Abstract
In recent years, Additive Manufacturing (AM), commonly referred to as 3D printing, has garnered the attention of the scientific community due to its capacity to transform ordinary and traditional items into customized materials at an affordable cost through various AM processes. Antimicrobial/antibiofilm 3D printed materials are one of the most trending research topics, owing to the growing concerns over the emergence of complex microbial structures called “biofilms” on various surfaces. The review provides an overview of the evolution of additive manufacturing (AM) technologies and their various derivatives, along with a brief description of their materials and applications. It also introduces how biofilms can represent an advantageous lifestyle for microbial populations. The primary objective of this research was to conduct a systematic review of the development of planctonic or biofilm forms of microorganisms on 3D-printed materials. The article summarizes commonly studied microorganisms on these materials and presents their 3D printing process, materials, as well as the fields covered by each of the analyzed papers. To the best of our knowledge, this is the first all-inclusive systematic review that amalgamates research conducted in diverse fields to assess the development of biofilms on surfaces produced through three-dimensional printing. Most notably, this review presents a comprehensive account of sustainable approaches for producing antimicrobial materials through 3D printing. Additionally, we assess their advancements in various fields such as medicine, environment, agri-food, and other relevant sectors. The findings of our literature review can be used to recommend appropriate microorganisms, 3D printing materials, and technologies for academic and industrial research purposes, focusing on the development of microbial biofilms on 3D-printed surfaces. Furthermore, it highlights the potential of environmentally friendly modified AM technologies to combat biofilms in clinical and non-clinical areas. Our goal with this review is to help readers gain a better understanding of fundamental concepts, inspire new researchers, and provide valuable insights for future empirical studies focused on eradicating biofilms from 3D-printed materials.
1. Introduction
In a world that is undergoing continual change, human civilization has an increased desire to research and explore new technologies that are superior in both their application and overall process. In fact, developing antimicrobial materials thanks to technological advancements could be of great interest in many fields and can greatly aid in producing sustainable solutions in the fight against some of society’s biggest threats, such as antibiotic resistance and emerging novel pathogens. Additionally, with the researcher’s focus shifting more toward multifunctional antimicrobial materials, modern technologies have recently increased their efforts to boost the effectiveness of such materials, notably 3D printing technologies. By definition and according to the ISO/ASTM (International Organization for Standardization/American Society for Testing and Materials) standards, 3D printing is described as “the act of combining materials typically layer by layer to produce objects using 3D model data [1]. This technology involves the overlapping of materials in order to produce components with intricate shapes promptly by precisely accumulating materials based on a computer-aided design (CAD) model or computed tomography (CT) scan under computer control [2]. Additive manufacturing is reportedly a fast-growing technology, applied in a broad range of disciplines, including tissue engineering, regenerative medicine, aerospace engineering, and even food industry and construction fields [3]. As stated by Campbell and Ivanova [4], AM is now increasingly considered a revolutionary innovation that provides a different concept for engineering design and production, with substantial impacts on economics, geopolitics, ecology, intellectual property, and safety. In their assessment of printable and multipurpose polymer composites, Bekas et al. (2019) mentioned that additive manufacturing can provide a number of benefits, including minimal material consumption, reduced waste, customization flexibility, and geometric sophistication, which explains the urgent demand to create antifouling materials using 3D-printing processes [5]. To produce such materials, multiple studies followed, for instance, simple methods of coating polymer filaments with nanoparticles, fibers, or metal flakes readily accessible [6]. However, the number of papers dedicated to studying biofilm adhesion on 3D-printed interfaces or understanding the development of antibacterial qualities in 3D-printed materials is not that large. As far as we know, a comprehensive exploration of the techniques utilized to produce 3D-printed surfaces with antimicrobial/antibiofilm properties has not been conducted yet, emphasizing the novelty of the present work.
One of the most prevalent 3D printing technologies are those based on the extrusion of materials, especially bio-plotting or more commonly fused deposition modeling (FDM) [7], thanks to their ability to produce components using a variety of biocompatible or biodegradable materials, and in certain circumstances even the printing of living cells or bacteria can be successfully achieved [8]. Besides fused deposition modeling (FDM) techniques, the rest of AM technologies can be broadly classified into the following types based on their printing principles: direct ink writing (DIW), photocuring (SLA, DLP), laminated object manufacturing (LOM), laser sintering and laser melting (SLS, SLM), photopolymer jetting (Ployjet), and binder jetting (3DP). These 3D printing technologies provide a variety of pricing, performance, and material alternatives. When it comes to 3D printing materials, polymers, metals and ceramics are by far the most widely used, as are hybrids, composites, and functionally graded materials (FGM) [9].
The number of research papers incorporating 3D printing has risen significantly in recent years. In the year 2013, the number of articles identified on Web of Science using (WOS) the search phrases “3D printing” or “additive manufacturing” skyrocketed by thousands each year, reaching a total of 10,000 in 2016. Since 3D printing is increasingly being integrated in many areas, namely biomedicine, which explains the pressing demand for antimicrobial 3DP materials, smart materials, nanomaterials, functional materials, biomaterials, composites and many others have been developed and highly investigated for 3D printability in order for them to acquire product complexity, multi-performance ability [10], and more importantly, antimicrobial activity and overall efficiency.
The possibility of pathogen contamination, especially through the development of biofilms, poses a serious threat to 3D-printed materials. Biofilms are renowned for their naturally occurring resistance to antibiotics, which can be 10 to 1000 times higher than that of planktonic cells [11]. This makes it difficult to manage biofilm contamination, particularly in therapeutic settings [12]. Statistically, biofilm production is involved in up to 80% of microbial infections, increasing dramatically the occurrence rates and morbidity [13]. Which calls for urgent solutions to inhibit the adhesion of these communities on all surfaces, including three-dimensionally printed ones [14].
To overcome the shortcomings of antimicrobial remedies, researchers have been working on various strategies these past few years to develop surfaces made of modified materials that are antimicrobial by nature. This antimicrobial activity can be achieved by either killing microorganisms as soon as they adhere to the surface or by preventing the formation of microbial colonies [15,16]. By producing materials that are microbe-repellent, microbe-killing, anti-adhesive, or biocide-releasing, antimicrobial surfaces can be successfully generated. Coating, vapor deposition, sol-gel, plasma deposition, laser-mediated techniques, electrochemical methods, etc. are often used to generate such surfaces [17]. Moreover, recently, 3D printing has been employed for this purpose by directly incorporating the antimicrobial substance during the printing process in order to provide either a microbiostatic or microbicidal activity to the desired material. Small molecules, macromolecules, polymers, ceramics, metals, and nanocomposites exhibiting microbicidal properties against bacteria, fungus, and viruses are the ones referred to as antimicrobial substances [18]. In this context, all of the reviewed research utilized precise 3D printing technology in accordance with a single or multiple antimicrobials. The materials used in these studies were precisely defined to create 3D-printed surfaces with microbicidal properties, specifically for use in fields such as healthcare, food, or environmental sectors.
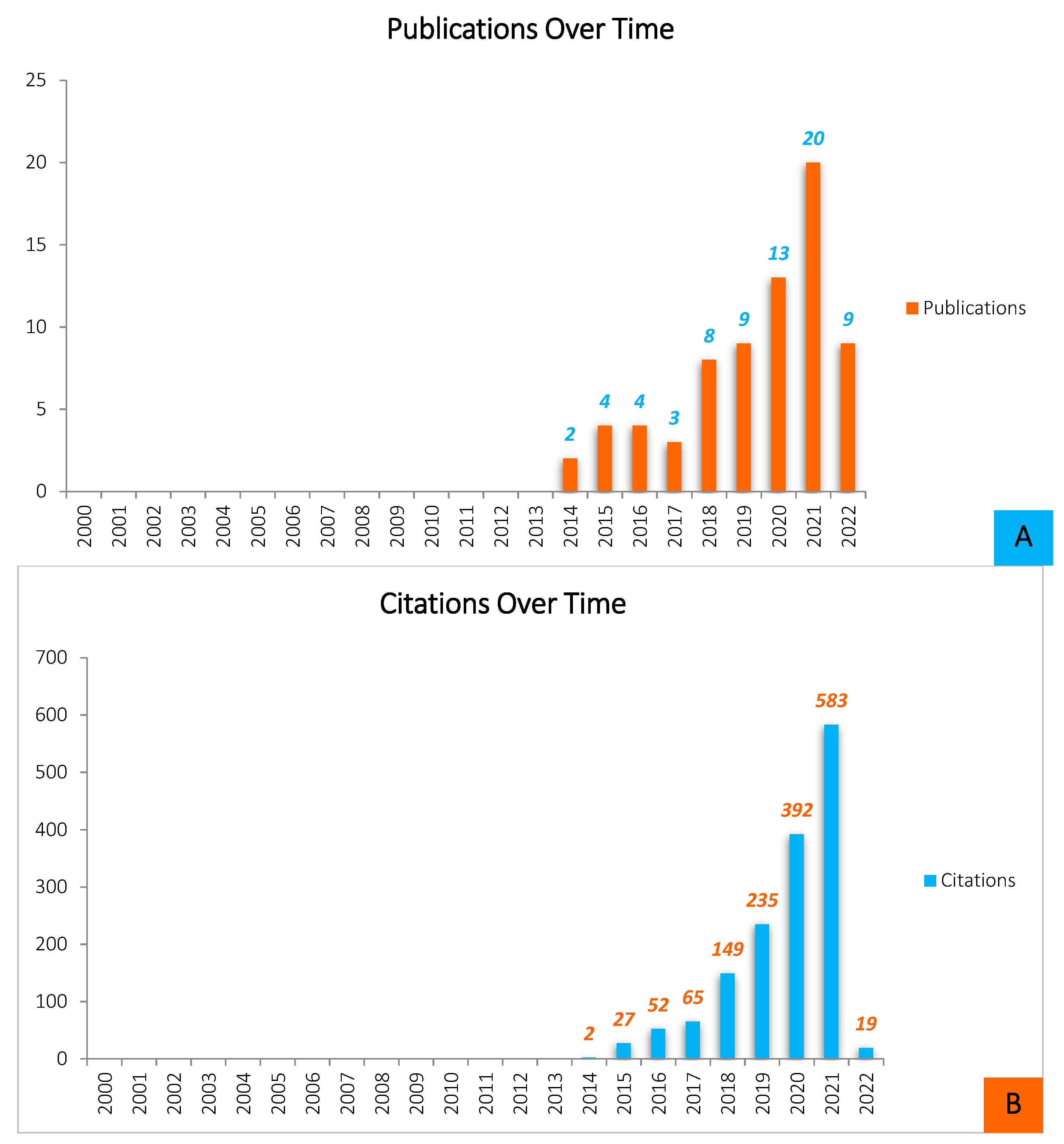
Throughout the years leading up to 2022, and based on WOS results, there was a significant rise in studies published on the formation of biofilms on 3D-printed materials, with particularly substantial increases in the last 3–4 years (Figure 1).
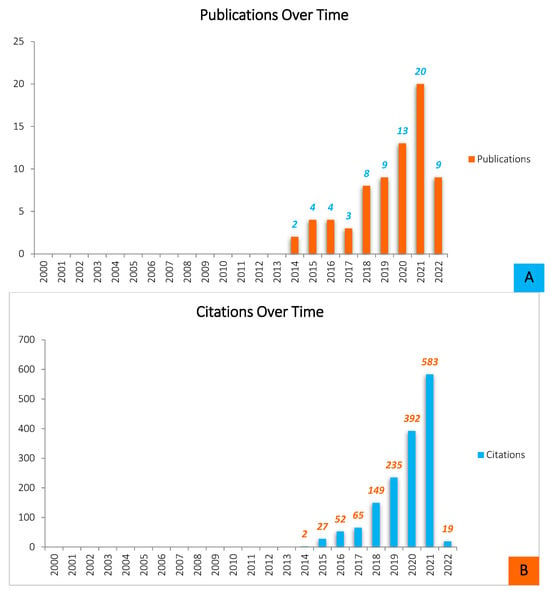
Figure 1.
Quantitative analysis of the scientific literature on Biofilm formation on 3D-printed materials (source: ISI WEB OF SCIENCE, May 2022). (A)—Shows the distribution of publications by year of release for papers written and produced throughout a ten-year period (2012–2022). The overall pattern of Biofilm development on 3D-printed materials-related article publication demonstrates that the most productive year was 2021, with twenty (n = 20) documents submitted, preceded by thirteen (n = 13) articles reported in 2020, nine (n = 9) published articles in 2019, and the same number of papers was also published in 2022. Nevertheless, it is worthy to note that the 2022 statistic represents articles made over a five-month timeframe, and therefore the amount of literature is likely to rise by the end of the year. The study of biofilm growth on 3D-printed objects is increasingly gaining popularity. In 2017, however, there was a slight decrease in the number of publications. This illustrates that, while the total number of publications in this field is expanding, research interest is always fluctuating. (B)—This figure also represents the average number of citations each year. Although the number of citations is constantly increasing, the expansion is not as steady.
Nonetheless, the integration of 3D printing in biotechnology and antimicrobial bioengineering fields is still relatively uncommon. Most reviews primarily emphasize 3D printing as a groundbreaking technique and describe its applications in a specific field, such as medical, agri-food, or environmental. Several other reviews solely focus on advancements in 3D printing for producing internal and external devices and equipment designed to combat biofouling problems, with its potential uses mainly in healthcare, food industries, or other areas.
This review article provides an in-depth overview of the development of 3D printing as a pervasive technology, all while detailing the wide range of processes it offers and also listing the variety of materials that are suitable for additive manufacturing, along with their various potential uses. Further, the susceptibility of 3D-printed materials to contamination was similarly covered. Based on the reviewed research articles, we were able to collect, classify, and quantify each Biofilm-forming microorganism within a well-defined application category. To the best of our knowledge, this analysis appears to be the first of its kind. Most significantly, and in a manner unparalleled by any other paper, our review highlighted the current understanding of 3DP material biofouling, all while delivering an extensive list of green strategies used in recent research articles in order to inhibit and prevent the formation of biofilm on 3D-printed surfaces, not only in clinical settings but also in every other field of research. All of this was performed while precisely detailing the different AM technologies used, and by providing a brief description of the adopted antimicrobial approaches, the effectiveness of our study’s demonstration of the antibacterial and anti-adhesive procedures adds to its originality and authenticity.
2. Methods: Search Strategy and Data Sources
A comprehensive literature search was conducted starting 14 February 2022, using the Web of Science, Scopus, and Science Direct databases. On 30 May 2022, the data collection process ended. The following keywords were used in the title or the summary to find articles of interest: “3D printing” OR “additive manufacturing”, AND “biofilm” OR “bacteria”, AND “3D printed Materials”, AND “3D printing technologies”. The search was restricted to papers written in English. Data that did not report on a microbiologic investigation of 3D-printed materials, particularly research on their antimicrobial characteristics, was omitted. Some of the relevant publications referenced in the chosen articles were also included. The search findings were logically selected and neatly summarized, then represented through matrices or illustrated in the form of descriptive graphics. In this case, Mendeley was selected to serve as the reference manager.
The extraction of data from the preselected articles was conducted with the following details in mind: (i) 3D printing technology description, printable materials and their potential applications; (ii) microorganisms, factors behind their adhesion to surfaces and their ability to form biofilms on 3D-printed interfaces; (iii) investigations on the development of biofilms on 3D-printed materials; and (iv) recent antimicrobial greener strategies tested on surfaces obtained by AM technologies.
3. 3D Printing/Additive Manufacturing
3.1. 3D Printing Technologies
Over the years, there have been several improvements and advancements in 3D printing technologies. In the field of additive manufacturing, achieving the desired shape of a product involves a layer-by-layer deposition of coatings during the printing process [19]. AM processes could be categorized into seven classes based on ASTM Standards. These technologies could be liquid, solid, or powder-based [20]. In a typical AM procedure, many characteristics should be taken into consideration since they highly affect the process, including manufacturing speed, resolution, quality, affordability, build volume, interface finish, and product strength [21,22] (Table 1). Nevertheless, printing speed and resolution have been the most important parameters for each of the 3D printing technologies [21].
In general, and based on the information presently analyzed, the major widely commercialized three-dimensional printing technologies are: (i) fused filament fabrication (FFF), also known as fused deposition modeling (FDM); (ii) selective layer/laser sintering (SLS); (iii) stereolithography (SLA); (iv) digital light processing (DLP); (v) polyjet/inkjet 3D printing; and (vi) electronic beam melting (EBM) [23]. Table 1 provides a more detailed description of some of these prominent strategies.
3.2. 3D Printing Materials
Three-dimensional printing, or additive manufacturing, is the process that uses computer-aided design (CAD) to create fully functional, three-dimensional items through a layering method utilizing a variety of materials [19], namely polymers, metals, ceramics, as well as hybrids, composites, and functionally graded materials (FGM) [9]. 3D printable materials usually have several specific attributes well-suited for the application in order to achieve design goals. The most important characteristics cited in the reviewed papers are: (i) tensile strength, (ii) elongation, (iii) hardness, (iv) thermal stability, (v) biocompatibility, (vi) environmental friendliness, and (vii) low cost. Overall, Table 2 gives a summary of the distinctive properties of some 3D printing materials, along with the industries and application domains that each of the material categories covers.

Table 2.
Characteristics of popular 3D printing materials, as well as their applications. Inspired by [20,40,41,42,43] and information supplied by the following websites; 3Dnatives; 3D printing industry Formlabs and Sculpteo [44,45,46,47,48,49].

Table 1.
Summary of 3D printing technologies, their principles, the corresponding materials, as well as their advantages and disadvantages. Based on [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Table 1.
Summary of 3D printing technologies, their principles, the corresponding materials, as well as their advantages and disadvantages. Based on [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
| Family | Technique | Principals/Process | Material Condition | Activation Source | Typically Used Materials | Advantages | Inconveniences |
|---|---|---|---|---|---|---|---|
| Molten Materials Deposition | FDM (Fused Deposition Modelling) | Extrusion through a preheated nozzle, and deposition in thin layers that bind and fully solidify by cooling on the substrate | Filaments | Heat | - Ceramics - Edible materials, - Thermoplastics. | - Good resistance, - Low cost, - Multi-material capability, - Production of complex 3D structures. | - Clogging of the nozzle, - High roughness of the printed objects, - Layer by layer appearance, - Poor surface quality. |
| FFF (Fused Filament Fabrication) | |||||||
| Photo-Polymerization | SLA (Stéréo-lithography) | Photosensitive liquid polymer exposed to laser (mainly UV) or free radicals solidifies through photopolymerization | Thermoset liquids | UV, LED | - Photocurable resin, - Photopolymers, - Thermoplastic polymers. | - High printing resolution, - Precise geometries, - Reproducibility, - Smooth surface finish. | - High cost, - Limitation of materials, - Relatively slow printing process, - Requires post-processing, - Release of toxic fumes during printing. |
| DLP (Digital light processing) | The polymer exposed to light projections (mainly UV) emitted by a digital projector solidifies by photopolymerization. | Soft materials | UV, LED | - Resins, - Waxes. | -Cost effective, - High precision, - Reduced time compared to SLA, - Simultaneous printing of several compact objects with less detail. | - Limited range of materials, - Need for adapted systems (ventilation, etc.), - SLA generally provides higher resolution and better surface finish than DLP technology, - Thickness limit, | |
| Material Jetting | 3D InkJet | The drops of photopolymer deposited on the working platform are exposed to UV light and solidified by light curing. | Inks | UV | - Gypsum, - Photo-polymers, - Polymers, - Waxes. | - High level of precision and complexity, - Possibility of using several materials. | - Expensive materials and printers, - Fixed resolution, - Long processing time, - Need for a material support. |
| Poly/Multijet | Printing layer by layer, projection of microdroplets and photopolymerization with UV light | Inks | UV | - Polymer resins. | - Advanced inkjet technology, - No post-processing, - Printing of objects combining several materials and colors, - Relatively low cost and printing time, - Smooth finish. | - Highly sensitive to sun and temperature, - Slow process, - Weak finished products. | |
| Powder Binding | SLS (Selective laser sintering) | Using a highly powered CO2-laser beam to sinter the powder particles, another powder coating is then added and smoothed using a recoater. | Powder | Heat | - Ceramics, - Metals, - Polymers (especially polyamides and derivatives). | - Ability to build articulated parts with various characteristics, - High level of complexity, - Good resistance, - Wide range of materials, - No need for support. | - Accuracy limited to the fineness of the powder, - Rough and slightly granular finish, - Limited material range, - Powdery surface, - Requires post-processing, - High cost |
| 3DP (Agglomeration of powder bonding) | Application of little colored glue droplets in various sizes to powdery layers until the desired effect is achieved. | Powder | Chemical | - All materials supplied in powder form are used. | - Ambient processing environment, - Easy removal of carrier powder, - Low cost, - Multi-material capability, - Low installation cost. | - Binder contamination, - Binder jet clogging, - Limited volume constructed, - Poor surface quality, - Poor porosity of the final product. | |
| DMLS (Direct Metal Laser Sintering) | A laser to deposit and fuse a metallic powder is used allowing for a layer-by-layer printing. | Powder | Heat | - Metals | - Complex geometries, - Dense components usage, - High construction speeds - Large objects production, - Remarkable objects strength, - Possibility of combining materials. | - High cost, - Less complex and detailed objects, - Mandatory polishing step, - Use of X-rays. |
3.3. 3D Printing Materials and Technologies Commonly Employed Based on the Reviewed Literature
The following section offers an overview of the research articles that were reviewed. Table 3 and Table 4 were created to simplify the analysis of the data by summarizing the AM techniques, materials (both before and after the application of antimicrobial strategies), microorganisms, and fields of study that were mentioned in the literature that was reviewed.
3.3.1. Reviewed 3D Printing Technologies
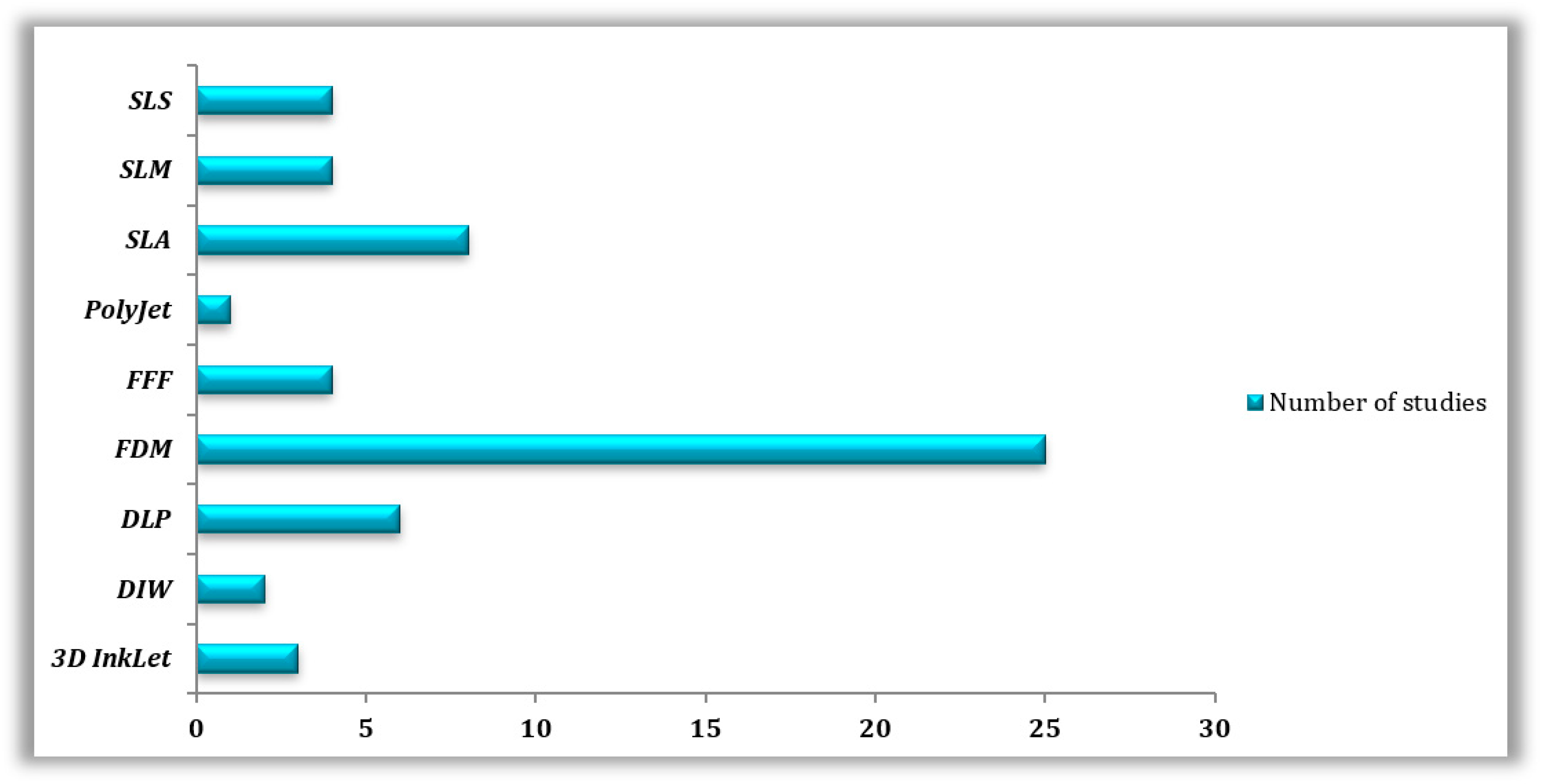
Molten polymer deposition (n = 29) was the most prevalent AM method in the reviewed publications, more specifically, (n = 4) for FFF and (n = 25) for FDM, followed by SLA (n = 8), DLP (n = 6), SLS (n = 4), SLM (n = 4), 3D InkJet (n = 4), and DIW (n = 2) (Table 3 and Table 4). A few articles did not specify the technology utilized, whereas (n = 2) papers mentioned using SLA and SLS for the printing of the same materials (Figure 2).
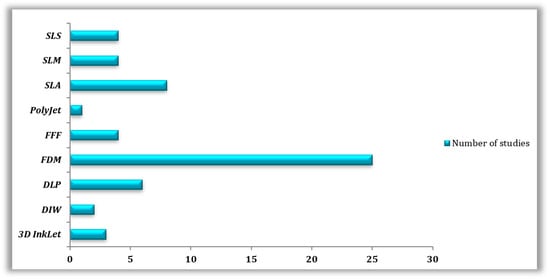
Figure 2.
Types of 3D printing technologies used in the reviewed studies.
3.3.2. Reviewed 3D-Printed Materials
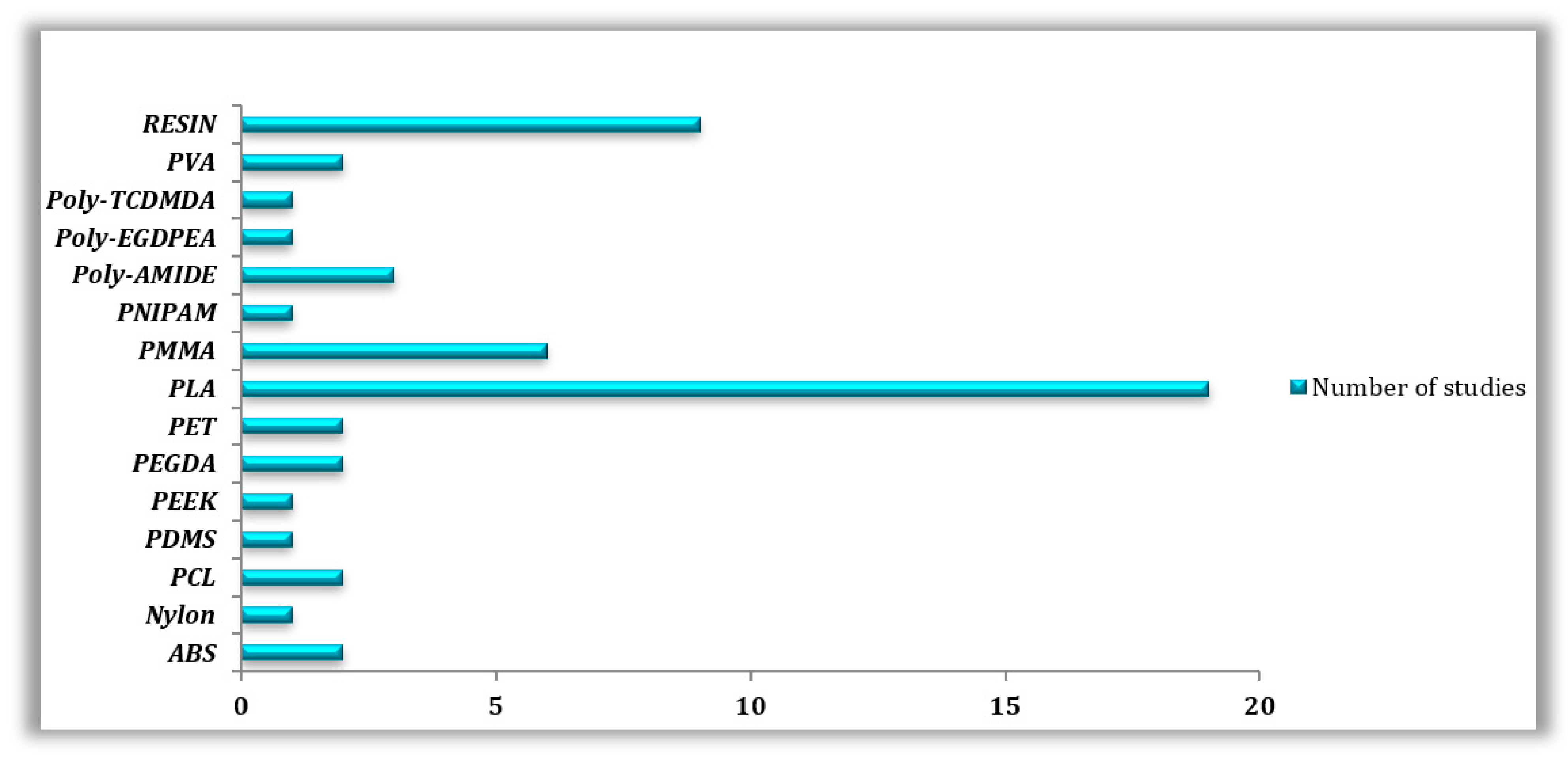
Overall, and according to our research, the most utilized 3D printing material is PLA and its variations, with (n = 19) out of (n = 50) articles dedicated to this popular thermoplastic. To be more precise, (n = 6) papers tested PLA material in its normal state; furthermore, in (n = 4) out of (n = 6) cases, FDM/FFF was the printing technology adopted by the researchers, whereas the (n = 2) left papers printed PLA using SLA/SLS techniques. The other (n = 10) articles tested PLA material in its composite form, (n = 9) articles treated PLA using FDM/FFF processes, while only (n = 1) paper utilized SLA technology. Lastly, (n = 3) works combined the study of PLA in its normal as well as in its composite forms, in which the printing was realized with FDM/FFF techniques. Second place was taken by resin materials with a total of (n = 9) studies, four of which used DLP printing technology (n = 4), followed by SLA (n = 3), and finally FDM (n = 2). Another exploited material is called PMMA, with a total of six articles (n = 6), half of them studied normal PMMA material (n = 3) using SLA (n = 2) and InkJetting (n = 1) technologies. On the other hand, PMMA composites (n = 3) were obtained either with FDM (n = 1), SLA (n = 1), or InkJetting (n = 1) approaches. SLS technology was best suited to materials such as polyamide (n = 3) and Nylon (n = 1). PCL (n = 2) and PDMS (n = 1) were both printed using three different manufacturing techniques: FDM, SLA and PolyJetting. Many other materials were studied, including PET (n = 2), PVA (n = 2), PEGDA (n = 2), ABS (n = 2), PEEK (n = 1), PNIPAM (n = 1), Poly-TCDMDA (n = 1), and Poly-EGDPEA (n = 1) (Figure 3).
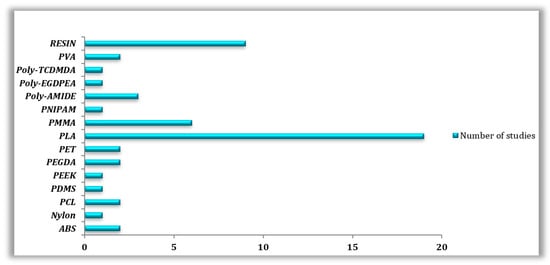
Figure 3.
Types of 3D-printed Polymers used in the reviewed studies.
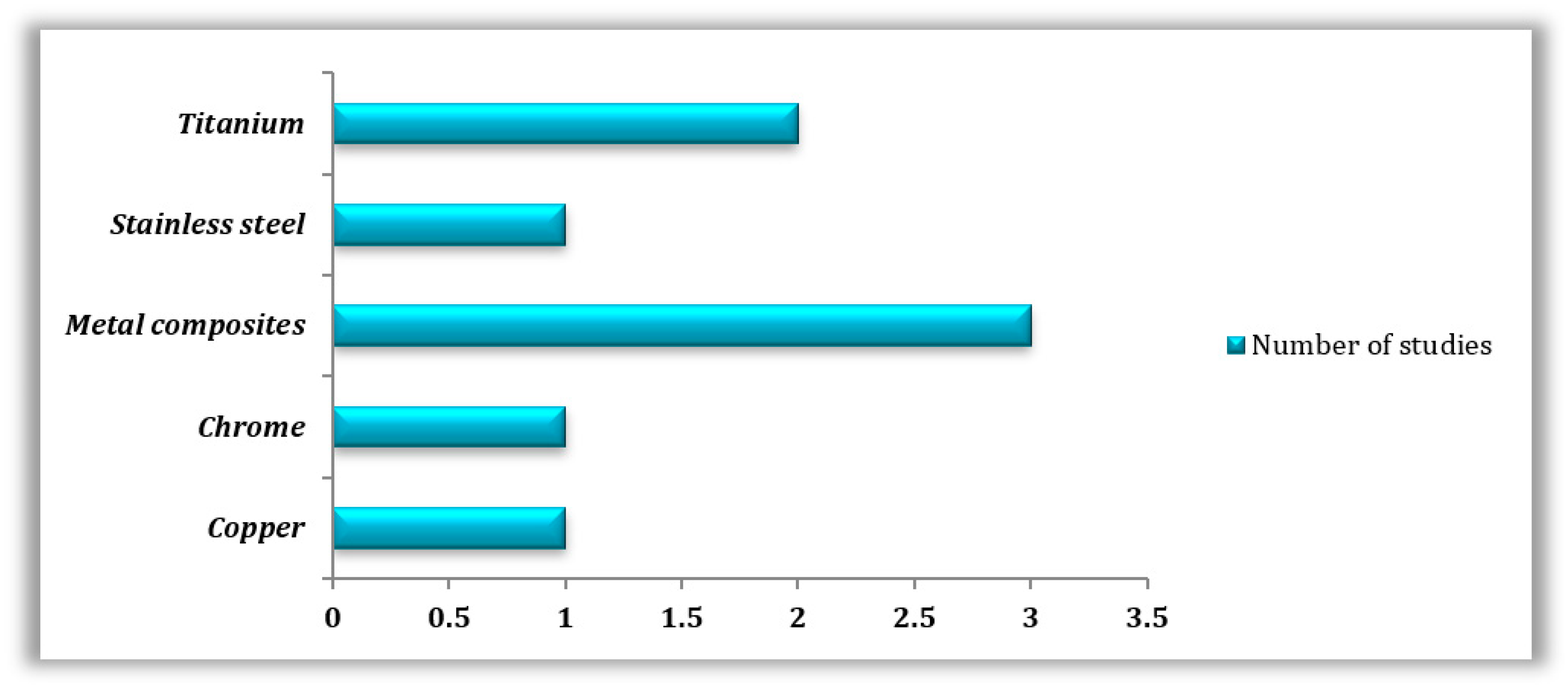
Metals were also highly studied, especially in their pure form (n = 5); in this case, titanium (n = 2), cobalt-chrome (n = 1), copper (n = 1), and stainless steel (n = 1) were tested, employing techniques such as SLM (n = 2), SLS (n = 1), and FDM (n = 1). Only three studies focused on metal composites utilizing different manufacturing techniques, which are FDM (n = 1), SLA (n = 1), and SLM (n = 1) (Figure 4).
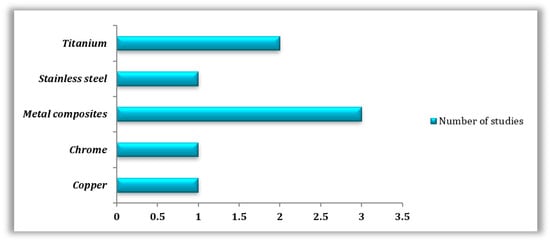
Figure 4.
Types of 3D-printed metals used in the reviewed studies.
FDM/FFF was by far the most prevalent AM technology according to this investigation, with PLA and resin as the most popular materials. PLA is highly selected due to its biodegradability, low cost, non-toxicity, adaptability, strength, resistance to corrosion, and longer lifespan [50,51,52,53]. Compared to ABS and polyamides, the low melting point of PLA is one of its biggest advantages when 3D printing [54]. Yet, its low temperature resistance and brittleness limit its use in comparison to ABS [55]. Its biodegradability, on the contrary, makes it a stronger candidate for use in a variety of biological applications, including bone fixation, drug delivery microspheres, and biomedical engineering [56]. All of the above explains why PLA material was the subject of the majority of analyzed studies in the medical field (n = 19).

Table 3.
Microorganisms often investigated on different 3D printing materials, along with their corresponding 3D printing technologies.
Table 3.
Microorganisms often investigated on different 3D printing materials, along with their corresponding 3D printing technologies.
| 3D Printing Technology | 3D Printing Material | Microorganism Studied | References |
|---|---|---|---|
| FDM | PLA | Escherichia coli | [57] |
| ND | [58] | ||
| Staphylococcus aureus | [59] | ||
| Staphylococcus epidermidis | |||
| Escherichia coli | [60] | ||
| Pseudomonas aeruginosa | |||
| Listeria monocytogenes | |||
| N/A | [61] | ||
| N/A | [62] | ||
| PLA 3D850 | N/A | [62] | |
| Staphylococcus aureus | [63] | ||
| PLA resin | Staphylococcus aureus | [64] | |
| Escherichia coli | |||
| DMHB resin | Escherichia coli | [65] | |
| Bacillus subtilis | |||
| PET | Escherichia coli | ||
| Bacillus subtilis | |||
| PCL | Marine Flora | [66] | |
| PVA | Mycobacterium abscessus | [67] | |
| Mycobacterium bovis | |||
| Mycobacterium smegmatis | |||
| PDMS | Marine Flora | [66] | |
| PEEK | Escherichia coli | [68] | |
| Staphylococcus aureus | |||
| SEBS | N/A | [58] | |
| Metal (Cu) | Escherichia coli | [64] | |
| Staphylococcus aureus | |||
| SLA | PLA Giahntarm | Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa | [69] |
| Plactive™ | Escherichia coli Staphylococcus aureus Pseudomonas aeruginosa | ||
| PCL | Marine Flora | [66] | |
| PDMS | Marine Flora | ||
| ABS (VisiJet®) | Marine Flora | ||
| VeroClear™ (Similair to PMMA) | Marine Flora | ||
| Elastic resin | N/A | [70] | |
| Acrylic resin | Buccal Flora | [71] | |
| Bisacrylic resin | |||
| Resin | Escherichia coli | [72] | |
| Bacillus cereus | |||
| PNIPAM Hydrogel | Escherichia coli | [73] | |
| PMMA | Candida albicans | [74] | |
| SLM | Titanium alloys | Staphylococcus aureus | [75] |
| Staphyloccocus epidermidis | |||
| Streptococcus mutans | |||
| Titanium Ti6Al4V | Staphylococcus aureus | [76] | |
| [77] | |||
| Staphylococcuspseudintermedius | [76] | ||
| Stainless steel | Staphylococcus aureus | ||
| Staphylococcuspseudintermedius | |||
| Cobalt-Chrome | Staphylococcuspseudintermedius | ||
| SLS | Acrylic resin | Buccal Flora | [71] |
| Bisacrylic resin | |||
| Stainless Steel Alloys | Escherichia coli Bacillus cereus | [72] | |
| PLA | Escherichia coli Bacillus cereus | ||
| Polyamide | Escherichia coli Bacillus cereus | ||
| Nylon | Escherichia coli Bacillus cereus | ||
| Polyamide12 | Saccharomyces cerevisiae | [78] | |
| DLP | PEGDA 575 | Marine Bacteria | [79] |
| Resin (PMMA based) | Streptococcus mutans | [80] | |
| Flexible resin | Pseudomonas aeruginosa Staphylococcus aureus | [81] | |
| Hard ENG resin | Pseudomonas aeruginosa Staphylococcus aureus | ||
| PEGDA | N/A | [82] | |
| InkJet | PEGDMA | N/A | |
| InkJet | Poly-TCDMDA | Pseudomonas aeruginosa Staphylococcus aureus | [83] |
| Poly-EGDPEA | Pseudomonas aeruginosa Staphylococcus aureus | ||
| PET | Staphylococcus aureus | [84] | |
| Pseudomonas aeruginosa | |||
| PMMA | Staphylococcus aureus | [85] | |
| PolyJet | PCL | Marine Flora | [66] |
| PDMS | Marine Flora | ||
| ABS (VisiJet®) | Marine Flora | ||
| VeroClear™ (Similair to PMMA) | Marine Flora |
ND: Not Determined.

Table 4.
Microorganisms frequently studied on antimicrobial 3D-printed materials developed using greener techniques plus the appropriate 3D printing technology.
Table 4.
Microorganisms frequently studied on antimicrobial 3D-printed materials developed using greener techniques plus the appropriate 3D printing technology.
| 3D Printing Technology | 3D-Printed Material | Studied Microorganisms | References |
|---|---|---|---|
| FDM | PLA (+Antimicrobials) | Escherichia coli | [11] |
| Staphylococcus aureus | |||
| Pseudomonas aeruginosa | |||
| PLA (+Graphene) | Pseudomonas aeruginosa | [86] | |
| PLA COS, (+COS + ZnHNTs + Ag) | Staphylococcus aureus | [59] | |
| Staphylococcus epidermidis | |||
| PLA (+AcAc) | Staphylococcus aureus | [60] | |
| Pseudomonas aeruginosa | |||
| PLA (+Ag) | Escherichia coli | [87] | |
| Staphylococcus aureus | |||
| Pseudomonas aeruginosa | |||
| PLA (+Ag NW) | Staphylococcus aureus | [88] | |
| Escherichia coli | |||
| PLA (+Col) PLA (+MH) PLA (+cHA) | Staphylococcus aureus | [89] | |
| PLA (+NF) PLA (+HA) | Staphylococcus aureus | [90] | |
| PCL (+ASA) | Staphylococcus aureus | [91] | |
| PMMA (+ATB) | Escherichia coli | [92] | |
| PLGA/HA (+HACC) | Staphylococcus aureus | [93] | |
| Metal (Cu + PLA resin) | Escherichia coli | [64] | |
| Staphylococcus aureus | |||
| Metal (Polished Bronze + PLA resin) | Escherichia coli | [64] | |
| Staphylococcus aureus | |||
| FFF | ABS (+AgNPs) | Acinetobacter baumannii | [94] |
| Escherichia coli | |||
| Pseudomonas aeruginosa | |||
| Staphylococcus aureus | |||
| Candida albicans | |||
| PLA (+AcAc) PLA (+TEOS) | Pseudomonas aeruginosa | [95] | |
| Staphylococcus aureus | |||
| Listeria monocytogenes | |||
| PLA (+Graphene) | ND | [62] | |
| PLA (+Lignin) | Staphylococcus aureus | [63] | |
| SLA | PMMA (+Nitrides) | Staphyloccocus epidermidis | [96] |
| Escherichia coli | |||
| Elastic resin (+Hydrochloride Lidocaine) | N/A | [70] | |
| PNIPAM (+CNF) | Escherichia coli | [73] | |
| Nanomodified Alumina | Listeria monocytogenes | [97] | |
| Staphylococcus aureus | |||
| Staphylococcus epidermidis | |||
| Escherichia coli | |||
| PLA (+NF) | Staphylococcus aureus | [14] | |
| SLM | Titanium (+HACC) | Staphylococcus aureus | [98] |
| SLS | Polyamide 12 (+ 1%B65003) | Staphylococcus aureus | [99] |
| Pseudomonas aeruginosa | |||
| Polyamide 12 (+UV stabilizer) | Saccharomyces cerevisiae | [78] | |
| DLP | GGMMA (+LNP™ + AgNP) | Escherichia coli | [100] |
| Staphylococcus aureus | |||
| Resin (+QAC) Resin (+SH-QAC) | Escherichia coli | [101] | |
| Staphyloccocus epidermidis | |||
| DIW | Ceramic (+3Y-TZP) | Escherichia coli | [102] |
| Streptococcus salivarius | |||
| MG-PVA MG(+LEV)-PVA(+VAN) G(+RIF)MG(+LEV) PVA(+VAN) | Escherichia coli | [103] | |
| Staphylococcus aureus | |||
| Inkjet | PMMA (+MPC) | Staphylococcus aureus | [104] |
| Streptococcus mutans | |||
| Klebsiella oxytoca | |||
| Klebsiella pneumonia | |||
| PMMA (+SB) | Staphylococcus aureus | ||
| Streptococcus mutans | |||
| Klebsiella oxytoca | |||
| Klebsiella pneumonia | |||
| Plastic (+Gel +ATB) | Escherichia coli | [105] |
ND: Not Determined.
3.4. Microorganisms Involved in Biofilm Formation on 3D-Printed Materials
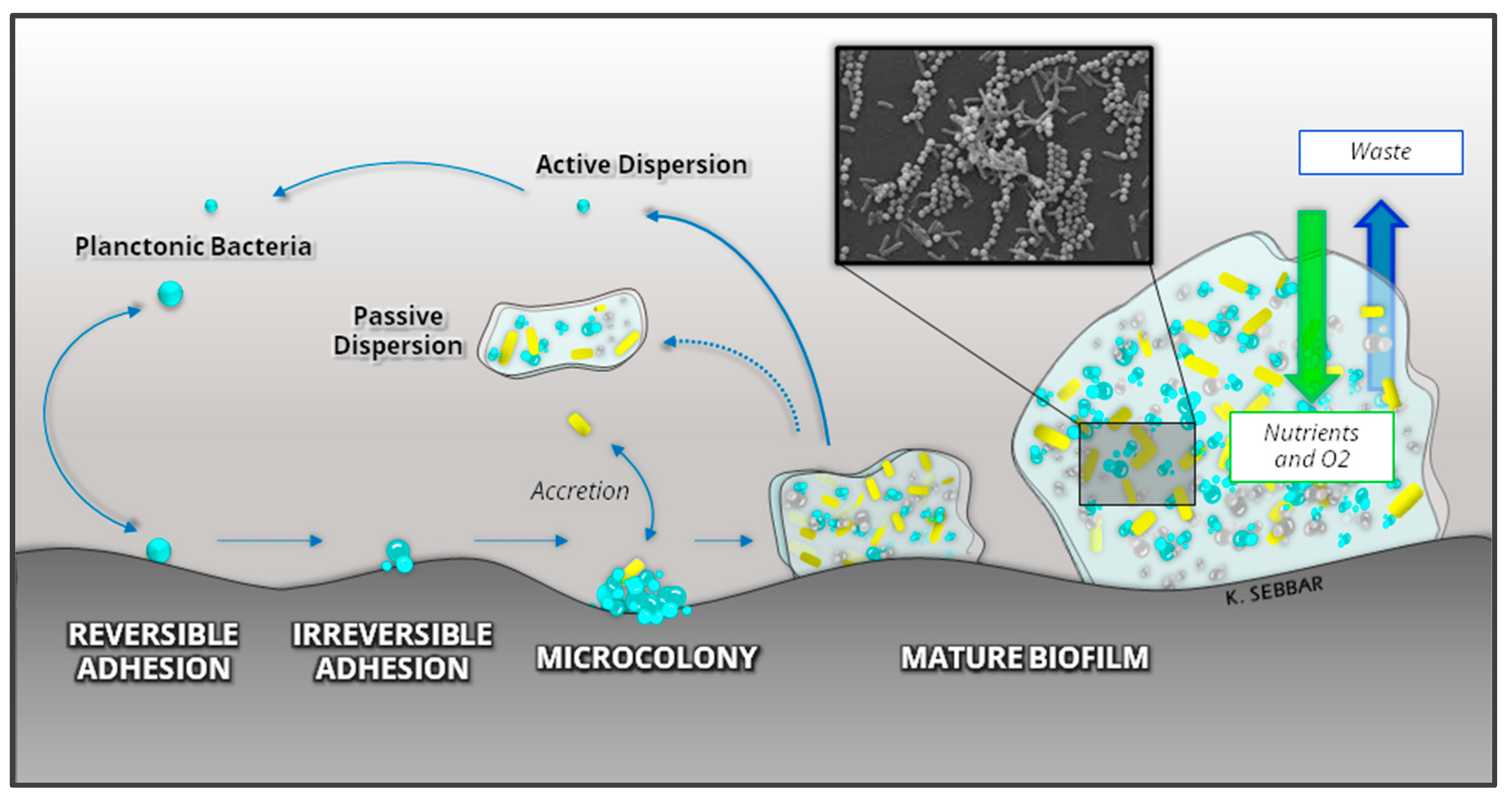
Bacteria are present in the environment in two forms: planktonic and sessile, both of which have existed on earth since the manifestation of the earliest microbial species [106]. In their planktonic form, microorganisms are isolated in suspension in a liquid medium. In sessile form, they are associated with a complex structure called Biofilm [107]. Biofilm formation is quite advantageous and beneficial for almost all microorganisms, especially bacteria, on any biotic or abiotic surface, including 3D-printed materials. Biofilm formation can be divided into five stages: (i) initial attachment, (ii) reversible adhesion, (iii) irreversible adhesion, (iv) maturation, and (v) dispersion, as illustrated in Figure 5.
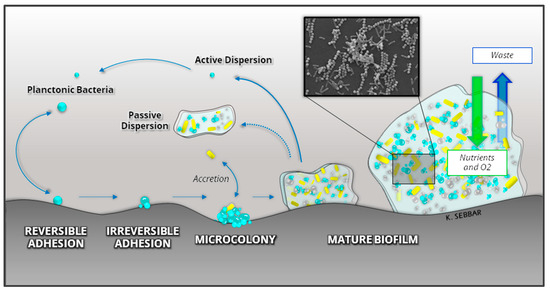
Figure 5.
Development and structure of a bacterial Biofilm.
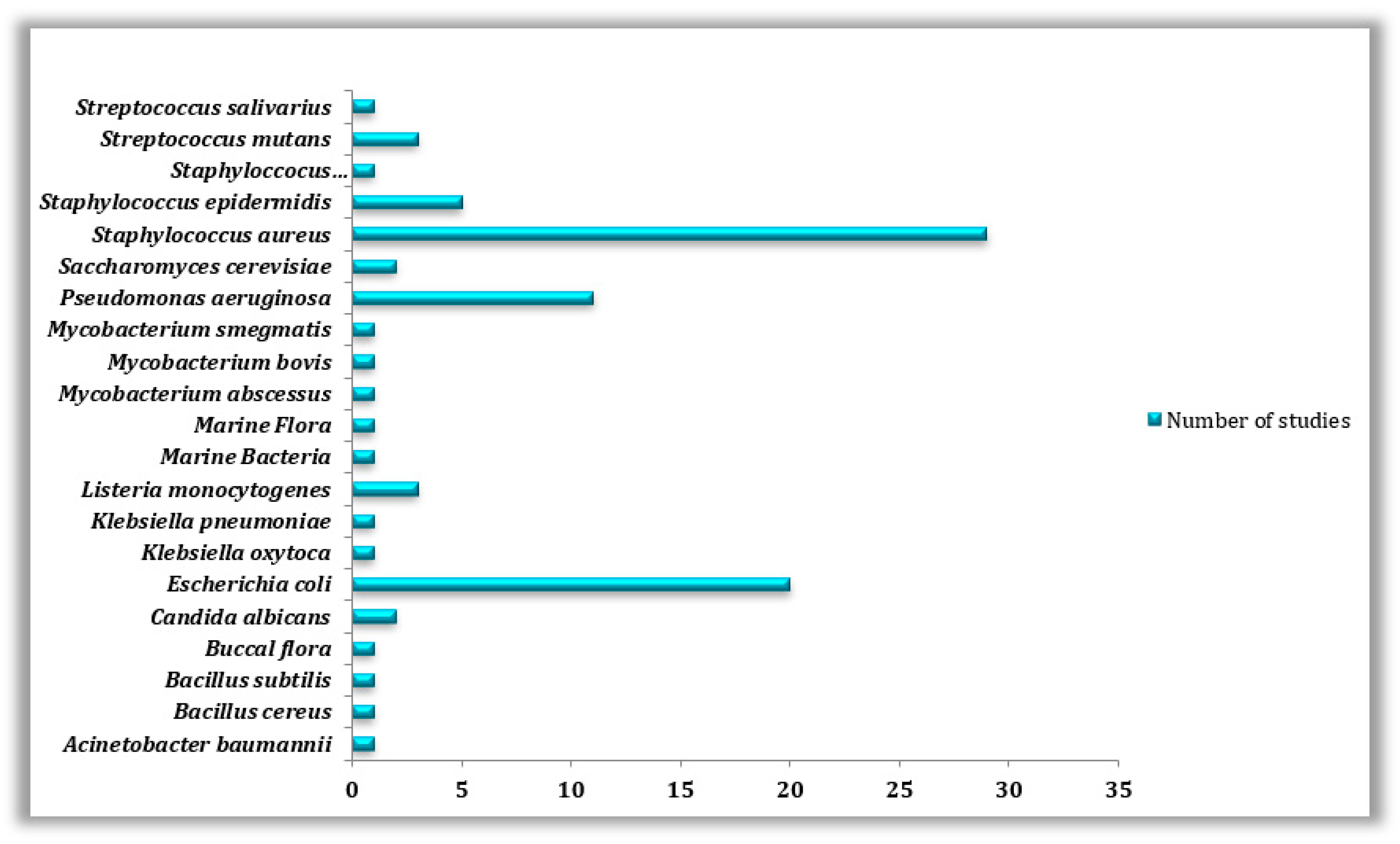
Therefore, according to data obtained from scientific literature regarding the biofouling of 3D-printed surfaces and also according to our collected data on the antibacterial properties of such materials, special attention was paid to Staphylococcus aureus (n = 29), Escherichia coli (n = 20), and Pseudomonas aeruginosa (n = 11) being the top three most commonly studied bacterial species on 3D-printed surfaces, in both forms, whether as planktonic cells or as biofilms (Table 5) and (Figure 6).

Table 5.
Microorganisms often studied on 3D-printed materials, their fields of use, and the number of studies dedicated to each microorganism collected from the examined publications.
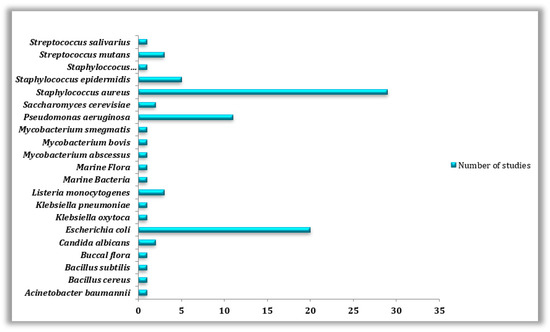
Figure 6.
Types of microorganisms used in the reviewed studies.
E. coli is a Gram-negative rod-shaped bacterium best known for its biofilm-forming capabilities. E. coli can secrete toxins, polysaccharides, and biofilms, making their eradication and treatment quite difficult [108,109]. In spite of that, Staphylococcus aureus was found to be the model studied bacteria (n = 29) because of its well-known involvement in various diseases linked to biofilm formation on different types of surfaces [110]. Other bacteria such as Staphyloccocus epidermidis (n = 5), Listeria monocytogenes (n = 3), Bacillus cereus (n = 1), B. subtilis (n = 1), Klebsiella oxytoca (n = 1), K. pneumonia (n = 1), Mycobacterium smegmatis (n = 1), M. abscessus (n = 1), M. bovis (n = 1), Acinetobacter baumannii (n = 1) and Streptococcus mutans (n = 3), S. salivarus (n = 1) as well as fungi species such as, Saccharomyces cerevisiae (n = 2) and Candida albicans (n = 2) have been the subject of other studies conducted on the biofouling of 3D manufactured materials. Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli are frequently studied as they are among the most prevalent bacteria found on human skin and hair [111].
Furthermore, they are responsible for many diseases, such as hospital-acquired pneumonia, nosocomial bloodstream infections, diarrheal infections, meningitis, wound infections, and septicemia [112,113]. Through our research, we were also able to notice that most of the microorganisms studied belong to the medical field, where (n = 14) out of the (n = 20) species most commonly investigated for their formation on 3D-printed surfaces were stains isolated in clinical environments or studied on some specific medical devices (Table 5) and (Figure 6). This predominance could be simply explained by the fact that bacterial contamination and biofilm infections, in particular, are extremely challenging to treat in a clinical setting since the germs that reside within are significantly more resistant to antibiotics and disinfectants [13], which calls for more studies and solution searching in this field.
4. Antimicrobial Approaches against the Microbial Proliferation or the Formation of Biofilms Adopted by Recent Publications
This section provides a summary of the recently reviewed articles. Table 6 summarizes some existing natural or synthetic control approaches against the proliferation of microorganisms and surface biofouling of AM-manufactured surfaces.

Table 6.
Summary of antimicrobial approaches used against the proliferation of microorganisms and surface biofouling of AM manufactured surfaces.
First of all, it is worth mentioning that (n = 37) out of the (n = 50) investigated papers were articles that addressed the biofouling problem in 3D-printed objects, in which a variety of green approaches in order to avoid microbial attacks on such surfaces were introduced and evaluated. Once more, we noticed a predominance of medical applications when it comes to this type of research, with a total of (n = 28) articles out of (n = 37). Then, in the agri-food domain, with only two studies (n = 2), the same number of studies belonged to the environmental field (n = 2), whereas the last five articles involved a variety of fields (n = 5). The data presented in Table 6 indicates that PLA was the most extensively researched antimicrobial material produced using 3D printing, with (n = 11) studies conducted on it. In the upcoming sections, we will delve into the various treatments and modifications that have been applied to this material, and the same goes for the rest of the materials. Additionally, the most commonly studied microorganism was Staphylococcus aureus, with (n = 28) studies conducted on this well-known bacterium frequently used as a model species due to its involvement in numerous biofilm-related infections [110]. Furthermore, the examinations that were carried out concluded that the integrity of papers adopted one out of the four major approaches: (i) antimicrobial 3D-printed materials (n = 2), (ii) printing process modification (n = 5), (iii) surface coatings (n = 19), (iv) 3D-printed composites with antimicrobial properties (n = 8), and rarely (v) combined/hurdle therapy (n = 3) (Table 6).
Due to their inherent antibacterial qualities, synthetic polymers are without a doubt one of the most employed materials in this context without the need for eluting antibiotics or coatings (n = 2). For instance, Walsh et al. (2016) have presented a method for assessing the microbial liquid culture potential of ten 3D-printable polymeric materials. They found six 3D-printable materials that created tubes suited for dealing with aqueous liquids, yet during a mass spectrometry analysis used to identify leached chemicals that inhibited bacterial growth, only three materials, the FormLabs, “Flexible” and “ClearV2”, and the Stratasys “TangoPlus” materials, were able to inhibit bacterial growth to varying degrees [115]. In a similar study, where both surface coating and antibiotic elution are not necessary, He et al. (2022) created bespoke devices employing ink-jetting and bacterial biofilm inhibitory compositions. The ability of the 3D-printed poly-TCDMDA and poly-EGDPEA to inhibit bacterial biofilms in vitro was assessed. The growth of Pseudomonas aeruginosa biofilms on poly-TCDMDA was 99% less than that on medical-grade silicone [83]. On another note, surface modification of 3D-printed parameters (n = 5) represents an interesting approach adopted in recent studies. In a study led by Sarker et al. (2019), in order to modify the surface topography of metallic implants for directed Staphylococcus aureus biofilm restriction, they proved that reducing the angle during SLM printing resulted in metallic surfaces with lower roughness, relatively low hydrophobicity, increased surface energy, and fewer partially melted metal particles without changing the bulk surface chemistry, which was directly correlated with considerably lower biofilm adhesion and microbial biomass minimization [77]. In this context, Shim also reported an illustrative strategy of this approach et al. (2019) where they described that printing orientation affects the microbiological reaction of 3D-printed denture base material PMMA, a denture foundation, which was utilized to print samples in three distinct printing orientations (0°, 45°, and 90°). The reaction of Candida albicans was studied. It was found that the specimens printed at 90°< 45°< 0° orientation degrees had a higher proportion of C. albicans. More interestingly and contrasting to other methods, where the generation of AgNPs is usually carried out at a post-treatment stage, this work introduced a substitute strategy to carry out photopolymerization of the resin and photogeneration of AgNPs within the same impression process [74]. With the same goal, which is surface treatment, Er-rahmani et al. (2022) have used the contact angle technique to assess the influence of thymol and carvacrol on the physicochemical features of DMHB resin and PET. A considerable change in the physicochemical properties of both surfaces was observed following treatment. Finally, it was suggested that the key molecules analyzed could be included in the composition of PET and resin materials for future uses in the food industry [65]. The results demonstrated in a study by González Flores et al. (2022) were attained by arbitrarily changing the printing settings during the printing procedure, which allowed for the selective photogeneration of silicon nanoparticles while still in the stage of 3D light printing. Finally, they showed that these 3D-printed items with silver patterns have shown antibacterial efficacy against environmental germs, including the maritime environment, in order to regulate biofilm growth and proliferation [79].
An additional procedure for the manufacturing of antimicrobial AM products involves applying surface coatings using multiple antimicrobial agents. Many illustrative applications of this technique were recently reported (n = 19), especially those involving silver nanoparticle coatings (n = 4). One of the most recent publications in this context was realized by Wang et al. (2022), in which a bio-based antimicrobial resin was developed for DLP printing, engaging GGMMA as a polymeric matrix and the nanocomposite lignin nanoparticles surface-embedded with silver nanoparticles (LNP@Ags) as a high-performance antimicrobial reagent. The antibacterial activity of the GGMMA(+LNP +Ag) hydrogel is enhanced by the bactericidal capacity of Ag+, which was seeped out of the hydrogel continuously over time [100]. Another form of coating calls for antibiotics (ATBs), natural compounds, and other chemical substances (n = 15). Furthermore, Shen et al. (2021) were able to achieve porous wound dressing material with phage-embedded hydrogel fibers. This antibacterial dressing was created with the capability to release lytic phages (HZJ phage) slowly but surely and efficiently inhibit the bacterial growth of Escherichia coli for up to 24 h. This concept appears to be a viable option for reducing the reliance on antibiotics and other substances in conventional coatings [114]. Additionally, as demonstrated by Muro-Fraguas et al. (2020), organic compounds can also serve as another form of coating. Acrylic acid (AcAc) coatings obtained by plasma polymerization were deposited on 3D-printed polylactic acid (PLA) Petri plates to prevent the formation of biofilms and to avoid serious infections. When compared to untreated plates, AcAc coatings with fewer plasma passes were more effective, showing up to a 50% reduction in relative biofilm [60].
Last but not least, antimicrobial 3D-printed devices have also been developed exploiting composites. Since composite manufacturing is a relatively novel approach, this explains the limited work conducted in this area of 3D printing [116]. Based on our findings, the development of antimicrobial composites was the focus of eight reviewed papers (n = 8). In general, metals like zinc, aluminum, or silver, among others [88,117], ceramics [102], chitosan [93], and many more have all been used to generate some of the antimicrobial composites. According to a study by Marin et al. (2021), PMMA/nitrides composites were able to increase the antibacterial characteristics of PMMA implants. The use of nitride-PMMA composite coatings on 3D-printed items made them more resistant to bacterial colonization. Silicon, zirconium, hafnium, and aluminum were the four nitrides studied. When compared to controls, all composite materials demonstrated antibacterial properties, with hafnium nitride being the most effective against E. coli and aluminum nitride being the most effective against S. epidermidis [96]. He et al. (2021) have shown how to make personalized MM-IJ3DP devices using generative design-guided ink co-deposition to generate functional composites that are resistant to bacterial biofilm development and have a customized deformation profile. When compared to regularly used silicone rubbers, the bacterial biofilm coverage of the resultant composites is reduced by up to 75%, plus no bioactives were required or necessary [84].
Finally, combined therapy involves the fusion of a number of strategies to maximize the level of antimicrobial activity. In this review (n = 3), investigated studies combined two or more of the previously discussed solutions. Garcia-Alvarez and colleagues did put this theory to the test by adopting rapid prototyping to create hierarchical 3D multidrug scaffolds based on nanocomposite bioceramic and polyvinyl alcohol (PVA) with an exterior coating using gelatin-glutaraldehyde (Gel-Glu). Three antibacterial agents were included in these AM scaffolds (RIF, LEV, and VAN). This combination treatment was able to kill and limit the growth of both Gram-positive and Gram-negative bacteria biofilms [103]. Humayun et al. (2020) reported that a zinc/HNTs-ag-chitosan oligosaccharide lactate solution was employed to functionalize the 3D-printed polylactic acid (PLA) structures after they were alkali treated to increase their hydrophilicity (ZnHNTs-Ag-COS). The resultant PLA construction’s anti-biofouling ability was validated by antibacterial testing utilizing Staphylococcus aureus cultures during the agar diffusion technique [59]. Lastly, and according to a study conducted in 2019 by Dominguez-Robles et al., PLA/LIG composite materials were used to create 3D-printed meshes. One of this study’s major objectives was to combine an antibiotic (TC) with lignin (LIG), which has been shown to have significant antibacterial action against Gram-positive and Gram-negative bacteria. As a result, S. aureus adherence to the materials was effectively reduced in the materials containing 2% (w/w) of TC (tetracycline). However, LIG did not exhibit any antibacterial action in this instance. As a result, the amount of germs adhered to the 3D-printed material’s surface can be highly reduced employing PLA/LIG/TC composites [63].
5. Conclusions
Numerous review articles have been published on additive manufacturing, covering a range of topics related to 3D-printed materials and the potential risks of contamination associated with them. Some of these reviews even discussed methods for manufacturing antimicrobial 3DP surfaces. However, most of these articles tend to provide a broad overview of AM processes and their applications in a particular field, or they focus on only one aspect mentioned above without addressing the other.
As the authors of this article, our goal was to demonstrate that while there are promising developments in the use of antimicrobial 3D-printed objects, it is crucial to assess the current state of research critically and realistically in various fields such as medicine, the environment, and the food industry. To achieve this, we gathered information on antimicrobial approaches that have been tested on 3D-printed materials, analyzed the AM technologies that have been utilized thus far, identified suitable materials, and highlighted the germs that are typically targeted across all fields that have been studied to date. The aim of this work is to showcase the efficacy of greener methods in inhibiting biofilm formation on 3D-printed surfaces. We also believe that in the near future, through such in-depth investigations, we can promote the safe incorporation of antimicrobial/antibiofilm 3D-printed surfaces into our day-to-day practices.
Based on our observations, planctonic microorganisms were the most widely studied form of microbes on 3D-printed materials, followed by biofilms, and only a few studies have investigated both forms concurrently. It was also noted that the medical field has been the most active in researching microbial infections due to the increasing incidence rate. Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli were the top three most commonly tested bacteria on 3D-printed surfaces, mainly due to their involvement in the development of multi-resistant biofilms responsible for the majority of incurable infection cases. Furthermore, we observed that FDM technology and PLA materials, whether pure or modified, were the most extensively researched as they provide a durable and cost-effective approach for developing user-specific products.
Our findings indicate that the majority of antimicrobial approaches studied on 3D-printed materials employed either a “before printing” approach, which involved incorporating the antimicrobial substance with the 3D printing material, or an “after printing” approach, which involved applying a variety of surface treatments or a simple modification of 3D printing parameters. In some cases, a “combination of multiple strategies” was also used simultaneously.
This review article draws its conclusions and observations from the searches conducted on Web of Science, Scopus, and ScienceDirect. Although the number of published experimental articles is limited, the existing literature still provides strong groundwork for future research. Exploring 3D printing and antimicrobial processes further may uncover new insights that could broaden the scope of this review and its applicability to various fields of study.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank the Moroccan Ministry of Higher Education, Scientific Research and Innovation and the OCP Foundation who funded this work through the APRD research program.
Conflicts of Interest
The authors have stated that they do not have any financial interests or personal relationships that could have potentially influenced the findings presented in this paper.
Abbreviations
| 3DP | Three Dimensional Printing |
| 3Y-TZP | Yttrium-stabilized tetragonal zirconia polycrystal |
| AcAc | Acrylic acid |
| AgNP | Silver nanoparticle |
| AgNW | Silver nanowire |
| Al | Aluminum |
| AM | Additive Manufacturing |
| ASA | Acetylsalicylic acid |
| ATB | Antibiotic |
| Cipro | Ciprofloxacin |
| Col | Collagen |
| CPS | Calcium phosphate scaffolds |
| CUR | Curcumin |
| DLP | Digital Light Processing |
| EBM | Electron Beam Melting |
| EPS | Extracellular Polymeric Substances |
| FA | Fluocinolone Acetonide |
| FDM | Fused Deposition Modelling |
| Gel-Glu | Gelatin-glutaraldehyde |
| Gen | Gentamicin |
| GGMMA | Methacrylated O-acetyl-galactoglucomannan |
| HA | Hydroxyapatite |
| HACC | Quaternized chitosan |
| Hf | Hafnium |
| HZJ | Bacteriophage |
| LIG | Lignin |
| LNP | Lignin nanoparticle |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| NDs (A-ND) | Amine-functionalized |
| NDs (ND) | Non-functionalized |
| NF ou NIT | Nitrofurantoin |
| PBF | Powder Bed Fusion |
| PEEK | Polyetheretherketone |
| PICN | Polymer-infiltrated ceramic network |
| PLGA | Polylactide-co-glycolide |
| Poly-EGDPEA | Polyethylene glycol dicyclopentenyl ether acrylate |
| Poly-TCDMDA | Pol-mers contained monomer D, tricylodecane-dimethanol diacrylate |
| RIF | Rifampin/Rifampicin |
| SB | Sulfobetaine methacrylate |
| Si | Silicon |
| SLA | Stereolithography |
| SLM | Selective Laser Melting |
| SLS | Selective Laser Sintering |
| TEOS | Tetraethyl orthosilicate |
| Ti | Titanium |
| TOB | Tobramycin |
| VAN | Vancomycin |
| WOS | Web of science |
| Zr | Zirconium |
References
- Gibson, I.; Rosen, D. Additive Manufacturing Technologies: 3D Printing, Rapid Prototyping, and Direct Digital Manufacturing; Springer: New York, NY, USA, 2015. [Google Scholar]
- Yan, Q.; Dong, H.; Su, J.; Han, J.; Song, B.; Wei, Q.; Shi, Y. A Review of 3D Printing Technology for Medical Applications. Engineering 2018, 4, 729–742. [Google Scholar] [CrossRef]
- Shi, G.; Wang, Y.; Derakhshanfar, S.; Xu, K.; Zhong, W. Materials Science & Engineering C Biomimicry of oil infused layer on 3D printed poly (dimethylsiloxane): Non-fouling, antibacterial and promoting infected wound healing. Mater. Sci. Eng. C 2019, 100, 915–927. [Google Scholar] [CrossRef]
- Campbell, T.A.; Ivanova, O.S. Additive Manufacturing as a Disruptive Technology: Implications of Three-Dimensional Printing. Technol. Innov. 2013, 15, 67–79. [Google Scholar] [CrossRef]
- Bekas, D.G.; Hou, Y.; Liu, Y.; Panesar, A. 3D printing to enable multifunctionality in polymer-based composites: A review. Compos. Part B 2019, 179, 107540. [Google Scholar] [CrossRef]
- Zuniga, J.M. 3D Printed Antibacterial Prostheses. Appl. Sci. 2018, 8, 1651. [Google Scholar] [CrossRef]
- Jung, J.W.; Lee, J.; Cho, D. Computer-aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. Nat. Publ. Gr. 2016, 6, 21685. [Google Scholar] [CrossRef] [PubMed]
- Melocchi, A.; Parietti, F.; Maroni, A.; Foppoli, A.; Gazzaniga, A.; Zema, L. Hot-melt extruded fi laments based on pharmaceutical grade polymers for 3D printing by fused deposition modeling. Int. J. Pharm. 2016, 509, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tofail, S.A.M.; Koumoulos, E.P.; Bandyopadhyay, A.; Bose, S.; O’Donoghue, L.; Charitidis, C. Additive manufacturing: Scientific and technological challenges, market uptake and opportunities. Mater. Today 2017, 21, 22–37. [Google Scholar] [CrossRef]
- Chua, C.K.; Wong, C.H.; Yeong, W.Y. Standards, Quality Control, and Measurement Sciences in 3D Printing and Additive Manufacturing; Brian Guer. Matthew Deans; Elsevier Ltd.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hall, D.C.; Palmer, P.; Ji, H.F.; Ehrlich, G.D.; Król, J.E. Bacterial Biofilm Growth on 3D-Printed Materials. Front. Microbiol. 2021, 12, 646303. [Google Scholar] [CrossRef]
- Hawas, S.; Verderosa, A.D.; Totsika, M. Combination Therapies for Biofilm Inhibition and Eradication: A Comparative Review of Laboratory and Preclinical Studies. Front. Cell. Infect. Microbiol. 2022, 12, 850030. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Asif, M.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Salmela, I.; Fallarero, A.; Rosling, A.; Khajeheian, M.; Kolakovic, R.; Genina, N.; Nyman, J.; Vuorela, P. Towards fabrication of 3D printed medical devices to prevent biofilm formation. Int. J. Pharm. 2014, 459, 62–64. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, M.; Misra, T.K.; Roy, D.N. In vitro anti-biofilm activity of 14-deoxy-11,12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa. Braz. J. Microbiol. 2020, 51, 15–27. [Google Scholar] [CrossRef]
- Gottenbos, B.; Grijpma, D.W.; Van Der Mei, H.C.; Feijen, J.; Busscher, H.J. Antimicrobial effects of positively charged surfaces on adhering Gram-positive and Gram-negative bacteria. J. Antimicrob. Chemother. 2001, 48, 7–13. [Google Scholar] [CrossRef]
- Kumar, S.; Roy, D.N.; Dey, V. A comprehensive review on techniques to create the anti-microbial surface of biomaterials to intervene in biofouling. Colloids Interface Sci. Commun. 2021, 43, 100464. [Google Scholar] [CrossRef]
- Duan, S.; Wu, R.; Xiong, Y.; Ren, H.; Lei, C.; Zhao, Y.; Zhang, X.; Xu, F. Progress in Materials Science Multifunctional antimicrobial materials: From rational design to biomedical applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Zivanovic, S.T.; Popovic, M.D.; Vorkapic, N.M.; Pjevic, M.D.; Slavkovic, N.R. An overview of rapid prototyping technologies using subtractive, additive and formative processes. FME Trans. 2020, 48, 246–253. [Google Scholar] [CrossRef]
- Wong, K.V.; Hernandez, A. A Review of Additive Manufacturing. Int. Sch. Res. Netw. 2012, 2012, 1–10. [Google Scholar] [CrossRef]
- Lee, J.; An, J.; Chua, C.K. Fundamentals and applications of 3D printing for novel materials. Appl. Mater. Today 2017, 7, 120–133. [Google Scholar] [CrossRef]
- Imširović, A.; Kumnova, G. Utilizing 3D Printing to Provide Customized Joysticks Designed to Function Universally across Volvo’ s Bachelor Thesis within the Program Design and Product Development; Chalmers University of Technology: Gothenburg, Sweden, 2021. [Google Scholar]
- Wu, G.; Hsu, S. Review: Polymeric-Based 3D Printing for Tissue Engineering. J. Med. Biol. Eng. 2015, 35, 285–292. [Google Scholar] [CrossRef]
- Aspar, G. L’ Impression 3D Polymère Appliquée au Packaging en Microélectronique tel-02148205; Université Grenoble Alpes: Saint-Martin-d’Hères, France, 2019. [Google Scholar]
- Barnatt, C. 3D Printing: The Next Industrial Revolution; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2013. [Google Scholar]
- Blyweert, P.; Nicolas, V.; Fierro, V.; Celzard, A. 3D printing of carbon-based materials: A review. Carbon N. Y. 2021, 183, 449–485. [Google Scholar] [CrossRef]
- Centre d’etude et de Prospective Industrielle, Note Sur L’ Impression 3D. 2018. Available online: https://www.tunisieindustrie.nat.tn/fr/home.asp (accessed on 22 May 2022).
- Chastand, V. Etude du Comportement Mécanique et des Mécanismes d’Endommagement de Pièces Métalliques Réalisées par Fabrication Additive. 2016. Available online: https://theses.hal.science/tel-01484725 (accessed on 22 May 2022).
- De Leon, A.C.; Chen, Q.; Palaganas, N.B.; Palaganas, J.O.; Manapat, J.; Advincula, R.C. High performance polymer nanocomposites for additive manufacturing applications. React. Funct. Polym. 2016, 103, 141–155. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2017, 20, 44–67. [Google Scholar] [CrossRef]
- Gebhardt, A.; Fateri, M. 3D Printing and Its Applications. Lizenznehmer RTejournal. 2013, 1–12. [Google Scholar] [CrossRef]
- Goodridge, R.D.; Shofner, M.L.; Hague, R.J.; McClelland, M.; Schlea, M.R.; Johnson, R.B.; Tuck, C.J. Processing of a Polyamide-12/carbon nanofibre composite by laser sintering. Polym. Test. 2011, 30, 94–100. [Google Scholar] [CrossRef]
- Jacques-hulin, M. Développement d’une Méthode de Conception de Moules Hybrides en Fonderie; Université de Reims Champagne-Ardenne—Ecole Supérieure d'ingénieurs de Reims: Reims, France, 2019. [Google Scholar]
- Kim, G.D.; Oh, Y.T. A benchmark study on rapid prototyping processes and machines: Quantitative comparisons of mechanical properties, accuracy, roughness, speed, and material cost. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2008, 222, 201–215. [Google Scholar] [CrossRef]
- Manapat, J.Z.; Chen, Q.; Ye, P.; Advincula, R.C. 3D Printing of Polymer Nanocomposites via Stereolithography. Macromol. Mater. Eng. 2017, 302, 1600553. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Moghaddam, K.M.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2018, 45, 148–163. [Google Scholar] [CrossRef]
- Sood, A.K.; Ohdar, R.K.; Mahapatra, S.S. Parametric appraisal of mechanical property of fused deposition modelling processed parts. Mater. Des. 2010, 31, 287–295. [Google Scholar] [CrossRef]
- Vyavahare, S.; Teraiya, S.; Panghal, D.; Kumar, S. Fused deposition modelling: A review. Rapid Prototyp. J. 2020, 26, 176–201. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, M.; Zhou, Z.; Gou, J.; Hui, D. 3D printing of polymer matrix composites: A review and prospective. Compos. Part B Eng. 2017, 110, 42–458. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive manufacturing (3D printing): A review of materials, methods, applications and challenges. Compos. Part B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Pereira, G.R.; Gasi, F.; Lourenço, S.R. Review, Analysis, and Classification of 3D Printing Literature: Types of Research and Technology Benefits. Int. J. Adv. Eng. Res. Sci. (IJAERS) 2019, 6, 167–187. [Google Scholar] [CrossRef]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. ScienceDirect ScienceDirect ScienceDirect An Overview on 3D Printing Technology: Technological, Materials, and Technology: Applications Technological, Materials, An Overview on 3D Printing and Applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- 3Dnatives. All about 3D Metal Printing. 2022. Available online: https://www.3dnatives.com/impression-3d-metal/ (accessed on 15 June 2022).
- Everett Hayley. 3D Printing Industry Year in Review: December 2021. Available online: https://3dprintingindustry.com/news/3dprinting-industry-year-in-review-december-2021-201491/ (accessed on 30 April 2022).
- Formlabs. Guide to 3D Printing Materials: Types, Applications, and Properties. 2022. Available online: https://formlabs.com/fr/blog/materiaux-impression-3d/ (accessed on 9 June 2022).
- Gaget Lucie, 2017. Archive 2017 Sculpteo Blog on 3D Printing. Available online: https://www.sculpteo.com/blog/fr/2017/ (accessed on 20 April 2022).
- Sculpteo Resin 3D Printing: The Complete Guide. 2022. Available online: https://www.sculpteo.com/en/3d-learning-hub/3d-printing-materials-guide/3d-printing-resin/ (accessed on 17 April 2022).
- Sculpteo. The Ultimate Guide: What is 3D Printing? 2022. Available online: https://www.sculpteo.com/en/3d-learning-hub/basics-of-3d-printing/what-is-3d-printing/ (accessed on 21 April 2022).
- Harris, A.M.; Lee, E.C. Improving Mechanical Performance of Injection Molded PLA by Controlling Crystallinity. J. Appl. Polym. Sci. 2008, 107, 2246–2255. [Google Scholar] [CrossRef]
- Benwood, C.; Anstey, A.; Andrzejewski, J.; Misra, M.; Mohanty, A.K. Improving the Impact Strength and Heat Resistance of 3D Printed Models: Structure, Property, and Processing Correlationships during Fused Deposition Modeling (FDM) of Poly(Lactic Acid). ACS OMEGA 2018, 3, 4400–4411. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Plastic biodegradation: Frontline microbes and their enzymes. Sci. Total Environ. 2021, 759, 143536. [Google Scholar] [CrossRef] [PubMed]
- Shah, R.; Nguyen, T.V.; Marcora, A.; Ruffell, A.; Hulthen, A.; Pham, K.; Wijffels, G.; Paull, C.; Beale, D.J. Exposure to polylactic acid induces oxidative stress and reduces the ceramide levels in larvae of greater wax moth (Galleria mellonella). Environ. Res. 2023, 220, 115137. [Google Scholar] [CrossRef]
- Wittbrodt, B.; Pearce, J.M. The effects of PLA color on material properties of 3-D printed components. Addit. Manuf. 2015, 8, 110–116. [Google Scholar] [CrossRef]
- Yang, T. Effect of Extrusion Temperature on the Fiber-Reinforced Polylactic Acid Composite (WFRPC). Polymers 2018, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef]
- Järvenpää, J.; Perkkiö, M.; Laitinen, R.; Lahtela-Kakkonen, M. PE and PET oligomers’ interplay with membrane bilayers. Sci. Rep. 2022, 12, 2234. [Google Scholar] [CrossRef] [PubMed]
- Foresti, R.; Ghezzi, B.; Vettori, M.; Bergonzi, L.; Attolino, S.; Rossi, S.; Tarabella, G.; Vurro, D.; Von Zeppelin, D.; Iannotta, S.; et al. 3D Printed Masks for Powders and Viruses Safety Protection Using Food Grade Polymers: Empirical Tests. Polymers 2021, 13, 617. [Google Scholar] [CrossRef]
- Humayun, A.; Luo, Y.; Elumalai, A.; Mills, D.K.; Humayun, A. 3D printed antimicrobial PLA constructs functionalised with zinc-coated halloysite nanotubes-Ag-chitosan oligosaccharide lactate ABSTRACT ARTICLE HISTORY. Mater. Technol. 2020, 1, 28–35. [Google Scholar] [CrossRef]
- Muro-Fraguas, I.; Sainz-García, A.; López, M.; Rojo-Bezares, B.; Múgica-Vidal, R.; Sainz-García, E.; Toledano, P.; Sáenz, Y.; González-Marcos, A.; Alba-Elías, F. Antibiofilm coatings through atmospheric pressure plasma for 3D printed surgical instruments. Surf. Coatings Technol. 2020, 399, 126163. [Google Scholar] [CrossRef]
- Beniak, J.; Krizan, P.; Matus, M. Mechanical properties of biodegradable pla plastic parts produced by 3d printing. MM Sci. J. 2019, 2746–2750. [Google Scholar] [CrossRef]
- Caminero, M.Á.; Chacón, J.M.; García-Plaza, E.; Núñez, P.J.; Reverte, J.M.; Becar, J.P. Additive Manufacturing of PLA-Based Composites Using Fused Filament Fabrication: Effect of Graphene Nanoplatelet Reinforcement on Mechanical Properties, Dimensional Accuracy and Texture. Polymers 2019, 11, 799. [Google Scholar] [CrossRef]
- Dominguez-Robles, J.; Martin, N.K.; Fong, M.L.; Stewart, S.A.; Irwin, N.J.; Donnelly, R.F.; Larrañeta, E. Antioxidant PLA Composites Containing Lignin for 3D Printing Applications: A Potential Material for Healthcare Applications. Pharmaceutics 2019, 11, 165. [Google Scholar] [CrossRef]
- Sato, T.; Wakabayashi, T.; Saitoh, K. Evaluation of Antibacterial and Mechanical Properties of 3D Shaped Metal-containing PLA resin. In Proceedings of the 10th International Conference on Leading Edge Manufacturing in 21st Century (LEM21), Kitakyushu, Japan, 14–18 November 2021; p. 6. Available online: http://hdl.handle.net/10112/00025656 (accessed on 2 May 2022).
- Er-rahmani, S.; Errabiti, B.; Er, S.; Elharchli, E.; Elaabedy, A.; Ibnsouda, S. Reduction of biofilm formation on 3D printing materials treated with essential oils major compounds. Ind. Crop. Prod. 2022, 182, 114864. [Google Scholar] [CrossRef]
- Ryley, M.; Carve, M.; Piola, R.; Scardino, A.J.; Shimeta, J. Comparison of biofouling on 3D-printing materials in the marine environment. Int. Biodeterior. Biodegrad. 2021, 164, 105293. [Google Scholar] [CrossRef]
- Al-Taie, A.; Han, X.; Williams, C.M.; Abdulwhhab, M.; Abbott, A.P.; Goddard, A.; Wegrzyn, M.; Garton, N.J.; Barer, M.R.; Pan, J. 3-D printed polyvinyl alcohol matrix for detection of airborne pathogens in respiratory bacterial infections. Microbiol. Res. 2020, 241, 126587. [Google Scholar] [CrossRef]
- Deng, L.; Deng, Y.; Xie, K. Colloids and Surfaces B: Biointerfaces AgNPs-decorated 3D printed PEEK implant for infection control and bone repair. Colloids Surf. B Biointerfaces 2017, 160, 483–492. [Google Scholar] [CrossRef]
- Kiel, A.; Kaltschmidt, B.P.; Asghari, E.; Hütten, A.; Kaltschmidt, B.; Kaltschmidt, C. Bacterial Biofilm Formation on Nano-Copper Added PLA Suited for 3D Printed Face Masks. Microorganisms 2022, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Goyanes, A.; Trenfield, S.J.; Diaz-Gomez, L.; Alvarez-Lorenzo, C.; Gaisford, S.; Basit, A.W. Stereolithography (SLA) 3D printing of a bladder device for intravesical drug delivery. Mater. Sci. Eng. C 2021, 120, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Simoneti, D.M.; Pereira-Cenci, T.; dos Santos, M.B.F. Comparison of material properties and biofilm formation in interim single crowns obtained by 3D printing and conventional methods. J. Prosthet. Dent. 2020, 127, 168–172. [Google Scholar] [CrossRef]
- Wilson, L.; Iqbal, K.M.; Simmons-Ehrhardt, T.; Bertino, M.F.; Shah, M.R.; Yadavalli, V.K.; Ehrhardt, C.J. Customizable 3D printed di ff usion chambers for studies of bacterial pathogen phenotypes in complex environments. J. Microbiol. Methods 2019, 162, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tyagi, P.; Agate, S.; Mccord, M.G.; Lucia, L.A.; Pal, L. Highly tunable bioadhesion and optics of 3D printable PNIPAm/cellulose nano fi brils hydrogels. Carbohydr. Polym. 2020, 234, 115898. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, J.; Jeong, H.; Choi, J.; Ryu, J.J. Printing accuracy, mechanical properties, surface characteristics, and microbial adhesion of 3D-printed resins with various printing orientations. J. Prosthet. Dent. 2020, 124, 468–475. [Google Scholar] [CrossRef]
- Mazurek-Popczyk, J.; Palka, L.; Arkusz, K.; Dalewski, B.; Baldy-Chudzik, K. Personalized, 3D-printed fracture fixation plates versus commonly used orthopedic implant materials- biomaterials characteristics and bacterial biofilm formation. Injury 2021, 53, 938–946. [Google Scholar] [CrossRef]
- Linden, M.; Bachynski, N.; Oblak, M.; James, F.; Weese, J.S. Manual polishing of 3D printed metals produced by laser powder bed fusion reduces biofilm formation. PLoS ONE 2019, 14, e0212995. [Google Scholar] [CrossRef]
- Sarker, A.; Tran, N.; Rifai, A.; Brandt, M.; Tran, P.A.; Leary, M. Rational design of additively manufactured Ti6Al4V implants to control Staphylococcus aureus biofilm formation. Materialia 2019, 5, 100250. [Google Scholar] [CrossRef]
- Lücking, T.H.; Sambale, F.; Schnaars, B.; Bulnes-Abundis, D.; Beutel, S.; Scheper, T. 3D-printed individual labware in biosciences by rapid prototyping: In vitro biocompatibility and applications for eukaryotic cell cultures. Eng. Life Sci. 2015, 15, 57–64. [Google Scholar] [CrossRef]
- González Flores, G.A.; Bertana, V.; Chiappone, A.; Roppolo, I.; Scaltrito, L.; Marasso, S.L.; Cocuzza, M.; Massaglia, G.; Quaglio, M.; Pirri, C.F.; et al. Single-Step 3D Printing of Silver-Patterned Polymeric Devices for Bacteria Proliferation Control. Macromol. Mater. Eng. 2022, 307, 2100596. [Google Scholar] [CrossRef]
- Mangal, U.; Min, Y.J.; Seo, J.; Kim, D.; Cha, J.; Kwon, J.; Choi, S. Changes in tribological and antibacterial properties of poly(methyl methacrylate)-based 3D-printed intra-oral appliances by incorporating nanodiamonds. J. Mech. Behav. Biomed. Mater. 2020, 110, 103992. [Google Scholar] [CrossRef] [PubMed]
- Vivero-Lopez, M.; Xu, X.; Muras, A.; Otero, A.; Concheiro, A.; Gaisford, S.; Basit, A.W.; Alvarez-Lorenzo, C.; Goyanes, A. Anti-biofilm multi drug-loaded 3D printed hearing aids. Mater. Sci. Eng. C 2020, 119, 111606. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Wadnap, S.; Xu, C.; Ahsan, F. Digital light processing (DLP) 3D-printing technology and photoreactive polymers in fabrication of modified-release tablets. Eur. J. Pharm. Sci. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- He, Y.; Luckett, J.; Begines, B.; Dubern, J.F.; Hook, A.L.; Prina, E.; Rose, F.R.A.J.; Tuck, C.J.; Hague, R.J.M.; Irvine, D.J.; et al. Ink-jet 3D printing as a strategy for developing bespoke non-eluting biofilm resistant medical devices. Biomaterials 2022, 281, 121350. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Abdi, M.; Trindade, G.F.; Begines, B.; Dubern, J.-F.; Prina, E.; Hook, A.L.; Choong, G.Y.H.; Ledesma, J.; Tuck, C.J.; et al. Exploiting Generative Design for 3D Printing of Bacterial Biofilm Resistant Composite Devices. Adv. Sci. 2021, 8, 2100249. [Google Scholar] [CrossRef]
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cells Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef]
- Slate, A.J.; Hickey, N.A.; Butler, J.A.; Wilson, D.; Liauw, C.M.; Banks, C.E.; Whitehead, K.A. Additive manufactured graphene-based electrodes exhibit beneficial performances in Pseudomonas aeruginosa microbial fuel cells. J. Power Sources 2021, 499, 229938. [Google Scholar] [CrossRef]
- Tzounis, L.; Bangeas, P.I.; Exadaktylos, A.; Petousis, M. Three-Dimensional Printed Polylactic Acid (PLA) Surgical Retractors with Sonochemically Immobilized Silver Nanoparticles: The Next Generation of Low-Cost Antimicrobial Surgery Equipment. Nanomaterials 2020, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Bayraktar, I.; Doganay, D.; Coskun, S.; Kaynak, C.; Akca, G.; Unalan, H.E. 3D printed antibacterial silver nanowire/polylactide nanocomposites. Compos. Part B 2019, 172, 671–678. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Claudio, R.A.; Grenho, L.; Maria, H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Water, J.J.; Bohr, A.; Boetker, J.; Aho, J.; Sandler, N.; Nielsen, H.M.; Rantanen, J. Three-dimensional printing of drug-eluting implants: Preparation of an antimicrobial polylactide feedstock material. Pharm. Drug Deliv. Pharm. Technol. Three-Dimensional. 2015, 104, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Diaz-Gomez, L.; Utomo, E.; Shen, T.; Picco, C.J.; Alvarez-Lorenzo, C.; Concheiro, A.; Donnelly, R.F.; Larrañeta, E. Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals 2021, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.K.; Jammalamadaka, U.; Tappa, K.; Weisman, J. Studies on the cytocompatibility, mechanical and antimicrobial properties of 3D printed poly(methyl methacrylate) beads. Bioact. Mater. 2018, 3, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chu, L.; Yang, S.; Zhang, H.; Qin, L.; Eglin, D.; Richards, R.G.; Tang, T. Dual-functional 3D-printed composite scaffold for inhibiting bacterial infection and promoting bone regeneration in infected bone defect models. Acta Biomater. 2018, 79, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Tse, I.; Jay, A.; Na, I.; Murphy, S.; Niño-mart, N.; Alejandro, G.; Magrill, J.; Bach, H. Antimicrobial Activity of 3D-Printed Acrylonitrile Butadiene Styrene (ABS) Polymer-Coated with Silver Nanoparticles. Materials 2021, 14, 7681. [Google Scholar] [CrossRef]
- Muro-Fraguas, I.; Sainz-García, A.; Fernández, P.; López, M.; Múgica-Vidal, R.; Sainz-García, E.; Toledano, P.; Sáenz, Y.; López, M.; Prieto, M.; et al. Atmospheric pressure cold plasma anti-biofilm coatings for 3D printed food tools. J. Pre-Proof 2020, 64, 102404. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Zanocco, M.; Honma, T.; Zhu, W.; Pezzotti, G. Explorative study on the antibacterial effects of 3D-printed PMMA/nitrides composites. Mater. Des. 2021, 206, 109788. [Google Scholar] [CrossRef]
- Feng, G.; Cheng, Y.; Wang, S.; Borca-Tasciuc, D.A.; Worobo, R.W.; Moraru, C.I. Bacterial attachment and bio film formation on surfaces are reduced by small-diameter nanoscale pores: How small is small enough? Nat. Publ. Gr. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Kodama, J.; Chen, H.; Zhou, T. Antibacterial efficacy of quaternized chitosan coating on 3D printed titanium cage in rat intervertebral disc space. Spine J. 2021, 21, 1217–1228. [Google Scholar] [CrossRef]
- Turner, R.D.; Wingham, J.R.; Paterson, T.E.; Shepherd, J.; Majewski, C. Use of silver-based additives for the development of antibacterial functionality in Laser Sintered polyamide 12 parts. Sci. Rep. 2020, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Slita, A.; Backman, O.; Gounani, Z.; Rosqvist, E.; Peltonen, J.; Willför, S.; Xu, C.; Rosenholm, J.M.; et al. Digital light processing (DLP) 3D-fabricated antimicrobial hydrogel with a sustainable resin of methacrylated woody polysaccharides and hybrid silver-lignin nanospheres. Green Chemestry 2022, 24, 2129–2145. [Google Scholar] [CrossRef]
- Li, Z.; Wang, C.; Qiu, W.; Liu, R. Antimicrobial Thiol–ene–acrylate Photosensitive Resins for DLP 3D Printing. Photochem. Photobiol. 2019, 95, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Hodasova, L.; Sans, J.; Molina, B.G.; An, C.A.; Llanes, L.; Fargas, G.; Armelin, E. Polymer infiltrated ceramic networks with biocompatible adhesive and 3D-printed highly porous scaffolds. Addit. Manuf. 2021, 39, 101850. [Google Scholar] [CrossRef]
- García-Alvarez, R.; Izquierdo-Barba, I.; Vallet-Regí, M. 3D scaffold with effective multidrug sequential release against bacteria biofilm. Acta Biomater. 2017, 49, 113–126. [Google Scholar] [CrossRef]
- Kwon, J.S.; Kim, J.Y.; Mangal, U.; Seo, J.Y.; Lee, M.J.; Jin, J.; Yu, J.H.; Choi, S.H. Durable oral biofilm resistance of 3d-printed dental base polymers containing zwitterionic materials. Int. J. Mol. Sci. 2021, 22, 417. [Google Scholar] [CrossRef]
- Han, R.S.; Haghiashtiani, G.; Mcalpine, M.C. 3D Printing a Susceptibility Assay for Multidrug-Resistant Bacteria. Chem Cell Press 2016, 1, 346–348. [Google Scholar] [CrossRef][Green Version]
- Caselli, E. Hygiene: Microbial strategies to reduce pathogens and drug resistance in clinical settings. Microb. Biotechnol. 2017, 10, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Khan, M.; Kleiman, M.; Hochbaum, A.I. Effects of Growth Surface Topography on Bacterial Signaling in Coculture Biofilms Effects of Growth Surface Topography on Bacterial Signaling in Coculture Biofilms. ACS Appl. Mater. Interfaces 2017, 9, 18531–18539. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef]
- Grenho, L.; Manso, M.C.; Monteiro, F.J.; Ferraz, M.P. Adhesion of Staphylococcus aureus, Staphylococcus epidermidis, and Pseudomonas aeruginosa onto nanohydroxyapatite as a bone regeneration material. J. Biomed. Mater. Res.-Part A 2012, 100, 1823–1830. [Google Scholar] [CrossRef]
- Reffuveille, F.; Josse, J.; Vallé, Q.; Josse, J.; Gangloff, S.C.; Mongaret, C.; Gangloff, S.C. Staphylococcus aureus Biofilms and their Impact on the on the Medical Field. INTECH 2018, 11, 187–214. [Google Scholar] [CrossRef]
- Wang, Y.; Blache, R.; Xu, X. Selection of additive manufacturing processes. Rapid Prototyp. J. 2017, 23, 1–29. [Google Scholar] [CrossRef]
- Fujitani, S.; Sun, H.Y.; Yu, V.L.; Weingarten, J.A. Pneumonia due to pseudomonas aeruginosa: Part I: Epidemiology, clinical diagnosis, and source. Chest 2011, 139, 909–919. [Google Scholar] [CrossRef]
- Driscoll, J.A.; Brody, S.L.; Kollef, M.H. The epidemiology, pathogenesis and treatment of Pseudomonas aeruginosa infections. Drugs 2007, 67, 351–368. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, Z.; Hong, J.; Wu, M.; Shiue, S.; Lin, H. Controlled-release of free bacteriophage nanoparticles from 3D-plotted hydrogel fibrous structure as potential antibacterial wound dressing. J. Control Release 2021, 331, 154–163. [Google Scholar] [CrossRef]
- Walsh, M.E.; Ostrinskaya, A.; Sorensen, M.T.; Kong, D.S.; Carr, P.A. 3D-printable materials for microbial liquid culture. 3D Print. Addit. Manuf. 2016, 3, 113–118. [Google Scholar] [CrossRef]
- Yuan, S.; Bai, J.; Chua, C.K.; Wei, J.; Zhou, K. Material Evaluation and Process Optimization of CNT-Coated Polymer Powders for Selective. Polymers 2016, 8, 370. [Google Scholar] [CrossRef] [PubMed]
- Tiimob, B.J.; Mwinyelle, G.; Abdela, W.; Samuel, T.; Jeelani, S.; Rangari, V.K. Nano-engineered eggshell-silver tailored co-polyester polymer blend film with antimicrobial properties. Am. Chem. Soc. 2017, 65, 1967–1976. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).