Abstract
TiN-Ag ceramic coatings deposited by magnetron sputtering on silicon wafers and AISI F138 stainless-steel substrates with different Ag concentrations were evaluated for their application as decorative coatings. The results obtained indicated an almost linear increase in the thickness and roughness of the film as a function of the increase in the silver content of the film. For Ag concentrations greater than (8.3 ± 0.5) at %, a matte/satin finish was observed, i.e., a dull surface, produced by the agglomeration of particulates and the increase in roughness, respectively, which was corroborated by SEM and AFM analyses. The EDS analyses indicated particles with a high concentration of silver, but the elements titanium and nitrogen were also observed, indicating the formation of the TiN-Ag coating. The L*a*b* parameters in the CIELab color space were evaluated. No major variations were observed for coatings A and B (Ag concentrations of (4.1 ± 0.4) and (6.3 ± 1.2) at %, respectively). When the Ag content increased substantially, there was a corresponding decrease in L* values, as well as a shift in red reflectance. Furthermore, unwanted changes in the visual appearance and resistance to accelerated corrosion (salt spray) were also analyzed, as these factors compromised the film’s aesthetics in decorative applications.
1. Introduction
Thin titanium nitride (TiN) coatings are well-established and widely used in a variety of industries [1,2,3]. Titanium nitride is a very hard and wear-resistant material known for its excellent chemical stability, high melting point, and low coefficient of friction. One of the most common applications of TiN coatings is in cutting tools, such as drills and milling cutters, as they can significantly improve the performance and tool life of these components [4].
Another important application segment is decorative coatings. The use of different TiN, CrN, and other multilayer films is reported in the literature [5,6]. This segment has shown significant growth in recent decades, with projections of annual growth of 8% in the coming years [7]. The metal sanitary, faucet, and accessories industry has followed this evolution.
The universe of decorative application is closely linked to the fashion industry, which is dynamic, volatile, and constantly changing. A great variability in aesthetic requirements is demanded, which makes the offer and variability of colors an important prerequisite, as they must reflect the trends of the present, as well as the preferences and demands of the final consumer. Within this context, color is probably one of the most important parameters in this segment, and one of the main objectives is to add value to the component; that is, to increase the perceived value to convert it into a desired luxury object. New challenges and demands, however, are being encountered, and the need for surfaces with functional characteristics is becoming increasingly frequent [8,9,10]. Surface modification becomes one of the strategies that should be investigated, with the objective, for example, of preventing or reducing bacterial adhesion [11,12,13,14,15,16]. Surfaces can be modified by physical or chemical processes [4,8,11]. Examples of the chemical approach include the pre-incorporation of pharmacologically active bactericidal agents, such as antibiotics, antiseptics, metallic ions, or other organic and inorganic substances. The literature reports physical strategies that involve the use of metals based on silver or copper [12]. This is not the only strategy, however; aluminum/TiO2 coatings reported in the literature show promising results, as they promote photocatalytic antibacterial effects, together with scratch-resistant and self-cleaning characteristics [17]. The release of silver ions from materials produced by plasma deposition processes at atmospheric pressure, PVD (Physical vapor deposition) and MOCVD (Metalorganic Chemical Vapor Deposition) have been reported and their action against the formation of biofilms confirmed [18].
Studies involving TiN/Ag multilayers deposited on titanium alloys showed the formation of a film with antibacterial properties [19,20,21,22]. In this context, the formation of decorative TiN films with silver becomes interesting and can play an important role in protecting sanitary metals from the formation of biofilms. Therefore, for this work, TiN-Ag coatings with different silver concentrations were deposited by RF magnetron sputtering on silicon (100) wafers and AISI F138 stainless-steel substrates. The objective was to investigate the influence of the different silver concentrations on the morphological and structural behavior of the deposited films. The possible application of the films as decorative coatings was assessed based on their associated color parameters (L, a*, and b*). Salt spray (ASTM B117) [23] corrosion resistance was also evaluated.
2. Experimental Section
2.1. Deposition of TiN-Ag Coatings
For deposition of TiN-Ag films, three-inch-diameter silicon wafers, with resistivity between 1.0 and 10.0 Ω cm and thickness of 381 ± 25 µm, were made from (100) p-type silicon. Prior to the deposition process, the silicon substrates were placed in a beaker and cleaned as follows: Deionized water (resistivity of 18 MΩ.cm)—wash for 5 min; 4H2SO4 + 1H2O2 solution—10 min at 115 °C; deionized water (resistivity of 18 MΩ.cm)—wash 5 min; 1HF + 20H2O solution. Stainless steel plates measuring 60 mm × 100 mm were prepared. One of the sides was mechanically polished to generate a mirror-like surface. Prior to the deposition process, the stainless-steel substrates were cleaned by an ultrasonic system in isopropyl alcohol for 10 min and dried with nitrogen. The titanium layer (60 nm) was deposited on top to promote adhesion of the TiN-Ag film.
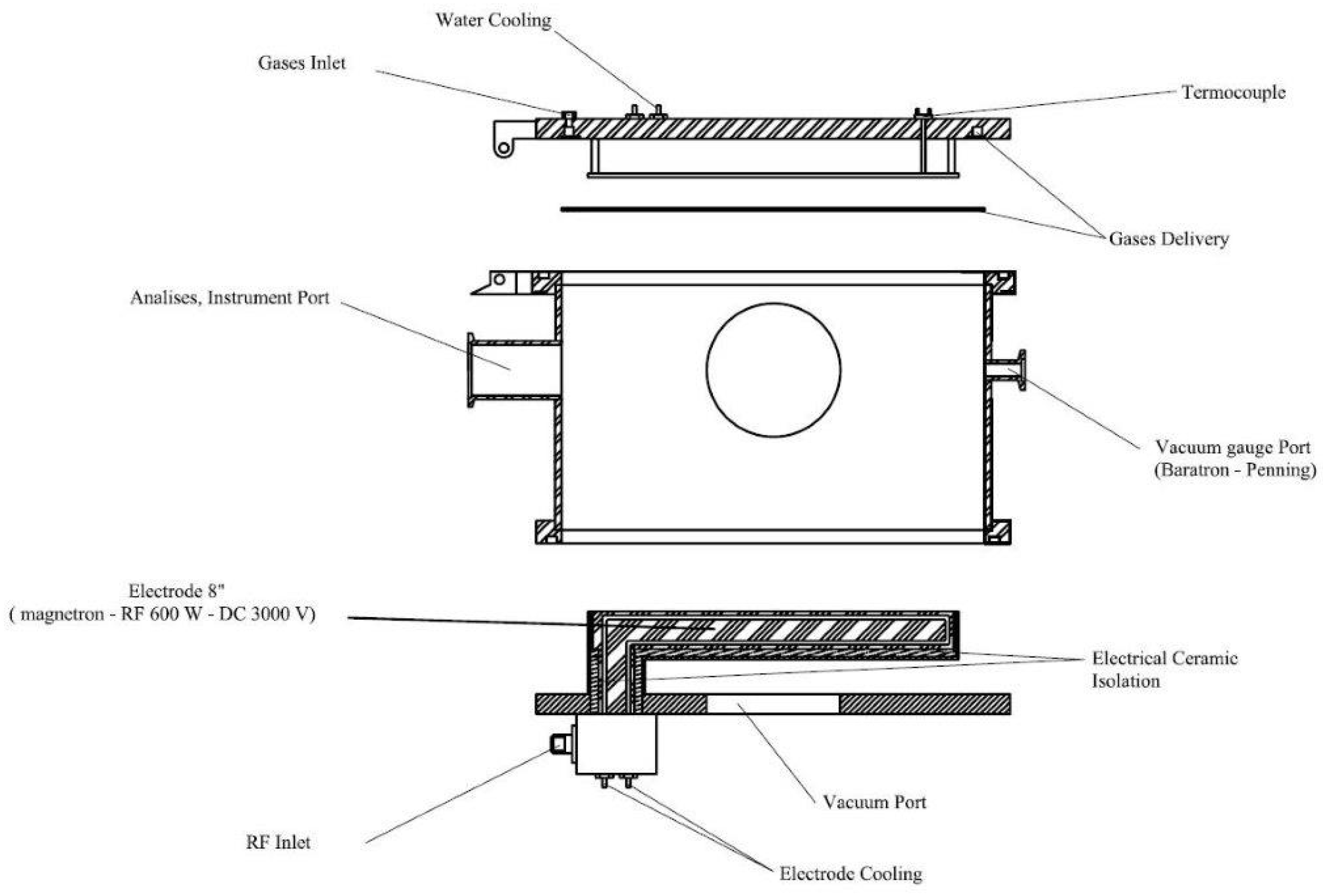
The TiN-Ag films studied in this work were produced in a planar diode-type RF (13.56 MHz) magnetron sputtering reactor, designed and built at the the Laboratório de Sistemas Integraveis da Escola Politécnica—USP (LSI-EPUSP). Figure 1 shows a schematic diagram of the reaction chamber. In all deposition processes, the base pressure was 1.0 × 10−5 Torr. This pressure was reached using a set of pumps (turbomolecular and mechanical) connected in series. The targets used were discs of titanium, 152 mm in diameter and 3 mm thick, from the Kurt J. Lesker Company, Jefferson Hills, PA, USA, (99.99% pure). A silver target (from Testbourne Ltd., Basingstoke, UK, with a purity of 99.99%) that was 25.4 mm in diameter and 3.18 mm thick was used. Both the target and the sample holder were refrigerated to maintain a constant temperature during depositions. The distance between the substrate and the target was fixed at 100 mm. For depositions, argon and nitrogen gases, both with a purity of 99.999%, were injected into the chamber using mass flow controllers.
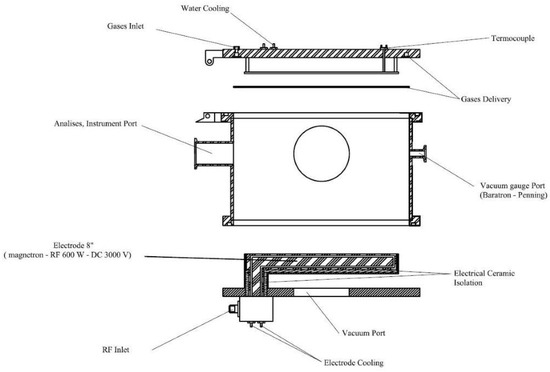
Figure 1.
Schematic diagram of the RF magnetron sputtering system.
The total pressure was maintained at 8.0 mTorr, and partial pressures in the reaction chamber were adjusted to 50%Ar: 50%N2. Films were deposited for 90 min and the applied power was 350 W. The temperature of the sample holder (substrate) remained constant (100 ± 5 °C).
2.2. Characterization of TiN-Ag Coatings
The thickness of the deposited films was measured using a profilometer—Dektak 6M Stylus Profiler from Veeco, Tucson, AZ, USA. Atomic Force Microscopy (AFM) was used to assess the topography and measure the root mean square (RMS) roughness of the film surfaces. A Nanosurf—SPM S200 microscope from Liestal, Switzerland was used in contact mode to produce images over a 10 μm × 10 μm region. The film morphology was evaluated using a scanning electron microscope (JEOL-MEV JSM-6010LA) from Tokio Japan. Integrated into this equipment was an Energy Dispersive X-Ray Spectrometer, from Tokio, Japan, (DRY SD Hyper (EX 94410T1L11)), which allows for elemental mapping of the films. For the X-ray diffraction analyses, a PAnalytical model Empyrean diffractometer from Malvern, UK, was used, with a copper X-ray source and a monochromator adjusted for Cu Kα radiation. The distribution angles ranged from 5 to 80°.
The wettability of the TiN-Ag films was evaluated by measuring the surface contact angle with a goniometer (KRÜSS DSA25E) from Hamburg, Germany. A drop of deionized water (polar component) with a volume of 4 μL was placed on the surface of the deposited film, and the angle formed between the plane tangent to a drop of liquid and the plane containing the surface where the liquid was deposited was measured. The study of the contact angle was also carried out with a non-polar component (diiodomethane). A drop of 2 μL volume was placed on the surface of the film. By measuring the contact angle of two liquids with known surface tension and different polarities [24], it was possible to calculate the surface energy.
The surface color of TiN-Ag films deposited on stainless steel was measured using a Minolta CM-26d (Konica Minolta from Tokyo, Japan) portable spectrophotometer describing the color in L*, a*, b* color space. The color of the coating can be described in the CIE L a*b* color space, where L represents the lightness of color with values ranging from 0 (black) to 100 (white). Parameters a* and b* describe the change in color from red (positive values) to green (negative values), and from yellow (positive values) to blue (negative values), respectively [25,26]. The (a*, b*) point (0,0) is defined as colorless. The chromaticity (c), which describes the hue and saturation of color, is calculated using Equation (1) [25] and it increases when the absolute values of a* and b* increase.
The indoor accelerated test method adopted the NSS test, which referred to ISO 10289 [23]. The salt (NaCl) spray concentration was 5 wt%, with a pH of 6.2–7.2, and a temperature of 35 °C. TIN-Ag films were deposited onto polished F138 stainless-steel plates measuring 60 mm × 100 mm. The visual assessments and photographic records of the surfaces were carried out at intervals of 24, 96 and 200 h.
3. Results and Discussion
3.1. XRD Patterns of TiN-Ag Films
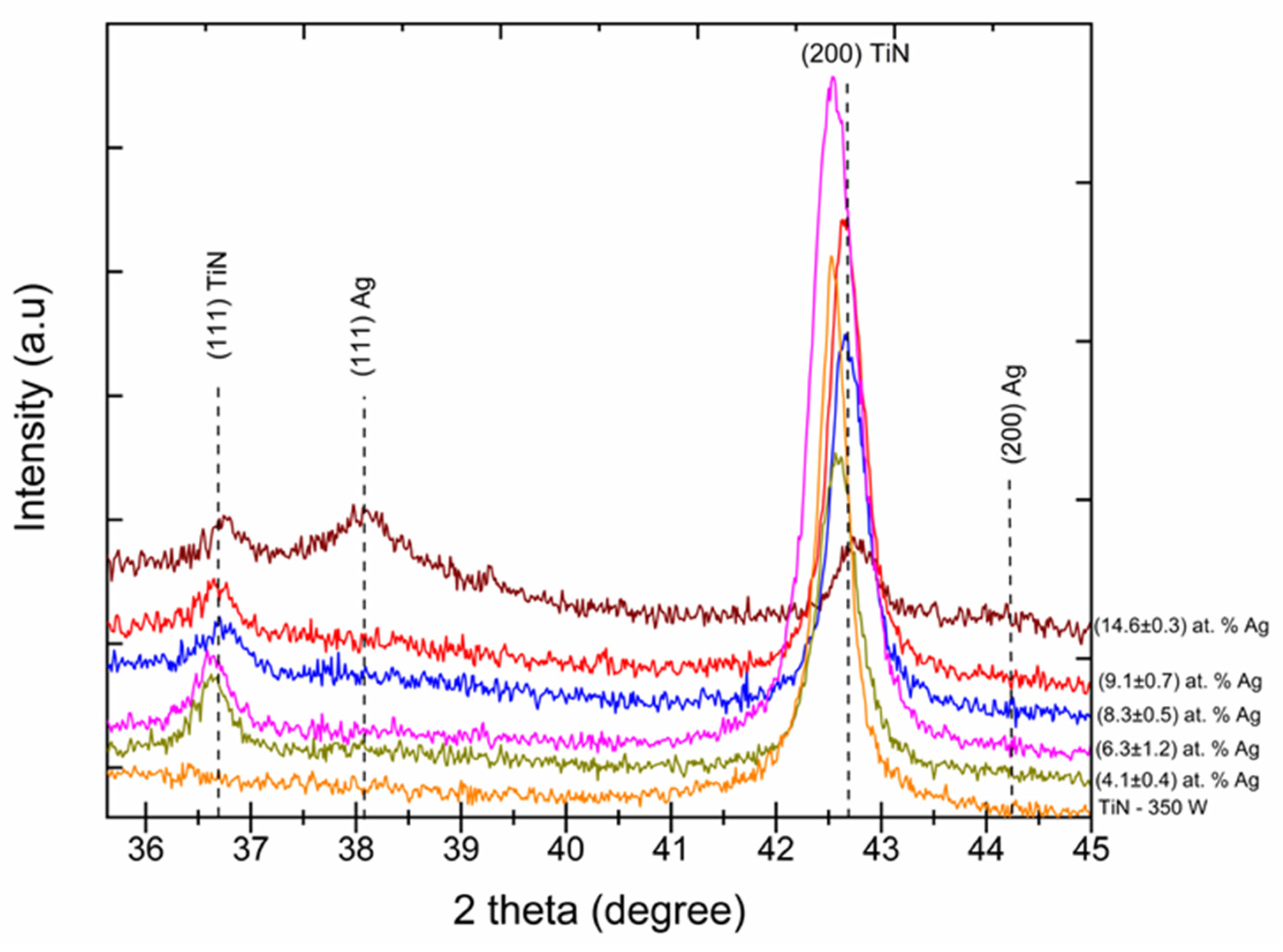
Figure 2 shows the XRD analysis performed to identify the phase composition of TiN-Ag coatings with different Ag concentrations. The ICDD charts 00-004-0783 and 00-038-142 were used to identify the peaks and their respective crystallographic orientations. Observation of the XRD pattern revealed that the most intense peaks were located at 36.6°, 42.7° and 61.7°, and were attributed to TiN (111), TiN (200) and TiN (220), respectively. This suggests that the preferred crystallographic orientation is TiN (200). The presence of the TiN-Ag structure was identified by the presence of two crystalline phases at positions 38.1° and 44.3°. The positions 36.6° and 38.1° were identified for the crystalline structures TiN (111) and TiN-Ag (111), respectively, and positions 42.7° and 44.3° were identified for TiN (200) and TiN-Ag (111), respectively. A reduction in intensity and a shift of the TiN peaks (111) and (200) was observed as the Ag concentration increased. For the samples where the silver concentrations were (9.1 ± 0.7) and (14.6 ± 0.3) at % Ag, both with a Ag (111) crystalline phase, we can observe at the 2θ angle of 38.1° that there was a reduction in intensity of the TiN peaks (111) and (200), the latter being more significant.
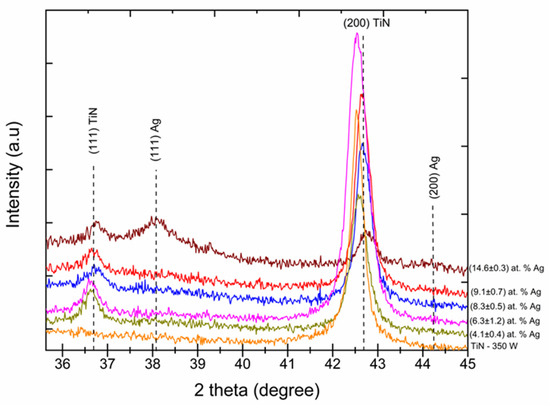
Figure 2.
XRD patterns of TiN-Ag coatings deposited with different silver content (Cu Kα radiation).
The incorporation of silver into the matrix becomes even more evident when compared to the TiN–350 W condition. It is noted that the addition of silver promoted the formation of the TiN (111) phase and a gradual reduction in the TiN (200) phase is detected. The diffractograms presented allow us to infer that the change in the microstructure of the TiN-Ag films is related to the processes of generation, transport and deposition of pulverized Ag particles, which in turn are closely related to the exposed area of Ag. The diffraction patterns show that the intensities of the TiN (111) peaks are lower than those of the TiN (200) peaks, suggesting that the preferred orientation of the film is (200). The overlapping of TiN and Ag peaks occurs gradually as the film silver density increases [22].
The observed decrease in the intensities of the (200) TiN peaks suggests a lower degree of crystallinity or smaller crystal size [27]. Another possibility is that film stress was increased by the incorporation of silver particulates, which can produce peak widening, thus reducing peak intensity. No significant (111) Ag peaks are observed in the patterns of Figure 2, except for that with the greatest [Ag], namely 14.6 at %. A similar observation was reported by Ju et al. 2017 [28]. The observed shift to the right (i.e., to greater angles) of the (200) TiN peaks of Figure 2 as the [Ag] increases may have been caused by the rearrangement of the deposited crystals, which created compressive stress.
3.2. Composition, Microstructure and Morphology of TiN-Ag Coatings
The mapping of the elemental composition of TiN-Ag films deposited on silicon is presented in Table 1, revealing that the film nitrogen concentration remained practically constant; that is, it did not undergo significant change caused by variations in [Ag]. In this work, the pressure, power and process time remained constant, as reported in Section 2.1. The variation in silver concentration was obtained by changing the exposed area of silver within the reaction chamber. For samples A, B, C, D and E, the area of the silver target was 32, 64, 127, 254 and 507 mm2, respectively. Under these conditions, a competition between the evaporation of Ti and Ag elements was observed, resulting in a reduction in the Ti/Ag ratio as the Ag concentration increased. The results obtained are satisfactory and coherent, considering that the evaporation rate of silver is significantly greater than that of titanium. Wasa [29] highlights that the evaporation rate (sputter yield) of Ag is greater than 2.6 atoms/ion, while for Ti it is approximately 0.4 atoms/ion of argon (400 eV).

Table 1.
The chemical composition TiN-Ag films.
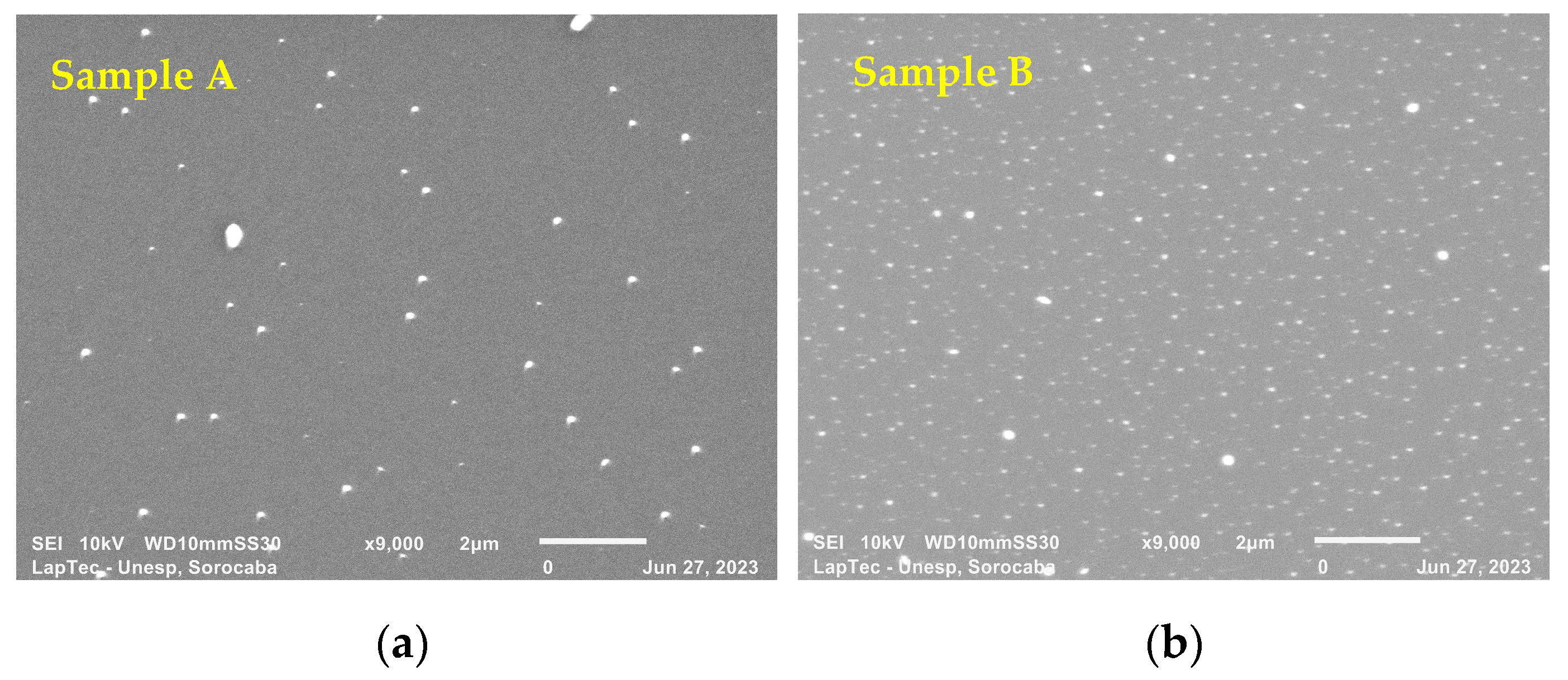
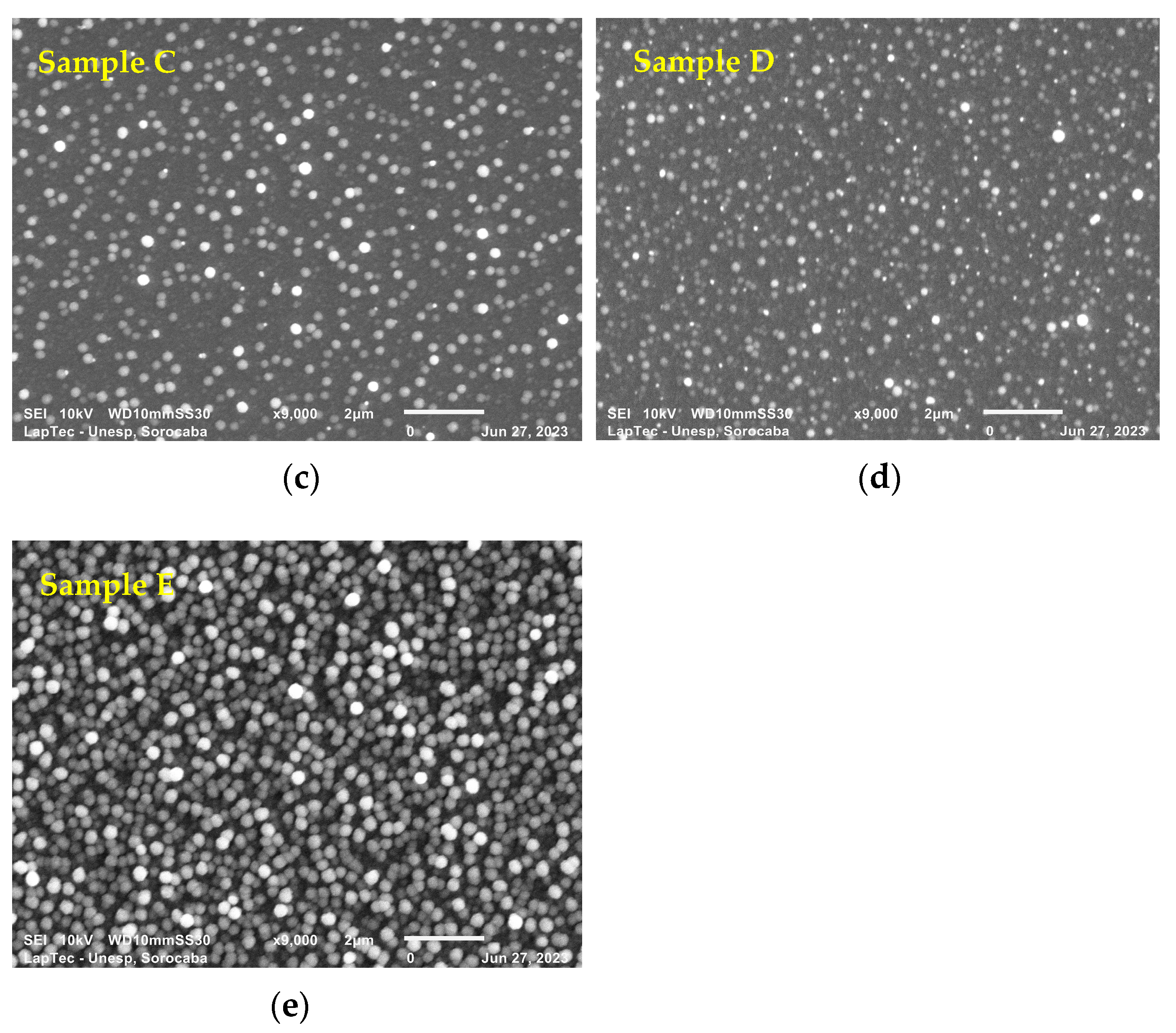
Figure 3a–e show the surface morphology of TiN-Ag films deposited on Si under the conditions described in Section 2.1. SEM analyses were performed to assess the surface characteristics and the presence of nanoparticles, possibly of Ag. Different authors [19,30] report that TiN-Ag coatings are composed of Ag clusters segregated at the TiN grain boundaries and are revealed as bright spots in the SEM micrographs. This is confirmed in the present study.
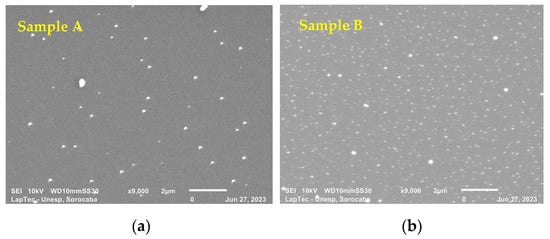
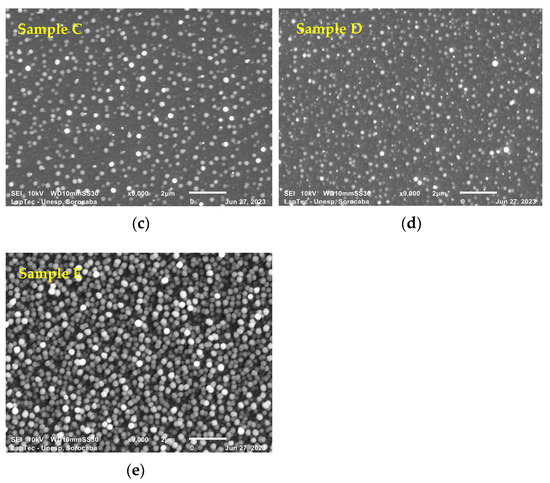
Figure 3.
(a–e) show the surface morphology of TiN-Ag films deposited on Si at a partial pressure of 4.0 mTorr argon and 4.0 mTorr nitrogen at 350 W with different Ag contents.
As observed with the aid of a SEM with a magnification of 9000×, the particles showed sizes ranging from 95 to 280 nm. Even smaller particles, however, can easily be detected along the deposited film but cannot be measured owing to the resolution of the equipment. A greater spacing between the particulates is observed in Figure 3a,b. As the exposed area of the Ag target increases, however, a denser grouping of Ag particulates with predominant spherical characteristics is observed (see Figure 3c–e). These results were expected owing to the greater sputtering rate of Ag compared with that of titanium.
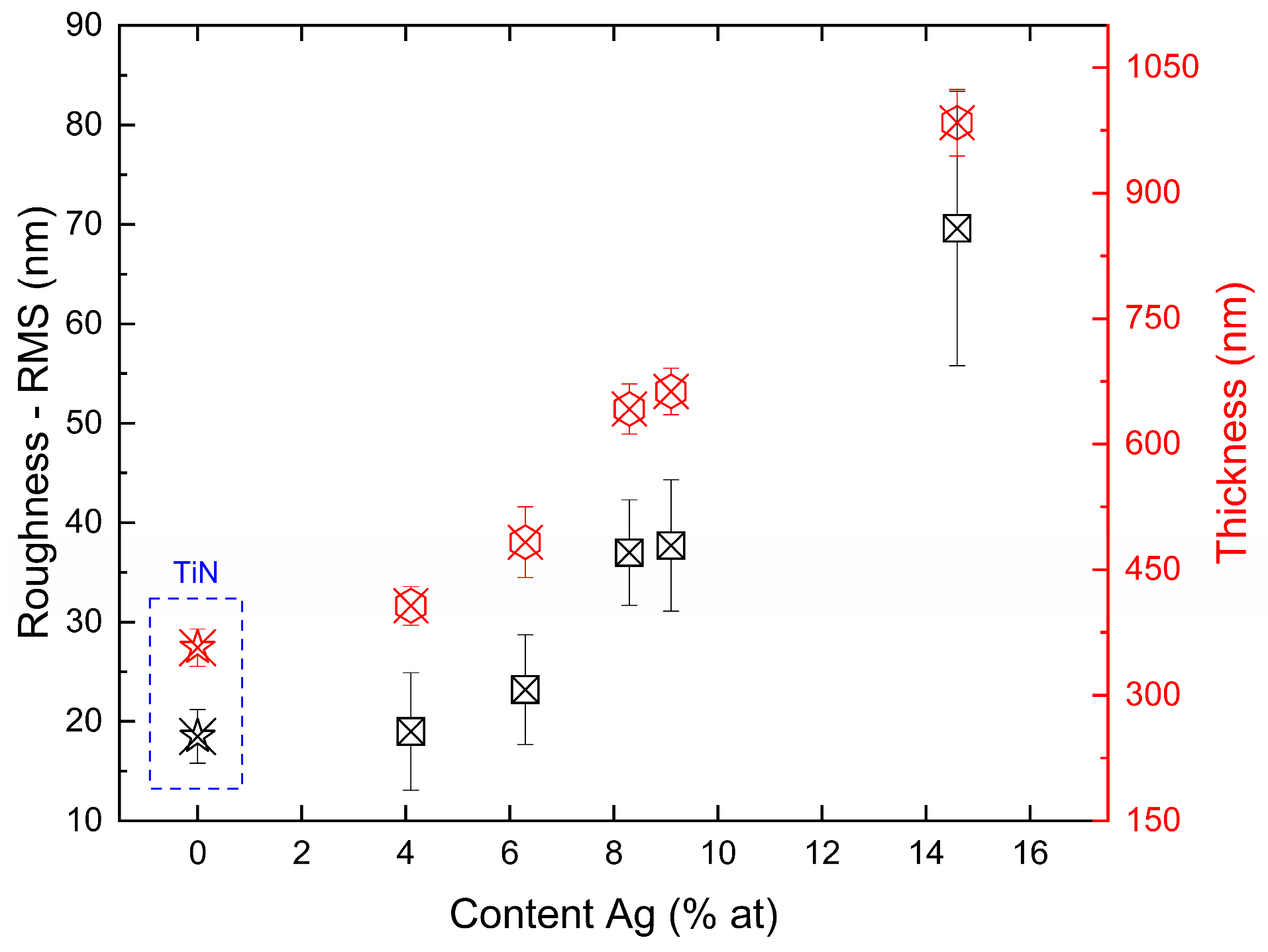
3.3. The Roughness—RMS and Thickness of TiN-Ag Coatings
Figure 4 shows the variation in RMS and thickness as a function of silver content for films on silicon substrates. The increase in the deposition rate is largely associated with the silver evaporation rate, which increased as the area of the silver target in the reactor increased. Consequently, as the evaporation rate of silver is significantly higher than that of titanium, a more intense formation of silver clusters is observed as the exposed area increases. As a result, an almost linear behavior is observed between roughness and deposition rate over the range of parameters studied.
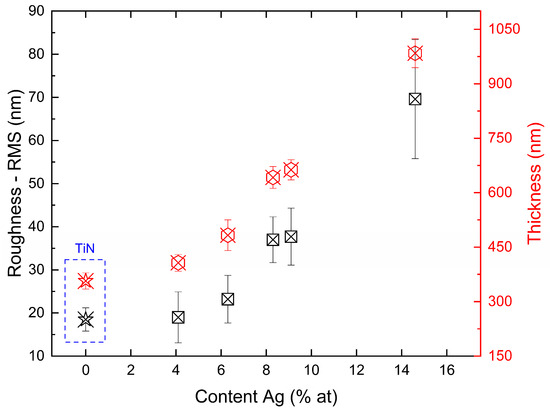
Figure 4.
RMS and thickness of TiN-Ag films deposited on Si at with different Ag content.
Roughness variations and topographical changes in the TiN-Ag films are associated with the incorporation of silver. The results obtained are consistent with those observed in the literature; for example, in the work presented by Velasco et al. [31], the changes in the surface of TiN-Ag films are associated with the incorporation and diffusion of silver. For a sufficiently large silver content, an increase in surface roughness will be observed, which is caused by silver segregation on the surface. However, when the silver content is insufficient to form large agglomerates, the incorporation of silver can produce smoother surfaces owing to the refinement of the structure.
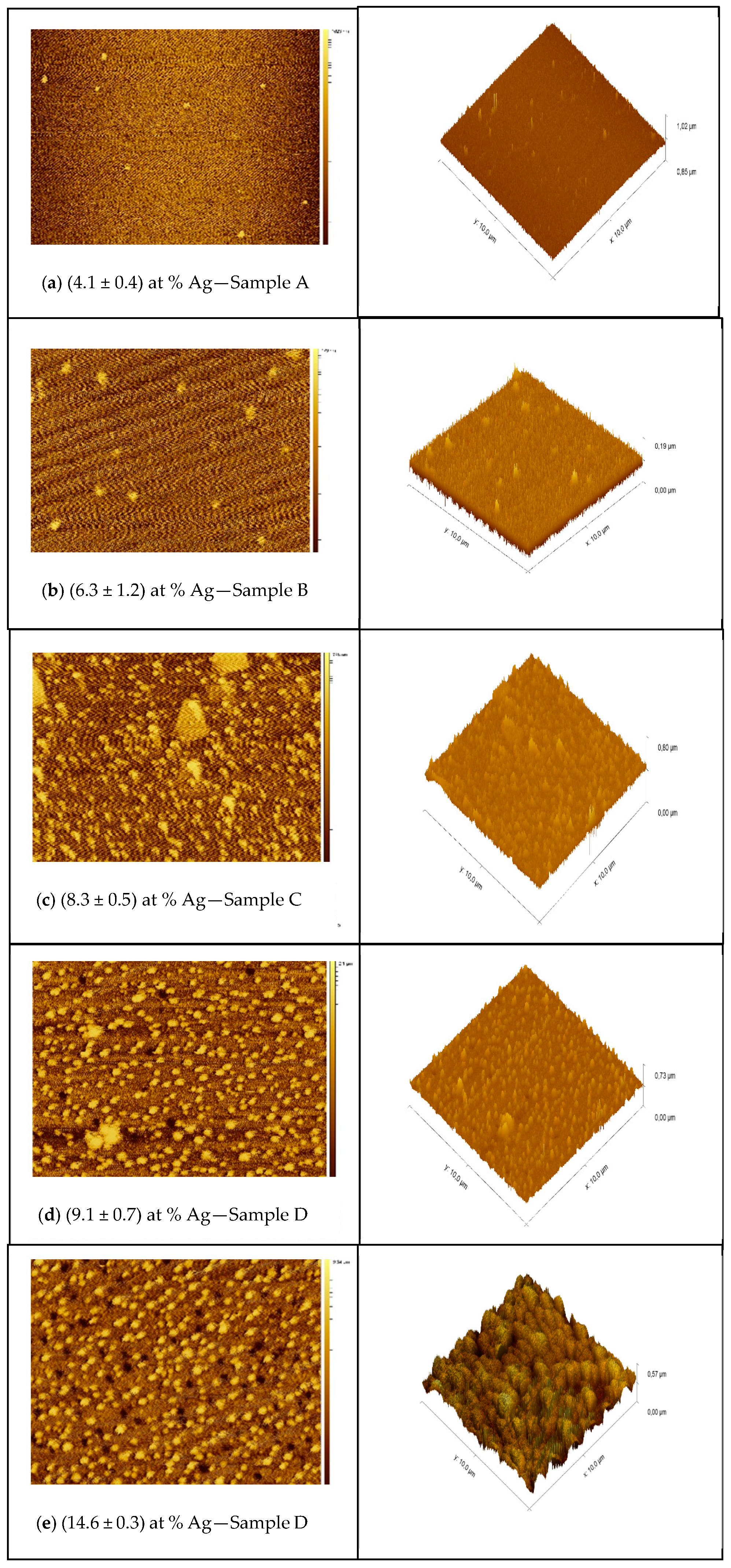
The 2D and 3D AFM images presented in Figure 5a–e show the effect of thickness and Ag incorporation on the surface topography. The AFM analyses showed the same characteristic observed in the SEM analyses already presented and those reported in previous studies [31,32], where a more intense distribution of particulates is noted at high deposition rates.
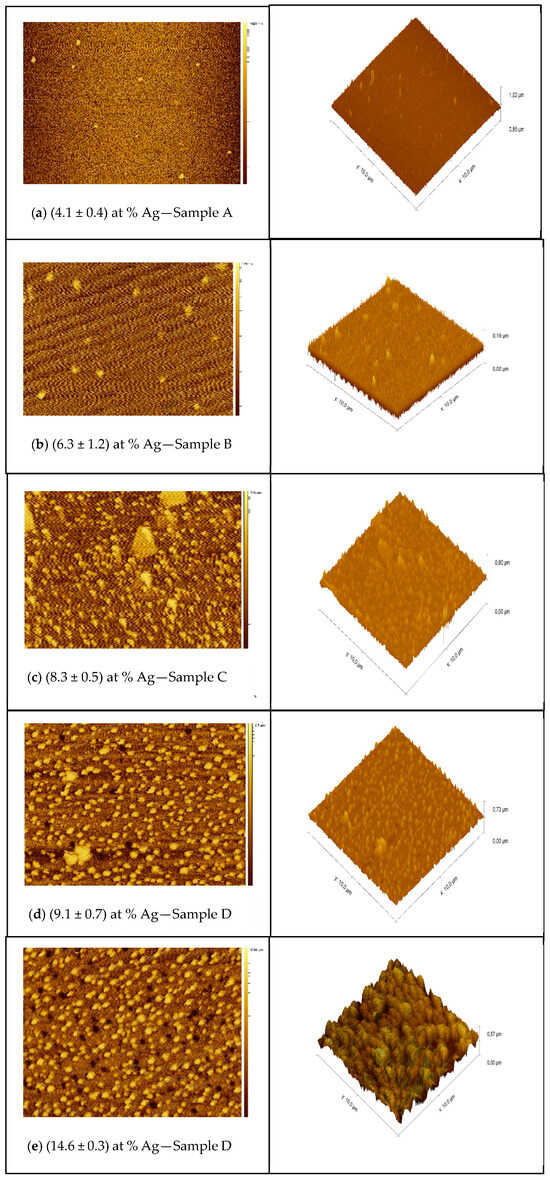
Figure 5.
(a–e) AFM topography of the surface of TiN-Ag films of different silver content.
The results are compatible with those of studies presented in the literature The agglomeration of particulates promoted by the increase in the Ag evaporation rate promotes an increase in the root mean square roughness caused by silver segregation on the surface.
3.4. Wettability (Contact Angle)
Studies report that the contact angle of a solid surface is sensitive to many factors, such as surface geometry, roughness, contamination, deformation, chemical composition, and thickness [33,34,35]. In this work, the effect of adding silver reflected in an almost linear way on the thickness and RMS roughness of the deposited coating. These variations were also reflected in the contact angle measurements. Table 2 shows the contact angle values as a function of Ag concentration and film roughness for films deposited on silicon substrates. An increase in the contact angle is caused by an increase in film roughness and in the silver concentration. For sample D, a film with hydrophobic behavior is observed, that is, a contact angle > 90°.

Table 2.
RMS roughness and contact angle of TiN-Ag coatings deposited with different silver contents on silicon substrates.
Samples C and D presented contact angles < 90°, which were hydrophilic, despite being at the threshold of hydrophobic behavior. For samples A and B, where the silver concentrations in the coating were (4.1 ± 0.4) and (6.3 ± 1.2) at %, hydrophilic behavior (>90) was more pronounced. It was not possible to evaluate the surface energy of the deposited films as a chemical reaction between silver and diiodomethane was observed, which made the measurement inaccurate (Figure 6).

Figure 6.
Drop of distilled water and diidomethane on the surface of the TiN-Ag film—sample B, deposited on a silicon substrate.
3.5. The L, a*, b* Color Space Analysis
The L*a*b* parameters give us an understanding of color, the L parameter represents the brightness of the color and indicates how close it is to black or white. This parameter varies between 0 (zero) and 100 (one hundred), that is, if L is zero, we have total black color, whereas if it is 100, we have total white color.
Each of the parameters a* and b* has a color range between (−120) and (+120). For parameter a* the range is between green (−120) and red (+120).
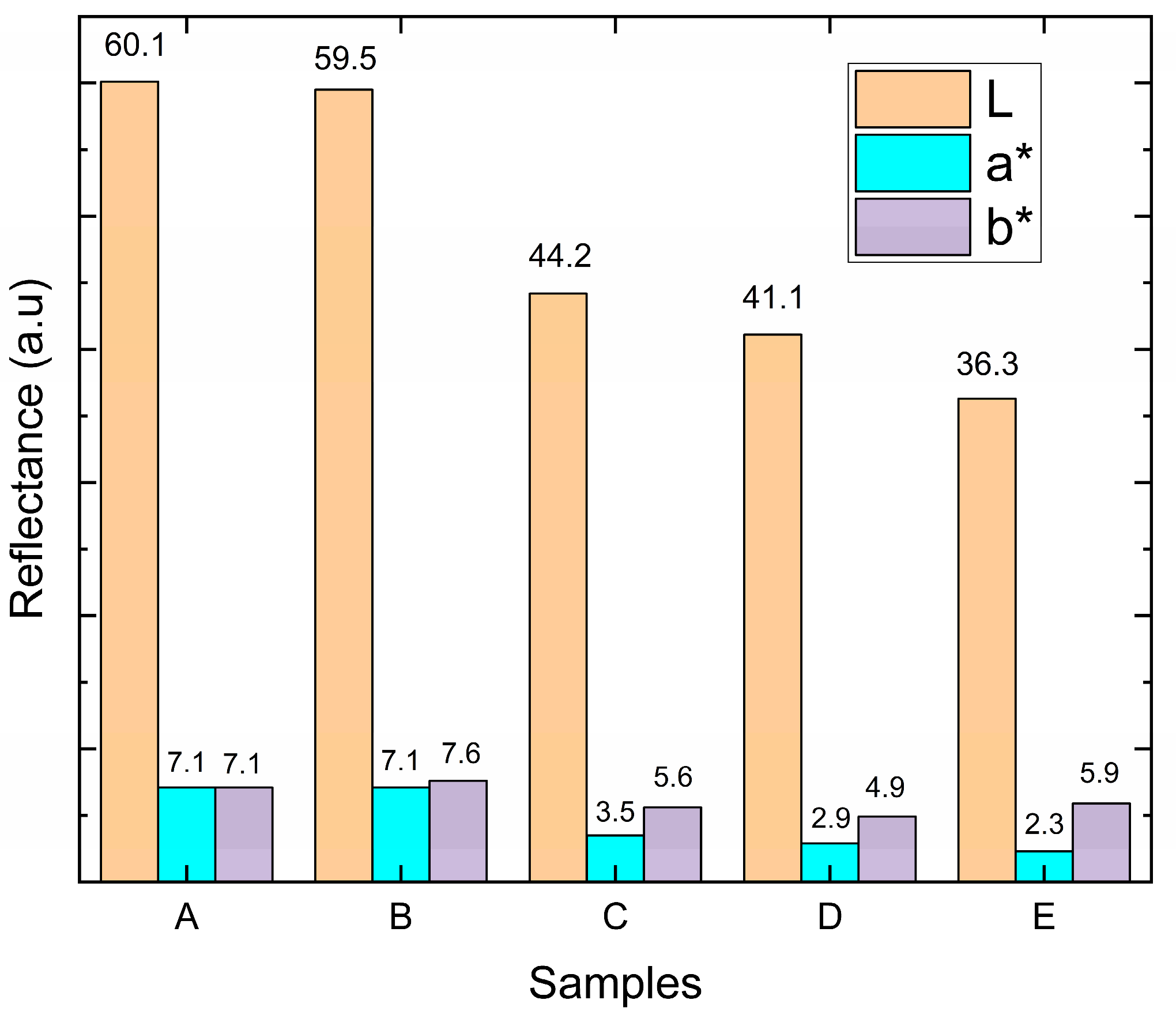
As for the parameter for b, the variation occurs between blue (−120) and yellow (+120). These parameters account for both the reflectivity spectra of the materials and the sensitivity of the human eye. In the present work, the appearance of TiN-Ag films with different silver concentrations is evaluated in the CIELab color space. Figure 7 presents the results for the different Ag concentrations used. No large variation is observed for L*, a*, and b* for coatings A and B (Ag concentrations of (4.1 ± 0.4) and (6.3 ± 1.2) at %, Ag, respectively). The L* value > 60 is observed and the a* and b* values simultaneously remain positive with high N content because the coating shows stronger reflectivity for red and yellow light than for green and blue light.
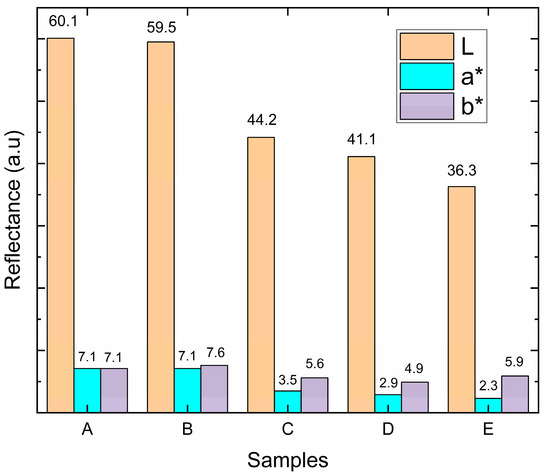
Figure 7.
The L, a* and b* parameters of TiN-Ag films deposited on stainless-steel substrates with different concentrations of Ag.
When the concentration of Ag is high (samples C, D, and E) a matte surface is observed through visual inspection, becoming more homogeneous for sample E. The increase in the matte coating is accompanied by an increase in the concentration of Ag present in the coating, and thus an increase in roughness, presented in Section 3.3, which in turn reflects a significant and decreasing reduction in L* values and also in the change in red reflectance.
3.6. Salt Spray Analysis
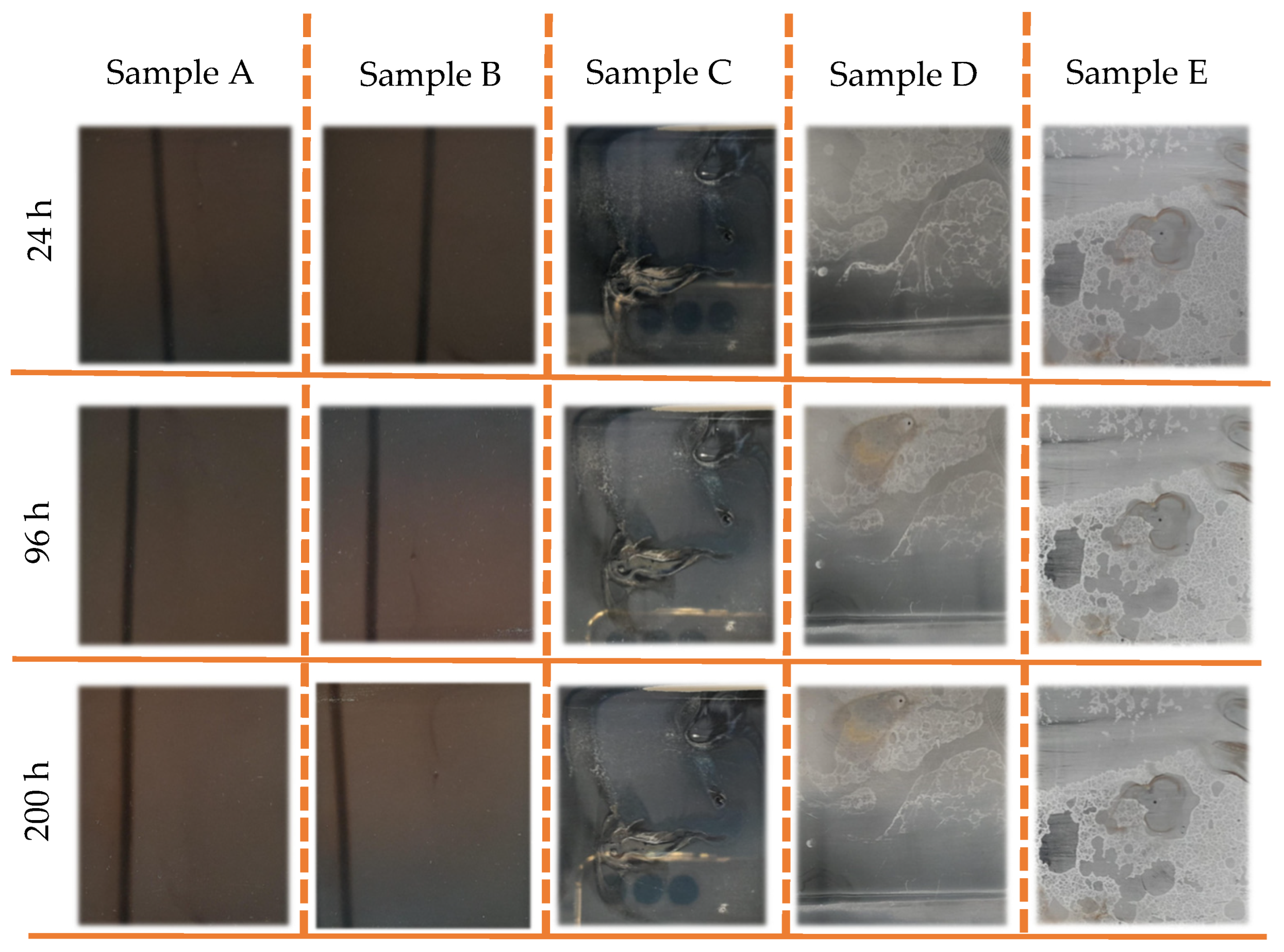
The TiN-Ag coatings deposited on F138 stainless steel were subjected to the salt spray test, and the results are presented in Figure 8. After a period of 24 h in salt spray, the coatings on samples C, D and E showed changes on the surface of the film, indicating the presence of regions with aspects of coating delamination. During this period, no surface variation was observed in samples A and B. At 96 and 200 h for sample D, the presence of red corrosion spots was observed. The coatings with the lowest concentrations of Ag presented more satisfactory results when evaluating the aesthetic aspect, as no corrosion points or points of peeling and/or delamination of the TiN-Ag film deposited on the stainless steel were visually observed. This high corrosion resistance of TiN–Ag films in samples A and B can be attributed to the low defect density of the films prepared by magnetron sputtering. On the other hand, the effect on morphology promoted by the increase in silver concentration (samples C, D and E) in the coating reflected in the increase in roughness and in the formation of more intense peaks and valleys. As a result, liquid from salt spray is more easily condensed and trapped in these valleys, which can lead to poor corrosion resistance.
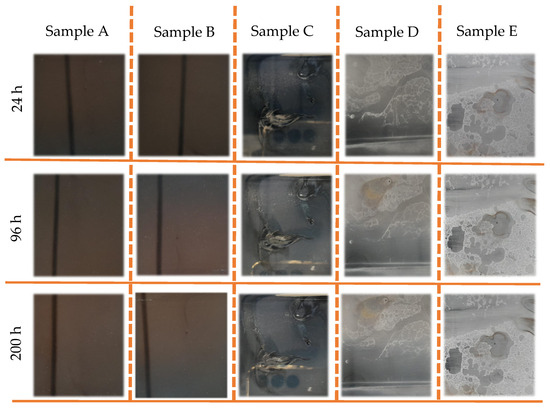
Figure 8.
Images of sample A–E show the evolution of TiN-Ag coatings deposited on F138 stainless steel with different Ag concentrations under salt spray test.
4. Conclusions
The TiN-Ag coatings developed in this work exhibited different concentrations of silver, which significantly influenced the structure, RMS roughness, texture, appearance, and corrosion resistance. It was observed that the increase in roughness was associated with the increase in the incorporation of silver into the film, which in turn influenced the thickness of the deposited coating in an almost linear way. The presence of silver in the concentrations used in this work made it impossible to measure the surface energy. A chemical reaction with diiodomethane was observed; therefore, the use of an alternative test liquid is necessary to evaluate the surface energy without chemical interference. However, a hydrophobic coating (Φ > 90°) was observed for samples with a silver concentration above (8.3 ± 0.5) at %, whereas a hydrophilic behavior (Φ < 90°) was observed for coatings with Ag concentrations lower than (6.3 ± 1.2) at %.
When evaluated from an aesthetic perspective, which is the objective of this work, we can see that samples C, D and E, which presented the highest concentrations of silver, did not present satisfactory results. Delamination and corrosion pits, as well as significant variation in the colors (represented by the variations observed in L*, a* and b*), together with the differences in the matte appearance of these samples, are not ideal for decorative applications. However, samples A and B, whose Ag concentrations were (4.1 ± 0.4) and (6.3 ± 1.2) at % Ag, respectively, were more promising for application as decorative coatings with stable behavior for corrosion and color.
Even after salt spraying, good color stability was observed for samples A and B. Variations in the behavior of parameters L*, a* and b* as a function of time are worthy of future investigation. The replacement of silver with copper or a Ag/Cu mixture also merits further study. Additional studies might include the tribological performance of films with different silver concentrations.
Author Contributions
A.C.S.d.A. was involved in conceptualization, methodology, investigation, validation, data curation, writing—original draft preparation, writing—review and editing. R.D.M. was involved in supervision, writing—review and editing. N.O. executed the DRX analyses and data curation. R.R. executed the Salt Spray and color space analyses and data curation, was involved in conceptualization and writing. S.F.D. was involved in supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Original data available from the corresponding author upon reasonable request.
Acknowledgments
Laboratório de Sistemas Integraveis—LSI-USP—SP. Laboratório de Plasmas Tecnológicos (LaPTec)—Unesp—CAPES, CNPq and Fapesp. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES)–Finance Code 001, Dexco Testing Laboratory.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Sola, R.; Veronesi, P.; Zardin, B.; Borghi, M. A Study on PVD Coatings for Reduction of Friction and Wear of Swashplate Axial Piston Pumps and Motors. Metall. Ital. 2020, 112, 14–23. [Google Scholar]
- Santecchia, E.; Hamouda, A.M.S.; Musharavati, F.; Zalnezhad, E.; Cabibbo, M.; Spigarelli, S. Wear Resistance Investigation of Titanium Nitride-Based Coatings. Ceram. Int. 2015, 41, 10349–10379. [Google Scholar] [CrossRef]
- Silva, F.; Martinho, R.; Andrade, M.; Baptista, A.; Alexandre, R. Improving the Wear Resistance of Moulds for the Injection of Glass Fibre-Reinforced Plastics Using PVD Coatings: A Comparative Study. Coatings 2017, 7, 28. [Google Scholar] [CrossRef]
- Van Stappen, M.; Stals, L.M.; Kerkhofs, M.; Quaeyhaegens, C. State of the Art for the Industrial Use of Ceramic PVD Coatings. Surf. Coat. Technol. 1995, 74, 629–633. [Google Scholar] [CrossRef]
- Arrousse, N.; Ferreira, J.; Carvalho, S.; Andritschky, M. PVD Black Coating for Decorative Applications. Coatings 2023, 13, 1838. [Google Scholar] [CrossRef]
- Vorobyova, M.; Biffoli, F.; Giurlani, W.; Martinuzzi, S.M.; Linser, M.; Caneschi, A.; Innocenti, M. PVD for Decorative Applications: A Review. Materials 2023, 16, 4919. [Google Scholar] [CrossRef]
- Fortune Business Insights Physical—Vapor Deposition (PVD) Market Size & Growth 2023–2028. Page. Available online: https://www.fortunebusinessinsights.com/physical-vapour-deposition-pvd-market-102364 (accessed on 14 January 2024).
- Arul, S.; Easwaramoorthi, M.; Meikandan, M. Different Types of PVD Coatings and Their Demands—A Review. Int. J. Appl. Eng. Res. 2014, 9, 26417–26430. [Google Scholar]
- Popović, M.; Novaković, M.; Vaňa, D.; Ronning, C.; Jugović, D.; Rajić, V.; Noga, P. Structure-Dependent Optical Properties of Au/Ag Irradiated TiN Thin Films. Opt. Mater. 2023, 138, 113684. [Google Scholar] [CrossRef]
- Novaković, M.; Popović, M.; Noga, P.; Vaňa, D.; Ronning, C. Low Optical Losses in Plasmonic TiN Thin Films Implanted with Silver and Gold. Opt. Mater. 2022, 123, 111936. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Chen, N.H.; Chung, C.J.; Chiang, C.C.; Chen, K.C.; He, J.L. Antimicrobial Copper-Containing Titanium Nitride Coatings Co-Deposited by Arc Ion Plating/Magnetron Sputtering for Protective and Decorative Purposes. Surf. Coat. Technol. 2014, 253, 83–88. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Qiu, W.; Fang, F. Overview of Antibacterial Strategies of Dental Implant Materials for the Prevention of Peri-Implantitis. Bioconjug. Chem. 2021, 32, 627–638. [Google Scholar] [CrossRef]
- Osés, J.; Fuentes, G.G.; Palacio, J.F.; Esparza, J.; García, J.A.; Rodríguez, R. Antibacterial Functionalization of PVD Coatings on Ceramics. Coatings 2018, 8, 197. [Google Scholar] [CrossRef]
- Sarraf, M.; Dabbagh, A.; Abdul Razak, B.; Nasiri-Tabrizi, B.; Hosseini, H.R.M.; Saber-Samandari, S.; Abu Kasim, N.H.; Yean, L.K.; Sukiman, N.L. Silver Oxide Nanoparticles-Decorated Tantala Nanotubes for Enhanced Antibacterial Activity and Osseointegration of Ti6Al4V. Mater. Des. 2018, 154, 28–40. [Google Scholar] [CrossRef]
- Cloutier, M.; Mantovani, D.; Rosei, F. Antibacterial Coatings: Challenges, Perspectives, and Opportunities. Trends Biotechnol. 2015, 33, 637–652. [Google Scholar] [CrossRef]
- Mesbah, M.; Sarraf, M.; Dabbagh, A.; Nasiri-Tabrizi, B.; Paria, S.; Banihashemian, S.M.; Bushroa, A.R.; Faraji, G.; Tsuzuki, T.; Madaah Hosseini, H.R. Synergistic Enhancement of Photocatalytic Antibacterial Effects in High-Strength Aluminum/TiO2 Nanoarchitectures. Ceram. Int. 2020, 46, 24267–24280. [Google Scholar] [CrossRef]
- Liu, H.; Battiato, S.; Pellegrino, A.L.; Paoli, P.; Rossi, P.; Jiménez, C.; Malandrino, G.; Muñoz-Rojas, D. Deposition of Metallic Silver Coatings by Aerosol Assisted MOCVD Using Two New Silver β-Diketonate Adduct Metalorganic Precursors. Dalton Trans. 2017, 46, 10986–10995. [Google Scholar] [CrossRef]
- Braceras, I.; Brizuela, M.; Álvarez, N.; Martínez Van Geeteruyen, M.; Azkona, I. TiN-Ag as an Antimicrobial and Wear Resistant Coating. Biotribology 2021, 28, 100192. [Google Scholar] [CrossRef]
- Marques, S.M.; Carvalho, I.; Leite, T.R.; Henriques, M.; Carvalho, S. Antimicrobial TiN-Ag Coatings in Leather Insole for Diabetic Foot. Materials 2022, 15, 2009. [Google Scholar] [CrossRef] [PubMed]
- Kelly, P.J.; Li, H.; Whitehead, K.A.; Verran, J.; Arnell, R.D.; Iordanova, I. A Study of the Antimicrobial and Tribological Properties of TiN/Ag Nanocomposite Coatings. Surf. Coat. Technol. 2009, 204, 1137–1140. [Google Scholar] [CrossRef]
- Du, D.; Liu, D.; Zhang, X.; Tang, J.; Xiang, D. Characterization and Mechanical Properties Investigation of TiN-Ag Films onto Ti-6Al-4V. Appl. Surf. Sci. 2016, 365, 47–56. [Google Scholar] [CrossRef]
- JIS Z 2801; Antibacterial Products–Test for Antibacterial Activity and Efficacy. Japanese Industrial Standard: Tokyo, Japan, 2012.
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Skowroński, Ł.; Antończak, A.J.; Trzcinski, M.; Łazarek, Ł; Hiller, T.; Bukaluk, A.; Wronkowska, A.A. Optical Properties of Laser Induced Oxynitride Films on Titanium. Appl. Surf. Sci. 2014, 304, 107–114. [Google Scholar] [CrossRef]
- Klein, G.A. Springer Series in Optical Sciences 154 Industrial Color Physics; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Pandey, A.; Dalal, S.; Dutta, S.; Dixit, A. Structural Characterization of Polycrystalline Thin Films by X-Ray Diffraction Techniques. J. Mater. Sci. Mater. Electron. 2021, 32, 1341–1368. [Google Scholar] [CrossRef]
- Ju, H.; Yu, L.; Yu, D.; Asempah, I.; Xu, J. Microstructure, Mechanical and Trobological Properties of TiN-Ag Films Deposited by Reactive Magnetron Sputtering. Vacuum 2017, 141, 82–88. [Google Scholar] [CrossRef]
- Wasa, K. 2-Sputtering Phenomena. In Handbook of Sputtering Technology, 2nd ed.; Wasa, K., Kanno, I., Kotera, H., Eds.; William Andrew Publishing: Oxford, UK, 2012; pp. 41–75. ISBN 978-1-4377-3483-6. [Google Scholar]
- Kışla, D.; Gökmen, G.G.; Akdemir Evrendilek, G.; Akan, T.; Vlčko, T.; Kulawik, P.; Režek Jambrak, A.; Ozogul, F. Recent Developments in Antimicrobial Surface Coatings: Various Deposition Techniques with Nanosized Particles, Their Application and Environmental Concerns. Trends Food Sci. Technol. 2023, 135, 144–172. [Google Scholar] [CrossRef]
- Calderon Velasco, S.; Cavaleiro, A.; Carvalho, S. Functional Properties of Ceramic-Ag Nanocomposite Coatings Produced by Magnetron Sputtering. Prog. Mater. Sci. 2016, 84, 158–191. [Google Scholar] [CrossRef]
- Ning, Z.; Wang, Y.; Li, S.; Tang, K.; Wen, M. The Sputtering Performance of Ag Sputtering Targets with Different Microstructure. Vacuum 2023, 210, 111888. [Google Scholar] [CrossRef]
- Marmur, A.; Volpe, C.D.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact Angles and Wettability: Towards Common and Accurate Terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Lugscheider, E.; Bobzin, K. Wettability of PVD Compound Materials by Lubricants. Surf. Coat. Technol. 2003, 165, 51–57. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2013; Volume 51, pp. 3–34. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).