Analysis of Causes and Protective Measures against Corrosion Perforation in the Shell-Side Outlet Flange of a Sour Water Steam Heater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
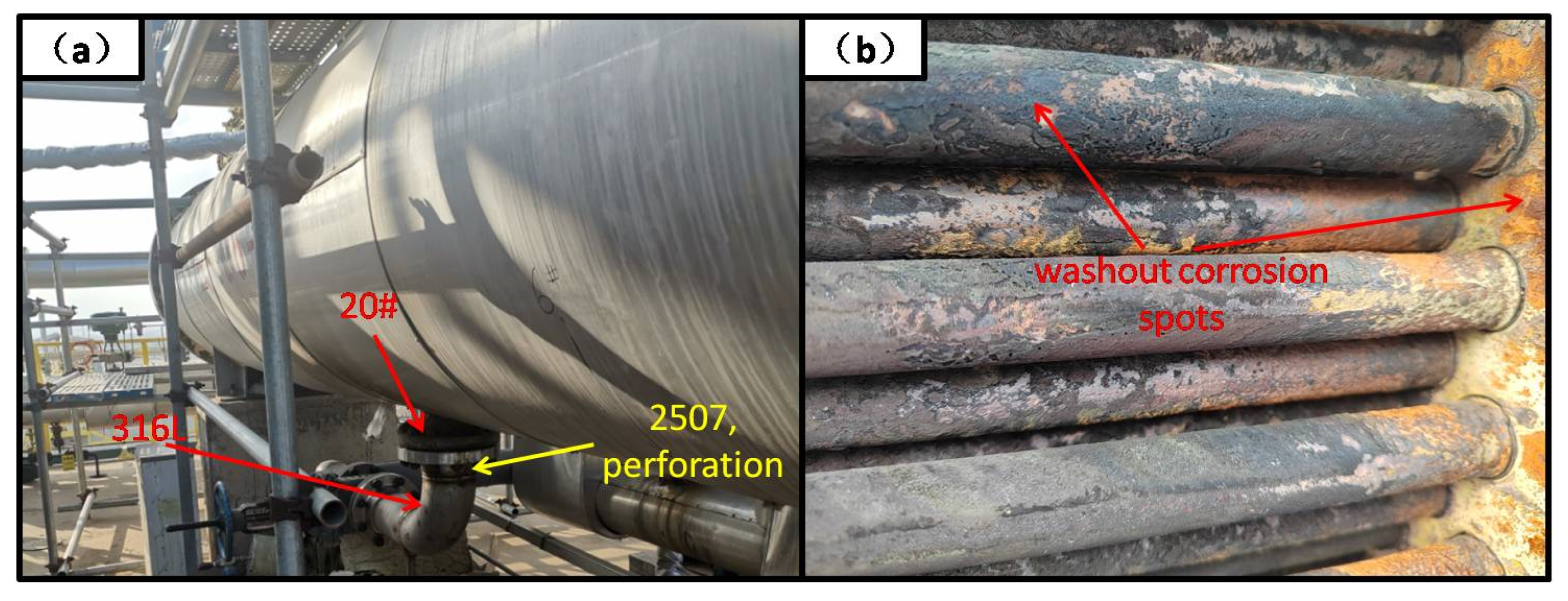
2.2. Visual Examination
2.3. Physical and Chemical Testing
2.4. Intergranular Corrosion Test
2.5. Flow Accounting
3. Results and Discussion
- (1)
- Under all reference conditions, the corrosion rate of carbon steel is generally less than 0.64 mm/y.
- (2)
- When the concentration of NH4HS is 25 wt% and the flow rate is 24 m/s, the corrosion rate of 316L exceeds 1 mm/y, which is twice the corrosion rate of carbon steel under the same conditions.
- (3)
- Alloys 2205 and 2507 have similar corrosion curves to 316L. Under the most severe conditions (an NH4HS concentration of 25%, a flow rate of 24 m/s, and PNH3 = 620 kPa absolute), the corrosion rate of alloys 2205 and 2507 exceeds 1.5 mm/y, which is 1.5 times that of 316L under the same conditions and 3 times that of carbon steel.
- (4)
- Under the most severe conditions (an NH4HS concentration of 25%, a flow rate of 24 m/s, and PNH3 = 620 kPa absolute), the corrosion rate of alloys 825 and C-276 is less than 0.05 mm/y.
- (5)
- Nickel-based alloys have shown excellent corrosion resistance in highly alkaline NH4HS solutions.
4. Conclusions and Recommendations
- (1)
- In NH3-dominated NH4HS water-solution corrosion environments, it is not recommended to use 300 series austenitic stainless-steel materials (such as 304, 316L, etc.) and duplex stainless steels (such as 2205, 2507, etc.). It is recommended to use carbon steel with enhanced supervision or to upgrade to corrosion-resistant materials such as alloys 825, C-276, etc.
- (2)
- Depending on the processing capacity of the equipment and the amount of gas, it is recommended to increase the diameter of the E107-II shell-side outlet pipe accordingly to reduce the flow velocity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ou, G.; Gu, Y.; Yu, C.; Jin, H. Failure analysis of ammonium chloride salt coagulation corrosion of U-tube heat exchanger in diesel hydrogenation unit. Eng. Fail. Anal. 2022, 137, 106264. [Google Scholar] [CrossRef]
- Jin, H.; Chen, X.; Ren, J.; Wu, X.; Zheng, Z.; Ou, G.; Ye, Y. Modeling of multiphase flow in an air-cooling system using the CFD-FSCA approach. Braz. J. Chem. Eng. 2018, 35, 1051–1061. [Google Scholar] [CrossRef]
- Rezaei, M.; Mahidashti, Z.; Eftekhari, S.; Abdi, E. A corrosion failure analysis of heat exchanger tubes operating in petrochemical refinery. Eng. Fail. Anal. 2021, 119, 105011. [Google Scholar] [CrossRef]
- Juchno, W. Global Cost of Corrosion Over $2 Trillion. Cool. J. Serv. Glob. Automot. Ind. Heat Transf. Ind. 2018, 61, 14. [Google Scholar]
- Ma, S.-Y.; Bi, T.-T.; Gong, Y.; Yang, Z.-G. Failure analysis on abnormal leakage of shell and tube heat exchanger in specialty chemical plant. Eng. Fail. Anal. 2023, 143, 106859. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Chen, C.; Liu, F.; Shi, Q. Molecular investigation into the transformation of recalcitrant dissolved organic sulfur in refinery sour water during stripping process. Pet. Sci. 2024, online. [Google Scholar] [CrossRef]
- Zhu, M.; Sun, L.; Ou, G.; Wang, K.; Wang, K.; Sun, Y. Erosion corrosion failure analysis of the elbow in sour water stripper overhead condensing reflux system. Eng. Fail. Anal. 2016, 62, 93–102. [Google Scholar] [CrossRef]
- Zahid, U. Techno-economic evaluation and design development of sour water stripping system in the refineries. J. Clean. Prod. 2019, 236, 117633. [Google Scholar] [CrossRef]
- Abolghasem, K.; Mehrabani-Zeinabad, A.; Beheshti, M. Development of a novel processing system for efficient sour water stripping. Energy 2017, 125, 449–458. [Google Scholar]
- Zhao, H.; Yue, Q.; Xie, F.; Wang, D.; Sun, D.; Wu, M.; Pan, Y.; Yang, S. Analysis of heat exchanger corrosion failure in 800,000 light hydrocarbon plant in Liaohe Oilfield. Eng. Fail. Anal. 2023, 151, 107348. [Google Scholar] [CrossRef]
- Lachowicz, M.M. A metallographic case study of formicary corrosion in heat exchanger copper tubes. Eng. Fail. Anal. 2020, 1111, 104502. [Google Scholar] [CrossRef]
- Wang, B.; Arsenyeva, O.; Zeng, M.; Klemeš, J.J.; Varbanov, P.S. An advanced Grid Diagram for heat exchanger network retrofit with detailed plate heat exchanger design. Energy 2022, 248, 123485. [Google Scholar] [CrossRef]
- Diaz-Bejarano, E.; Coletti, F.; Macchietto, S. Modeling and prediction of shell-side fouling in shell-and-tube heat exchangers. Heat Transf. Eng. 2018, 40, 845–861. [Google Scholar] [CrossRef]
- Babuška, I.; Silva, R.S.; Actor, J. Break-off model for CaCO3 fouling in heat exchangers. Int. J. Heat Mass Transf. 2018, 116, 104–114. [Google Scholar] [CrossRef]
- Jin, H.-Z.; Gu, Y.; Ou, G.-F. Corrosion risk analysis of tube-and-shell heat exchangers and design of outlet temperature control system. Pet. Sci. 2021, 18, 1219–1229. [Google Scholar] [CrossRef]
- Prithiraj, A.; Otunniyi, I.O.; Osifo, P.; van der Merwe, J. Corrosion behaviour of stainless and carbon steels exposed to sulphate—Reducing bacteria from industrial heat exchangers. Eng. Fail. Anal. 2019, 104, 977–986. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Z.; Wang, Z.; Shi, Q.; Xu, B.; Zhou, C.; Zhang, L. The influence of copper on the stress corrosion cracking of 304 stainless steel. Appl. Surf. Sci. 2019, 478, 492–498. [Google Scholar] [CrossRef]
- Dan, N.E.; Bin Hussain, P.; Shaik, N.B.; Bakthavatchalam, B.; Mohapatra, R.K.; Behera, A. Improved Surface Morphology and Corrosion Resistance Performance of 2205 Duplex Stainless Steel by Low Temperature Gas Nitriding. J. Bio- Tribo-Corros. 2022, 8, 100. [Google Scholar] [CrossRef]
- Deen, K.; Virk, M.; Haque, C.; Ahmad, R.; Khan, I. Failure investigation of heat exchanger plates due to pitting corrosion. Eng. Fail. Anal. 2010, 17, 886–893. [Google Scholar] [CrossRef]
- Corleto, C.R.; Argade, G.R. Failure analysis of dissimilar weld in heat exchanger. Case Stud. Eng. Fail. Anal. 2017, 9, 27–34. [Google Scholar] [CrossRef]
- Klein, U.; Zunkel, A.; Eberle, A. Breakdown of heat exchangers due to erosion corrosion and fretting caused by inappropriate operating conditions. Eng. Fail. Anal. 2014, 43, 271–280. [Google Scholar] [CrossRef]
- Yang, B.; Huai, X.; Zhao, Y.; Xiu, M. Failure analysis on outer tube breaks of a double-sleeve rapid cooling heat exchanger. Eng. Fail. Anal. 2023, 153, 107540. [Google Scholar] [CrossRef]
- Nicacio, J.A.P.; Oliveira, F.C.; Dumont, M.R. Failure analysis and electrochemical testing of ammonium chloride corrosion in a heat exchanger in a diesel hydrotreating unit of a petroleum refinery. Eng. Fail. Anal. 2023, 156, 107758. [Google Scholar] [CrossRef]
- Fan, Z.D.; Du, J.S.; Zhang, Z.B.; Ma, Y.C.; Cao, S.Y.; Niu, K.; Liu, C.X. Internal leakage of plate heat exchangers caused by cooperation of pitting, crevice corrosion, and fretting. Eng. Fail. Anal. 2019, 96, 340–347. [Google Scholar] [CrossRef]
- Liu, Y.; Qu, Y.; Chang, F.; Li, S. Failure analysis of heat exchange tubes in hydrogenation unit. Eng. Fail. Anal. 2021, 129, 105718. [Google Scholar] [CrossRef]
- GB/T 11170-2008; Stainless Steel-Determination of Multi-Element Contents—Spark Discharge Atomic Emission Spectrometric Method (Routinemethod). Standardization Administration of China: Beijing, China, 2008.
- GB/T 231.1—2018; Metallic Materials—Brinell Hardness Test—Part 1: Test Method. Standardization Administration of China: Beijing, China, 2018.
- GB/T 229.1—2007; Metallic Materials—Charpy Pendulum Impact Test Method. Standardization Administration of China: Beijing, China, 2007.
- GB/T 13298—2015; Inspection Methods of Microstructure for Metal. Standardization Administration of China: Beijing, China, 2015.
- Elhoud, A.; Ezuber, H.; Deans, W. Influence of cold work and sigma phase on the pitting corrosion behavior of 25 chromium super duplex stainless steel in 3.5% sodium chloride solution. Mater. Corros. 2010, 61, 199–204. [Google Scholar] [CrossRef]
- Maehara, Y.; Masao, K.; Fujino, N. Precipitation of σ Phase in a 25Cr-7Ni-3Mo Duplex Phase Stainless Steel. Trans. Iron Steel Inst. Jpn. 1983, 23, 240–246. [Google Scholar] [CrossRef]
- Maehara, Y.; Fujino, N.; Kunitake, T. Effects of Plastic Deformation and Thermal History on σ Phase Precipitation in Duplex Phase Stainless Steels. Trans. Iron Steel Inst. Jpn. 1983, 23, 247–255. [Google Scholar] [CrossRef]
- GB/T 4334-2020; Corrosion of Metals and Alloys—Intergranular Corrosion Test for Austenitic and Ferritic-Austenitic (Duplex) Stainless Steels—Ferric Chloride Solution Method (F Method). Standardization Administration of China: Beijing, China, 2020.
- API RP 932-B-2019; Design, Materials, Fabrication, Operation, and Inspection Guidelines for Corrosion Control in Hydroprocessing Reactor Effluent Air Cooler (REAC) Systems. American Petroleum Institute (API): Washington, DC, USA, 2019.
- GB/T 8570-2008; Determination of Liquefied Anhydrous Ammonia. Standardization Administration of China: Beijing, China, 2008.
- HJ/T 195-2005; Water Quality—Determination of Ammonia-Nitrogen Gas-Phase Molecular Absorption Spectrometry. Ministry of Ecology and Environment of the People’s Republic of China: Beijing, China, 2005.
- SY/T 6537-2016; Analysis Methods of Gas and Solution for Natural Gas Treating Plant. National Energy Administration: Beijing, China, 2016.
- Bastos, P.D.; Bastos, A.C.; Ferreira, M.G.; Santos, M.A.; Carvalho, P.J.; Crespo, J.G. A corrosion evaluation of mild carbon steel in reclaimed refinery stripped sour water. J. Environ. Manag. 2020, 272, 111080. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, E.E.; Rodríguez, M.V.; Chavarín, J.U.; Cruz, V.R. Corrosion of Carbon Steel in Sour Water From the Oil Industry: The Effect of Temperature. Int. J. Electrochem. Sci. 2011, 6, 5016–5030. [Google Scholar] [CrossRef]
- Obot, I.B.; Sorour, A.A.; Verma, C.; Al-Khaldi, T.A.; Rushaid, A.S. Key parameters affecting sweet and sour corrosion: Impact on corrosion risk assessment and inhibition. Eng. Fail. Anal. 2023, 145, 107008. [Google Scholar] [CrossRef]
- Srinivasan, S.; Lagad, V.; Kane, R.D. Evaluation of Prediction Tool for Sour Water Corrosion Quantification and Management in Refineries. In Proceedings of the CORROSION 2009, Atlanta, GA, USA, 22–26 March 2009. [Google Scholar]






| Design Pressure (MPa) | Design Temperature (°C) | Operating Pressure (MPa) | Operating Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|
| Shell Range | Tube Range | Shell Range | Tube Range | Shell Range | Tube Range | Shell Range | Tube Range |
| 0.83 | 0.67 | 183 | 80 | 0.39 | 0.3 | 35 | 30 |
| Element | C | Si | Mn | P | S | Cr | Mo | Ni | N |
|---|---|---|---|---|---|---|---|---|---|
| Corroded flanges | 0.024 | 0.56 | 0.92 | 0.035 | 0.001 | 24.57 | 3.59 | 6.94 | 0.26 |
| New flanges | 0.025 | 0.55 | 0.93 | 0.034 | 0.001 | 24.66 | 3.55 | 6.98 | 0.25 |
| S2507 | ≤0.03 | ≤0.08 | ≤1.20 | ≤0.035 | ≤0.020 | 24.00~26.00 | 3~5 | 6~8 | 0.24~0.32 |
| Element | C | O | Na | Si | P | Mo | S | Ca | Cr | Mn | Fe | Ni | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 29.55 | 15.19 | 0.87 | 0.35 | 0.51 | 3.8 | 2.73 | 0.47 | 10.88 | 0.65 | 32.55 | 2.44 | - |
| 2 | 35.92 | 13.88 | 0.67 | 0.37 | 0.56 | 4.34 | 6.05 | - | 7.48 | 0.67 | 27.63 | 1.99 | 0.46 |
| 3 | 16.97 | 4.36 | - | 0.83 | - | 2.72 | - | - | 20.04 | 1.1 | 48.66 | 5.32 | - |
| Test Sample | Number of Samples | Sample Size (mm) | Test Solution | Sensitization System | Testing Time/h | Bending Angle/° |
|---|---|---|---|---|---|---|
| Corrosion flange | 4 | 58×20×3 | Cu-CuSO4−35%H2SO4 (microboil) | 700 °C Holding 30 min, water cooling | 20 | 90 |
| New flange | 4 | 80×16×3 |
| Medium | NH3 | H2S | CO2 | H2O | Hydrocarbons |
|---|---|---|---|---|---|
| Crude ammonia (inlet) | 80.86 | 8.94 | 0 | 10.20 | 0 |
| Crude ammonia (exits) | 95.04 | 3.92 | 0 | 1.04 | 0 |
| Condensate (exits) | 36.74 | 24.56 | 0 | 38.70 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, H.; Duan, Z.; Li, W. Analysis of Causes and Protective Measures against Corrosion Perforation in the Shell-Side Outlet Flange of a Sour Water Steam Heater. Coatings 2024, 14, 306. https://doi.org/10.3390/coatings14030306
Liang H, Duan Z, Li W. Analysis of Causes and Protective Measures against Corrosion Perforation in the Shell-Side Outlet Flange of a Sour Water Steam Heater. Coatings. 2024; 14(3):306. https://doi.org/10.3390/coatings14030306
Chicago/Turabian StyleLiang, Haiming, Zhihong Duan, and Weiming Li. 2024. "Analysis of Causes and Protective Measures against Corrosion Perforation in the Shell-Side Outlet Flange of a Sour Water Steam Heater" Coatings 14, no. 3: 306. https://doi.org/10.3390/coatings14030306
APA StyleLiang, H., Duan, Z., & Li, W. (2024). Analysis of Causes and Protective Measures against Corrosion Perforation in the Shell-Side Outlet Flange of a Sour Water Steam Heater. Coatings, 14(3), 306. https://doi.org/10.3390/coatings14030306





