Effect of Cl− on Passivation Properties of Fe-20Cr-20Mn-0.75N High Nitrogen Austenitic Stainless Steel
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Material and Solution Preparation
2.2. Corrosion Measurements
3. Results and Discussion
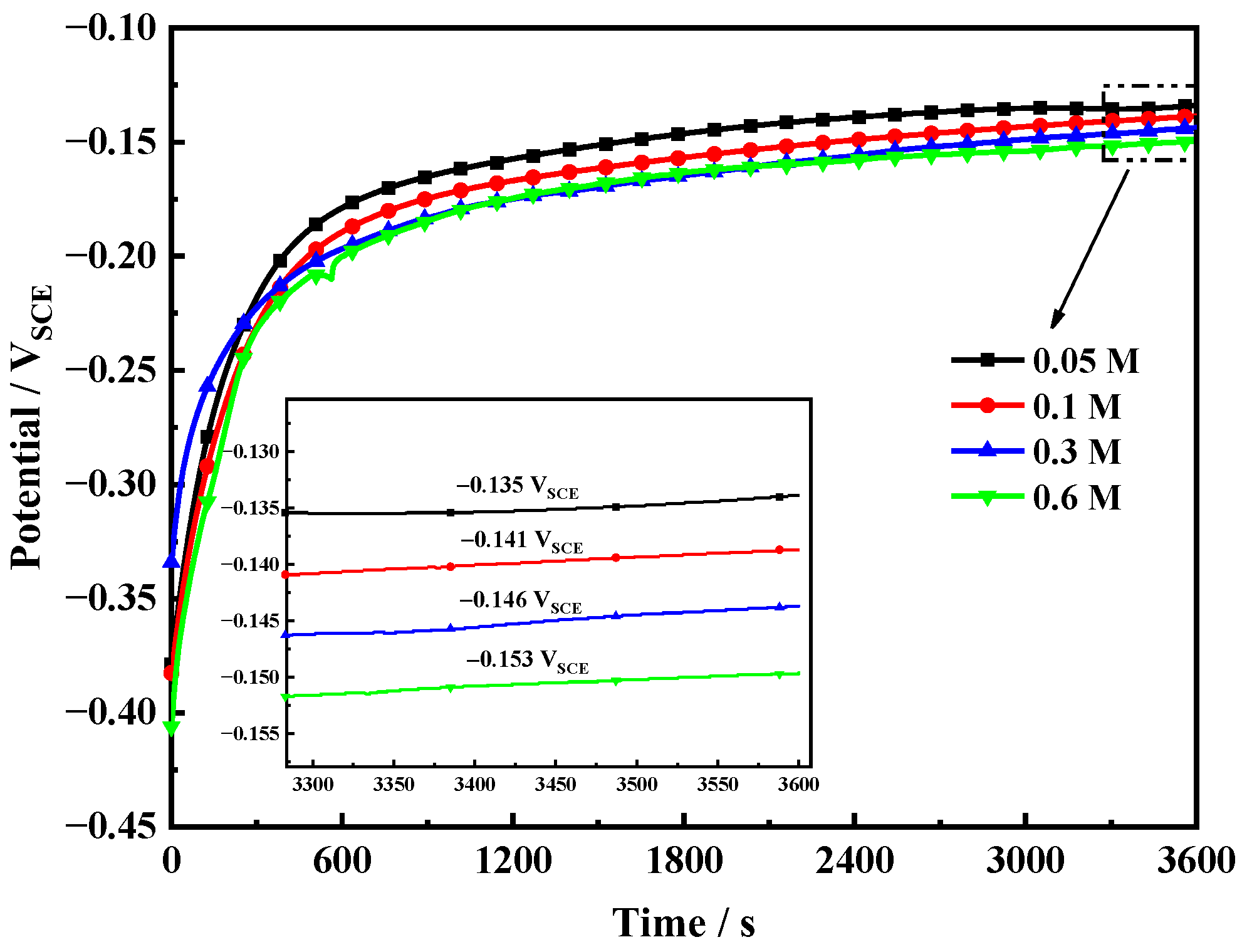
3.1. OCP Measurements
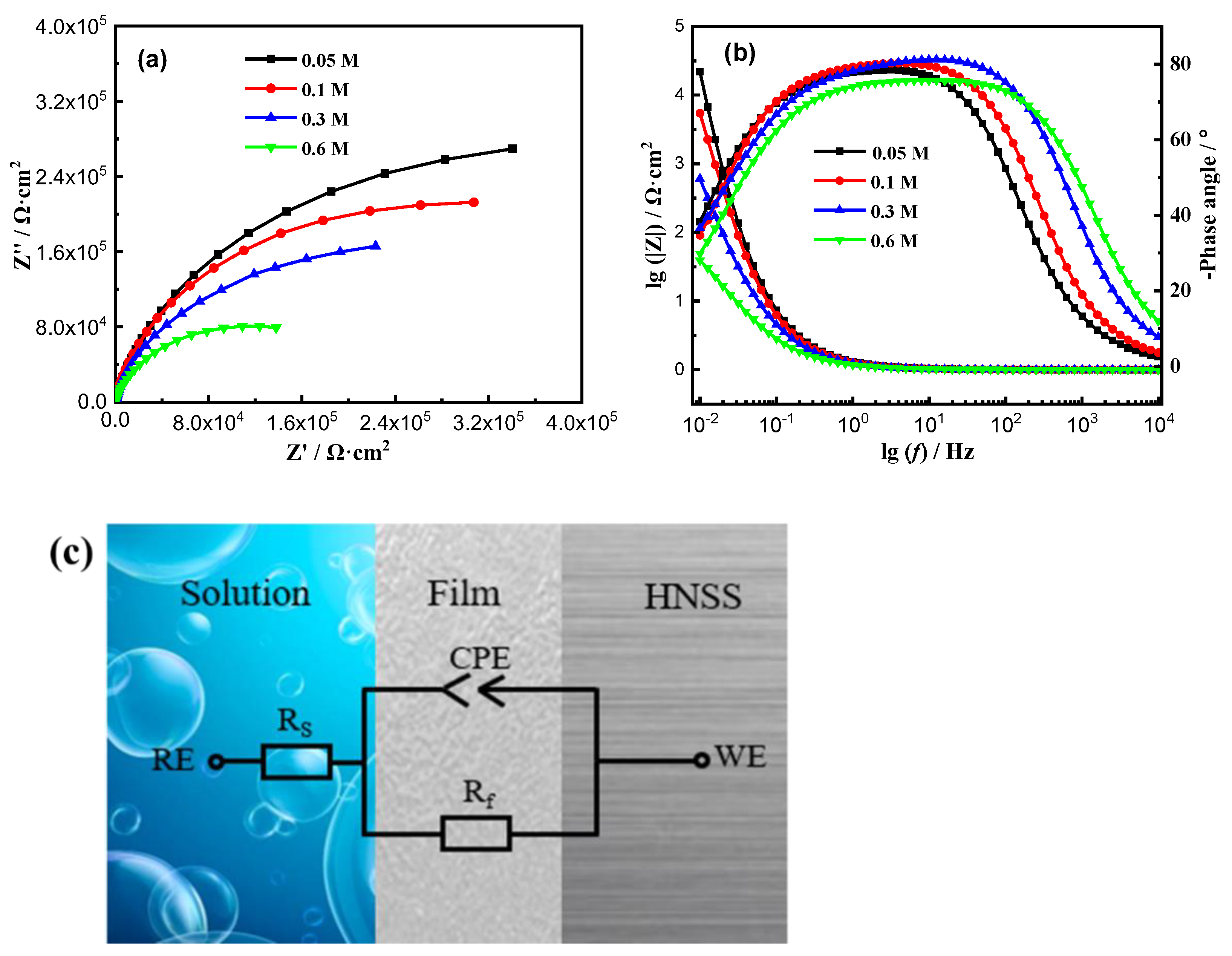
3.2. Electrochemical Impedance Spectroscopy (EIS)
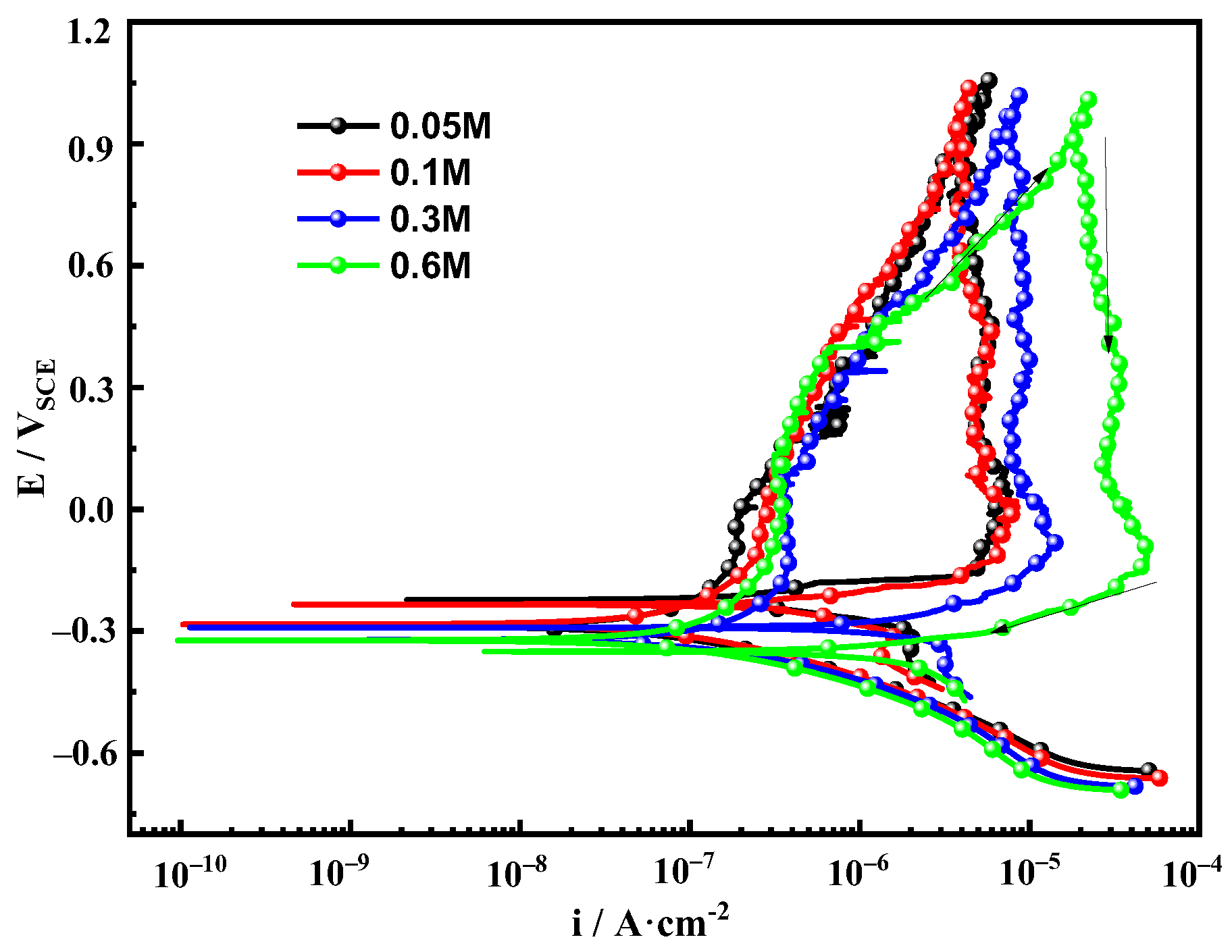
3.3. Potentiodynamic Cyclic Polarization Curve
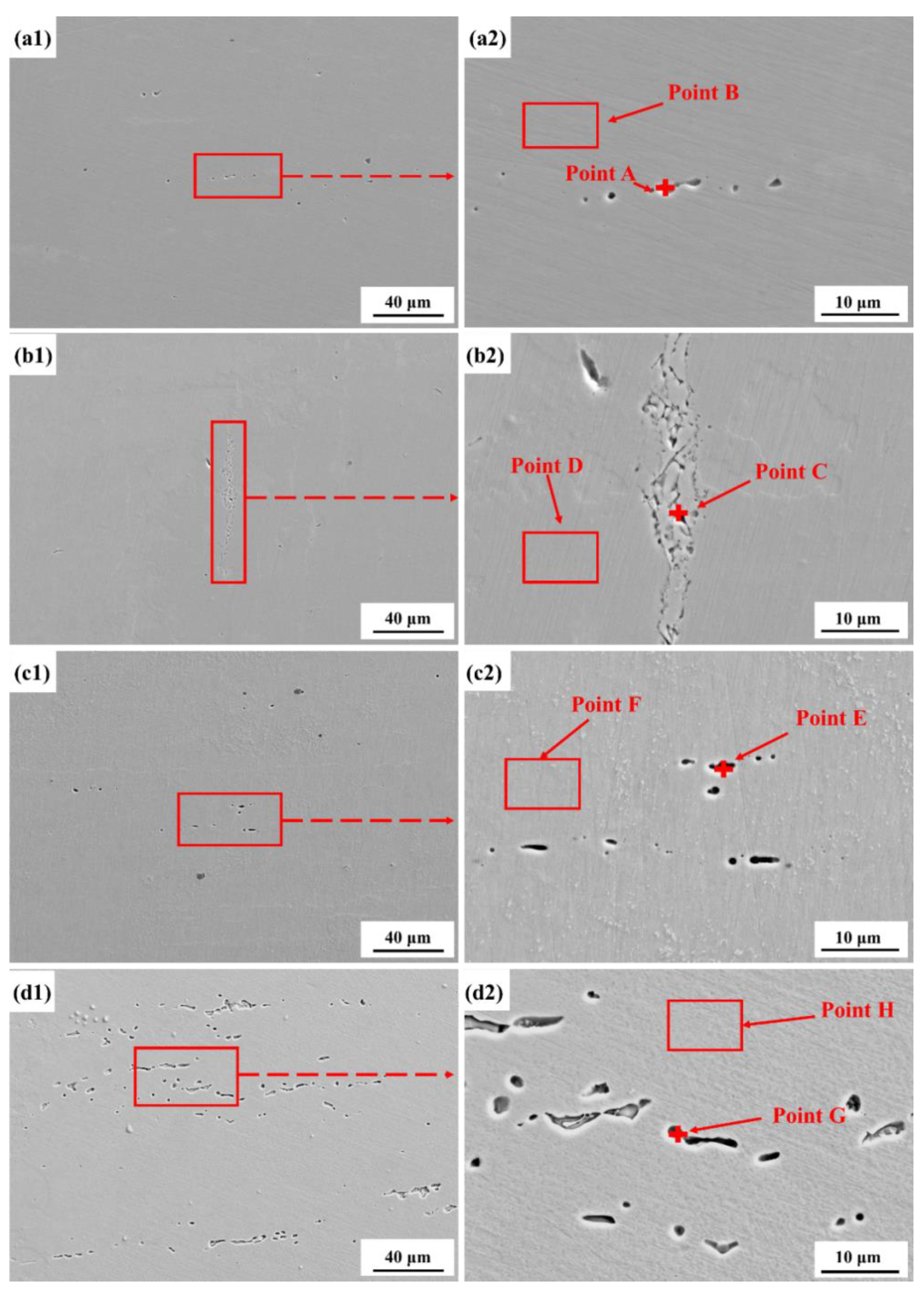
3.4. Corrosion Morphologies
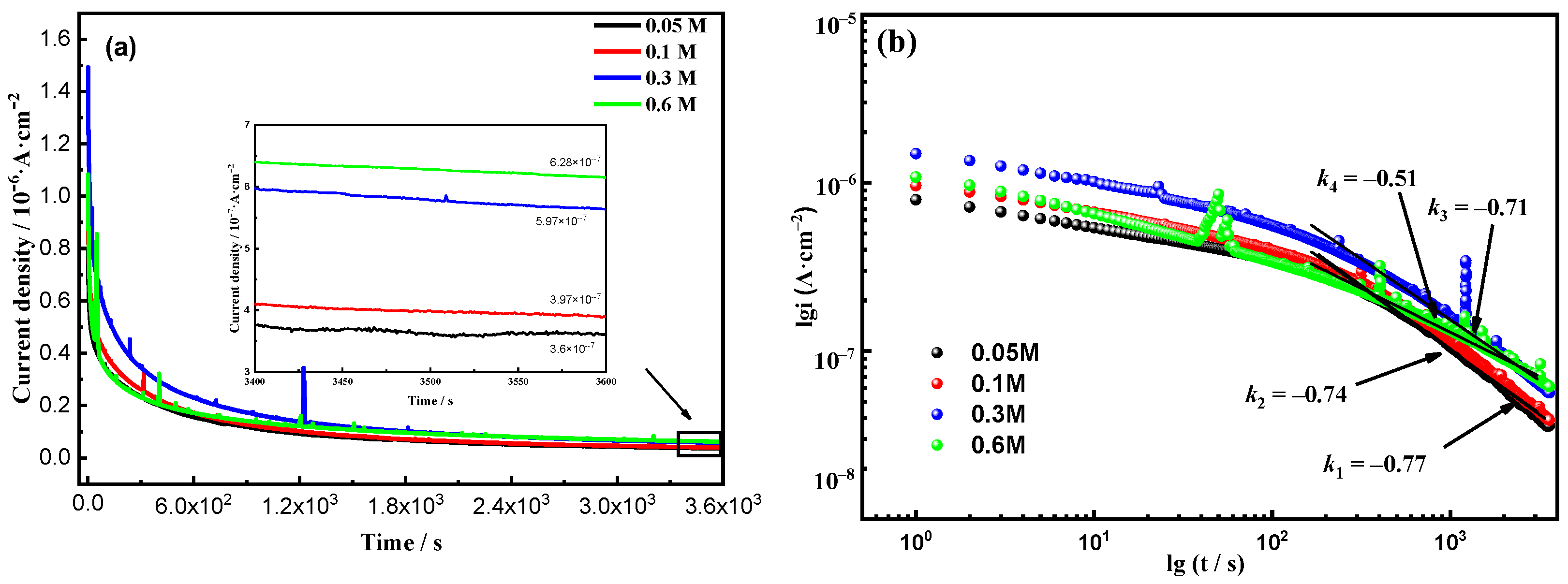
3.5. Potentiostatic Polarization Analysis
3.6. Mott–Schottky Analysis
3.7. Point Defect Model (PDM) of Fe-20Cr-20Mn-0.75N
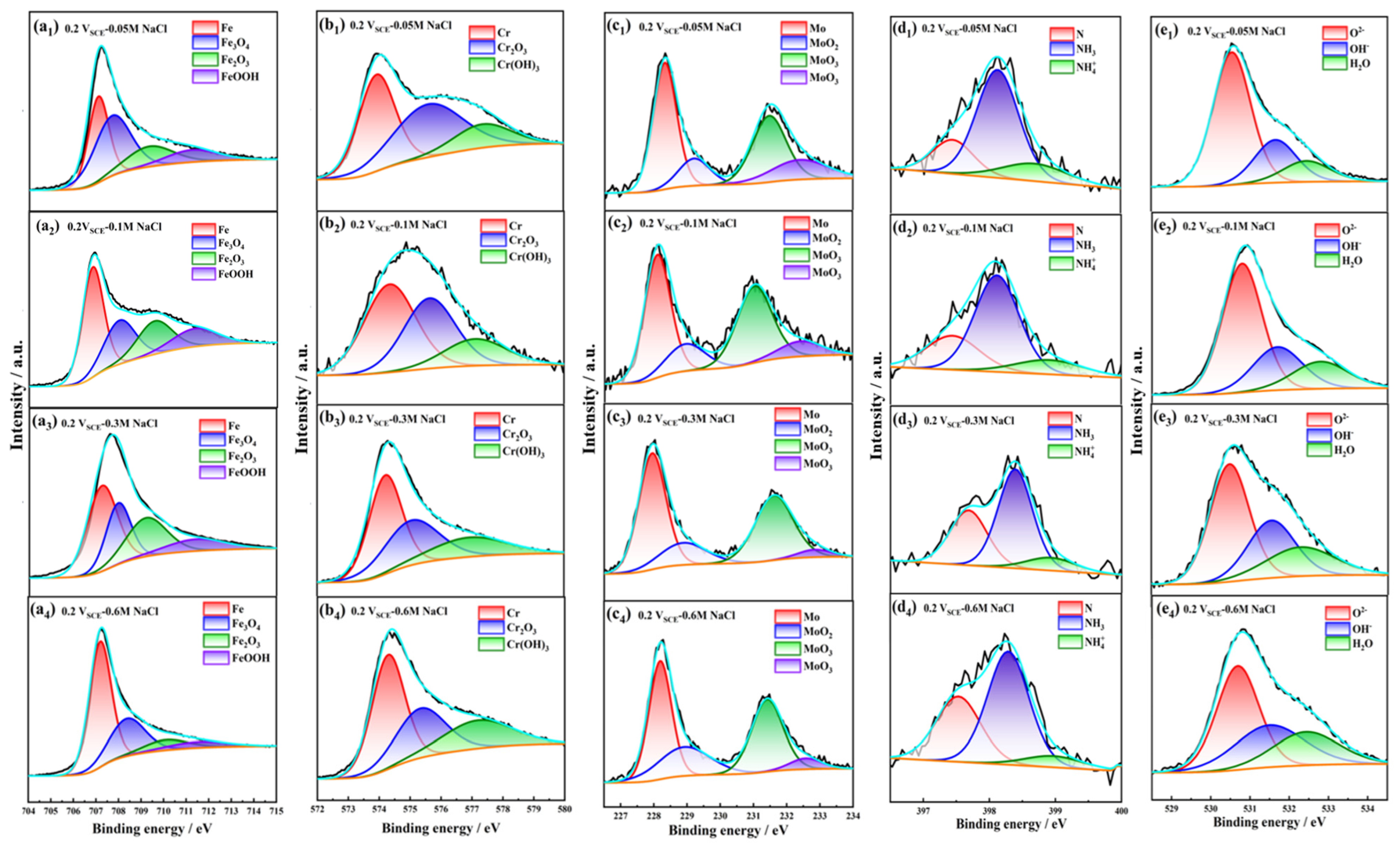
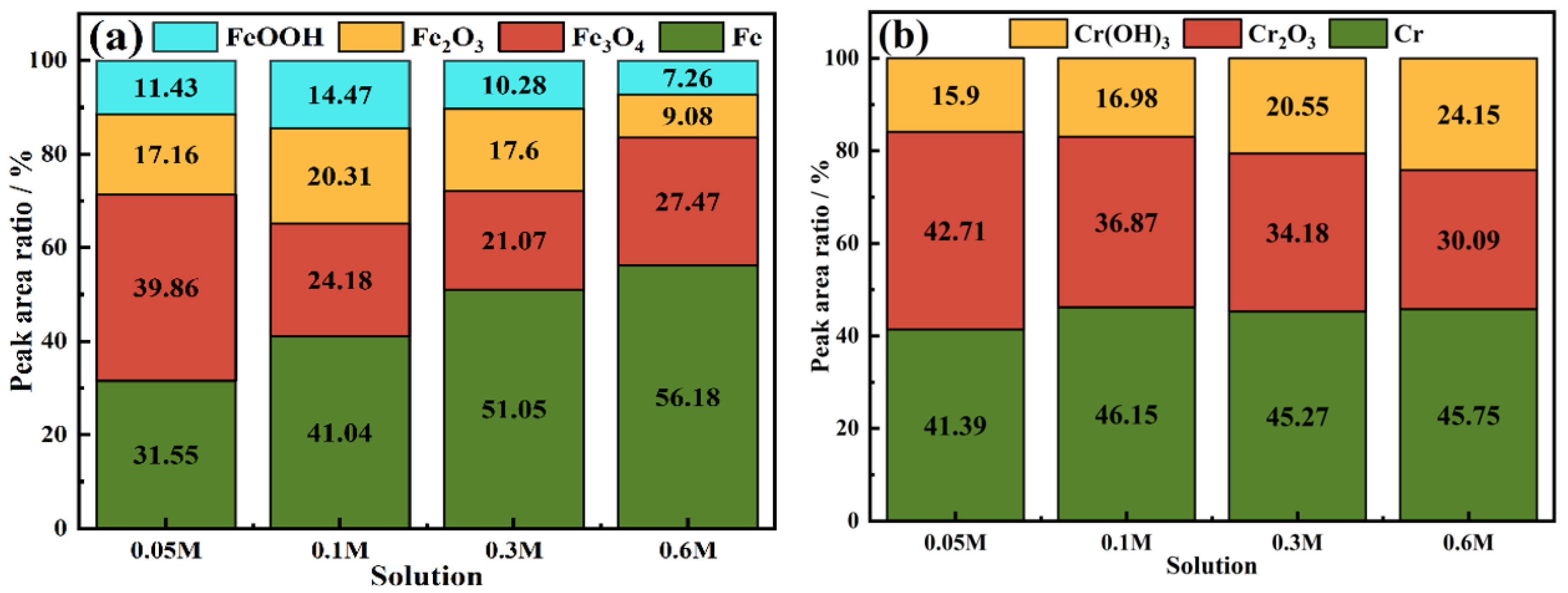
3.8. XPS of the Passive Film
4. Conclusions
- The corrosion tendency and corrosion rate of Fe-20Cr-20Mn-0.75N HNSS increased with increasing Cl− concentration.
- Fe-20Cr-20Mn-0.75N HNSS showed passive behavior in different concentrations of NaCl solutions. The anodic region of the polarization curves showed activation–passivation–activation behaviors, and all of them had wide passive regions, but the passive zones fluctuated due to the increase in the concentration, the poor corrosion environment, and the unstable corrosion. In 0.6 mol/L NaCl solution, Fe-20Cr-20Mn-0.75N HNSS had the highest corrosion current density, largest passive film dissolution rate, and lowest corrosion resistance.
- With the increase in Cl− concentration, the stable corrosion current density increased; at this time, the passive film’s resistance to Cl− ’s erosion ability became poor, and Fe-20Cr-20Mn-0.75N HNSS’s corrosion resistance decreased.
- In the solution with a low Cl− concentration, the passive film was thicker, and the surface was more stable. And the high content of NH4+ inhibited the rupture of the passive film; hence, the passive film was more dense, and the corrosion resistance increased.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilevbare, G.O.; Burstein, G.T. The inhibition of pitting corrosion of stainless steels by chromate and molybdate ions. Corros. Sci. 2003, 45, 1545–1569. [Google Scholar] [CrossRef]
- Bairi, L.R.; Ningshen, S.; Mudali, U.K.; Raj, B. Microstructural analysis and corrosion behaviour of D9 stainless steel-zirconium metal waste form alloys. Corros. Sci. 2010, 52, 2291–2302. [Google Scholar] [CrossRef]
- Hu, S.F.; Sun, Z.Z.; Shen, F.H.; Deng, J.; Yang, W.P.; Yang, H.K. Carbon fiber breakage mechanism in aluminum (Al)/carbon fibers (CFs) composite sheet during accumulative roll bonding (ARB) process. J. Wuhan Univ. Technol. 2024, 39, 167–173. [Google Scholar] [CrossRef]
- Chen, S.S.; Yao, Z.F.; Guan, Y.B.; Yang, H.; Shahzad, M.B.; Wu, Y.Z.; Zhang, B.C.; Shen, L.; Yang, K. High nitrogen stainless steel drug-eluting stent-Assessment of pharmacokinetics and preclinical safety in vivo. Bioact. Mater. 2020, 5, 779–786. [Google Scholar] [CrossRef]
- Zhou, R.; Northwood, D.O.; Liu, C. On nitrogen diffusion during solution treatment in a high nitrogen austenitic stainless steel. J. Mater. Res. Technol. 2020, 9, 2331–2337. [Google Scholar] [CrossRef]
- Fossati, A.; Borgioli, F.; Galvanetto, E.; Bacci, T. Corrosion resistance properties of glow-discharge nitrided AISI 316L austenitic stainless steel in NaCl solutions. Corros. Sci. 2006, 48, 1513–1527. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, Z.B.; Long, J.; Wang, J.; Zheng, K.H.; Ke, Z.M.; Luo, Z.C.; Pokrovsky, A.I.; Khina, B.B. Recent advances in wear-resistant steel matrix composites: A review of reinforcement particle selection and preparation processes. J. Mater. Res. Technol. 2024, 29, 1779–1797. [Google Scholar] [CrossRef]
- Maznichevsky, A.N.; Sprikut, R.V.; Goikhenber, Y.N. Investigation of nitrogen containing austenitic stainless steel. Mater. Sci. Forum. 2020, 989, 152–159. [Google Scholar] [CrossRef]
- Gao, F.Y.; Qiao, Y.X.; Chen, J.; Yang, L.L.; Zhou, H.L.; Zheng, Z.B.; Zhang, L.M. Effect of nitrogen content on corrosion behavior of high-nitrogen austenitic stainless steel. NPJ Mater. Degrad. 2023, 7, 75. [Google Scholar] [CrossRef]
- Satpati, A.K.; Phadnis, S.V.; Sundaresan, R.I. Electrochemical and XPS studies and the potential scan rate dependent pitting corrosion behavior of Zircaloy-2 in 5% NaCl solution. Corros. Sci. 2005, 47, 1445–1458. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Effect of surface machining and cold working on the ambient temperature chloride stress corrosion cracking susceptibility of AISI 304L stainless steel. Mater. Sci. Eng. A 2010, 527, 679–683. [Google Scholar] [CrossRef]
- Wang, X.L.; Hu, Q.X.; Liu, W.K.; Yuan, W.; Shen, X.W.; Gao, F.Y.; Tang, D.X.; Hu, Z.C. Microstructure and corrosion properties of wire arc additively manufactured multi-trace and multilayer stainless steel 321. Metals 2022, 12, 1039. [Google Scholar] [CrossRef]
- Mu, J.; Li, Y.Z.; Wang, X. Crevice corrosion behavior of X70 steel in NaCl solution with different pH. Corros. Sci. 2021, 182, 109310. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhang, B.C.; Ren, Y.B.; Yang, K. Eliminating detrimental effect of cold working on pitting corrosion resistance in high nitrogen austenitic stainless steels. Corros. Sci. 2017, 123, 351–355. [Google Scholar] [CrossRef]
- Abdelfatah, A. Corrosion Characteristics of 304 stainless steel in sodium chloride and sulfuric acid solutions. Int. J. Electrochem. Sci. 2022, 17, 220417. [Google Scholar] [CrossRef]
- Li, S.H.; Li, J.; Zhang, J.; Shi, C.B. Effect of nitrogen on microstructure and microsegregation of martensitic stainless steel 4Cr13 produced by electroslag remelting. J. Iron Steel Res. Int. 2022, 30, 1854–1861. [Google Scholar] [CrossRef]
- Li, H.B.; Jiang, Z.H.; Yang, Y.; Cao, Y.; Zhang, Z.R. Pitting corrosion and crevice corrosion behaviors of high nitrogen austenitic stainless steels. Int. J. Miner. Metall. Mater. 2009, 16, 517–524. [Google Scholar] [CrossRef]
- Deyab, M.A.; Mohamed, N.H.; Moustafa, Y.M. Corrosion protection of petroleum pipelines in NaCl solution by microcrystalline waxes from waste materials: Electrochemical studies. Corros. Sci. 2017, 122, 74–79. [Google Scholar] [CrossRef]
- Dai, N.W.; Zhang, L.C.; Zhang, J.X.; Chen, Q.M.; Wu, M.L. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Sci. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Li, J.Q.; Han, Q.L.; Zou, Y.B.; Yu, Z.X.; Zhu, G.X.; Xu, W.; Shi, S.H. Electrochemical behavior of additive manufactured TC4 alloy in different concentrated NaCl solutions. J. Appl. Electrochem. 1022, 52, 1419–1431. [Google Scholar] [CrossRef]
- Leng, C.; Yang, H.; Yi, G.; Lo, H.; Li, J.; Li, Y. The effect of carbon addition on the mechanical properties and fracture behaviors of cobalt-chromium-iron-manganese-nickel high entropy alloy. Materialwiss. Werkst. 2024, 55, 7–12. [Google Scholar] [CrossRef]
- Wang, M.Y.; Zhao, X.Y.; Gao, S.; Zhu, Y.S.; Zheng, Y.; Huang, Y.; Xu, Y.Z. Visualizing and understanding corrosion evolution beneath a condensed droplet using the multi-electrode array. Colloids Surf. A Physicochem. Eng. Asp. 2024, 684, 133252. [Google Scholar] [CrossRef]
- Cai, X.; Yang, M.M.; Rui, Y.P.; Wang, Z.; Zhou, J.; Xue, F. Investigation of mechanical and corrosion behavior of multi-pass nickel-aluminum bronze fabricated through wire-arc directed energy deposition. Addit. Manuf. 2024, 80, 103967. [Google Scholar] [CrossRef]
- Zou, Z.; Liu, Z.H.; Yang, L.L.; Tang, Y.B.; Qiao, Y.X.; Lu, D.H. Corrosion behavior of different building planes of selective laser melting 316L stainless steel in 0.1 M HCl solution. J. Mater. Res. Technol. 2024, 28, 4738–4753. [Google Scholar] [CrossRef]
- Nie, S.J.; Yi, X.N.; Zhou, H.L.; Zhu, H.J.; Yang, L.L.; Fu, F.L.; Li, J.Y.; Yang, H.K.; Xu, G.X.; Lu, S.; et al. Corrosion behavior of as-cast Al0.75CoFeCr1.25Ni high entropy alloy in 0.5 mol/L NaOH solution. J. Iron Steel Res. Int. 2024. [Google Scholar]
- Liao, X.Y.; Zheng, Z.B.; Liu, T.L.; Long, J.; Wang, S.; Zhang, H.Y.; Zheng, K.H. Achieving high impact–abrasion–corrosion resistance of high–chromium wear–resistant steel via vanadium additions. J. Mater. Res. Technol. 2024, 29, 2425–2436. [Google Scholar] [CrossRef]
- Zhang, Y.B.; Yang, X.L.; Tang, A. Corrosion behavior of nickel-based 718 alloy determined by in situ electrochemical methods at different partial pressures of H2S in 25 wt% NaCl solution at 150 °C. Rare Met. 2019, 38, 855–863. [Google Scholar] [CrossRef]
- Kong, W.C.; Li, M.K.; Hu, J. Immersion corrosion behavior, electrochemical performance and corrosion mechanism of subsonic flame sprayed FeCoCrMoSi amorphous coating in 3.5% NaCl solution. Int. J. Hydrogen Energy 2022, 47, 6911–6923. [Google Scholar] [CrossRef]
- Liu, Y.C.; Zheng, Z.B.; Xu, L.L.; Xu, Z.B.; Yin, F.X.; Zheng, K.H. Unraveling the interfacial structure of TA2 titanium-A36 steel composite plate and its corrosion behavior in marine environment. Corros. Sci. 2024, 230, 111923. [Google Scholar] [CrossRef]
- Zhao, Q.C.; Wang, X.F.; Pan, Z.M.; Wei, Y.; Cheng, H.X.; Ma, Y.C.; Luo, H.; Li, X.G. Effects of rare earth elements addition on mechanical properties and corrosion behavior of GCr15 bearing steel under different heat treatment conditions. Corros. Commun. 2023, 9, 65–76. [Google Scholar] [CrossRef]
- Grubac, Z.; Hukovic, M.M. EIS study of solid-state transformations in the passivation process of bismuth in sulfide solution. J. Electroanal. Chem. 2004, 565, 85–94. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Wang, X.Y.; Yang, L.L.; Wang, X.J.; Chen, J.; Wang, Z.B.; Zhou, H.L.; Zou, J.S.; Wang, F.H. Effect of aging treatment on microstructure and corrosion behavior of a Fe-18Cr-15Mn-0.66N stainless steel. J. Mater. Sci. Technol. 2022, 107, 197–206. [Google Scholar] [CrossRef]
- Moayed, M.H.; Newman, R.C. Evolution of current transients and morphology of metastable and stable pitting on stainless steel near the critical pitting temperature. Corros. Sci. 2006, 48, 1004–1018. [Google Scholar] [CrossRef]
- Galvele, J.R.; Torresi, R.M.; Carranza, R.M. Passivity breakdown its relation to pitting and stress corrosion cracking processes. Corros. Sci. 1990, 31, 563–571. [Google Scholar] [CrossRef]
- Zhang, L.N.; Ojo, O.A. Corrosion behavior of wire arc additive manufactured Inconel 718 superalloy. J. Alloys Compd. 2020, 829, 154455. [Google Scholar] [CrossRef]
- Genevieve, B.; Christine, B.; Nadine, P. AC impedance spectroscopy in characterizing time-dependent corrosion of AZ91 and AM50 magnesium alloys. J. Electrochem. Soc. 2001, 148, 489–496. [Google Scholar]
- Fattah-alhosseini, A.; Golozar, M.A.; Saatchi, A.; Raeissi, K. Effect of solution concentration on semiconducting properties of passive films formed on austenitic stainless steels. Corros. Sci. 2010, 52, 205–209. [Google Scholar] [CrossRef]
- Sikora, J.; Sikora, E.; Macdonald, D.D. The electronic structure of the passive film on tungsten. Electrochim. Acta 2000, 45, 1875–1883. [Google Scholar] [CrossRef]
- Sunseri, C.; Piazza, S.; Quarto, F.D. Photocurrent spectroscopic investigations of passive films on chromium. J. Electrochem. Soc. 1990, 137, 2411–2417. [Google Scholar] [CrossRef]
- Hamadou, L.; Kadri, A.; Benbrahim, N. Impedance investigation of thermally formed oxide films on AISI 304L stainless steel. Corros. Sci. 2010, 52, 859–864. [Google Scholar] [CrossRef]
- Jun, L.; Macdonald, D.D. The passivity of iron in the presence of ethylenediaminetetraacetic acid. II. The defect and electronic structures of the barrier layer. J. Electrochem. Soc. 2001, 148, 425–430. [Google Scholar]
- Zhou, Y.T.; Xu, A.N.; Mao, F.X.; Yu, J.K.; Kong, D.C.; Dong, C.F.; Macdonald, D.D. Passivity breakdown on copper: Influence of borate anion. Electrochim. Acta 2019, 320, 134545. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Ismail, K.M.; Sikora, E. Characterization of the passive state on zinc. J. Electrochem. Soc. 1998, 145, 3141–3149. [Google Scholar] [CrossRef]
- Cheng, H.X.; Luo, H.; Wang, X.F.; Pan, Z.M.; Jiang, Y.; Li, X.G. Electrochemical corrosion and passive behavior of a new high-nitrogen austenitic stainless steel in chloride environment. Mater. Chem. Phys. 2022, 292, 126837. [Google Scholar] [CrossRef]
- Xie, H.Y.; Chen, J.L.; Zhang, P.; Gao, L.K.; Liu, D.W.; Chen, L.Z. Separation of galena and chalcopyrite using the difference in their surface acid corrosion characteristics. Int. J. Miner. Metall. Mater. 2023, 30, 2157–2168. [Google Scholar] [CrossRef]
- Qiao, Y.X.; Qin, Y.; Zhou, H.L.; Yang, L.L.; Wang, X.J.; Wang, Z.B.; Liu, Z.G.; Zou, J.S. Electrochemical hydrogen charging on corrosion behavior of Ti-6Al-4V alloy in artificial seawater. Chin. J. Mech. Eng. 2024, 37, 2. [Google Scholar] [CrossRef]
- Wang, D.P.; Chen, G.; Wang, A.D.; Wang, Y.X.; Qiao, Y.X.; Liu, Z.G.; Qi, Z.X.; Liu, C.T. Corrosion behavior of single- and poly-crystalline dual-phase TiAl-Ti3Al alloy in NaCl solution. Int. J. Miner. Metall. Mater. 2023, 30, 689–696. [Google Scholar] [CrossRef]
- Sun, S.C.; Wei, S.F.; Wang, G.Y.; Jiang, Z.H.; Lian, J.S.; Ji, C.T. The synthesis and electrochemical behavior of high-nitrogen nickel-free austenitic stainless steel. J. Mater. Eng. Perform. 2014, 23, 3957–3962. [Google Scholar] [CrossRef]
- Leia, M.K.; Zhu, X.M. Role of Nitrogen in pitting corrosion resistance of a high-nitrogen face-centered-cubic phase formed on austenitic stainless steel. J. Electrochem. Soc. 2005, 152, 291–295. [Google Scholar] [CrossRef]
- Luo, H.; Su, H.Z.; Li, B.S.; Ying, G.B. Electrochemical and passive behaviour of tin alloyed ferritic stainless steel in concrete environment. Appl. Surf. Sci. 2018, 439, 232–239. [Google Scholar] [CrossRef]



| C | Si | Mn | N | Cr | Mo | S | P | Fe |
|---|---|---|---|---|---|---|---|---|
| 0.048 | 0.22 | 19.68 | 0.75 | 19.71 | 2.23 | 0.003 | ≤0.03 | Bal. |
| Cl− Concentration | Rs/Ω·cm2 | Q/Ω−1·sn·cm−2 | n | Rf/Ω·cm2 |
|---|---|---|---|---|
| 0.05 mol/L | 110.2 ± 0.05 | (1.67 ± 0.01) × 10−5 | 0.87 | (5.88 ± 0.10) × 105 |
| 0.1 mol/L | 56.26 ± 0.01 | (1.82 ± 0.02) × 10−5 | 0.89 | (4.68 ± 0.08) × 105 |
| 0.3 mol/L | 18.48 ± 0.02 | (2.62 ± 0.05) × 10−5 | 0.90 | (2.52 ± 0.05) × 105 |
| 0.6 mol/L | 12.49 ± 0.05 | (3.67 ± 0.01) × 10−5 | 0.90 | (2.17 ± 0.05) × 105 |
| Cl− Concentrations | Ecorr/VSCE | icorr/A·cm−2 |
|---|---|---|
| 0.05 mol/L | –0.29 | 2.39 × 10−8 |
| 0.1 mol/L | –0.28 | 2.45 × 10−8 |
| 0.3 mol/L | –0.32 | 2.56 × 10−8 |
| 0.6 mol/L | –0.33 | 3.28 × 10−8 |
| Cl− Concentration | Point | Fe | Cr | Mn | O | Mo |
|---|---|---|---|---|---|---|
| 0.05 M | A | 55.73 | 20.76 | 19.82 | 1.76 | 2.3 |
| B | 52.05 | 21.74 | 18.46 | 4.69 | 3.06 | |
| 0.1 M | C | 55.53 | 20.76 | 19.82 | 1.58 | 2.3 |
| D | 53.79 | 22.24 | 16.25 | 2.54 | 4.9 | |
| 0.3 M | E | 56.08 | 18.79 | 22.81 | 1.15 | 1.07 |
| F | 52.28 | 23.18 | 20.85 | 2.28 | 1.41 | |
| 0.6 M | G | 58.42 | 17.39 | 20.86 | 0.39 | 2.94 |
| H | 56.68 | 19.25 | 20.23 | 0.92 | 2.92 |
| Cl− Concentration | iss | ND/1020 cm−3 | D0 |
|---|---|---|---|
| 0.05 mol/L | 3.65 × 10−8 | 1.67 | 2.59 × 105 |
| 0.1 mol/L | 3.99 × 10−8 | 2.83 | 2.72 × 105 |
| 0.3 mol/L | 5.79 × 10−8 | 3.61 | 2.81 × 105 |
| 0.6 mol/L | 6.28 × 10−8 | 4.37 | 3.49 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Gao, F.; Zhou, H.; Li, C.; Liu, Z.; Yang, H.; Qiao, Y. Effect of Cl− on Passivation Properties of Fe-20Cr-20Mn-0.75N High Nitrogen Austenitic Stainless Steel. Coatings 2024, 14, 280. https://doi.org/10.3390/coatings14030280
Zhang W, Gao F, Zhou H, Li C, Liu Z, Yang H, Qiao Y. Effect of Cl− on Passivation Properties of Fe-20Cr-20Mn-0.75N High Nitrogen Austenitic Stainless Steel. Coatings. 2024; 14(3):280. https://doi.org/10.3390/coatings14030280
Chicago/Turabian StyleZhang, Wentao, Fengyin Gao, Huiling Zhou, Chengtao Li, Zhong Liu, Haokun Yang, and Yanxin Qiao. 2024. "Effect of Cl− on Passivation Properties of Fe-20Cr-20Mn-0.75N High Nitrogen Austenitic Stainless Steel" Coatings 14, no. 3: 280. https://doi.org/10.3390/coatings14030280





