Yinchun, H.; Xiaojie, L.; Song, C.; Weiyi, C. Ag nanoparticles incorporated tannic acid/nanoapatite composite coating on Ti implant surfaces for enhancement of antibacterial and antioxidant properties. Surf. Coat. Technol. 2020, 399, 126169. [Google Scholar] [CrossRef]
- Odatsu, T.; Kuroshima, S.; Sato, M.; Takase, K.; Valanezhad, A.; Naito, M.; Sawase, T. Antibacterial Properties of Nano-Ag Coating on Healing Abutment: An In Vitro and Clinical Study. Antibiotics 2020, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ueda, K.; Narushima, T. Fabrication of Ag and Ta co-doped amorphous calcium phosphate coating films by radiofrequency magnetron sputtering and their antibacterial activity. Mater. Sci. Eng. C 2019, 109, 110599. [Google Scholar] [CrossRef]
- Mokabber, T.; Cao, H.T.; Norouzi, N.; van Rijn, P.; Pei, Y.T. Antimicrobial Electrodeposited Silver-Containing Calcium Phosphate Coatings. ACS Appl. Mater. Interfaces 2020, 12, 5531–5541. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram, M.; Coryn, R.; Asadian, M.; Tabaei, P.S.E.; Rigole, P.; Rajendhran, N.; Nikiforov, A.; Sukumaran, J.; Coenye, T.; Van Der Voort, P.; et al. Fabrication of Microporous Coatings on Titanium Implants with Improved Mechanical, Antibacterial, and Cell-Interactive Properties. ACS Appl. Mater. Interfaces 2020, 12, 30155–30169. [Google Scholar] [CrossRef]
- Guo, C.; Cui, W.; Wang, X.; Lu, X.; Zhang, L.; Li, X.; Li, W.; Zhang, W.; Chen, J. Poly-l-lysine/Sodium Alginate Coating Loading Nanosilver for Improving the Antibacterial Effect and Inducing Mineralization of Dental Implants. ACS Omega 2020, 5, 10562–10571. [Google Scholar] [CrossRef]
- Chu, G.; Zhang, C.; Liu, Y.; Cao, Z.; Wang, L.; Chen, Y.; Zhou, W.; Gao, G.; Wang, K.; Cui, D. A Gold Nanocluster Constructed Mixed-Metal Metal–Organic Network Film for Combating Implant-Associated Infections. ACS Nano 2020, 14, 15633–15645. [Google Scholar] [CrossRef]
- Karthik, R.; Thambidurai, S. Synthesis of cobalt doped ZnO/reduced graphene oxide nanorods as active material for heavy metal ions sensor and antibacterial activity. J. Alloy Compd. 2017, 715, 254–265. [Google Scholar] [CrossRef]
- Abdulkareem, E.H.; Memarzadeh, K.; Allaker, R.; Huang, J.; Pratten, J.; Spratt, D. Anti-biofilm activity of zinc oxide and hydroxyapatite nanoparticles as dental implant coating materials. J. Dent. 2015, 43, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Dubey, P.; Ray, S.; Jayaganthan, R.; Pant, A.B.; Chandra, R. Co-sputtered Antibacterial and Biocompatible Nanocomposite Titania-Zinc Oxide thin films on Si substrates for Dental Implant applications. Mater. Technol. 2018, 34, 32–42. [Google Scholar] [CrossRef]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and Antibiofilm Coating of Dental Implants—Past and New Perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Abdulghafor, M.A.; Mahmood, M.K.; Tassery, H.; Tardivo, D.; Falguiere, A.; Lan, R. Biomimetic Coatings in Implant Dentistry: A Quick Update. J. Funct. Biomater. 2023, 15, 15. [Google Scholar] [CrossRef] [PubMed]
- Hickok, N.J.; Shapiro, I.M. Immobilized antibiotics to prevent orthopaedic implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Braun, B.M.; Skelly, J.D.; Ayers, D.C.; Song, J. Significant Suppression of Staphylococcus aureus Colonization on Intramedullary Ti6Al4V Implants Surface-Grafted with Vancomycin-Bearing Polymer Brushes. ACS Appl. Mater. Interfaces 2019, 11, 28641–28647. [Google Scholar] [CrossRef]
- Boot, W.; Vogely, H.; Nikkels, P.; Pouran, B.; van Rijen, M.; Ekkelenkamp, M.; Hänsch, G.; Dhert, W.; Gawlitta, D. Prophylaxis of implant-related infections by local release of vancomycin from a hydrogel in rabbits. Eur. Cells Mater. 2020, 39, 108–120. [Google Scholar] [CrossRef]
- Song, W.; Xiao, Y. Sequential drug delivery of vancomycin and rhBMP-2 via pore-closed PLGA microparticles embedded photo-crosslinked chitosan hydrogel for enhanced osteointegration. Int. J. Biol. Macromol. 2021, 182, 612–625. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, S.; Lan, S.; Xiong, H.; Tao, B.; Ding, Y.; Liu, Y.; Liu, P.; Cai, K. Surface engineering of titanium implants with enzyme-triggered antibacterial properties and enhanced osseointegration in vivo. J. Mater. Chem. B 2018, 6, 8090–8104. [Google Scholar] [CrossRef]
- Yang, K.; Xin, S.-S.; Qu, H.-Y.; An, G.; Wu, X.-F.; Li, S.-Q.; Gao, L.; Cui, L.-Y.; Zhi, K.-Q. Gentamicin loaded polyelectrolyte multilayers and strontium doped hydroxyapatite composite coating on Ti-6Al-4V alloy: Antibacterial ability and biocompatibility. Mater. Technol. 2021, 37, 1478–1485. [Google Scholar] [CrossRef]
- Wang, J.; Wu, G.; Liu, X.; Sun, G.; Li, D.; Wei, H. A decomposable silica-based antibacterial coating for percutaneous titanium implant. Int. J. Nanomed. 2017, 12, 371–379. [Google Scholar] [CrossRef]
- Stevanović, M.; Djošić, M.; Janković, A.; Kojić, V.; Vukašinović-Sekulić, M.; Stojanović, J.; Odović, J.; Sakač, M.C.; Yop, R.K.; Mišković-Stanković, V. Antibacterial graphene-based hydroxyapatite/chitosan coating with gentamicin for potential applications in bone tissue engineering. J. Biomed. Mater. Res. Part A 2020, 108, 2175–2189. [Google Scholar] [CrossRef]
- Ren, X.; van der Mei, H.C.; Ren, Y.; Busscher, H.J.; Peterson, B.W. Antimicrobial loading of nanotubular titanium surfaces favoring surface coverage by mammalian cells over bacterial colonization. Mater. Sci. Eng. C 2021, 123, 112021. [Google Scholar] [CrossRef]
- Geuli, O.; Metoki, N.; Zada, T.; Reches, M.; Eliaz, N.; Mandler, D. Synthesis, coating, and drug-release of hydroxyapatite nanoparticles loaded with antibiotics. J. Mater. Chem. B 2017, 5, 7819–7830. [Google Scholar] [CrossRef]
- Bourgat, Y.; Mikolai, C.; Stiesch, M.; Klahn, P.; Menzel, H. Enzyme-Responsive Nanoparticles and Coatings Made from Alginate/Peptide Ciprofloxacin Conjugates as Drug Release System. Antibiotics 2021, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Wongsuwan, N.; Dwivedi, A.; Tancharoen, S.; Nasongkla, N. Development of dental implant coating with minocycline-loaded niosome for antibacterial application. J. Drug Deliv. Sci. Technol. 2020, 56, 101555. [Google Scholar] [CrossRef]
- Kazek-Kęsik, A.; Nosol, A.; Płonka, J.; Śmiga-Matuszowicz, M.; Student, S.; Brzychczy-Włoch, M.; Krok-Borkowicz, M.; Pamuła, E.; Simka, W. Physico-chemical and biological evaluation of doxycycline loaded into hybrid oxide-polymer layer on Ti–Mo alloy. Bioact. Mater. 2020, 5, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Patianna, G.; Valente, N.A.; D’Addona, A.; Andreana, S. In vitro evaluation of controlled-release 14% doxycycline gel for decontamination of machined and sandblasted acid-etched implants. J. Periodontol. 2018, 89, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.F.; Babu, J.; Hamlekhan, A.; Patel, S.; Shokuhfar, T. Efficiency of Nanotube Surface-Treated Dental Implants Loaded with Doxycycline on Growth Re-duction of Porphyromonas gingivalis. Int. J. Oral Maxillofac. Implant. 2017, 32, 322–328. Available online: https://web.a.ebscohost.com/abstract?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=08822786&asa=Y&AN=122030482&h=%2bwxO73LRG66Spcm9OUZ3f2gftntSSfddVlAa3I9MiQsAyOSVSvyn0xkTafBj5Dd9O95Kupf1tRvZAglegNnFfQ%3d%3d&crl=c&resultNs=AdminWebAuth&resultLocal=ErrCrlNotAuth&crlhashurl=login.aspx%3fdirect%3dtrue%26profile%3dehost%26scope%3dsite%26authtype%3dcrawler%26jrnl%3d08822786%26asa%3dY%26AN%3d122030482 (accessed on 13 October 2021). [CrossRef] [PubMed]
- Nastri, L.; De Rosa, A.; De Gregorio, V.; Grassia, V.; Donnarumma, G. A New Controlled-Release Material Containing Metronidazole and Doxycycline for the Treatment of Periodontal and Peri-Implant Diseases: Formulation and In Vitro Testing. Int. J. Dent. 2019, 2019, 9374607. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, L.D.; Peres, B.U.; Maezono, H.; Shen, Y.; Haapasalo, M.; Jackson, J.; Carvalho, R.M.; Manso, A.P. Doxycycline release and antibacterial activity from PMMA/PEO electrospun fiber mats. J. Appl. Oral Sci. 2019, 27, e20180663. [Google Scholar] [CrossRef] [PubMed]
- de Avila, E.D.; Castro, A.G.B.; Tagit, O.; Krom, B.P.; Löwik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J.J.J.P. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Shahi, R.G.; Albuquerque, M.T.P.; Münchow, E.A.; Blanchard, S.B.; Gregory, R.L.; Bottino, M.C. Novel bioactive tetracycline-containing electrospun polymer fibers as a potential antibacterial dental implant coating. Odontology 2017, 105, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, J.; Sun, Z.; Zheng, F.; Liu, C.; Wang, C.; Hu, X.; Xia, L.; Liu, Z.; Xia, R. Decreased Porphyromonas gingivalis adhesion and improved biocompatibility on tetracycline-loaded TiO2 nanotubes: An in vitro study. Int. J. Nanomed. 2018, 13, 6769–6777. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-Y.; Kim, K.-M.; Kwon, J.-S. Antibacterial and Osteogenic Activity of Titania Nanotubes Modified with Electrospray-Deposited Tetracycline Nanoparticles. Nanomaterials 2020, 10, 1093. [Google Scholar] [CrossRef]
- Iwańczyk, B.; Wychowański, P.; Minkiewicz-Zochniak, A.; Strom, K.; Jarzynka, S.; Olędzka, G. Bioactive Healing Abutment as a Potential Tool for the Treatment of Peri-Implant Disease—In Vitro Study. Appl. Sci. 2020, 10, 5376. [Google Scholar] [CrossRef]
- Skelly, J.D.; Chen, F.; Chang, S.-Y.; Ujjwal, R.R.; Ghimire, A.; Ayers, D.C.; Song, J. Modulating On-Demand Release of Vancomycin from Implant Coatings via Chemical Modification of a Micrococcal Nuclease-Sensitive Oligonucleotide Linker. ACS Appl. Mater. Interfaces 2023, 15, 37174–37183. [Google Scholar] [CrossRef]
- Thamvasupong, P.; Viravaidya-Pasuwat, K. Controlled Release Mechanism of Vancomycin from Double-Layer Poly-L-Lactic Acid-Coated Implants for Prevention of Bacterial Infection. Polymers 2022, 14, 3493. [Google Scholar] [CrossRef]
- Alenezi, A. The Antibacterial Performance of Implant Coating Made of Vancomycin-Loaded Polymer Material: An In Vitro Study. Surfaces 2023, 6, 304–315. [Google Scholar] [CrossRef]
- Jackson, J.; Lo, J.; Hsu, E.; Burt, H.M.; Shademani, A.; Lange, D. The Combined Use of Gentamicin and Silver Nitrate in Bone Cement for a Synergistic and Extended Antibiotic Action against Gram-Positive and Gram-Negative Bacteria. Materials 2021, 14, 3413. [Google Scholar] [CrossRef]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The antimicrobial activity and biocompatibility of a controlled gentamicin-releasing single-layer sol-gel coating on hydroxyapatite-coated titanium. Bone Jt. J. 2021, 103-B, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.A.R.; Roohi, S.; Iqbal, A.; Sherazi, T.A.; Zahoor, A.F.; Imran, M. Ciprofloxacin: From infection therapy to molecular imaging. Mol. Biol. Rep. 2018, 45, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, J.; Jelonek, K.; Jaworska-Kik, M.; Musiał-Kulik, M.; Marcinkowski, A.; Szewczenko, J.; Kajzer, W.; Pastusiak, M.; Kasperczyk, J. Development of antibacterial, ciprofloxacin-eluting biodegradable coatings on Ti6Al7Nb implants to prevent peri-implant infections. J. Biomed. Mater. Res. Part A 2020, 108, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Hajinaebi, M.; Ganjali, M.; Nasab, N.A. Antibacterial Activity and Drug Release of Ciprofloxacin Loaded PVA-nHAp Nanocomposite Coating on Ti-6Al-4 V. J. Inorg. Organomet. Polym. Mater. 2022, 32, 3521–3532. [Google Scholar] [CrossRef]
- Yoon, S.-W.; Kim, M.-J.; Paeng, K.-W.; Yu, K.A.; Lee, C.-K.; Song, Y.W.; Cha, J.-K.; Sanz, M.; Jung, U.-W. Locally Applied Slow-Release of Minocycline Microspheres in the Treatment of Peri-Implant Mucositis: An Experimental In Vivo Study. Pharmaceutics 2020, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Bidell, M.R.; Lodise, T.P. Use of oral tetracyclines in the treatment of adult outpatients with skin and skin structure infections: Focus on doxycycline, minocycline, and omadacycline. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2021, 41, 915–931. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khanna, D.; Kalra, S. Minocycline and Doxycycline: More Than Antibiotics. Curr. Mol. Pharmacol. 2021, 14, 1046–1065. [Google Scholar] [CrossRef]
- Ding, L.; Zhang, P.; Wang, X.; Kasugai, S. A doxycycline-treated hydroxyapatite implant surface attenuates the progression of peri-implantitis: A radiographic and histological study in mice. Clin. Implant. Dent. Relat. Res. 2019, 21, 154–159. [Google Scholar] [CrossRef]
- Alécio, A.B.W.; Ferreira, C.F.; Babu, J.; Shokuhfar, T.; Jo, S.; Magini, R.; Garcia-Godoy, F. Doxycycline Release of Dental Implants with Nanotube Surface, Coated with Poly Lactic-Co-Glycolic Acid for Extended pH-Controlled Drug Delivery. J. Oral Implant. 2019, 45, 267–273. [Google Scholar] [CrossRef]
- Meng, M.; Chen, Y.; Ren, H.; Zhang, Q.; Chen, S.; Zhou, X.; Zou, J. Effect of tetracyclines on pulpal and periodontal healing after tooth replantation: A systematic review of human and animal studies. BMC Oral Health 2021, 21, 289. [Google Scholar] [CrossRef]
- Koev, K.; Donkov, N.; Stankova, N.; Moraliiski, E.; Najdenski, H.; Nurgaliev, T.; Zaharieva, M.; Avramov, L. Application of Silver Antibacterial and Antifungal Nanolayers for Ocular Prostheses Coating. Phys. Status Solidi A 2019, 216, 1800695. [Google Scholar] [CrossRef]
- Baino, F.; Perero, S.; Miola, M.; Ferraris, M. Antibacterial Nanocoatings for Ocular Applicationsin. In Advances in Science and Technology; Trans Tech Publ: Beach, Switzerland, 2017; pp. 24–28. [Google Scholar]
- Kazemzadeh-Narbat, M.; Cheng, H.; Chabok, R.; Alvarez, M.M.; de la Fuente-Nunez, C.; Phillips, K.S.; Khademhosseini, A. Strategies for antimicrobial peptide coatings on medical devices: A review and regulatory science perspective. Crit. Rev. Biotechnol. 2020, 41, 94–120. [Google Scholar] [CrossRef]
- Arshad, M.; Carnt, N.; Tan, J.; Ekkeshis, I.; Stapleton, F. Water exposure and the risk of contact lens–related disease. Cornea 2019, 38, 791–797. [Google Scholar] [CrossRef]
- Rad, M.S.; Khameneh, B.; Sabeti, Z.; Mohajeri, S.A.; Bazzaz, B.S.F. Antibacterial activity of silver nanoparti-cle-loaded soft contact lens materials: The effect of monomer composition. Curr. Eye Res. 2016, 41, 1286–1293. [Google Scholar]
- Casciaro, B.; Dutta, D.; Loffredo, M.R.; Marcheggiani, S.; McDermott, A.M.; Willcox, M.D.; Mangoni, M.L. Esculentin-1a derived peptides kill Pseudomonas aeruginosa biofilm on soft contact lenses and retain antibacterial activity upon immobilization to the lens surface. Pept. Sci. 2018, 110, e23074. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, J.; Qu, C.; Bo, G.; Jiang, L.; Zhao, H.; Zhang, J.; Lin, Y.; Hua, Y.; Yang, P.; et al. A mussel-inspired facile method to prepare multilayer-AgNP-loaded contact lens for early treatment of bacterial and fungal keratitis. ACS Biomater. Sci. Eng. 2018, 4, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Hoyo, J.; Ivanova, K.; Guaus, E.; Tzanov, T. Multifunctional ZnO NPs-chitosan-gallic acid hybrid nanocoating to overcome contact lenses associated conditions and discomfort. J. Colloid Interface Sci. 2019, 543, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Bin Sahadan, M.Y.; Tong, W.Y.; Tan, W.N.; Leong, C.R.; Bin Misri, M.N.; Chan, M.; Cheng, S.Y.; Shaharuddin, S. Phomopsidione nanoparticles coated contact lenses reduce microbial keratitis causing pathogens. Exp. Eye Res. 2018, 178, 10–14. [Google Scholar] [CrossRef]
- Jadhav, R.L.; Sonwalkar, S.G.; Patil, M.V.; Shaikh, S.N.; Belhekar, S.N. Formulation and Characterization of Poly Sulfoxyamine Grafted Chitosan Coated Contact Lens. J. Pharm. Res. Int. 2020, 32, 49–57. [Google Scholar] [CrossRef]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Serro, A.P.; Saramago, B. Antibacterial layer-by-layer coatings to control drug release from soft contact lenses material. Int. J. Pharm. 2018, 553, 186–200. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Mahmood, T.; Lee, C.-S. Contact lenses coated with hybrid multifunctional ternary nanocoatings (Phytomolecule-coated ZnO nanoparticles:Gallic Acid:Tobramycin) for the treatment of bacterial and fungal keratitis. Acta Biomater. 2021, 128, 262–276. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, K.; Wang, H.; Ma, L.; Yu, L.; Nie, Y. Stable Fabrication of Zwitterionic Coating Based on Copper-Phenolic Networks on Contact Lens with Improved Surface Wettability and Broad-Spectrum Antimicrobial Activity. ACS Appl. Mater. Interfaces 2020, 12, 16125–16136. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.; de Sousa, H.C.; Gil, M.H.; Santos, L.F.; Moutinho, G.M.; Salema-Oom, M.; Alvarez-Lorenzo, C.; Serro, A.P.; Saramago, B. Diclofenac sustained release from sterilised soft contact lens materials using an optimised layer-by-layer coating. Int. J. Pharm. 2020, 585, 119506. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef]
- Bachert, C.; Holtappels, G. Pathophysiology of chronic rhinosinusitis, pharmaceutical therapy options. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2015, 14, Doc09. [Google Scholar]
- Orlandi, R.R.; Kingdom, T.T.; Hwang, P.H.; Smith, T.L.; Alt, J.A.; Baroody, F.M.; Batra, P.S.; Bernal-Sprekelsen, M.; Bhattacharyya, N.; Chandra, R.K.; et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6 (Suppl. S1), S22–S209. [Google Scholar] [CrossRef]
- Lim, D.; Skinner, D.; Mclemore, J.; Rivers, N.; Elder, J.B.; Allen, M.; Koch, C.; West, J.; Zhang, S.; Thompson, H.M.; et al. In-vitro evaluation of a ciprofloxacin and azithromycin sinus stent for Pseudomonas aeruginosa biofilms. Int. Forum Allergy Rhinol. 2020, 10, 121–127. [Google Scholar] [CrossRef]
- Kumar, S.; Chandra, N.; Singh, L.; Hashmi, M.Z.; Varma, A. Biofilms in Human Diseases: Treatment and Control; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Natan, M.; Edin, F.; Perkas, N.; Yacobi, G.; Perelshtein, I.; Segal, E.; Homsy, A.; Laux, E.; Keppner, H.; Rask-Andersen, H.; et al. Two are Better than One: Combining ZnO and MgF2 Nanoparticles Reduces Streptococcus pneumoniae and Staphylococcus aureus Biofilm Formation on Cochlear Implants. Adv. Funct. Mater. 2016, 26, 2473–2481. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Z.; Chen, H.; Pang, B.; Cai, H.; Chen, Z.; Ning, C.; Ma, D.; Tang, J.; Zhang, H. Zwitterion modified cochlear implants resist postoperative infection and inflammation. Mater. Today Bio 2023, 23, 100856. [Google Scholar] [CrossRef]
- Cozma, V.; Rosca, I.; Radulescu, L.; Martu, C.; Nastasa, V.; Varganici, C.-D.; Ursu, E.-L.; Doroftei, F.; Pinteala, M.; Racles, C. Antibacterial Polysiloxane Polymers and Coatings for Cochlear Implants. Molecules 2021, 26, 4892. [Google Scholar] [CrossRef]
- Brady, A.J.; Laverty, G.; Gilpin, D.F.; Kearney, P.; Tunney, M. Antibiotic susceptibility of planktonic- and biofilm-grown staphylococci isolated from implant-associated infections: Should MBEC and nature of biofilm formation replace MIC? J. Med. Microbiol. 2017, 66, 461–469. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, H.; Guo, B.; Dong, R.; Qiu, Y.; Ma, P.X. Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 2017, 122, 34–47. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, X.; Lai, H.; Zhang, X. Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants. J. Funct. Biomater. 2022, 13, 173. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Elashnikov, R.; Ulbrich, P.; Vokatá, B.; Pavlíčková, V.S.; Švorčík, V.; Lyutakov, O.; Rimpelová, S. Physically Switchable Antimicrobial Surfaces and Coatings: General Concept and Recent Achievements. Nanomaterials 2021, 11, 3083. [Google Scholar] [CrossRef]
- Rajput, V.S.; Bhinder, J. Advanced Materials for Biomedical Applications: Development and Processing; Springer Nature: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Souza, J.G.S.; Bertolini, M.M.; Costa, R.C.; Nagay, B.E.; Dongari-Bagtzoglou, A.; Barão, V.A.R. Targeting implant-associated infections: Titanium surface loaded with antimicrobial. iScience 2020, 24, 102008. [Google Scholar] [CrossRef]
- Mas-Moruno, C.; Su, B.; Dalby, M.J. Multifunctional Coatings and Nanotopographies: Toward Cell Instructive and Antibacterial Implants. Adv. Health Mater. 2018, 8, 1801103. [Google Scholar] [CrossRef] [PubMed]
- Carnicer-Lombarte, A.; Chen, S.-T.; Malliaras, G.G.; Barone, D.G. Foreign Body Reaction to Implanted Biomaterials and Its Impact in Nerve Neuroprosthetics. Front. Bioeng. Biotechnol. 2021, 9, 622524. Available online: https://www.frontiersin.org/articles/10.3389/fbioe.2021.622524 (accessed on 14 February 2024). [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Dolan, E.B.; Varela, C.E.; Mendez, K.; Whyte, W.; Levey, R.E.; Robinson, S.T.; Maye, E.; O’dwyer, J.; Beatty, R.; Rothman, A.; et al. An actuatable soft reservoir modulates host foreign body response. Sci. Robot. 2019, 4, eaax7043. [Google Scholar] [CrossRef]
- Lamichhane, S.; Anderson, J.A.; Vierhout, T.; Remund, T.; Sun, H.; Kelly, P. Polytetrafluoroethylene topographies determine the adhesion, activation, and foreign body giant cell formation of macrophages. J. Biomed. Mater. Res. Part A 2017, 105, 2441–2450. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, Z.; Bai, T.; Carr, L.; Ella-Menye, J.-R.; Irvin, C.; Ratner, B.D.; Jiang, S. Zwitterionic hydrogels implanted in mice resist the foreign-body reaction. Nat. Biotechnol. 2013, 31, 553–556. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous Implants Modulate Healing and Induce Shifts in Local Macrophage Polarization in the Foreign Body Reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Doloff, J.C.; Ma, M.; Vegas, A.J.; Tam, H.H.; Bader, A.R.; Li, J.; Langan, E.; Wyckoff, J.; Loo, W.S.; et al. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- A Stewart, S.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Evaluation of sterilisation techniques for 3D-printed implantable devices. RPS Pharm. Pharmacol. Rep. 2023, 2, rqad003. [Google Scholar] [CrossRef]
- Tipnis, N.P.; Burgess, D.J. Sterilization of implantable polymer-based medical devices: A review. Int. J. Pharm. 2018, 544, 455–460. [Google Scholar] [CrossRef] [PubMed]


| Material | Advantage/Activity | Ref. |
|---|---|---|
| Calcium phosphate (CP) | Good osseointegration rate; corrosion resistance; cell adhesion | [58,59,60] |
| Hydroxyapatite (HA) | Cell adhesion and proliferation; enhanced osteo-conductivity and -integration | [61] |
| Bioactive glasses (BGs) | Excellent osteo-conductivity and -inductivity properties | [62] |
| Medical Device | Type of Infection | Common Pathogens | Concern | Ref. |
|---|---|---|---|---|
| Dental implants | Peri-implantitis, biofilm-associated infections, mucositis | S. aureus, P. aeruginosa, E. coli, Porphyromonas gingivalis | Biofilm formation leads to chronic infections, resistance to antibiotics, and challenges in achieving effective sterilization. | [64] |
| Ocular prostheses | Endophthalmitis, conjunctivitis | S. epidermidis, S. aureus, Serratia marcescens | Biofilm formation on prostheses surfaces leads to persistent infections and inflammation. | [65,66] |
| Contact lenses | Microbial keratitis, corneal ulcers | P. aeruginosa, S. aureus, Acanthamoeba spp., Fusarium spp. | The risk of infection increases with poor hygiene practices, overnight wear, and the use of contaminated solutions. | [67,68] |
| Sinus stents | Sinusitis, biofilm-associated infections | S. aureus, P. aeruginosa, S. epidermidis | Biofilms can form on stents, leading to chronic inflammation and the potential need for surgical intervention | [69] |
| Element | Modified Material | Coating Method | Pathogen(s) | Ref. |
|---|---|---|---|---|
| Silver (Ag) | Ag-hydroxyapatite-tannic acid | Immersion | E. coli S. aureus | [90] |
| Nano-Ag | Microwave-assisted synthesis | S. aureus | [91] | |
| Ag- and Ta-co-doped amorphous calcium phosphate | Radio frequency magnetron sputtering | E. coli | [92] | |
| Ag-containing calcium phosphate | Electrodeposition | S. aureus | [93] | |
| TiO2 coatings enriched with Ca, P, and Ag | Plasma electrolytic oxidation | E. coli | [94] | |
| Poly-L-lysine/sodium alginate loading nano-Ag | Polyelectrolyte electrostatic self-assembly and reduction of Ag with dopamine | S. aureus S. mutans | [95] | |
| Gold (Au) | Au nanocluster constructed mixed-metal metal–organic network | Metal−ligand coordination-driven and solvent evaporation-induced self-assembly | E. coli | [96] |
| Cobalt (Co) | Co-doped ZnO/reduced graphene oxide nanorods | Chemical co-precipitation | E. coli S. aureus | [97] |
| Zinc (Zn) | NPs of zinc oxide (nZnO) and hydroxyapatite (nHA) | Electrohydrodynamic deposition | Streptococcus spp. | [98] |
| Co-sputtered titania(Ti)-Zn-oxide nanocomposite | Sputtering | E. coli S. aureus | [99] |
| Drug | Tested Pathogen | Ref. |
|---|---|---|
| Vancomycin | S. aureus | [104,105,106,107] |
| Gentamicin | E. coli, S. aureus | [107,108] |
| S. aureus | [109,110] | |
| S. aureus, S. epidermidis, P. aeruginosa | [111] | |
| P. aeruginosa | [112] | |
| Ciprofloxacin | S. aureus | [113] |
| P.aeruginosa | [112] | |
| Minocycline | Porphylomonas gingivalis | [114] |
| Doxycycline | S. aureus, S. epidermidis | [115] |
| Streptococcus sanguinis | [116], p. 14 | |
| Porphyromonas gingivalis | [117] | |
| A. actinomycetemcomitans, S. sanguinis, P. micra, E. corrodens | [118] | |
| S. mutans | [119] | |
| Tetracycline | Porphyromonas gingivalis | [120] |
| Porphyromonas gingivalis, Fusobacterium nucleatum, Prevotella intermedia, Aggregatibacter actinomycetemcomitans | [121] | |
| Porphyromonas gingivalis | [122] | |
| S. aureus | [123] | |
| S. aureus, S. epidermidis | [124] |
| Coating | Substrate | Method | Microorganism | Ref. |
|---|---|---|---|---|
| Ag | Weflex 55 hydrogel | Adsorption | P. aeruginosa, S. aureus | [143,144] |
| Skin-derived antimicrobial peptide Esc(1–21) and its diastereomer Esc(1–21)-1c | Soft contact lenses | Covalent immobilization | P. aeruginosa | [80] |
| Ag NPs | Hydrogel (soft contact lens) | Incorporated collagen hydrogels | [145] | |
| ZnO, chitosan, and gallic acid | Comfilcon A (silicone-hydrogel) | Sonochemical coating | S. aureus | [146] |
| Phomopsidione NPs | ACUVUE® TrueEye™ (silicone hydrogel) | Soaking | S. marcescens, P. aeruginosa, MRSA, P. mirabilis, C. utilis | [147] |
| Chloro sulfoxy chitosan | Ophthalmic lenses | Soaking | P. aeruginosa | [148] |
| Moxifloxacin hydrochloride, chlorhexidine diacetate monohydrate, diclofenac sodium salt | Silicone-based hydrogel (soft contact lens) | Layer-by-layer deposition technique | P. aeruginosa, S. aureus | [149] |
| Gallic acid (GA) phytomolecule-coated zinc oxide NPs (ZN), phytomolecule-coated ZN + GA + tobramycin | Methafilcon A (CooperVision, San Ramon, CA, USA) | Sonochemical method | S. aureus, P. aeruginosa, E. coli, Aspergillus, fumigatus Fusarium solani | [150] |
| Zwitterionic metal–phenolic networks (MPNs) based on the coordination of copper ions (CuII) and a poly(carboxylbetaine-co-dopamine methacrylamide) copolymer | Aqua Moist (Hydron Contact Lens Co., Shanghai, China) | One-step method due to MPN structure with enhanced adhesive property bestowed by CuII cross-linked catechol groups | E. coli, P. aeruginosa S. aureus, | [151] |
| Ionic polysaccharides (chitosan, sodium alginate, sodium hyaluronate) and genipin (crosslinker) to sustain the release of diclofenac sodium salt | Silicone-based hydrogel SofLens Purevision | Layer-by-layer deposition technique | P. aeruginosa, S. aureus | [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negut, I.; Albu, C.; Bita, B. Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices. Coatings 2024, 14, 256. https://doi.org/10.3390/coatings14030256
Negut I, Albu C, Bita B. Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices. Coatings. 2024; 14(3):256. https://doi.org/10.3390/coatings14030256
Chicago/Turabian StyleNegut, Irina, Catalina Albu, and Bogdan Bita. 2024. "Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices" Coatings 14, no. 3: 256. https://doi.org/10.3390/coatings14030256
APA StyleNegut, I., Albu, C., & Bita, B. (2024). Advances in Antimicrobial Coatings for Preventing Infections of Head-Related Implantable Medical Devices. Coatings, 14(3), 256. https://doi.org/10.3390/coatings14030256







