Effect of Conformal Coating on Electrochemical Migration Behavior of Multi-Layer Ceramic Capacitor for Automotives Based on Water Drop Test
Abstract
:1. Introduction
2. Experimental Methods
2.1. Specimen
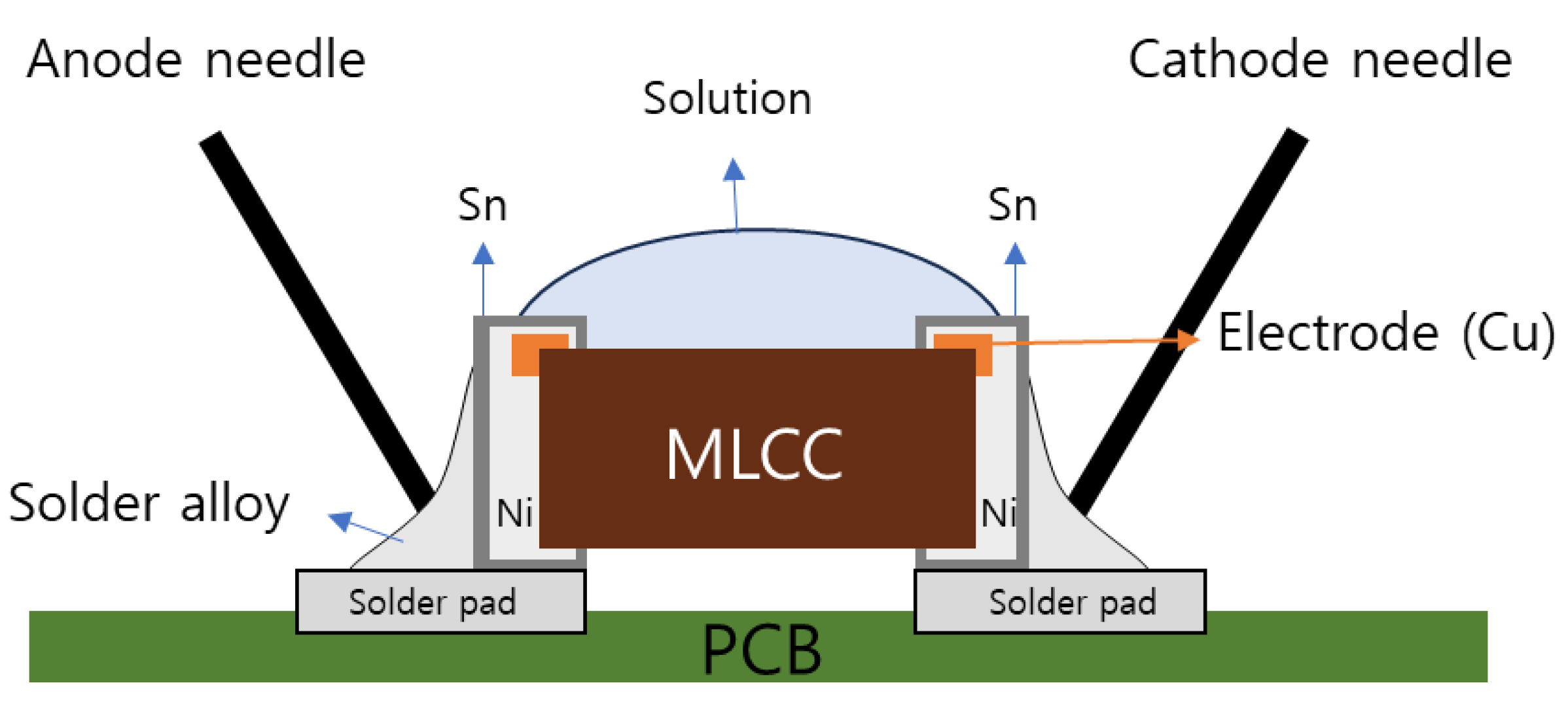
2.2. Water Drop Test
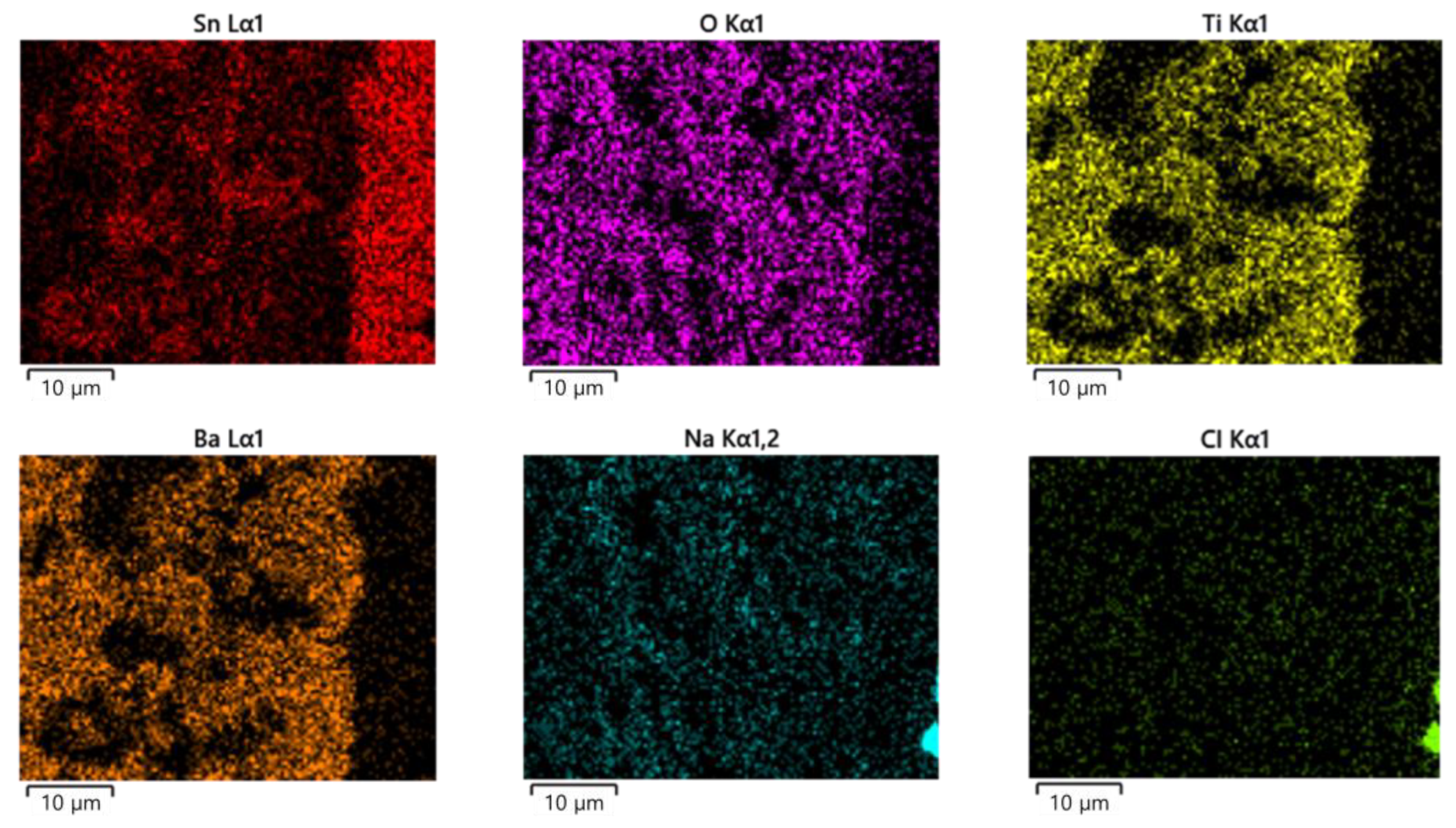
2.3. Surface Appearance Analysis
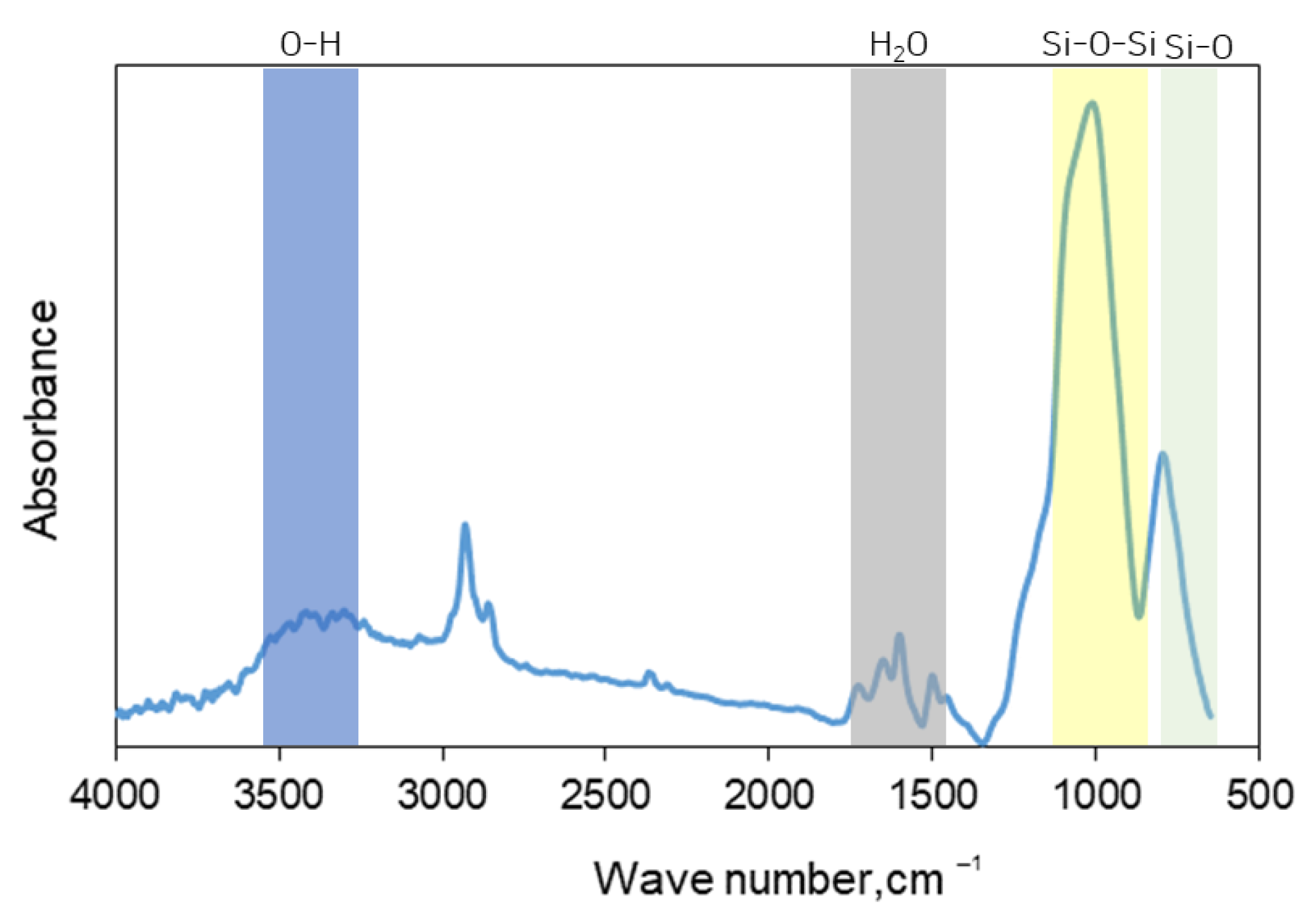
2.4. FT-IR Analysis
3. Results
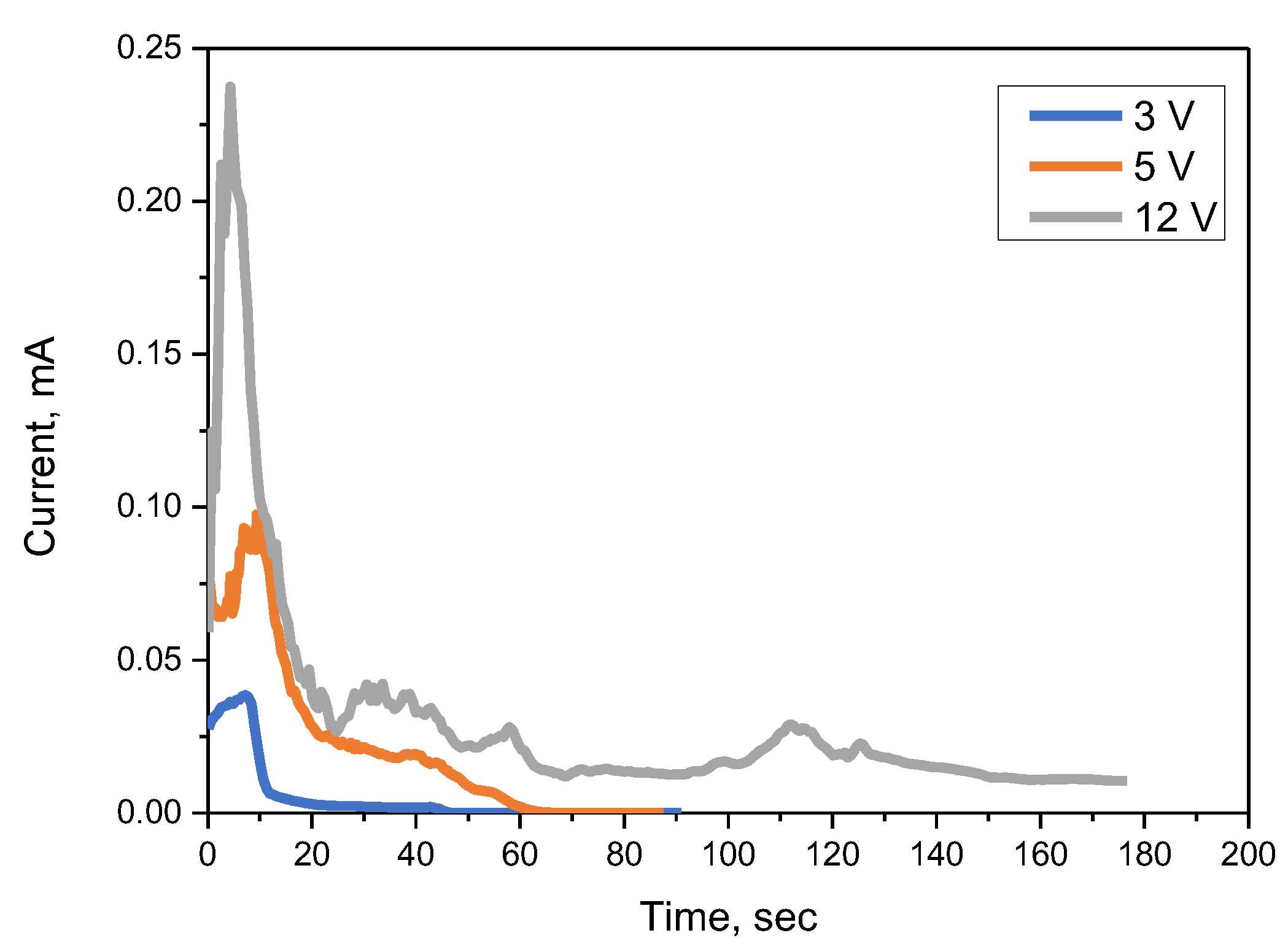
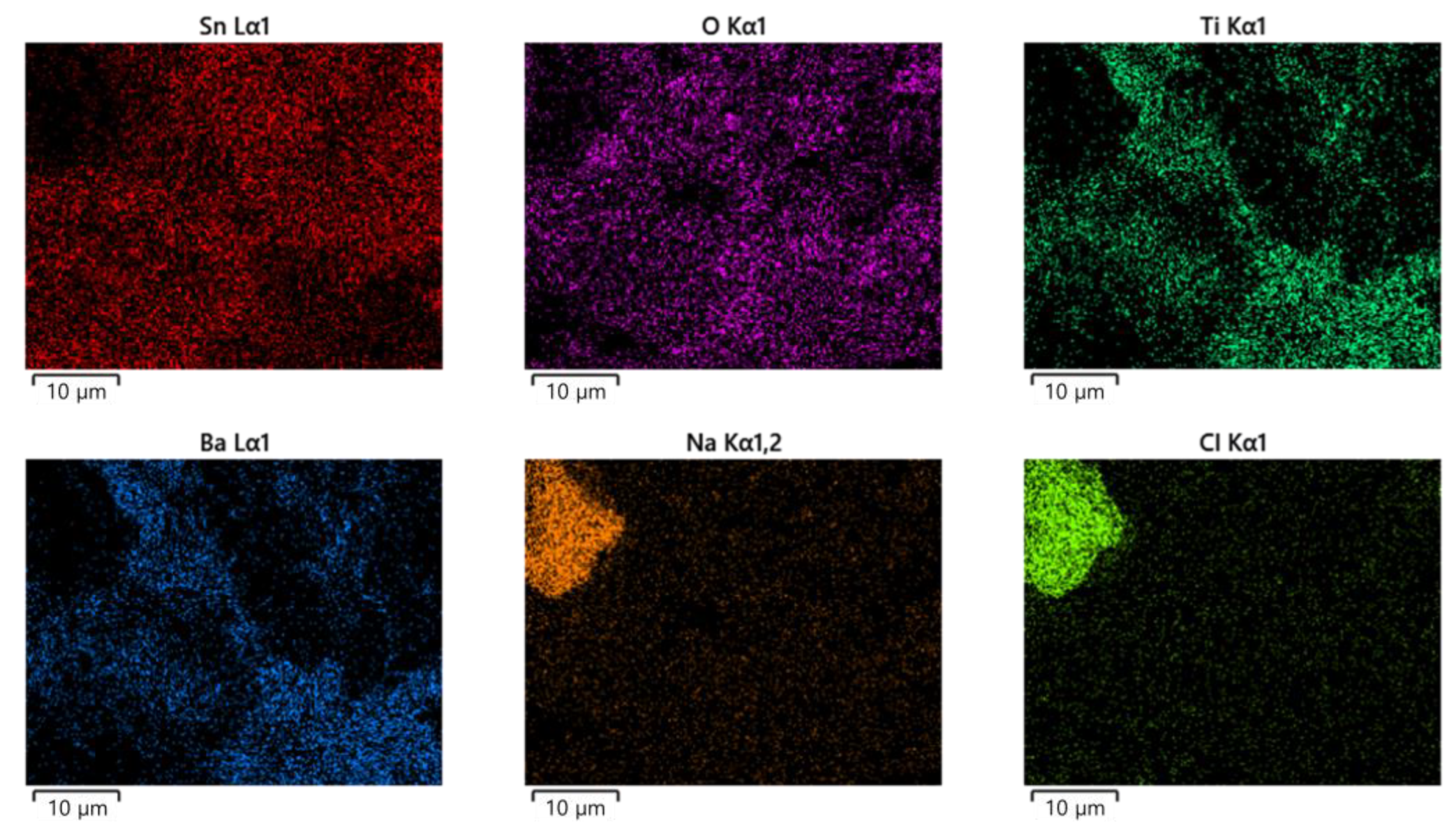
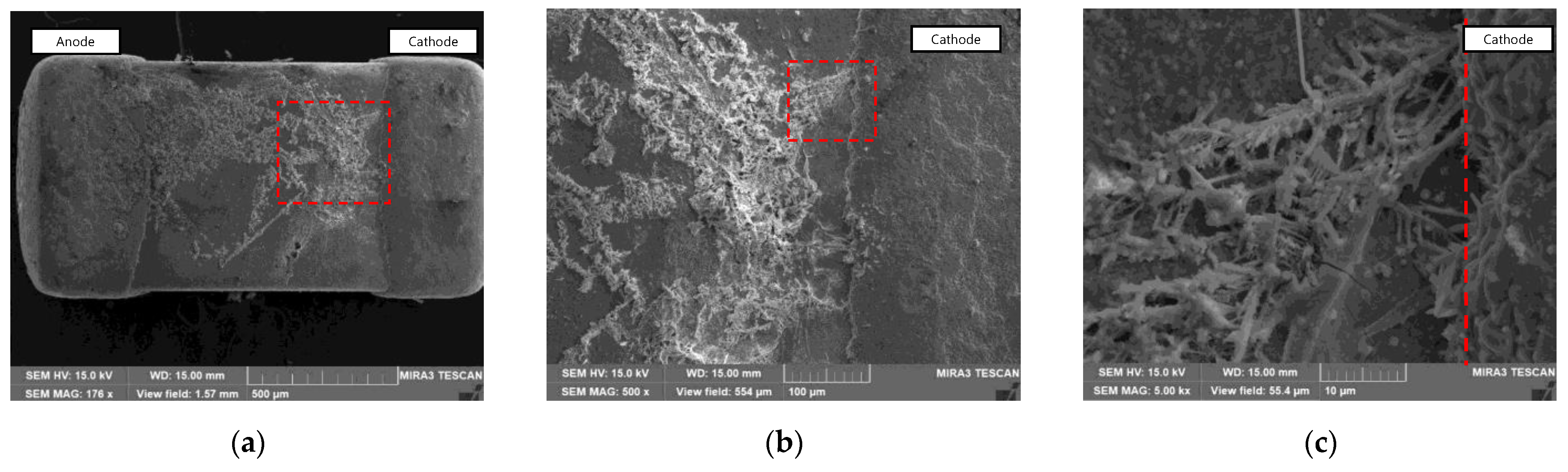
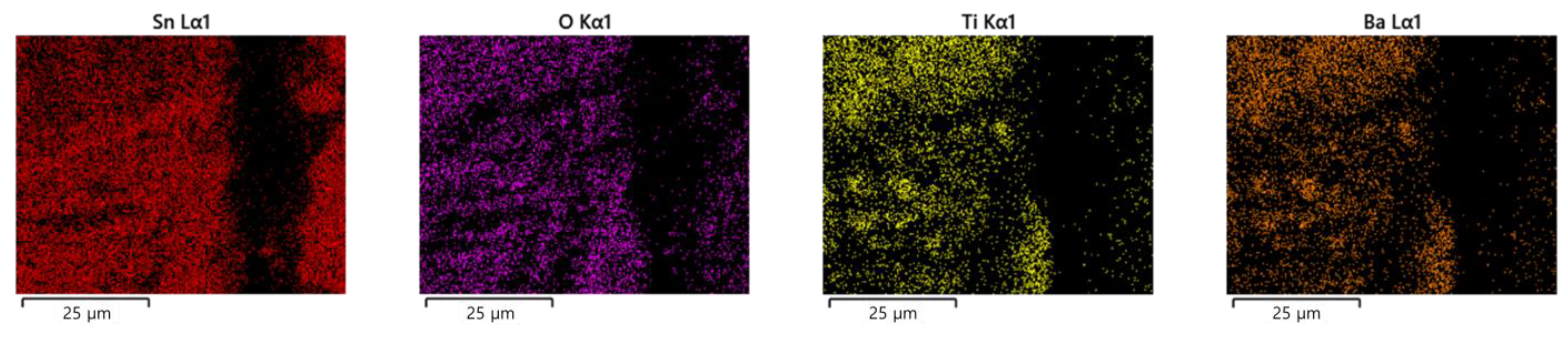
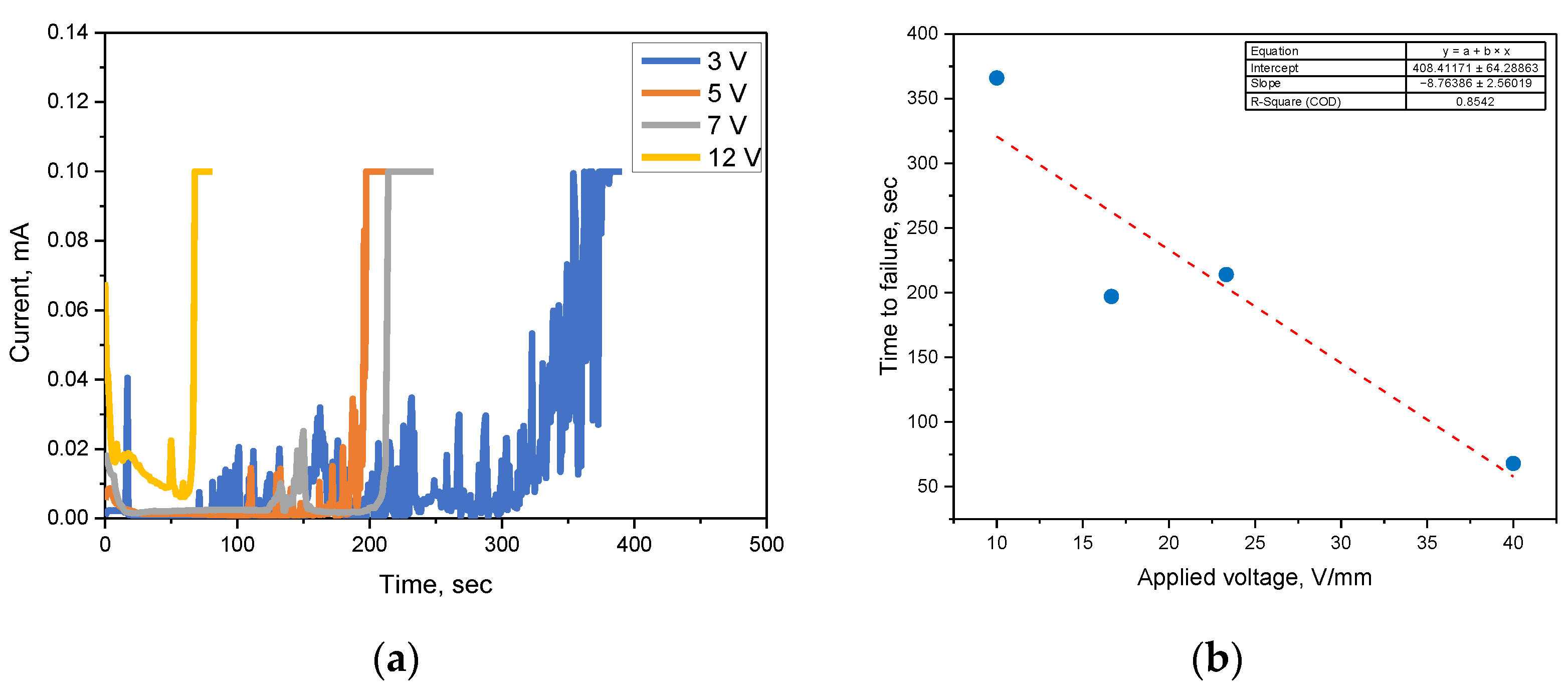
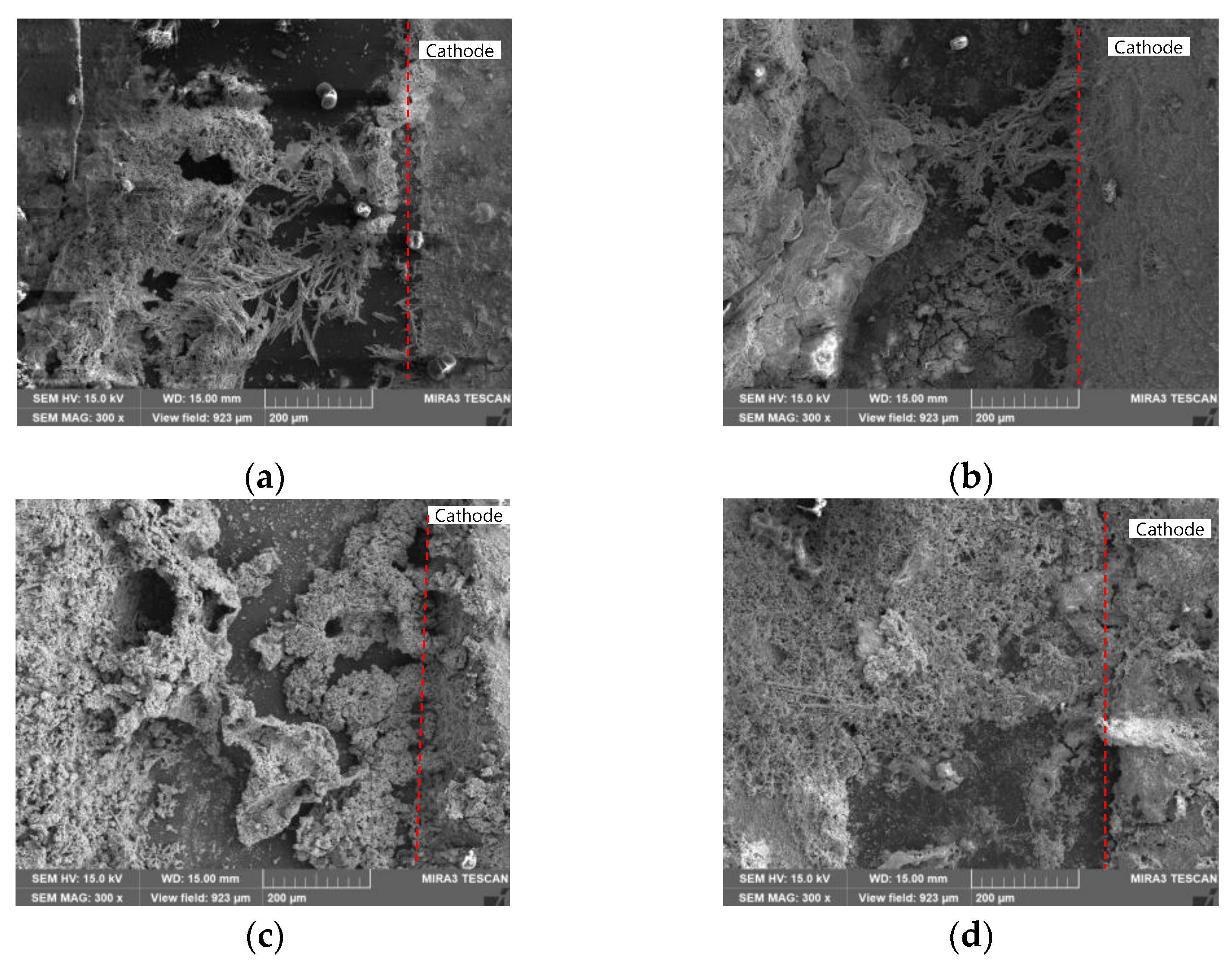
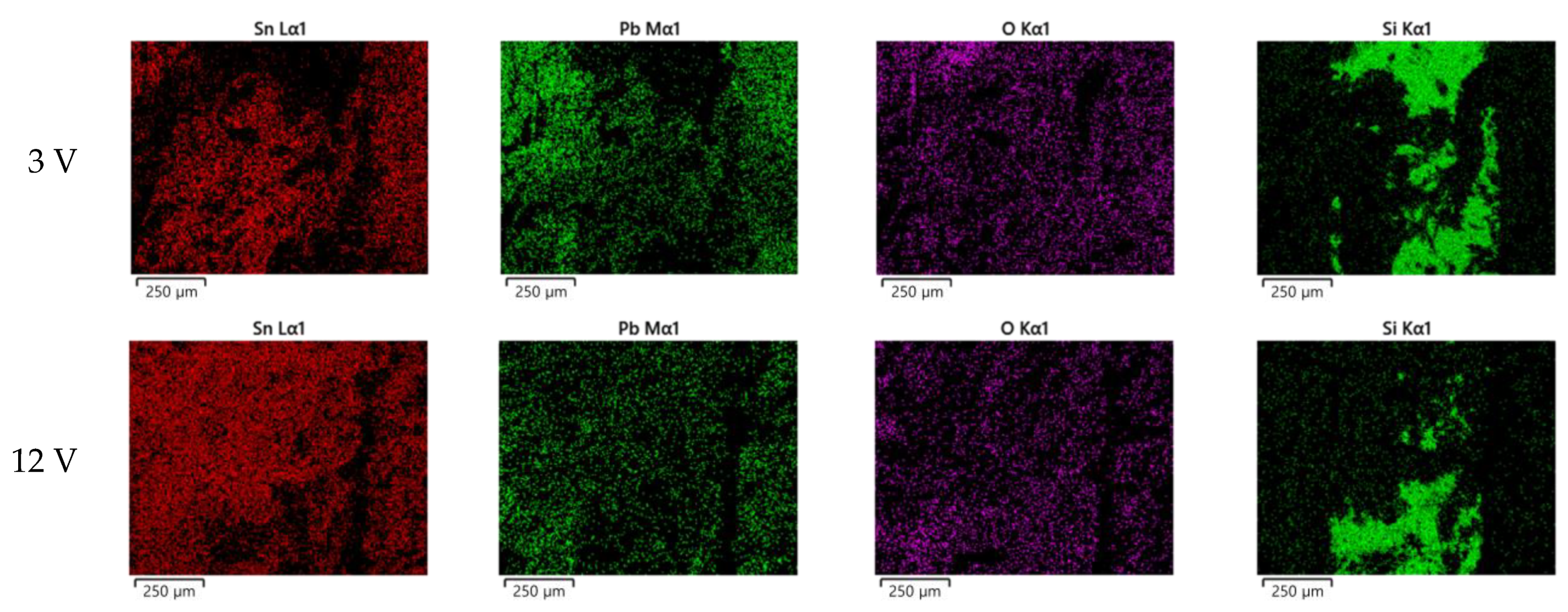
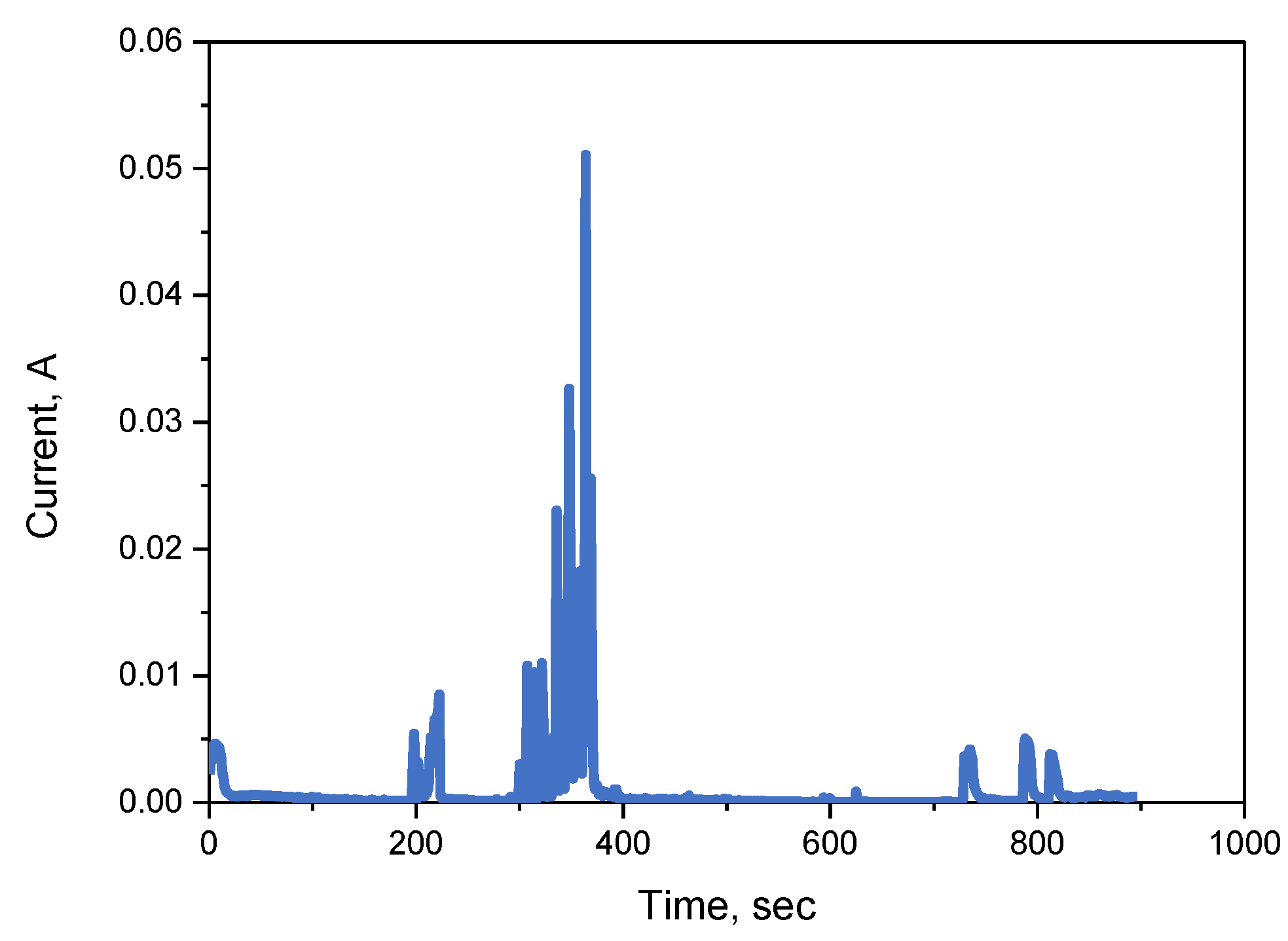
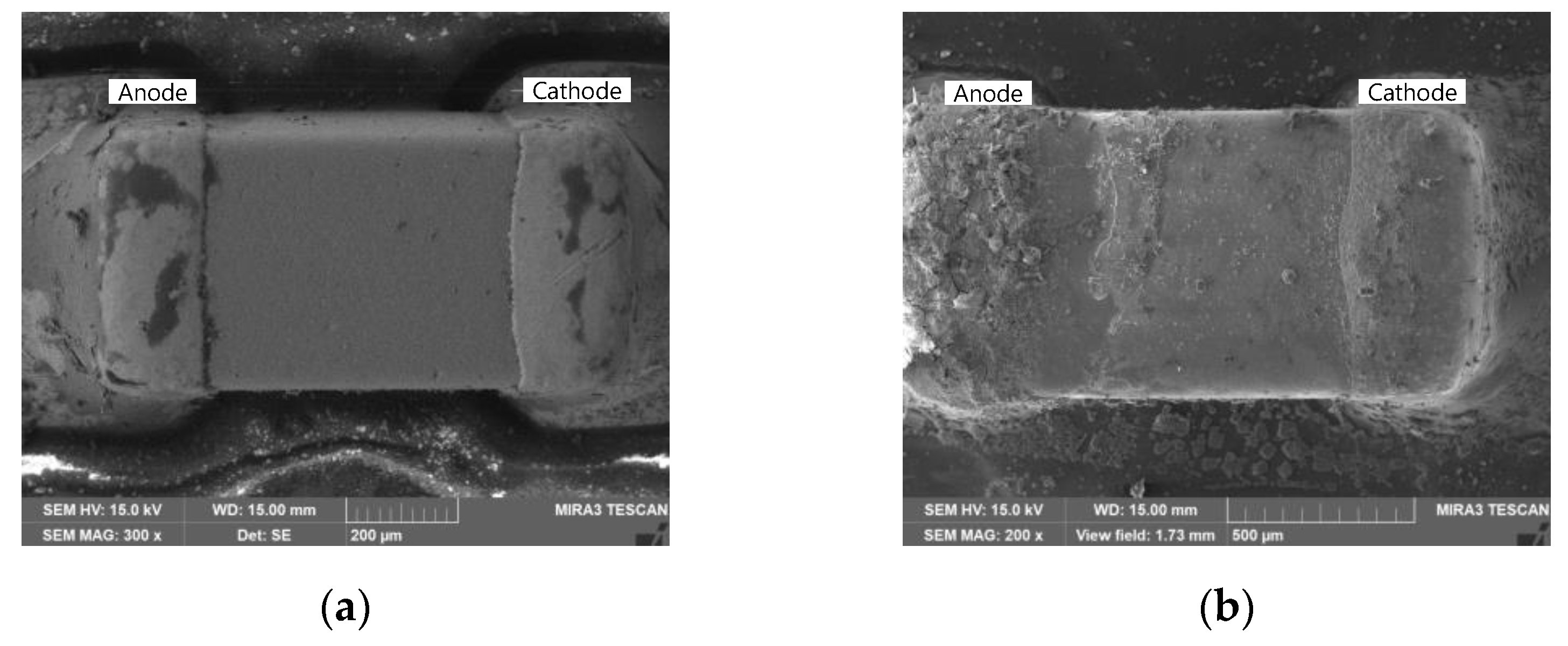
3.1. ECM Behavior of Surface Mount MLCC by Water Drop Test
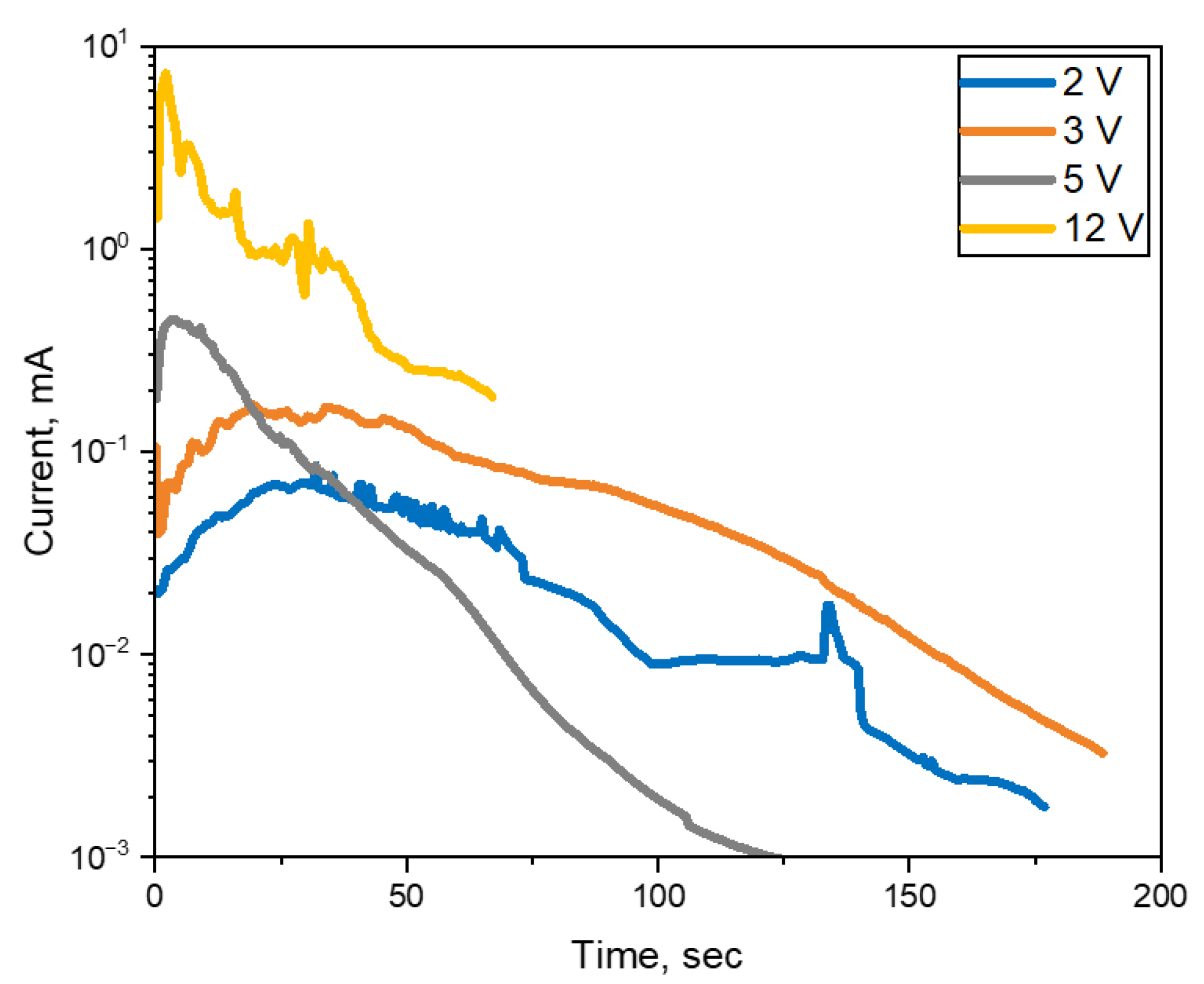
3.2. ECM Behavior of Non-Mount MLCC by Water Drop Test
4. Discussion
5. Conclusions
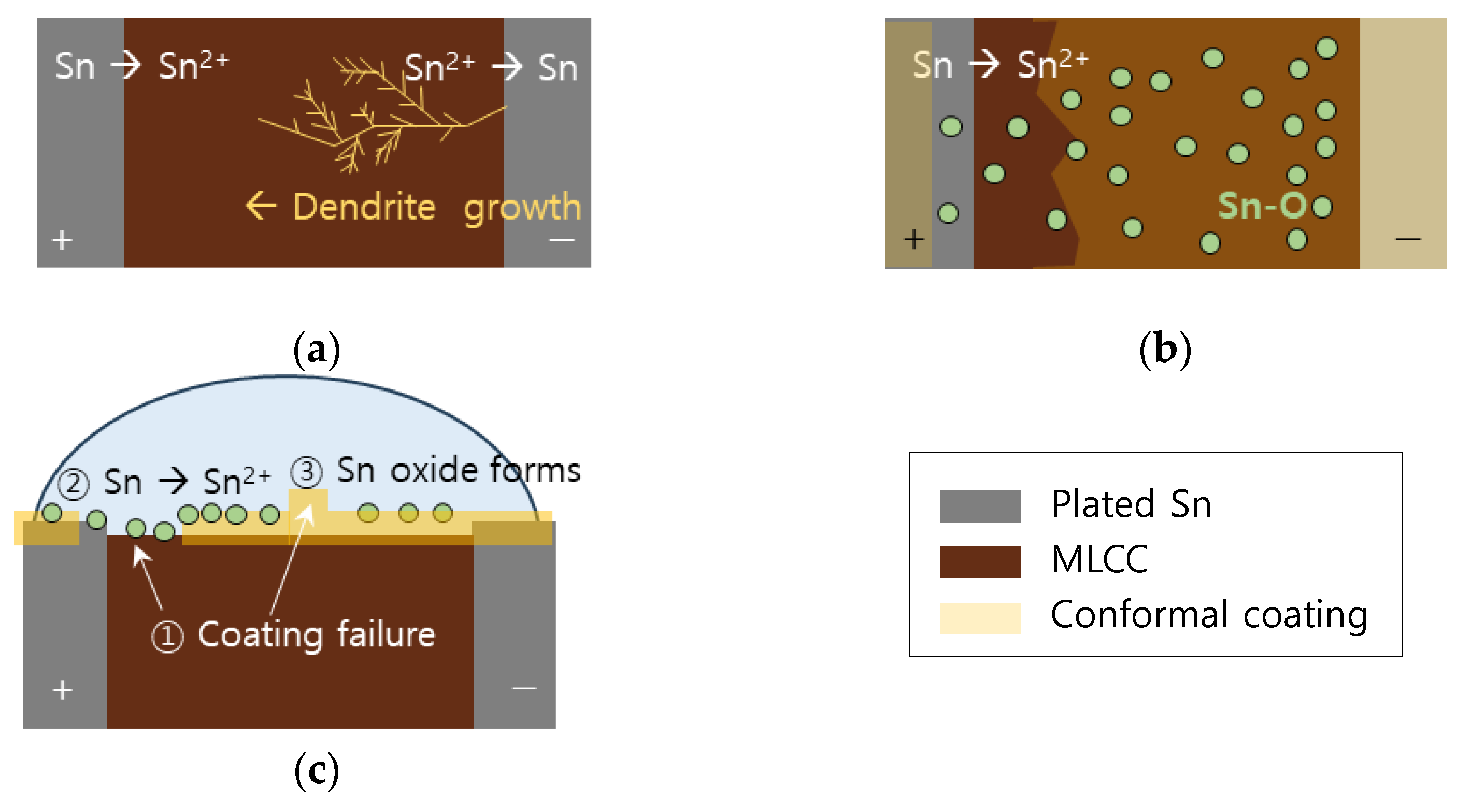
- In the case of a MLCC without conformal coating, regardless of the water drop test conditions, the Sn from the anode dissolved and migrated to the cathode, forming a metallic dendrite, resulting in the ECM phenomenon.
- In the case of a surface mount MLCC with conformal coating, ECM phenomenon by the water drop test did not occur due to the following mechanisms: ① When a voltage was applied, a part of the conformal coating on the anode was damaged by the applied voltage. ② Sn was ionized and dissolved in an aqueous solution by the applied voltage. It then migrated toward the cathode. ③ The integrity of the conformal coating on the cathode side was maintained with electrical insulation without metallic dendrite formation. However, it reacted with water present in the aqueous solution to form tin oxide. Thus, ECM occurrence became difficult.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marati, N.; Gupta, R.G.; Vaithilingam, B. Multilayer ceramic capacitors crisis management in automotive industry. In Proceedings of the 2020 IEEE International Conference on Power Electronics Smart Grid and Renewable Energy (PESGRE2020), Cochin, India, 2–4 January 2020. [Google Scholar] [CrossRef]
- Hong, K.; Lee, T.H.; Suh, J.M.; Yoon, S.-H.; Jang, H.W. Perspective and challenges in multilayer ceramic capacitors for next generation electronics. J. Mater. Chem. C 2019, 32, 1–21. [Google Scholar] [CrossRef]
- Xu, X.; Gurav, A.S.; Lessner, P.M.; Randall, C.A. Robust BME Class-I MLCCs for Harsh-Environment Applications. IEEE Trans. Ind. Electron. 2011, 58, 2636–2643. [Google Scholar] [CrossRef]
- IEC 60068-1; Environmental Testing—Part 1: General and Guidance. International Electrotechnical Commission: Geneve, Switzerland, 2023.
- Tan, C.M.; Chen, H.H.; Wu, J.P.; Sangwan, V.; Tsai, K.Y.; Huang, W.C. Root cause analysis of a printed circuit board (PCB) failure in a public transport communication system. Appl. Sci. 2022, 12, 640. [Google Scholar] [CrossRef]
- Kohman, G.T.; Hermance, H.W.; Downes, G.H. Silver migration in electrical insulation. Bell Syst. Tech. J. 1955, 34, 1115–1147. [Google Scholar] [CrossRef]
- Noh, B.I.; Jung, S.B. Behaviour of electrochemical migration with solder alloys on printed circuit boards (PCBs). Circuit World 2008, 34, 8–13. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.B.; Joo, Y.C.; Lee, H.Y.; Park, Y.B. Anodic dissolution characteristics and electrochemical migration lifetimes of Sn solder in NaCl and Na2SO4 solutions. Microelectron. Eng. 2008, 85, 1597–1602. [Google Scholar] [CrossRef]
- IPC-TM-650; Assessment of Susceptibility to Metallic Dendritic Growth: Uncoated Printed Wiring. Institute for Interconnecting and Packaging Electronic Circuits: Bannockburn, IL, USA, 2015.
- IPC-TM-650; Solder Mask—Resistance to Electrochemical Migration. Institute for Interconnecting and Packaging Electronic Circuits: Bannockburn, IL, USA, 2015.
- Krumbein, S.J. Metallic electromigration phenomena. IEEE Trans. Compon. Hybrids Manuf. Technol. 1988, 11, 5–15. [Google Scholar] [CrossRef]
- Minzari, D.; Jellesn, M.S.; Moller, P.; Ambat, R. On the electrochemical migration mechanism of tin in electronics. Corros. Sci. 2011, 53, 3366–3379. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, G.; Qiu, Y.; Chen, Z.; Zou, W.; Guo, X. In situ study the dependence of electrochemical migration of tin on chloride. Electrochem. Commun. 2013, 27, 63–68. [Google Scholar] [CrossRef]
- Yoo, Y.R.; Nam, H.S.; Jung, J.Y.; Lee, S.B.; Park, Y.B.; Joo, Y.C.; Kim, Y.S. Effect of Ag and Cu additions on the electrochemical migration susceptibility of Pb-free solder in Na2SO4 solution. Corros. Sci. Technol. 2007, 2, 50–55. [Google Scholar]
- Medgyes, B.; Szabo, P.; Tamasi, P.; Gal, L.; Harsanyi, G. Electrochemical migration of Cu and Sn in Na2SO4 environment. In Proceedings of the 2016 39th International Spring Seminar on Electronics Technology (ISSE), Pilsen, Czech Republic, 18–22 May 2016. [Google Scholar] [CrossRef]
- Vianco, P.T. Corrosion issues in solder joint design and service. Weld. J. 1999, 78, 39–46. [Google Scholar]
- Medgyes, B.; Kiss, R.; Szurdan, S.; Rigler, D.; Gal, L.; Berenyi, R.; Harsanyi, G. Electrochemical migration investigations on Sn-Sb solder alloys using 3.5wt% NaCl solution. In Proceedings of the 2017 40th International Spring Seminar on Electronics Technology (ISSE), Sofia, Bulgaria, 10–14 May 2017. [Google Scholar] [CrossRef]
- Yu, D.Q.; Jillek, W.; Schmitt, E. Electrochemical migration of Sn-Pb and lead free solder alloys under distilled water. J. Mater. Sci. Mater. Electron. 2006, 17, 219–227. [Google Scholar] [CrossRef]
- Ready, W.J.; Turbini, L.J.; Nickel, R.; Fischer, J. A novel test circuit for automatically detecting electrochemical migration and conductive anodic filament formation. J. Electron. Mater. 1999, 28, 1158–1163. [Google Scholar] [CrossRef]
- Yoo, Y.R.; Kim, Y.S. Difference of potential range formed at the anode between water drop test and temperature humidity bias test to evaluate electrochemical migration of solders for printed circuit board. Corros. Sci. Technol. 2023, 22, 153–163. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Z.; Zhao, N.; Yang, F. Migration behavior of indium atoms in Cu/Sn-52In/Cu interconnects during electromigration. J. Mater. Res. 2015, 30, 3316–3323. [Google Scholar] [CrossRef]
- Kang, H.; Rajendran, S.H.; Jung, J.P. Low melting temperature Sn-Bi solder: Effect of alloying and nanoparticle addition on the microstructural, thermal, interfacial bonding, and mechanical characteristics. Metals 2021, 11, 364. [Google Scholar] [CrossRef]
- Medgyes, B.; Roman, E.; Bohnert, A.; Szurdan, S.; Zhong, X.; Harsanyi, G. Electrochemical migration investigations on SAC-Bi-xMn solder alloys. In Proceedings of the 2018 IEEE 24th International Symposium for Design and Technology in Electronic Packaging (SIITME), Iasi, Romania, 25–28 October 2018. [Google Scholar] [CrossRef]
- Liao, B.; Jia, W.; Sun, R.; Chen, Z.; Guo, X. Electrochemical migration behavior of Sn-3.0Ag-0.5Cu solder alloy under thin electrolyte layers. Surf. Rev. Lett. 2019, 26, 1850208. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, S.B.; Joo, Y.C.; Lee, H.Y.; Park, Y.B. Effect of ionization characteristics on electrochemical migration lifetimes of Sn-3.0Ag-0.5Cu solder in NaCl and Na2SO4 solutions. J. Electron. Mater. 2008, 37, 1111–1118. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.; Liu, Z.; Jiu, Y.; Zhang, S.; Geng, J.; Chen, X.; Wu, S.; He, P.; Long, W. Corrosion behavior of Sn-based lead-free solder alloys: A review. J. Mater. Sci. Mater. Electron. 2020, 31, 9076–9090. [Google Scholar] [CrossRef]
- Pan, M.J.; Randall, C.A. A brief introduction to ceramic capacitors. IEEE Electr. Insul. Mag. 2010, 26, 44–50. [Google Scholar] [CrossRef]
- Verdingovas, V.; Jellsen, M.S.; Ambat, R. Influence of sodium chloride and weak organic acids (flux residues) on electrochemical migration of tin on surface mount chip components. Corros. Eng. Sci. Technol. 2010, 48, 426–435. [Google Scholar] [CrossRef]
- Laadjal, K.; Cardoso, A.J.M. Multilayer ceramic capacitors: An overview of failure mechanisms, perspectives, and challenges. Electronics 2023, 12, 1297. [Google Scholar] [CrossRef]
- Francisc, S. Study of ceramic capacitor technology link to electro chemical migration in automotive electronics. In Proceedings of the 2020 IEEE 26th International Symposium for Design and Technology in Electronic Packaging (SIITME), Pitesti, Romania, 21–24 December 2020. [Google Scholar] [CrossRef]
- Gu, J.; Azarian, M.H.; Pecht, M.G. Failure prognostics of multilayer ceramic capacitors in temperature-humidity-bias condition. In Proceedings of the 2008 International Conference on Prognostics and Health Management, Denver, CO, USA, 6–9 October 2008. [Google Scholar] [CrossRef]
- Takemoto, T.; Latanision, R.M.; Eagar, T.W.; Matsunawa, A. Electrochemical migration tests of solder alloys in pure water. Corros. Sci. 1997, 39, 1415–1430. [Google Scholar] [CrossRef]
- ISO 26262-2; Part 2: Management of Functional Safety. International Organization for Standardization: Geneve, Switzerland, 2018.
- IPC-6011; Generic Performance Specification for Printed Bards. International Organization for Standardization: Bannockburn, IL, USA, 1966.
- Byram, J. Conformal Coating, Printed Circuits Handbook, 6th ed.; Coombs, C.F., Ed.; McGraw Hill: New York, NY, USA, 2008; Volume 1, pp. 41.1–41.17. [Google Scholar]
- Vesely, P.; Klimtova, M.; Fros, D. Electrochemical migration issues related to improper solder mask application. In Proceedings of the 2022 45th International Spring Seminar on Electronics Technology (ISSE), Vienna, Austria, 11–15 May 2022. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-R.; Won, S.; Kim, Y.-S. Effect of Conformal Coating on Electrochemical Migration Behavior of Multi-Layer Ceramic Capacitor for Automotives Based on Water Drop Test. Coatings 2024, 14, 359. https://doi.org/10.3390/coatings14030359
Yoo Y-R, Won S, Kim Y-S. Effect of Conformal Coating on Electrochemical Migration Behavior of Multi-Layer Ceramic Capacitor for Automotives Based on Water Drop Test. Coatings. 2024; 14(3):359. https://doi.org/10.3390/coatings14030359
Chicago/Turabian StyleYoo, Young-Ran, Seokyeon Won, and Young-Sik Kim. 2024. "Effect of Conformal Coating on Electrochemical Migration Behavior of Multi-Layer Ceramic Capacitor for Automotives Based on Water Drop Test" Coatings 14, no. 3: 359. https://doi.org/10.3390/coatings14030359
APA StyleYoo, Y.-R., Won, S., & Kim, Y.-S. (2024). Effect of Conformal Coating on Electrochemical Migration Behavior of Multi-Layer Ceramic Capacitor for Automotives Based on Water Drop Test. Coatings, 14(3), 359. https://doi.org/10.3390/coatings14030359







