Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Processing of Carvacrol Active Coatings
2.2. Preparation of Turbot and Immersion Sample Treatment
2.3. Bacteria Analysis
2.4. Total Volatile Basic Nitrogen (TVB-N) Analysis
2.5. K Value Analysis
2.6. Thiobarbituric Acid Reactive Substances (TBARS) Analysis
2.7. Myofibrillar Protein (MP) Extraction
2.8. Measurement of Sulfydryl Groups (SH) and Carbonyl Groups
2.9. Measurement of Ca2+-ATPase Activity
2.10. Surface Hydrophobicity of Myofibrillar Protein (MP)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Microbiological Analysis
3.2. TVB-N Analysis
3.3. K Values Analysis
3.4. TBARS Value Analysis
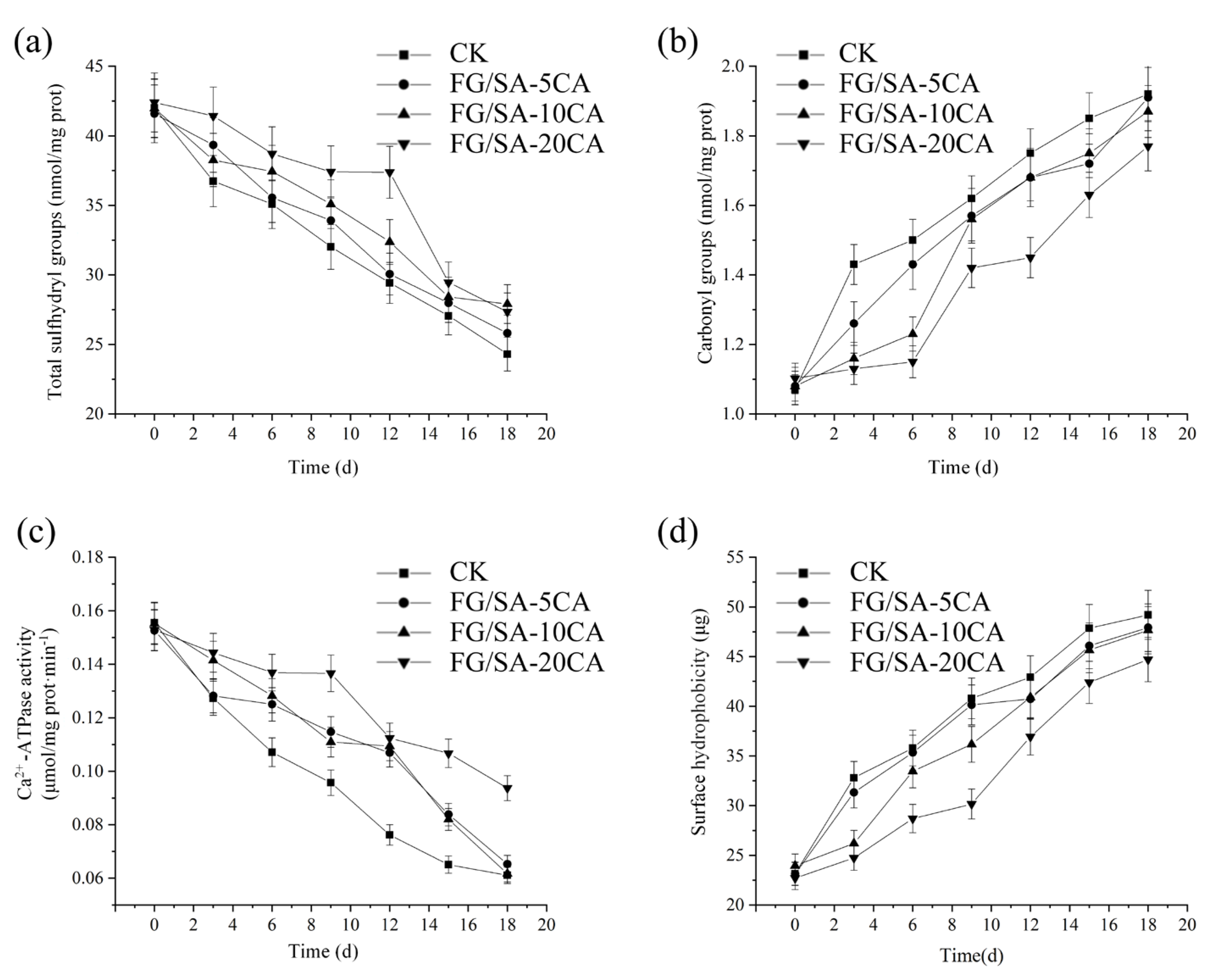
3.5. Changes of Residual Groups in Amino Acid Side-Chains
3.5.1. Total SH Counts Analysis
3.5.2. Carbonyl Counts Analysis
3.6. Ca2+-ATPase Activity Analysis
3.7. Surface Hydrophobicity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jie, C.; Meijie, G.; Weiqiang, Q.; Jun, M.; Jing, X. Effect of tea polyphenol-trehalose complex coating solutions on physiological stress and flesh quality of marine cultured turbot (Scophthalmus maximus) during waterless transport. J. Aquat. Anim. Health 2024. [Google Scholar] [CrossRef]
- Ertan, K.; Celebioglu, A.; Chowdhury, R.; Sumnu, G.; Sahin, S.; Altier, C.; Uyar, T. Carvacrol/cyclodextrin inclusion complex loaded gelatin/pullulan nanofibers for active food packaging applications. Food Hydrocoll. 2023, 142, 108864. [Google Scholar] [CrossRef]
- Chaparro-Hernández, S.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ocaño-Higuera, V.M.; Valenzuela-López, C.C.; Ornelas-Paz, J.D.; Del-Toro-Sánchez, C.L. Effect of chitosan-carvacrol edible coatings on the quality and shelf life of tilapia (Oreochromis niloticus) fillets stored in ice. Food Sci. Technol. 2015, 35, 734–741. [Google Scholar] [CrossRef]
- Alves, V.; Rico, B.P.M.; Cruz, R.M.S.; Vicente, A.A.; Khmelinski, I.; Vieira, M.C. Preparation and characterization of a chitosan film with grape seed extract-carvacrol microcapsules and its effect on the shelf-life of refrigerated Salmon (Salmo salar). LWT-Food Sci. Technol. 2018, 89, 525–534. [Google Scholar] [CrossRef]
- Guo, Q.; Li, S.; Du, G.A.; Chen, H.; Yan, X.H.; Chang, S.D.; Yue, T.L.; Yuan, Y.H. Formulation and characterization of microcapsules encapsulating carvacrol using complex coacervation crosslinked with tannic acid. LWT-Food Sci. Technol. 2022, 165, 113683. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Kovacevic, D.B.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Prasad, P.; Kochhar, A. Active Packaging in Food Industry: A Review. IOSR J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 01–07. [Google Scholar] [CrossRef]
- Remya, S.; Sivaraman, G.K.; Joseph, T.C.; Parmar, E.; Sreelakshmi, K.R.; Mohan, C.O.; Ravishankar, C.N. Influence of corn starch based bio-active edible coating containing fumaric acid on the lipid quality and microbial shelf life of silver pomfret fish steaks stored at 4 °C. J. Food Sci. Technol. 2022, 59, 3387–3398. [Google Scholar] [CrossRef]
- Yang, H.; Li, Q.; Xu, Z.; Ge, Y.; Zhang, D.; Li, J.; Sun, T. Preparation of three-layer flaxseed gum/chitosan/flaxseed gum composite coatings with sustained-release properties and their excellent protective effect on myofibril protein of rainbow trout. Int. J. Biol. Macromol. 2022, 194, 510–520. [Google Scholar] [CrossRef]
- Zhang, M.; Tao, N.; Li, L.; Xu, C.; Deng, S.; Wang, Y. Non-migrating active antibacterial packaging and its application in grass carp fillets. Food Packag. Shelf Life 2022, 31, 100786. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.T.; Zhuang, S.; Li, D.P.; Hong, H.; Lametsch, R.; Tan, Y.Q.; Luo, Y.K. Shelf life extension of chilled blunt snout bream fillets using coating based on chia seed gum and Oleum ocimi gratissimi. Food Biosci. 2023, 54, 102853. [Google Scholar] [CrossRef]
- Xu, Z.; Pei, J.; Mei, J.; Yu, H.; Chu, S.; Xie, J. Effect of gum tragacanth-sodium alginate coatings incorporated with epigallocatechin gallate on the quality and shelf life of large yellow croaker (Larimichthys crocea) during superchilling storage. Food Qual. Saf. 2023, 8, fyad039. [Google Scholar] [CrossRef]
- Pei, J.X.; Mei, J.; Yu, H.J.; Qiu, W.Q.; Xie, J. Effect of Gum Tragacanth-Sodium Alginate Active Coatings Incorporated with Epigallocatechin Gallate and Lysozyme on the Quality of Large Yellow Croaker at Superchilling Condition. Front. Nutr. 2022, 8, 812741. [Google Scholar] [CrossRef]
- Ren, X.J.; Meng, X.; Zhang, Z.; Du, H.Y.; Li, T.P.; Wang, N. Effects of Ultrasound-Assisted Extraction on Structure and Rheological Properties of Flaxseed Gum. Gels 2023, 9, 318. [Google Scholar] [CrossRef]
- Liu, J.; Shim, Y.Y.; Tse, T.J.; Wang, Y.; Reaney, M.J.T. Flaxseed gum a versatile natural hydrocolloid for food and non-food applications. Trends Food Sci. Technol. 2018, 75, 146–157. [Google Scholar] [CrossRef]
- Presenza, L.; Teixeira, B.F.; Galvao, J.A.; Vieira, T. Technological strategies for the use of plant-derived compounds in the preservation of fish products. Food Chem. 2023, 419, 136069. [Google Scholar] [CrossRef]
- Fang, S.Y.; Qiu, W.Q.; Mei, J.; Xie, J. Effect of Sonication on the Properties of Flaxseed Gum Films Incorporated with Carvacrol. Int. J. Mol. Sci. 2020, 21, 1637. [Google Scholar] [CrossRef]
- Fang, S.Y.; Zhou, Q.Q.; Hu, Y.; Liu, F.; Mei, J.; Xie, J. Antimicrobial Carvacrol Incorporated in Flaxseed Gum-Sodium Alginate Active Films to Improve the Quality Attributes of Chinese Sea bass (Lateolabrax maculatus) during Cold Storage. Molecules 2019, 24, 3292. [Google Scholar] [CrossRef]
- Saelens, G.; Houf, K. The involvement of Pseudoterranova decipiens fish infestation on the shelf-life of fresh Atlantic cod (Gadus morhua) fillet. Int. J. Food Microbiol. 2024, 410, 110426. [Google Scholar] [CrossRef]
- Cen, S.J.; Fang, Q.; Tong, L.; Yang, W.G.; Zhang, J.J.; Lou, Q.M.; Huang, T. Effects of chitosan-sodium alginate-nisin preservatives on the quality and spoilage microbiota of Penaeus vannamei shrimp during cold storage. Int. J. Food Microbiol. 2021, 349, 109227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.W.; Li, Y.X.; Wang, Y.F.; Yakubu, S.; Tang, H.B.; Li, L. Active polylactic acid/tilapia fish gelatin-sodium alginate bilayer films: Application in preservation of Japanese sea bass (Lateolabrax japonicus). Food Packag. Shelf Life 2022, 33, 100915. [Google Scholar] [CrossRef]
- Tan, M.T.; Ding, Z.Y.; Mei, J.; Xie, J. Effect of cellobiose on the myofibrillar protein denaturation induced by pH changes during freeze-thaw cycles. Food Chem. 2022, 373, 131511. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, X.; Yang, W.; Mei, J.; Xie, J. Effect of magnetic nano-particles combined with multi-frequency ultrasound-assisted thawing on the quality and myofibrillar protein-related properties of salmon (Salmo salar). Food Chem. 2024, 445, 138701. [Google Scholar] [CrossRef]
- Remya, S.; Mohan, C.O.; Venkateshwarlu, G.; Sivaraman, G.K.; Nagarajarao, C.; Ravishankar, C.N. Combined effect of O2 scavenger and antimicrobial film on shelf life of fresh cobia (Rachycentron canadum) fish steaks stored at 2 °C. Food Control. 2017, 71, 71–78. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef]
- Wenru, L.; Jun, M.; Jing, X. Effect of locust bean gum-sodium alginate coatings incorporated with daphnetin emulsions on the quality of Scophthalmus maximus at refrigerated condition. Int. J. Biol. Macromol. 2021, 170, 129–139. [Google Scholar] [CrossRef]
- Cheng, J.H.; Sun, D.W.; Wei, Q.Y. Enhancing Visible and Near-Infrared Hyperspectral Imaging Prediction of TVB-N Level for Fish Fillet Freshness Evaluation by Filtering Optimal Variables. Food Anal. Methods 2017, 10, 1888–1898. [Google Scholar] [CrossRef]
- Cai, L.Y.; Cao, A.L.; Li, T.T.; Wu, X.S.; Xu, Y.X.; Li, J.R. Effect of the Fumigating with Essential Oils on the Microbiological Characteristics and Quality Changes of Refrigerated Turbot (Scophthalmus maximus) Fillets. Food Bioprocess Technol. 2015, 8, 844–853. [Google Scholar] [CrossRef]
- Li, P.Y.; Peng, Y.F.; Mei, J.; Xie, J. Effects of microencapsulated eugenol emulsions on microbiological, chemical and organoleptic qualities of farmed Japanese sea bass (Lateolabrax japonicus) during cold storage. LWT-Food Sci. Technol. 2020, 118, 108831. [Google Scholar] [CrossRef]
- Bian, C.H.; Yu, H.J.; Yang, K.; Mei, J.; Xie, J. Effects of single-, dual-, and multi-frequency ultrasound-assisted freezing on the muscle quality and myofibrillar protein structure in large yellow croaker (Larimichthys crocea). Food Chem.-X 2022, 15, 100362. [Google Scholar] [CrossRef] [PubMed]
- Esua, O.J.; Sun, D.W.; Cheng, J.H.; Li, J.L. Evaluation of storage quality of vacuum-packaged silver Pomfret (Pampus argenteus) treated with combined ultrasound and plasma functionalized liquids hurdle technology. Food Chem. 2022, 391. [Google Scholar] [CrossRef]
- Lan, W.; Che, X.; Xu, Q.; Wang, T.; Du, R.; Xie, J.; Hou, M.; Lei, H. Sensory and chemical assessment of silver pomfret (Pampus argenteus) treated with Ginkgo biloba leaf extract treatment during storage in ice. Aquac. Fish. 2018, 3, 30–37. [Google Scholar] [CrossRef]
- Bazargani-Gilani, B.; Pajohi-Alamoti, M. The effects of incorporated resveratrol in edible coating based on sodium alginate on the refrigerated trout (Oncorhynchus mykiss) fillets’ sensorial and physicochemical features. Food Sci. Biotechnol. 2020, 29, 207–216. [Google Scholar] [CrossRef]
- Le, T.; Takahashi, K.; Okazaki, E.; Osako, K. Mitigation of lipid oxidation in tuna oil using gelatin pouches derived from horse mackerel (Trachurus japonicus) scales and incorporating phenolic compounds. LWT-Food Sci. Technol. 2020, 128, 109533. [Google Scholar] [CrossRef]
- Jonusaite, K.; Venskutonis, P.R.; Martínez-Hernández, G.B.; Taboada-Rodríguez, A.; Nieto, G.; López-Gómez, A.; Marín-Iniesta, F. Antioxidant and Antimicrobial Effect of Plant Essential Oils and Sambucus nigra Extract in Salmon Burgers. Foods 2021, 10, 776. [Google Scholar] [CrossRef]
- Kostaki, M.; Giatrakou, V.; Savvaidis, I.N.; Kontominas, M.G. Combined effect of MAP and thyme essential oil on the microbiological, chemical and sensory attributes of organically aquacultured sea bass (Dicentrarchus labrax) fillets. Food Microbiol. 2009, 26, 475–482. [Google Scholar] [CrossRef]
- Piccini, J.L.; Evans, D.R.; Quaranta, H.O. Comparison of TBA number of irradiated fish with sensory quality. Food Chem. 1986, 19, 163–171. [Google Scholar] [CrossRef]
- Gao, W.H.; Wu, X.R.; Ye, R.S.; Zeng, X.A.; Brennan, M.A.; Brennan, C.S.; Ma, J. Analysis of protein denaturation, and chemical visualisation, of frozen grass carp surimi containing soluble soybean polysaccharides. Int. J. Food Sci. Technol. 2022, 57, 5504–5513. [Google Scholar] [CrossRef]
- Zhang, L.T.; Li, Q.; Bao, Y.L.; Tan, Y.Q.; Lametsch, R.; Hong, H.; Luo, Y.K. Recent advances on characterization of protein oxidation in aquatic products: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 64, 1572–1591. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.C.; Yu, C.C.; Hsu, K.C. Changes in conformation and sulfhydryl groups of tilapia actomyosin by thermal treatment. LWT-Food Sci. Technol. 2007, 40, 1316–1320. [Google Scholar] [CrossRef]
- Pei, J.X.; Mei, J.; Wu, G.; Yu, H.J.; Xie, J. Gum tragacanth-sodium alginate active coatings containing epigallocatechin gallate reduce hydrogen peroxide content and inhibit lipid and protein oxidations of large yellow croaker (Larimichthys crocea) during superchilling storage. Food Chem. 2022, 397, 133792. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, Y.G.; Zhao, L.; Chen, L.; He, Y.; Yang, H.S. Vacuum impregnation of fish gelatin combined with grape seed extract inhibits protein oxidation and degradation of chilled tilapia fillets. Food Chem. 2019, 294, 316–325. [Google Scholar] [CrossRef]
- Chamba, M.V.M.; Hua, Y.F.; Katiyo, W. Oxidation and Structural Modification of Full-Fat and Defatted Flour Based Soy Protein Isolates Induced by Natural and Synthetic Extraction Chemicals. Food Biophys. 2014, 9, 193–202. [Google Scholar] [CrossRef]
- Yang, F.; Jia, S.N.; Liu, J.X.; Gao, P.; Yu, D.W.; Jiang, Q.X.; Xu, Y.S.; Yu, P.P.; Xia, W.S.; Zhan, X.B. The relationship between degradation of myofibrillar structural proteins and texture of superchilled grass carp (Ctenopharyngodon idella) fillet. Food Chem. 2019, 301, 125278. [Google Scholar] [CrossRef]
- Reza, M.S.; Bapary, M.A.J.; Ahasan, C.T.; Islam, M.N.; Kamal, M. Shelf life of several marine fish species of Bangladesh during ice storage. Int. J. Food Sci. Technol. 2009, 44, 1485–1494. [Google Scholar] [CrossRef]
- Kong, B.H.; Guo, Y.Y.; Xia, X.F.; Liu, Q.; Li, Y.Q.; Chen, H.S. Cryoprotectants Reduce Protein Oxidation and Structure Deterioration Induced by Freeze-Thaw Cycles in Common Carp (Cyprinus carpio) Surimi. Food Biophys. 2013, 8, 104–111. [Google Scholar] [CrossRef]
- Cui, H.Y.; Zhang, C.H.; Li, C.Z.; Lin, L. Antibacterial mechanism of oregano essential oil. Ind. Crops Prod. 2019, 139, 111498. [Google Scholar] [CrossRef]
- Chelh, I.; Gatellier, P.; Santé-Lhoutellier, V. Technical note: A simplified procedure for myofibril hydrophobicity determination. Meat Sci. 2006, 74, 681–683. [Google Scholar] [CrossRef] [PubMed]
- Benjakul, S.; Visessanguan, W.; Thongkaew, C.; Tanaka, M. Effect of frozen storage on chemical and gel-forming properties of fish commonly used for surimi production in Thailand. Food Hydrocoll. 2005, 19, 197–207. [Google Scholar] [CrossRef]
- Hu, Y.P.; Gao, Y.F.; Solangi, I.; Liu, S.C.; Zhu, J. Effects of tea polyphenols on the conformational, functional, and morphological characteristics of beef myofibrillar proteins. LWT-Food Sci. Technol. 2022, 154, 112596. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Fang, S.; Xie, Y.; Mei, J.; Xie, J. Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage. Coatings 2024, 14, 338. https://doi.org/10.3390/coatings14030338
Yang X, Fang S, Xie Y, Mei J, Xie J. Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage. Coatings. 2024; 14(3):338. https://doi.org/10.3390/coatings14030338
Chicago/Turabian StyleYang, Xinrui, Shiyuan Fang, Yao Xie, Jun Mei, and Jing Xie. 2024. "Preservative Effects of Flaxseed Gum-Sodium Alginate Active Coatings Containing Carvacrol on Quality of Turbot (Scophthalmus maximus) during Cold Storage" Coatings 14, no. 3: 338. https://doi.org/10.3390/coatings14030338




