Innovation and Evaluations of 3D Printing Resins Modified with Zirconia Nanoparticles and Silver Nanoparticle-Immobilized Halloysite Nanotubes for Dental Restoration
Abstract
:1. Introduction
2. Materials
2.1. Silver Nanoparticles and Zirconia Nanoparticles
2.2. Preparation of Zirconia Nanoparticles
2.3. Preparation of Silver Nanoparticle-ImmobilizedHalloysite Nanotubes
2.4. Preparation of Sample and 3D Printing
2.5. Flexural Strength and Modulus
2.6. Fracture Toughness Testing
2.7. Vickers Microhardness Test (VHN)
- VHN = p/d2 × C.
- VHN = Vickers microhardness number.
- P = Load applied equal 1000 gm.
- d2 = Diagonal length square of the indentation.
- C = Constant equals 1.854.
3. Result
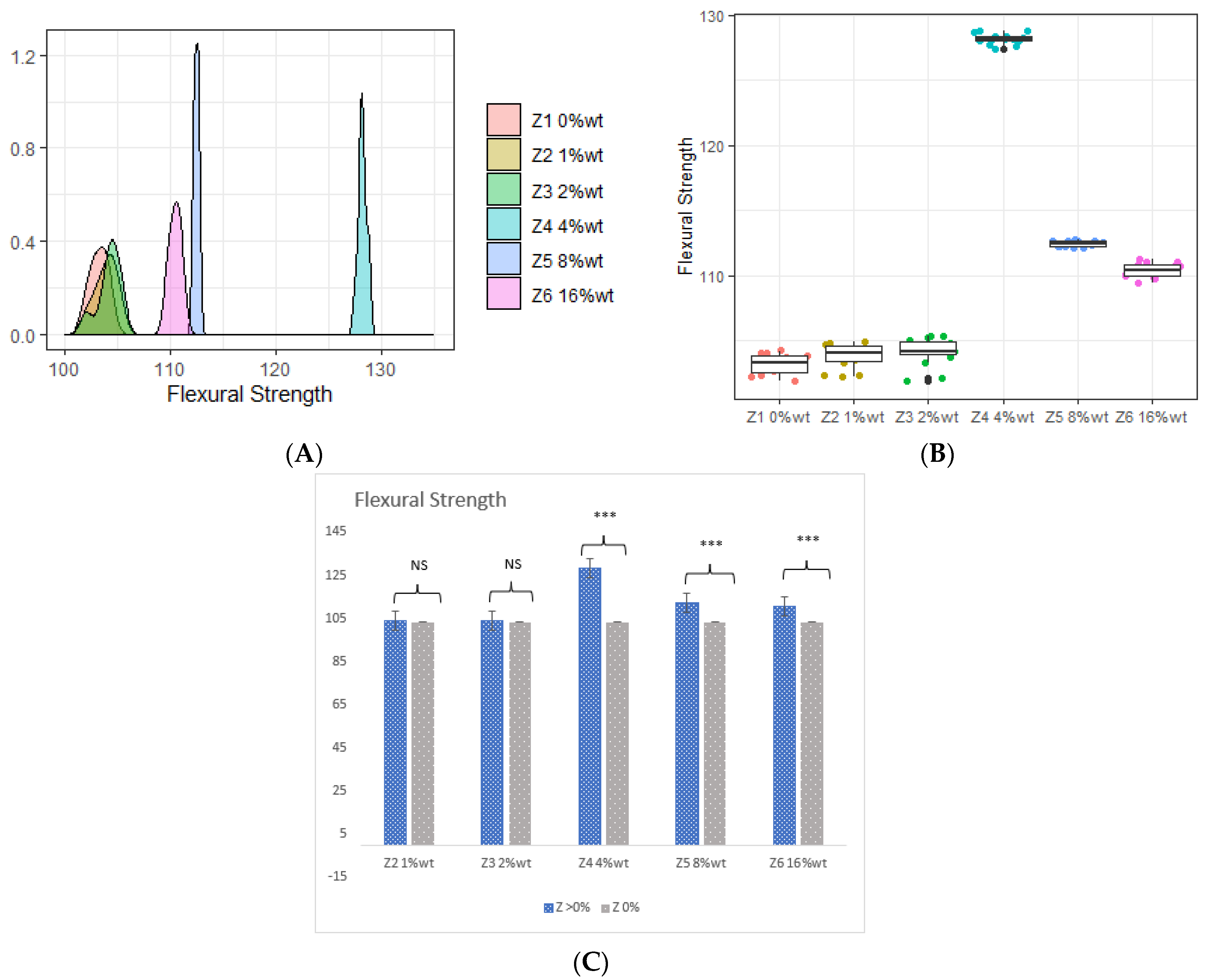
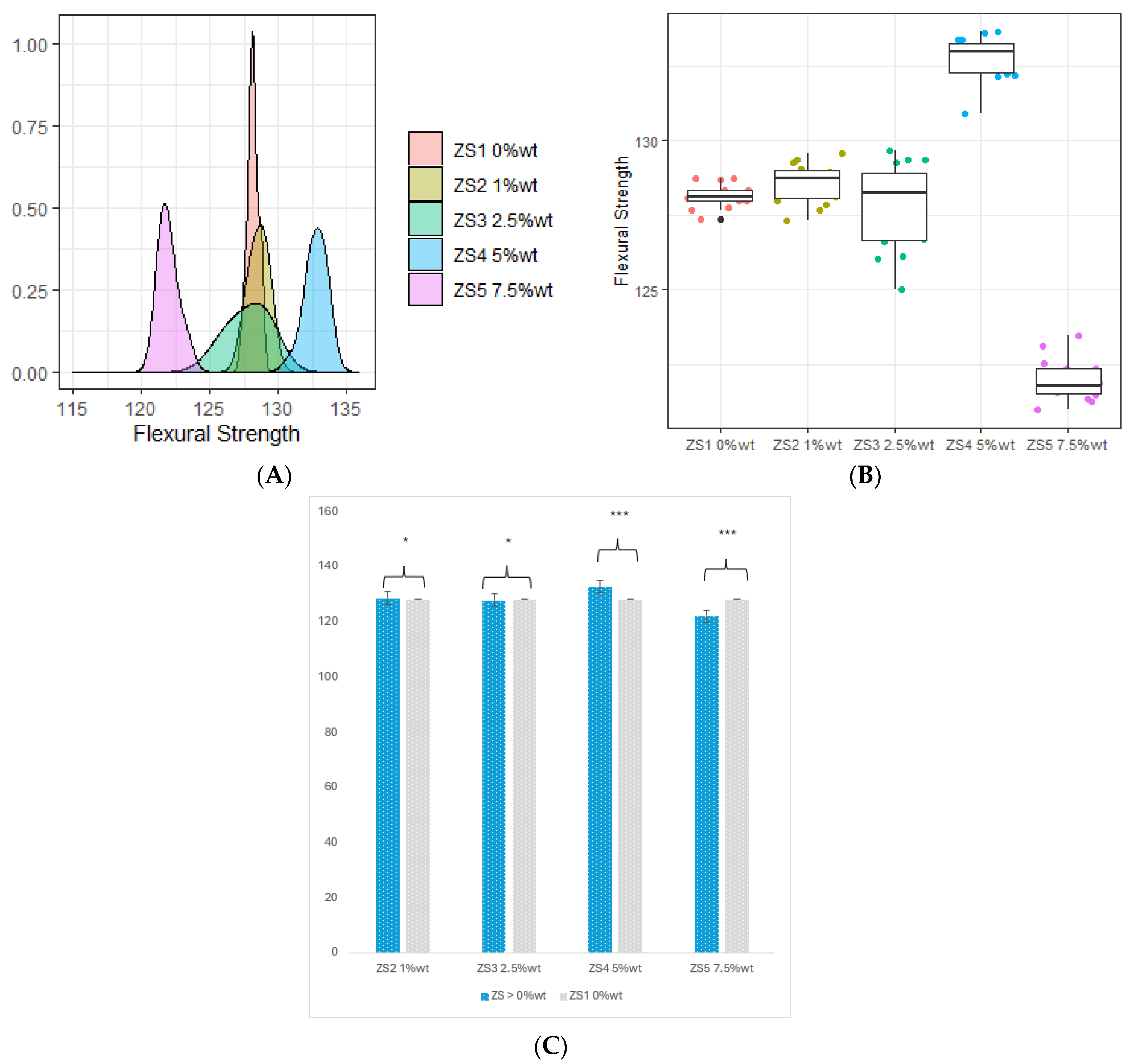
3.1. Flexural Strength (FS) and Flexural Modulus (FM)
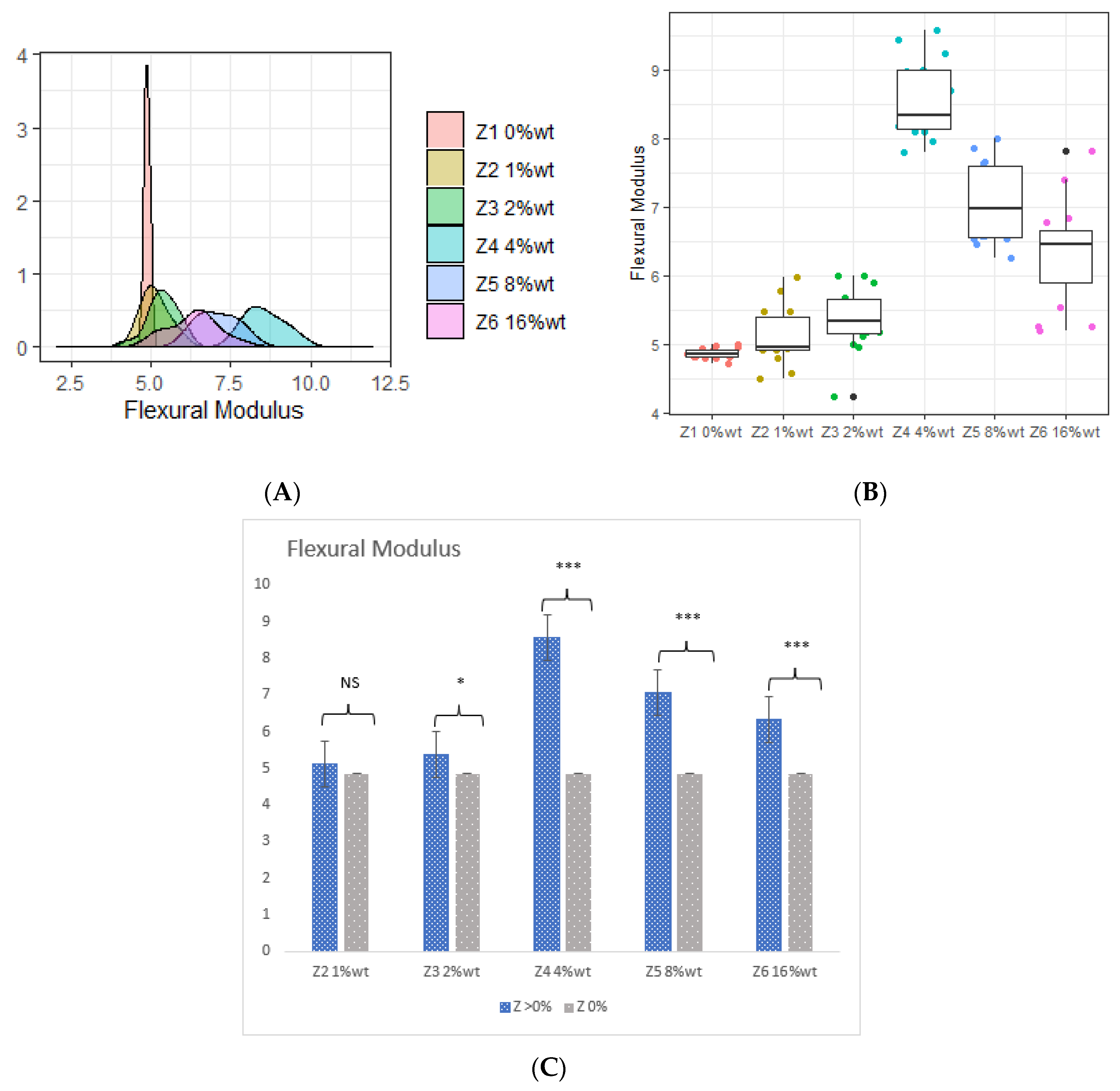
3.2. Flexural Modulus
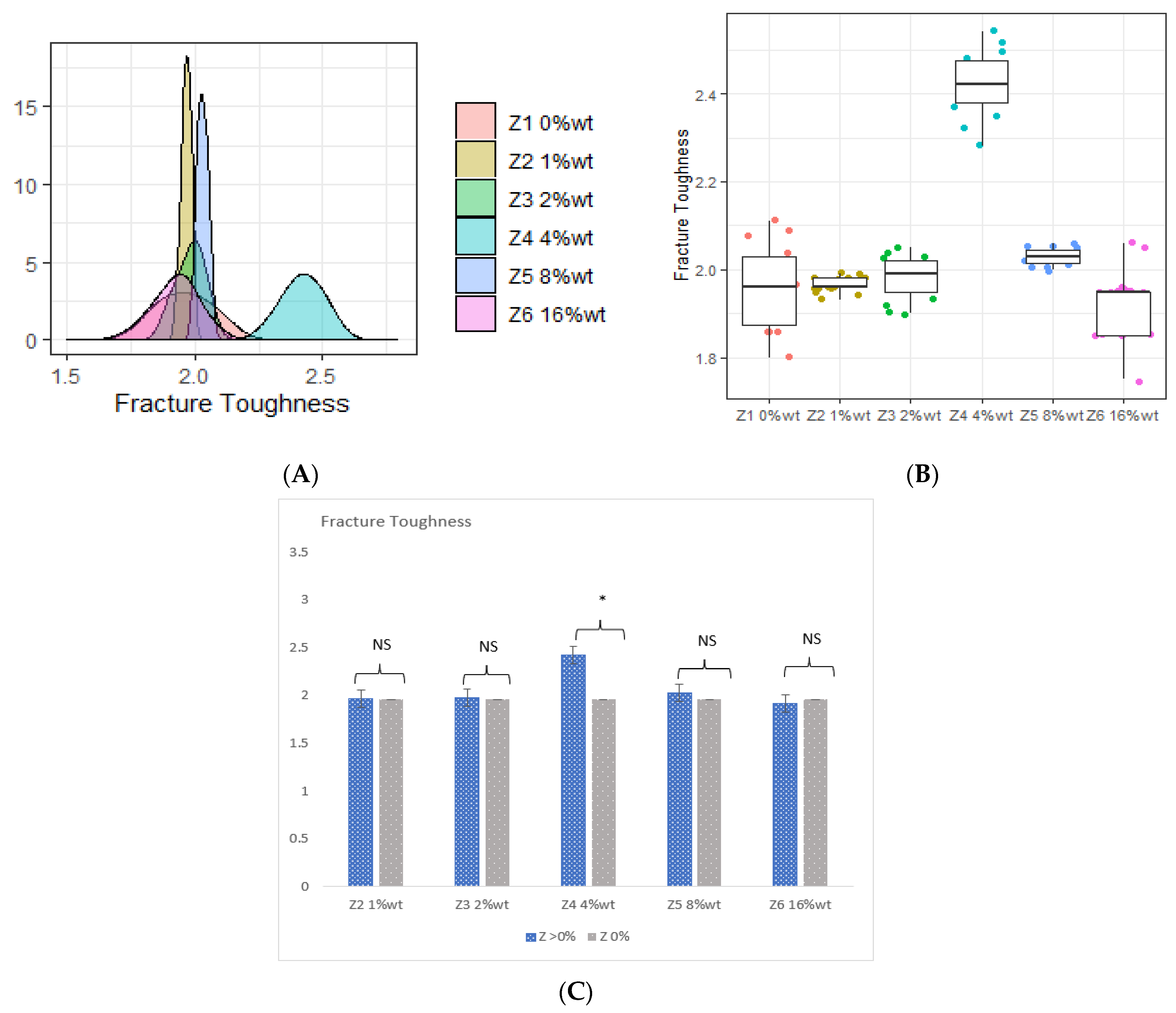
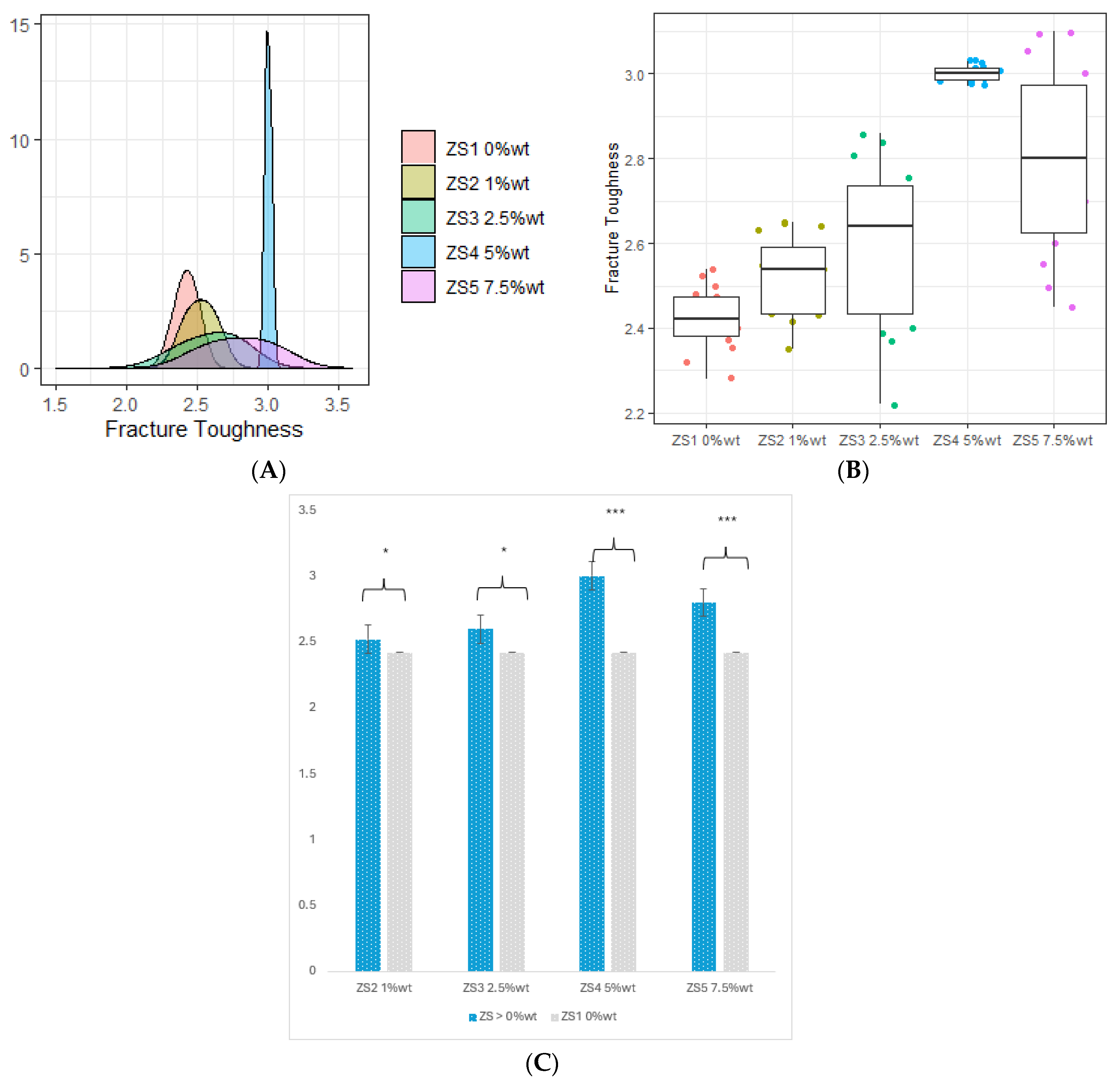
3.3. Fracture Toughness
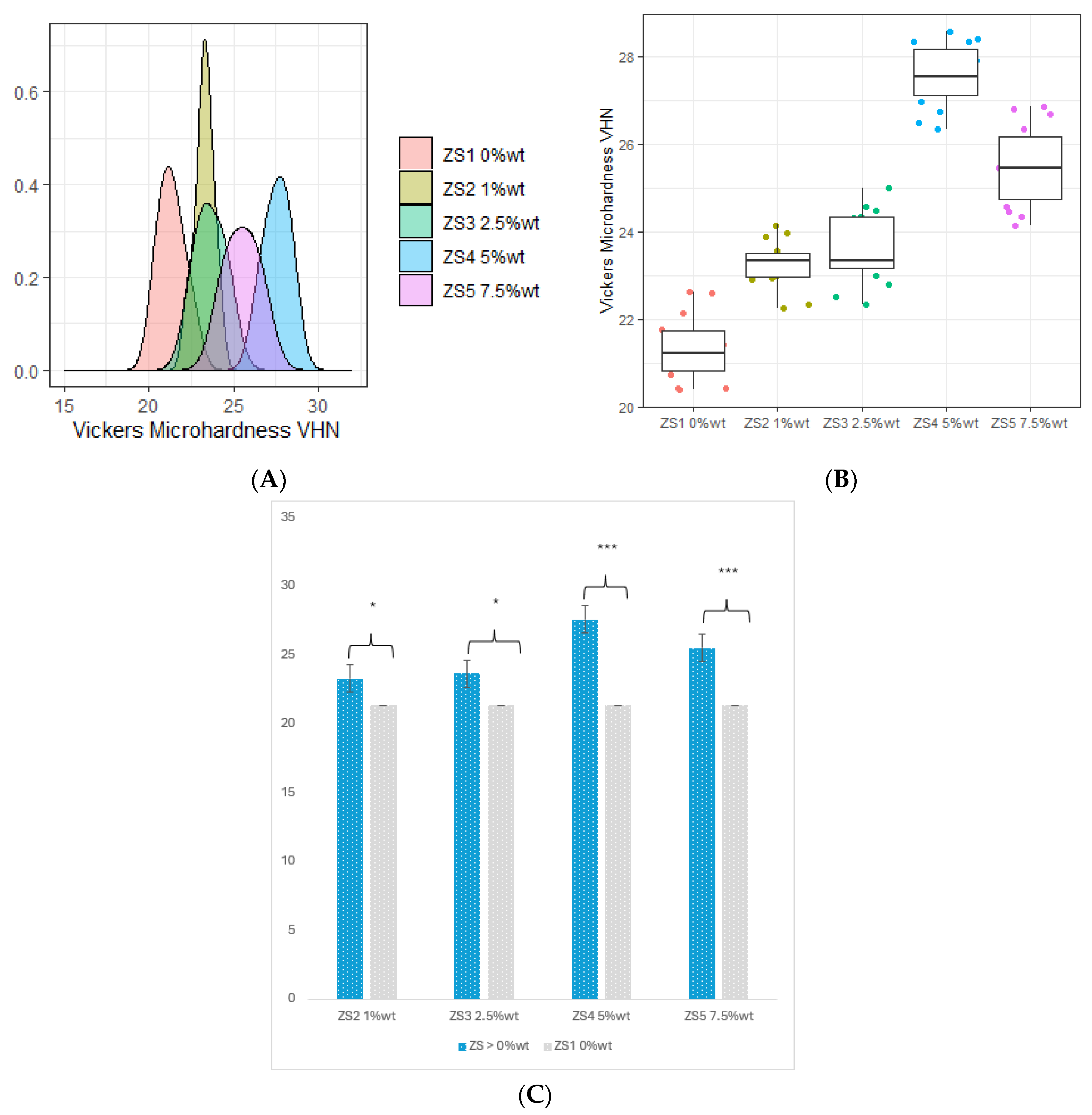
3.4. Vickers Microhardness (VHN)
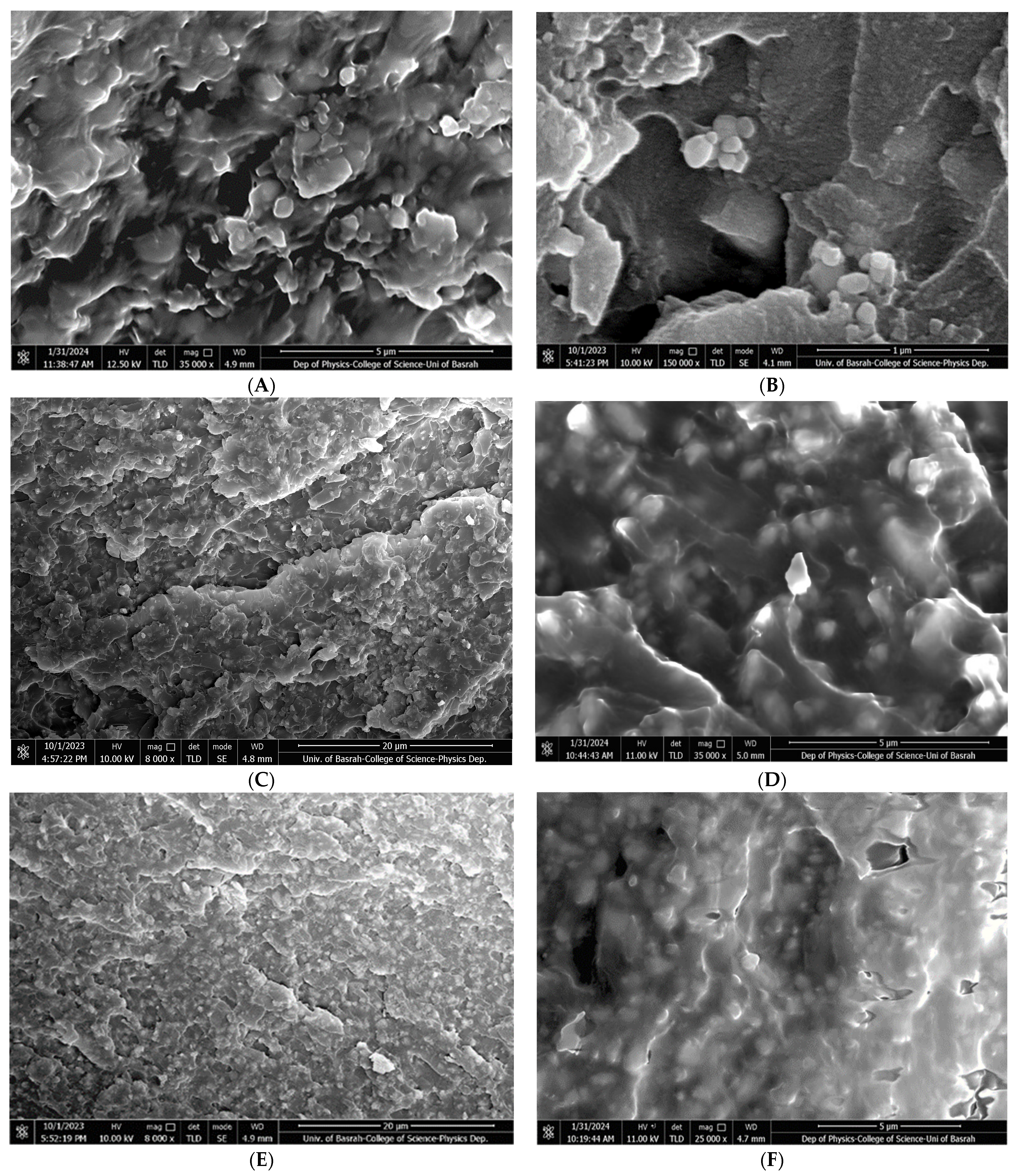
3.5. Microscopical Characterization
4. Discussion
5. Conclusions
- (A)
- The incorporation of 4 %wt. ZrO2, 8 %wt. ZrO2, 16 %wt. ZrO2, and 5 %wt. HNC/Ag nanoparticles significantly increased the flexural strength and flexural modulus of the 3D-printed resin, whereas the 7.5 %wt. HNC/Ag decreased the flexural strength and flexural modulus of the resin.
- (B)
- The incorporation of 4 %wt. ZrO2, 2.5 %wt. HNC/Ag, 5 %wt. HNC/Ag, and 7.5 %wt. HNC/Ag nanoparticles significantly increased the fracture toughness of the 3D-printed resin
- (C)
- All fractions of ZrO2 and HNC/Ag nanoparticles significantly increased the microhardness of the 3D-printed resin.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tabriz, A.G.; Viegas, B.; Okereke, M.; Uddin, M.J.; Lopez, E.A.; Zand, N.; Ranatunga, M.; Getti, G.; Douroumis, D. Evaluation of 3D Printability and Biocompatibility of Microfluidic Resin for Fabrication of Solid Microneedles. Micromachines 2022, 13, 1368. [Google Scholar] [CrossRef] [PubMed]
- Bellini, A.; Güçeri, S. Mechanical characterization of parts fabricated using fused deposition modeling. Rapid Prototype J. 2003, 9, 52–64. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 4. [Google Scholar] [CrossRef]
- Elahpour, N.; Pahlevanzadeh, F.; Kharaziha, M.; Bakhsheshi-Rad, H.R.; Ramakrishna, S.; Berto, F. 3D printed microneedles for transdermal drug delivery: A brief review of two decades. Int. J. Pharm. 2021, 597, 120301. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.; Alhotan, A.; Kelly, E.; Ellakwa, A. Mechanical and Biocompatibility Properties of 3D-Printed Dental Resin Reinforced with Glass Silica and Zirconia Nanoparticles: In Vitro Study. Polymers 2023, 15, 2523. [Google Scholar] [CrossRef]
- Jawahar, A.; Maragathavalli, G. Applications of 3D Printing in Dentistry—A Review. J. Pharm. Sci. Res. 2019, 11, 1670–1675. [Google Scholar]
- Anketa, J.; Ikshita, C.; Ishika, W.; Ankush, R.; Mir, I.H. 3D printing—A review of processes, materials, and applications in industry 4.0. Sustain. Oper. Comput. 2022, 3, 33–42. [Google Scholar]
- Konidena, A. 3D Printing: Future of dentistry? J. Indian Acad. Oral Med. Radiol. 2016, 28, 109. [Google Scholar] [CrossRef]
- Voet, V.S.D.; Strating, T.; Schnelting, G.H.M.; Dijkstra, P.; Tietema, M.; Xu, J.; Woortman, A.J.J.; Loos, K.; Jager, J.; Folkersma, R. Biobased Acrylate Photocurable Resin Formulation for Stereolithography 3D Printing. ACS Omega 2018, 3, 1403–1408. [Google Scholar] [CrossRef]
- Mosavari, M.; Khajehhaghverdi, A.; Aghdam, R. Nano- ZrO2: A review on synthesis methodologies. Inorg. Chem. Commun. 2023, 157, 111293. [Google Scholar] [CrossRef]
- Hu, C.; Sun, J.; Long, C.; Wu, L.; Zhou, C.; Zhang, X. Synthesis of nano zirconium oxide and its application in dentistry. Nanotechnol. Rev. 2019, 8, 396–404. [Google Scholar] [CrossRef]
- Son, M.; Raju, K.; Lee, J.; Jung, J.; Jeong, S.; Kim, J.I.; Cho, J. 3D Printing of CNT- and YSZ-Added Dental Resin-Based Composites by Digital Light Processing and Their Mechanical Properties. Materials 2023, 16, 1873. [Google Scholar] [CrossRef] [PubMed]
- El-Tamimi, K.M.; Bayoumi, D.A.; Ahmed, M.M.Z.; Albaijan, I.; El-Sayed, M.E. The Effect of Salinized Nano ZrO2 Particles on the Microstructure, Hardness, and Wear Behavior of Acrylic Denture Tooth Nanocomposite. Polymers 2022, 14, 302. [Google Scholar] [CrossRef] [PubMed]
- Abdulrazzaq, N.S.; Al-azzawi, M.A. Effect of Zirconium Oxide -Titanium Dioxide Nanoparticles on Mechanical and Physical Properties of Soft Denture Lining Materials. J. Nanostruct. 2022, 12, 34–44. [Google Scholar]
- Chen, S.; Yang, J.; Jia, Y.G.; Lu, B.; Ren, L. A Study of 3D-Printable Reinforced Composite Resin: PMMA Modified with Silver Nanoparticles Loaded Cellulose Nanocrystal. Materials 2018, 11, 2444. [Google Scholar] [CrossRef]
- Ivask, A.; ElBadawy, A.; Kaweeteerawat, C.; Boren, D.; Fischer, H.; Ji, Z.; Chang, C.H.; Liu, R.; Tolaymat, T.; Telesca, D.; et al. Toxicity Mechanisms in Escherichia coli Vary for Silver Nanoparticles and Differ from Ionic Silver. ACS Nano 2014, 8, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Chaubal, T.V.; Joshi, C.P.; Bapat, P.R.; Choudhury, H.; Pandey, M.; Gorain, B.; Kesharwani, P. An overview of the application of silver nanoparticles for biomaterials in dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 91, 881–898. [Google Scholar] [CrossRef]
- Kumari, N.; Sareen, S.; Verma, M.; Sharma, S.; Sharma, A.; Sohal, H.S.; Mehta, S.K.; Park, J.; Mutreja, V. Zirconia-based nanomaterials: Recent developments in synthesis and applications. Nanoscale Adv. 2022, 4, 4210–4236. [Google Scholar] [CrossRef]
- Dai, S.; Chen, Y.; Yang, J.; He, F.; Chen, C.; Xie, H. Surface Treatment Of Nanozirconia Fillers To Strengthen Dental Bisphenol A-Glycidyl Methacrylate-Based Resin Composites. Int. J. Nanomed. 2019, 14, 9185–9197. [Google Scholar] [CrossRef]
- Matos, Y.B.; Romanus, R.S.; Torquato, M.; de Souza, E.H.; Villanova, R.L.; Soares, M.; Viana, E.R. Silver nanoparticles nucleated in NaOH-treated halloysite: A potential antimicrobial material. Bilstein J. Nano Technol 2021, 12, 798–807. [Google Scholar] [CrossRef]
- Sypabekova, M.; Hagemann, A.; Rho, D.; Kim, S. Review: 3-Aminopropyltriethoxysilane (APTES) Deposition Methods on Oxide Surfaces in Solution and Vapor Phases for Biosensing Applications. Biosensors 2023, 13, 36. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Ngo, H.; Fawzy, A.S. Development of 3D printed resin reinforced with modified ZrO2 nanoparticles for long-term provisional dental restorations. Dent. Mater. 2021, 37, e360–e374. [Google Scholar] [CrossRef]
- ISO 4049; Dentistry—Polymer-Based Restorative Materials. International Standards Organization (ISO): Geneva, Switzerland, 2019.
- ISO 20795-1:2013; Dentistry—Base Polymers—Part 1: Denture Base Polymers. International Standards Organization (ISO): Geneva, Switzerland, 2013.
- ElGayar, M.; Moustafa, E.; Ghoneim, M. Microhardness & degree of conversion of recently introduced bulk-fill composite resin using different application techniques. Alex. Dent. J. 2023, 48, 126–136. [Google Scholar]
- ISO 10477; Dentistry—Polymer-Based Crown and Veneering Materials. International Standards Organization (ISO): Geneva, Switzerland, 2020.
- Prakash, J.; Shenoy, M.; Alhasmi, A.; Al Saleh, A.; Shivakumar, A.; Shivakumar, S. Biocompatibility of 3D-Printed Dental Resins: A Systematic Review. Cureus 2024, 1, e51721. [Google Scholar] [CrossRef]
- Alshaikh, A.A.; Khattar, A.; Almindil, I.A.; Alsaif, M.H.; Akhtar, S.; Khan, S.Q.; Gad, M.M. 3D-printed nanocomposite denture-base resins: Effect of ZrO(2) nanoparticles on the mechanical and surface properties in vitro. Nanomaterials 2022, 12, 2451. [Google Scholar] [CrossRef]
- Azmy, E.; Al-Kholy, M.R.; Fattouh, M.; Kenawi, L.M.; Helal, M.A. Impact of Nanoparticle Additions on the Strength of Dental Composite Resin. Int. J. Biomater. 2022, 2022, 1165431. [Google Scholar] [CrossRef] [PubMed]
- Hiers, R.D.; Huebner, P.; Khajotia, S.S.; Florez, F.L.E. Characterization of Experimental Nanoparticulated Dental Adhesive Resins with Long-Term Antibacterial Properties. Nanomaterials 2022, 12, 3732. [Google Scholar] [CrossRef] [PubMed]
- Al-Dulaijan, Y.A.; AlGhamdi, M.A.; Azmy, E.; Al-Kholy, M.R.Z.; Almulhim, K.S.; Helal, M.A. Color Stability of Nanoparticles-Modified Dental Resin-Based Composites. Appl. Sci. 2023, 13, 3870. [Google Scholar] [CrossRef]
- Hong, G.; Yang, J.; Jin, X.; Wu, T.; Dai, S.; Xie, H.; Chen, C. Mechanical Properties of Nanohybrid Resin Composites Containing Various Mass Fractions of Modified Zirconia Particles. Int. J. Nanomed. 2020, 15, 9891–9907. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.P.; Amar, B. Inder. The in vitro wear behavior of nano zirconia-filled dental composite in food slurry condition. Proc. Inst. Mech. Eng. J. J. Eng. Tribol. 2016, 231, 23–40. [Google Scholar] [CrossRef]
- Albadr, R. Effect of addition ZrO2 nanoparticles to dental composites on the physical and mechanical properties. Int. J. Sci. Eng. 2018, 9, 1288–1293. [Google Scholar]
- Barot, T.M.; Deepak, K.P. Physicochemical and biological assessment of silver nanoparticles immobilized Halloysite nanotubes-based resin composite for dental applications. Heliyon 2020, 6, e03601. [Google Scholar] [CrossRef]
- Jana, S.; Kondakova, A.V.; Shevchenko, S.N.; Sheval, E.V.; Gonchar, K.A.; Timoshenko, V.Y.; Vasiliev, A.N. Halloysite nanotubes with immobilized silver nanoparticles for anti-bacterial application. Colloids Surf. B Biointerfaces 2017, 151, 249–254. [Google Scholar] [CrossRef]
- Wang, B.; Arab, A.; Xie, J.; Chen, P. The Influence of Microstructure on the Flexural Properties of 3D Printed Zirconia Part via Digital Light Processing Technology. Materials 2022, 15, 1602. [Google Scholar] [CrossRef]
- Halim, S. Comparative Evaluation of Micro-hardness and Surface Roughness of Different Composites Resins and Polishing System (In-Vitro Study). Ahram Can. Dent. J. 2023, 2, 24–36. [Google Scholar] [CrossRef]
- Alla, R.K.; Guduri, V.; Tiruveedula, N.B.; Narasimha, R.G.; Swamy, K.R.; Vyas, R. Effect of silver nanoparticles incorporation on microhardness of Heat-cure denture base resins. Int. J. Dent. Mater. 2020, 2, 103–110. [Google Scholar] [CrossRef]
- Sokolowski, J.; Szynkowska, M.I.; Kleczewska, J.; Kowalski, Z.; Sobczak-Kupiec, A.; Pawlaczyk, A. Evaluation of resin composites modified with nanogold and nanosilver. Acta Bioeng. Biomech. 2014, 16, 1651–1661. [Google Scholar]
- Hada, T.; Kanazawa, M.; Miyamoto, N.; Liu, H.; Iwaki, M.; Komagamine, Y.; Minakuchi, S. Effect of Different Filler Contents and Printing Directions on the Mechanical Properties for Photopolymer Resins. Int. J. Mol. Sci. 2022, 23, 2296. [Google Scholar] [CrossRef]
- Gad, M.M.; Abualsaud, R.; Al-Thobity, A.M.; Baba, N.Z.; Al-Harbi, F.A. Influence of Addition of Different Nanoparticles on the Surface Properties of Poly(methylmethacrylate) Denture Base Material. J. Prosthodont. 2020, 29, 422–428. [Google Scholar] [CrossRef]
- Bottino, M.C.; Batarseh, G.; Palasuk, J.; Alkatheeri, M.S.; Windsor, L.J.; Platt, J.A. Nanotube-modified dentin adhesive—Physicochemical and dentin bonding characterizations. Dent. Mater. J. 2013, 29, 1158–1165. [Google Scholar] [CrossRef]
- Tejas, B.; Deepak, R.; Pratik, K.; Chaudhary, M.; Satyaprasad, A. Physicochemical and biological assessment of flowable resin composites incorporated with farnesol loaded halloysite nanotubes for dental applications. J. Mech. Behav. Biomed. Mater. 2020, 103675, 1751–6161. [Google Scholar]
- Jehan, A.; Chidambaranathan, A.; Balasubramanium, M. Effect of Nanoparticles on Mechanical Properties of Chemically Activated Provisional PMMA Resin: An In Vitro Study. World J. Dent. 2023, 14, 617–624. [Google Scholar] [CrossRef]
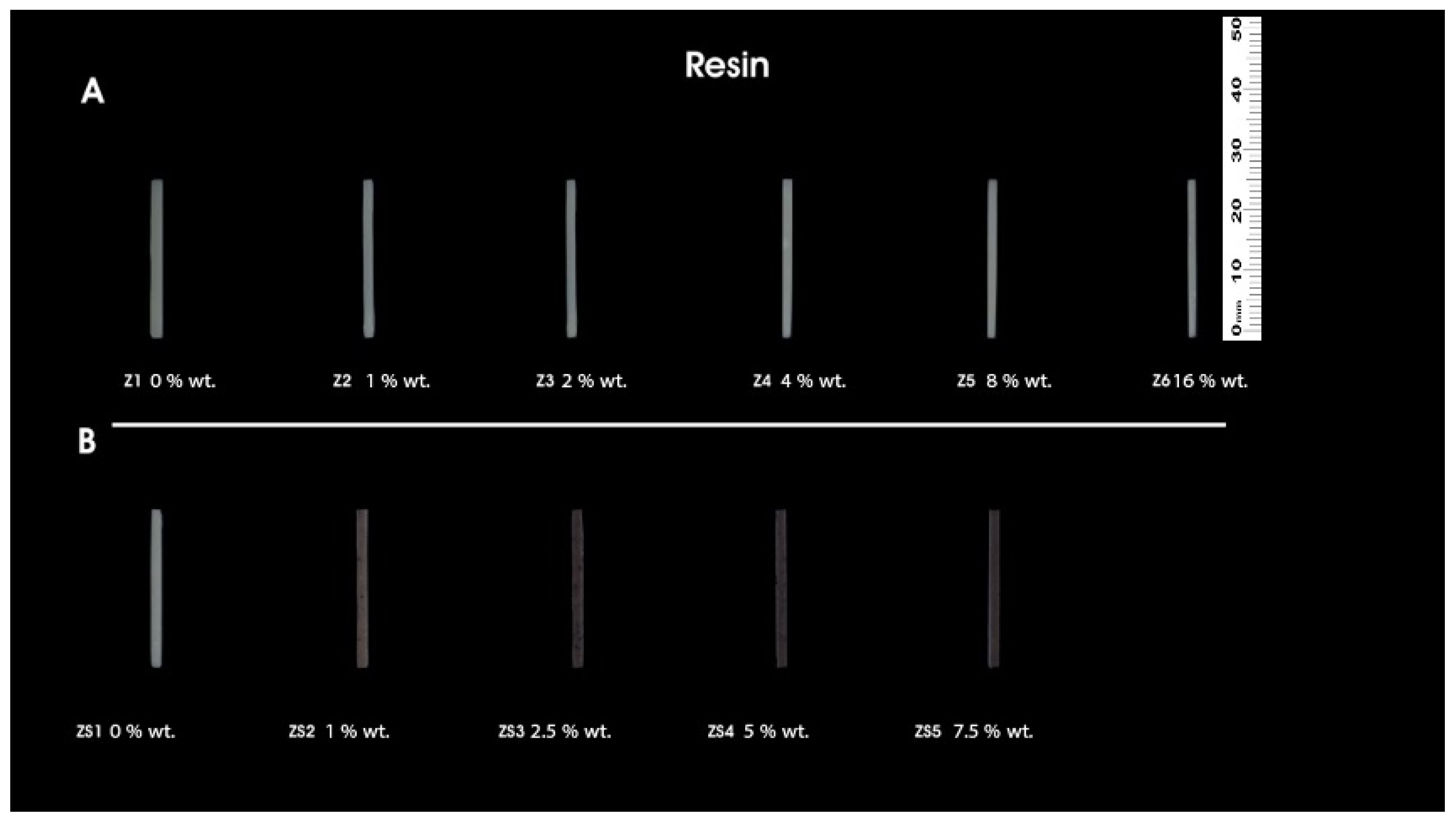




| Sample Code | %wt. of ZrO2 | Resin |
|---|---|---|
| Z1 | 0 | A1 saremco print CROWNTEC |
| Z2 | 1 | A1 saremco print CROWNTEC |
| Z3 | 2 | A1 saremco print CROWNTEC |
| Z4 | 4 | A1 saremco print CROWNTEC |
| Z5 | 8 | A1 saremco print CROWNTEC |
| Z6 | 16 | A1 saremco print CROWNTEC |
| Sample Code | %wt. of HNC/Ag | %wt. of ZrO2 | Resin |
|---|---|---|---|
| ZS1 | 0 | 4 | A1 saremco print CROWNTEC |
| ZS2 | 1 | 4 | A1 saremco print CROWNTEC |
| ZS3 | 2.5 | 4 | A1 saremco print CROWNTEC |
| ZS4 | 5 | 4 | A1 saremco print CROWNTEC |
| ZS5 | 7.5 | 4 | A1 saremco print CROWNTEC |
| Nanoparticles Portions | Flexural Strength (MPa) | Flexural Modulus (GPa) | Fracture Toughness (Mpa.m1.2) | Vickers Microhardness (HV0.05) |
|---|---|---|---|---|
| ZrO2 | ||||
| Z1 0 %wt. (Control) | 103.190 ± 0.769 | 4.860 ± 0.079 | 1.960 ± 0.096 | 16.090 ± 0.642 |
| Z2 1 %wt. | 103.840 ± 0.940 | 5.120 ± 0.419 | 1.970 ± 0.018 | 16.880 ± 0.576 |
| Z3 2 %wt. | 104.150 ± 1.054 | 5.360 ± 0.459 | 1.980 ± 0.049 | 17.030 ± 0.222 |
| Z4 4 %wt. | 128.140 ± 0.395 | 8.560 ± 0.562 | 2.420 ± 0.074 | 21.340 ± 0.730 |
| Z5 8 %wt. | 112.430 ± 0.217 | 7.070 ± 0.582 | 2.030 ± 0.018 | 23.440 ± 0.704 |
| Z6 16 %wt. | 110.410 ± 0.523 | 6.330 ± 0.764 | 1.920 ± 0.081 | 27.080 ± 0.391 |
| ZrO2/HNC/Ag | ||||
| ZS1 0 %wt. | 128.137 ± 0.395 | 8.557 ± 0.561 | 2.422 ± 0.074 | 21.339 ± 0.730 |
| ZS2 1 %wt. | 128.540 ± 0.668 | 8.853 ± 0.177 | 2.520 ± 0.097 | 23.272 ± 0.534 |
| ZS3 2.5 %wt. | 127.778 ± 1.438 | 8.856 ± 0.450 | 2.597 ± 0.194 | 23.621 ± 0.811 |
| ZS4 5 %wt. | 132.727 ± 0.731 | 9.903 ± 0.075 | 3.001 ± 0.020 | 27.560 ± 0.714 |
| ZS5 7.5 %wt. | 121.947 ± 0.690 | 6.993 ± 0.281 | 2.796 ± 0.217 | 25.511 ± 0.924 |
| ANOVA Test | <0.001 | <0.001 | <0.001 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darbandi, K.R.; Amin, B.K. Innovation and Evaluations of 3D Printing Resins Modified with Zirconia Nanoparticles and Silver Nanoparticle-Immobilized Halloysite Nanotubes for Dental Restoration. Coatings 2024, 14, 310. https://doi.org/10.3390/coatings14030310
Darbandi KR, Amin BK. Innovation and Evaluations of 3D Printing Resins Modified with Zirconia Nanoparticles and Silver Nanoparticle-Immobilized Halloysite Nanotubes for Dental Restoration. Coatings. 2024; 14(3):310. https://doi.org/10.3390/coatings14030310
Chicago/Turabian StyleDarbandi, Karwan Rashid, and Bassam Karem Amin. 2024. "Innovation and Evaluations of 3D Printing Resins Modified with Zirconia Nanoparticles and Silver Nanoparticle-Immobilized Halloysite Nanotubes for Dental Restoration" Coatings 14, no. 3: 310. https://doi.org/10.3390/coatings14030310
APA StyleDarbandi, K. R., & Amin, B. K. (2024). Innovation and Evaluations of 3D Printing Resins Modified with Zirconia Nanoparticles and Silver Nanoparticle-Immobilized Halloysite Nanotubes for Dental Restoration. Coatings, 14(3), 310. https://doi.org/10.3390/coatings14030310






