Influence of the Al Content on the Electrochemical Behavior of Zn-Al Cold-Sprayed Coatings in the Context of the Deep Geological Disposal of Radioactive Waste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cold Spray
2.3. Characterization of the Zn-Al Coatings before the Corrosion Experiments
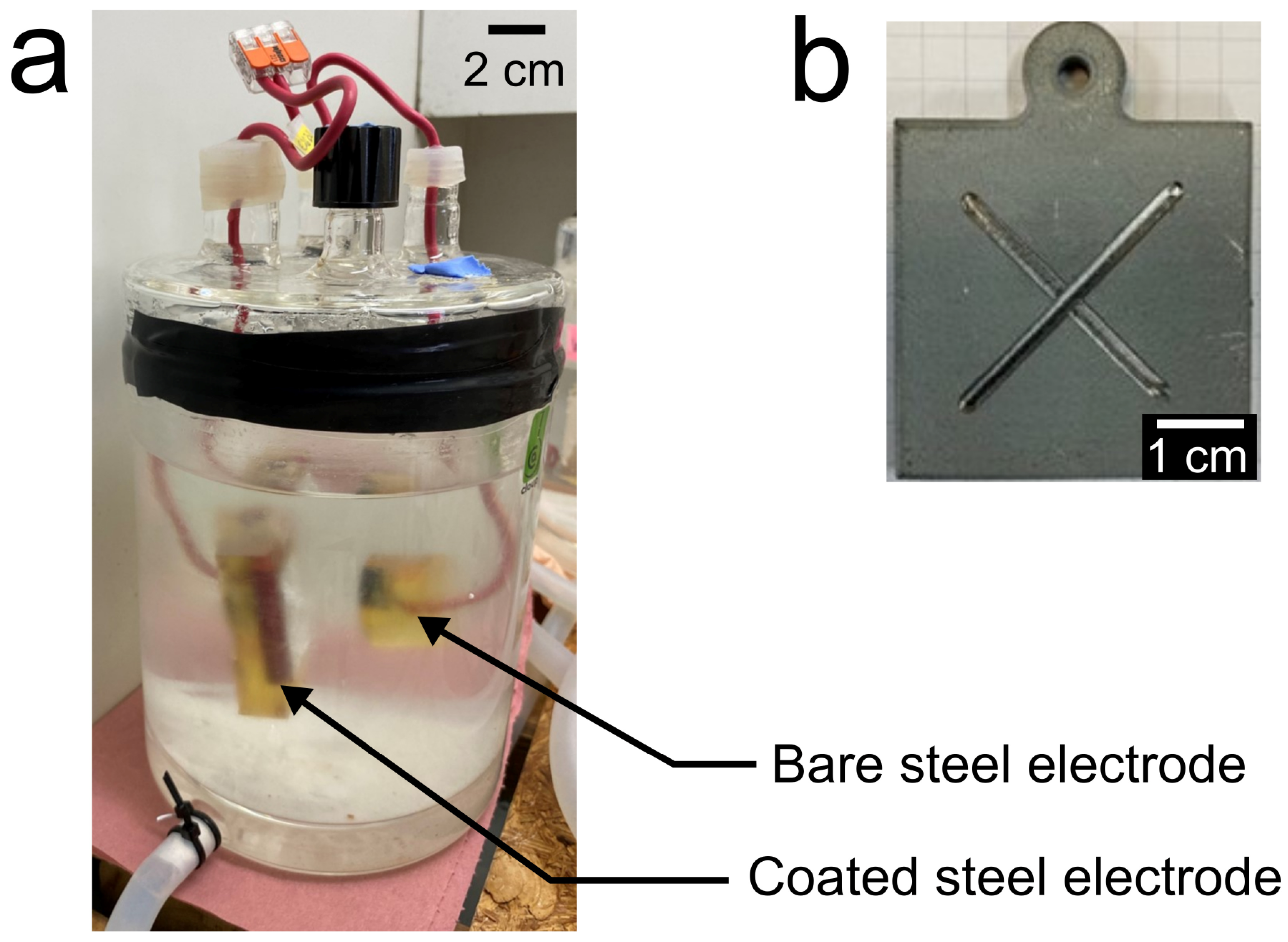
2.4. Electrochemical Set-Up and Methods
2.5. Characterization of Corrosion Products
3. Results
3.1. Characterization of the Zn-Al Coatings before the Corrosion Experiments
3.2. Corrosion Behavior of Zn-Al Coatings
3.3. Six Months of Corrosion Experiments
3.4. Characterization of the Corrosion Products after an Extended Immersion Duration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dupuis, M.C.; Gonnot, F.M. The Cigéo Project: Meuse/Haute-Marne Reversible Geological Disposal Facility for Radioactive Waste; Reference 504va; Andra: Châtenay-Malabry, France, 2013. [Google Scholar]
- Rincón, O.T.; Rincón, A.; Sánchez, M.; Romero, N.; Salas, O.; Delgado, R.; López, B.; Uruchurtu, J.; Marroco, M.; Panossian, Z. Evaluating Zn, Al and Al–Zn coatings on carbon steel in a special atmosphere. Construct. Buil. Mater. 2009, 23, 1465–1471. [Google Scholar] [CrossRef]
- Büteführ, M. Zinc-Aluminium-Coatings as Corrosion Protection for Steel. Mater. Corros. 2007, 58, 721–726. [Google Scholar] [CrossRef]
- Kaewmaneekul, T.; Lothongkum, G. Effect of aluminium on the passivation of zinc–aluminium alloys in artificial seawater at 80 °C. Corros. Sci. 2013, 66, 67–77. [Google Scholar] [CrossRef]
- Katayama, H.; Kuroda, S. Long-term atmospheric corrosion properties of thermally sprayed Zn, Al and Zn–Al coatings exposed in a coastal area. Corros. Sci. 2013, 76, 35–41. [Google Scholar] [CrossRef]
- Lee, H.-S.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C.; Seikh, A.H.; Alharthi, N.; Hussain, R.R. Corrosion mechanism and kinetics of Al-Zn coating deposited by arc thermal spraying process in saline solution at prolong exposure periods. Sci. Rep. 2019, 9, 3399. [Google Scholar] [CrossRef]
- Martin, A.; Texier-Mandoki, N.; Crusset, D.; Sabot, R.; Creus, J.; Refait, P. Corrosion Behavior and Sacrificial Properties of Zn and Zn-Al Coatings in Conditions Simulating Deep Geological Disposal of Radioactive Waste at 80 °C. Coatings 2022, 12, 1044. [Google Scholar] [CrossRef]
- Glass, G.K.; Ashworth, V. The corrosion behavior of the zinc-mild steel galvanic cell in hot sodium bicarbonate solution. Corros. Sci. 1985, 25, 971–983. [Google Scholar] [CrossRef]
- Fraunhofer, J.A.; Lubinski, A.T. Polarity reversal in the zinc-mild steel couple. Corros. Sci. 1974, 14, 225–232. [Google Scholar] [CrossRef]
- Refait, P.; Jeannin, M.; François, E.; Sabot, R.; Grolleau, A.-M. Galvanic corrosion in marine environments: Effects associated with the inversion of polarity of Zn/carbon steel couples. Mater. Corros. 2019, 70, 950–961. [Google Scholar] [CrossRef]
- Schmidt, T.; Assadi, H.; Gärtner, F.; Richter, H.; Stoltenhoff, T.; Kreye, H.; Klassen, T. From Particle Acceleration to Impact and Bonding in Cold Spraying. J. Therm. Spray Technol. 2009, 18, 794–808. [Google Scholar] [CrossRef]
- Azarmi, A.; Sevostianov, I. Comparative micromechanical analysis of alloys 625 coatings deposited by air plasma spraying, wire arc spraying, and cold spraying technologies. Mech. Mater. 2020, 144, 103345. [Google Scholar] [CrossRef]
- Assadi, H.; Gartner, F.; Stoltenhoff, T.; Kreye, H. Bonding mechanism in cold gas spraying. Acta Mater. 2003, 51, 4379–4394. [Google Scholar] [CrossRef]
- Raoelison, R.N.; Verdy, C.; Liao, H. Cold gas dynamic spray additive manufacturing today: Deposit possibilities technological solutions and viable applications. Mater. Des. 2017, 133, 266–287. [Google Scholar] [CrossRef]
- Van Steenkiste, T.H.; Smith, J.R.; Teets, R.E. Aluminum coatings via kinetic spray with relatively large powder particles. Surf. Coat. Technol. 2002, 154, 237–252. [Google Scholar] [CrossRef]
- Alkhimov, A.P.; Kosarev, V.F.; Papyrin, A.N. A Method of Cold Gas-Dynamic Deposition. Sov. Phys. Dokl. 1990, 35, 1047–1049. [Google Scholar]
- Champagne, V.; Helfritch, D. The unique abilities of cold spray deposition. Int. Mater. Rev. 2016, 61, 437–455. [Google Scholar] [CrossRef]
- Lupoi, R.; O’Neill, W. Deposition of metallic coatings on polymer surfaces using cold spray. Surf. Coat. Technol. 2010, 205, 2167–2173. [Google Scholar] [CrossRef]
- Wüstefeld, C.; Rafaja, D.; Motylenko, M.; Ullrich, C.; Drehmann, R.; Grund, T.; Lampke, T.; Wielage, B. Local heteroepitaxy as an adhesion mechanism in aluminium coatings cold gas sprayed on AlN substrates. Acta Mater. 2017, 128, 418–427. [Google Scholar] [CrossRef]
- Drehmann, R.; Grund, T.; Lampke, T.; Wielage, B.; Wüstefeld, C.; Motylenko, M.; Schreiber, G.; Rafaja, D. Investigation of the Bonding Mechanisms of Al Coatings on Ceramic Substrates Deposited by Cold Gas Spraying and Magnetron Sputtering. In Proceedings of the International Thermal Spray Conference, Long Beach, CA, USA, 11–14 May 2015; pp. 544–552. [Google Scholar]
- Li, W.Y.; Cao, C.C.; Yin, S. Solid-state cold spraying of Ti and its alloys: A literature review. Prog. Mater. Sci. 2020, 110, 100633. [Google Scholar] [CrossRef]
- Assadi, H.; Kreye, H.; Gärtner, F.; Klassen, T. Cold spraying—A materials perspective. Acta Mater. 2016, 116, 382–407. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Nelson, K.A.; Schuh, C.A. In-situ observations of single micro-particle impact bonding. Scr. Mater. 2018, 145, 9–13. [Google Scholar] [CrossRef]
- Hassani-Gangaraj, M.; Veysset, D.; Champagne, V.K.; Nelson, K.A.; Schuh, C.A. Adiabatic shear instability is not necessary for adhesion in cold spray, Acta. Mater. 2018, 158, 430–439. [Google Scholar]
- Grujicic, M.; Zhao, C.L.; DeRosset, W.S.; Helfritch, D. Adiabatic shear instability-based mechanism for particles/substrate bonding in the cold-gas dynamic-spray process. Mater. Des. 2004, 25, 681–688. [Google Scholar] [CrossRef]
- Saleh, M.; Luzin, V.; Spencer, K. Analysis of the residual stress and bonding mechanism in the cold spray technique using experimental and numerical methods. Surf. Coat. Technol. 2014, 252, 15–28. [Google Scholar] [CrossRef]
- Panossian, Z.; Mariaca, L.; Morcillo, M.; Flores, S.; Rocha, J.; Pena, J.J.; Herrera, F.; Corvo, F.; Sanchez, M.; Rincon, O.T.; et al. Steel cathodic protection afforded by zinc, aluminium and zinc/aluminium alloy coatings in the atmosphere. Surf. Coat. Technol. 2005, 190, 244–248. [Google Scholar] [CrossRef]
- Yadav, A.P.; Katayama, H.; Noda, K.; Masuda, H.; Nishikata, A.; Tsuru, T. Effect of Al on the galvanic ability of Zn–Al coating under thin layer of electrolyte. Electrochim. Acta 2007, 52, 2411–2422. [Google Scholar] [CrossRef]
- Necib, S.; Bumbieler, F.; Duret-Thual, C.; Bulidon, N.; Crusset, D.; Combrade, P. Assessment of the resistance to environmentally assisted cracking (EAC) of C-steel casing and overpack in the COx claystone. Corros. Eng. Sci. Technol. 2017, 52, 95–100. [Google Scholar] [CrossRef]
- ASTM C633-01; Standard Test Method for Adhesion or Cohesion Strength of Thermal Spray Coatings. ASTM International: West Conshohocken, PA, USA, 2008.
- Drapala, J.; Kostiukova, G.; Losertova, M. Contribution to the aluminum–tin–zinc ternary system. IOP Conf. Ser. Mater. Sci. Eng. 2017, 266, 012002. [Google Scholar] [CrossRef]
- Moreira, A.R.; Panossian, Z.; Camargo, P.L.; Ferreira Moreira, M.; Da Silva, I.C.; Ribeiro de Carvalho, J.E. Zn/55Al coating microstructure and corrosion mechanism. Corros. Sci. 2006, 48, 564–576. [Google Scholar] [CrossRef]
- Exbrayat, L.; Steyer, P.; Rébéré, C.; Berziou, C.; Savall, C.; Ayrault, P.; Tertre, E.; Joly-Pottuz, G.L.; Creus, J. Electrodeposition of zinc–ceria nanocomposite coatings in alkaline bath. J. Solid State Electrochem. 2014, 18, 223–233. [Google Scholar] [CrossRef]
- Aubanel, L.; Delloro, F. Tribological behavior of steel-based composite coatings produced by cold spray. Surf. Coat. Technol. 2023, 470, 129815. [Google Scholar] [CrossRef]
- Akiyama, E.; Zhang, Z.; Watanabe, Y.; Tsusaki, K. Effects of severe plastic deformation on the corrosion behavior of aluminum alloys. J. Solid State Electrochem. 2009, 13, 277–282. [Google Scholar] [CrossRef]
- Cui, Z.; Liu, Z.; Wang, L.; Du, C.; Wang, X. Effect of plastic deformation on the electrochemical and stress corrosion cracking behavior of X70 steel in near-neutral pH environment. Mater. Sci. Eng. A 2016, 677, 259–273. [Google Scholar] [CrossRef]
- Cachet, C.; Ganne, F.; Joiret, S.; Maurin, G.; Petitjean, J.; Vivier, V.; Wiart, R. EIS investigation of zinc dissolution in aerated sulphate medium. Part II: Zinc coatings. Electrochim. Acta 2002, 47, 3409–3422. [Google Scholar] [CrossRef]
- Vu, T.N.; Mokaddem, M.; Volovitch, P.; Ogle, K. The anodic dissolution of zinc and zinc alloys in alkaline solution. II. Al and Zn partial dissolution from 5% Al–Zn coatings. Electrochim. Acta 2012, 74, 130–138. [Google Scholar] [CrossRef]
- Vu, T.N.; Volovitch, P.; Ogle, K. The effect of pH on the selective dissolution of Zn and Al from Zn–Al coatings on steel. Corros. Sci. 2013, 67, 42–49. [Google Scholar] [CrossRef]
- Cui, Z.; Li, X.; Xiao, K.; Dong, C.; Liu, Z.; Wang, L. Corrosion behavior of field-exposed zinc in a tropical marine atmosphere. Corrosion 2014, 70, 731–748. [Google Scholar] [CrossRef] [PubMed]














| Composition/ Particle Size Repartition | d10 | d50 | d90 |
|---|---|---|---|
| Zn-5wt.%Al | 17.5 µm | 31.3 µm | 55.2 µm |
| Zn-15wt.%Al | 12.9 µm | 25.7 µm | 49.3 µm |
| Zn-25wt.%Al | 15 µm | 27.7 µm | 51.2 µm |
| Parameter | Value |
|---|---|
| Gun velocity | 100 mm s−1 |
| Spray bead | 2 mm |
| Stand-off distance | 25 mm |
| Injector–throat distance | 22 or 30 mm |
| Number of scans | 5–7 1 |
| Powder mass flow rate | 20 g min−1 |
| Process/carrier gas | Azote |
| Nozzle material | PBI (Polybenzimidazole) |
| Gas temperature (°C)/Pressure (MPa) | 320/1.8 or 350/2.0 |
| Component | Concentration (mol L−1) | Component | Concentration (mol L−1) |
|---|---|---|---|
| KCl | 1.0 × 10−5 | CaSO4∙2H2O | 8.0 × 10−3 |
| K2SO4 | 2.5 × 10−2 | Na2SiO3∙5H2O | 2.9 × 10−3 |
| Al2(SO4)3∙18H2O | 2.1 × 10−4 | NaHCO3 | 1.3 × 10−2 |
| Element/Coating | Zn-5wt.%Al | Zn-15wt.%Al | Zn-25wt.%Al | |||
|---|---|---|---|---|---|---|
| Zone | (1) | (2) | (3) | (4) | (5) | (6) |
| O | 1 | 0.7 | 1.40 | 2.2 | 0.8 | 0.6 |
| Al | 1.3 | 44.5 | 19.0 | 15.3 | 10.3 | 48.9 |
| Zn | 97.8 | 54.8 | 79.6 | 82.5 | 88.9 | 50.5 |
| Profile (0 µm = Interface)/ Hardness (HV) | Zn-15wt.%Al CS | Zn-25wt.%Al CS | Zn-15wt.%Al WA |
|---|---|---|---|
| 100 µm | 37 HV | 45 HV | 17 HV |
| 200 µm | 38 HV | 46 HV | 19 HV at 250 µm |
| 300 µm | 39 HV | 47 HV | |
| 400 µm | 41 HV | 48 HV | 24 HV |
| Parameter | Zn-5wt.%Al | Zn-15wt.%Al WA CS | Zn-25wt.%Al | |
|---|---|---|---|---|
| OCP at 1 h (V/SHE) | −0.80 | - | −0.80 | −1.22 |
| Ecor (V/SHE) | −0.79 | −0.79 | −0.75 | −1.11 |
| jcor (µA cm−2) | 3.6 | 4.5 | 11.5 | 57 |
| Eb (V/SHE) | - | - | - | −0.64 |
| Element | Zn-5wt.%Al | Zn-15wt.%Al | Zn-25wt.%Al | |||
|---|---|---|---|---|---|---|
| Platelets | Clusters | Platelets | Clusters | Platelets | Clusters | |
| C | 33.2 | 16.9 | - | - | 3.0 | 2.8 |
| O | 23.5 | 13.8 | 39.9 | 42.8 | 21.5 | 10.9 |
| Al | 0.2 | 0 | 7.8 | 8.7 | 0.6 | 0.5 |
| Si | 1.0 | 2.5 | 2.6 | 6.1 | 6.1 | 3.8 |
| S | 2.8 | 1.6 | 1.3 | 0.40 | 1.8 | 0.5 |
| K | 0.8 | 0.8 | 0.5 | 0.6 | 1.5 | 0.9 |
| Zn | 38.5 | 64.4 | 47.9 | 41.4 | 65.5 | 80.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, A.; Charrier, G.; Maillot, V.; Crusset, D.; Gouraud, F.; Verdy, C.; Conforto, E.; Sabot, R.; Creus, J.; Refait, P. Influence of the Al Content on the Electrochemical Behavior of Zn-Al Cold-Sprayed Coatings in the Context of the Deep Geological Disposal of Radioactive Waste. Coatings 2024, 14, 261. https://doi.org/10.3390/coatings14030261
Martin A, Charrier G, Maillot V, Crusset D, Gouraud F, Verdy C, Conforto E, Sabot R, Creus J, Refait P. Influence of the Al Content on the Electrochemical Behavior of Zn-Al Cold-Sprayed Coatings in the Context of the Deep Geological Disposal of Radioactive Waste. Coatings. 2024; 14(3):261. https://doi.org/10.3390/coatings14030261
Chicago/Turabian StyleMartin, Alice, Gaëlle Charrier, Valérie Maillot, Didier Crusset, Fanny Gouraud, Christophe Verdy, Egle Conforto, René Sabot, Juan Creus, and Philippe Refait. 2024. "Influence of the Al Content on the Electrochemical Behavior of Zn-Al Cold-Sprayed Coatings in the Context of the Deep Geological Disposal of Radioactive Waste" Coatings 14, no. 3: 261. https://doi.org/10.3390/coatings14030261






