Effect of Anodic Oxidation Pulse Voltage on Antibacterial Properties and Biocompatibility of Ti-Ag Alloy
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation and AO Treatment of Ti-Ag Samples
2.2. XRD and Microstructure Characterization
2.3. XPS Analysis
2.4. Corrosion Resistance
2.5. Surface Roughness and Water Wetting Angle
2.6. Ion Release
2.7. Antibacterial Property
2.7.1. Plate Count Method
2.7.2. Live/Dead Staining
2.8. Cell Compatibility In Vitro
2.8.1. Cell Viability and Cytotoxicity
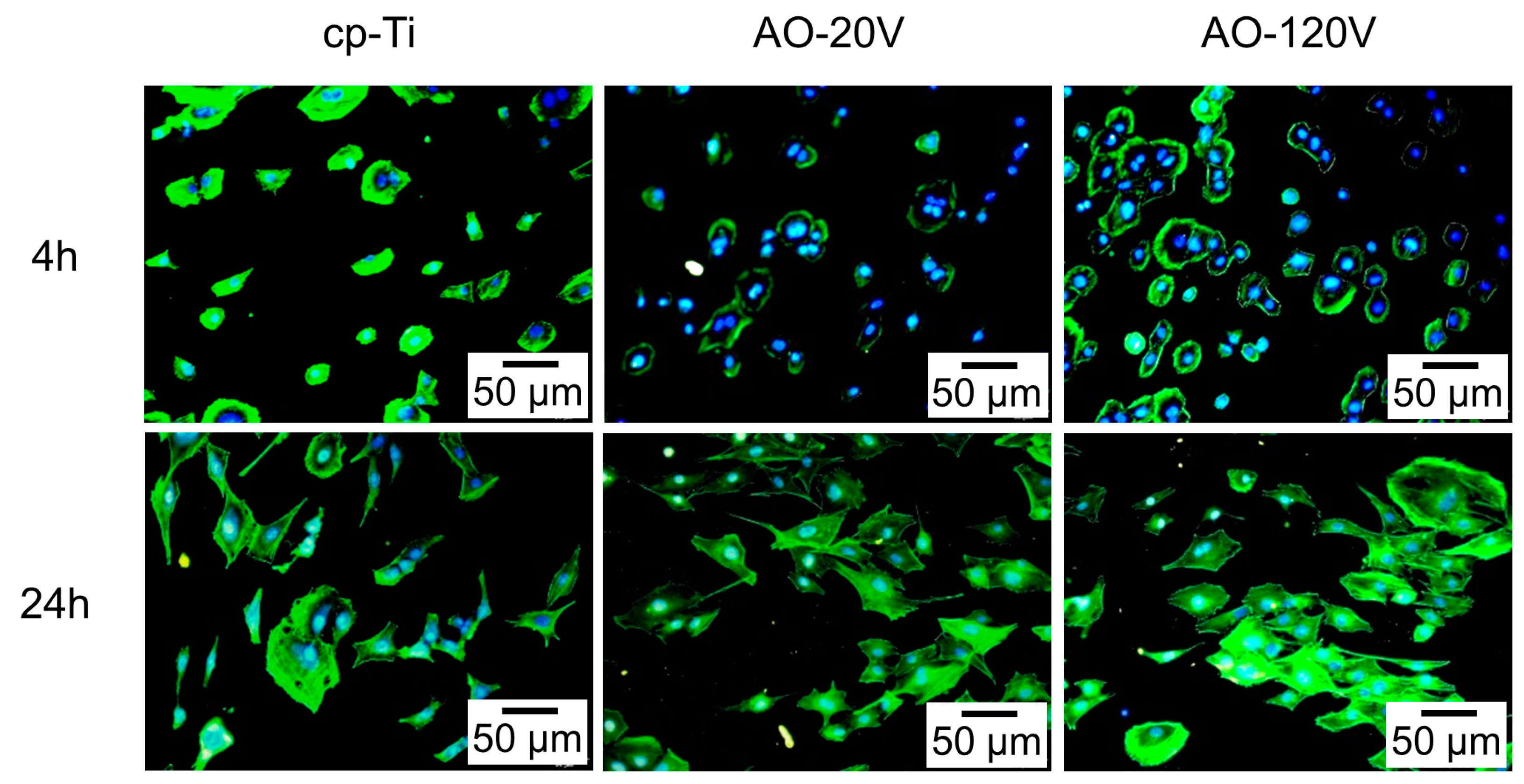
2.8.2. Cell Adhesion and Morphology
2.9. Statistical Analysis
3. Results
3.1. Surface Morphology
3.2. Phase Identification
3.3. Microstructure and EDS
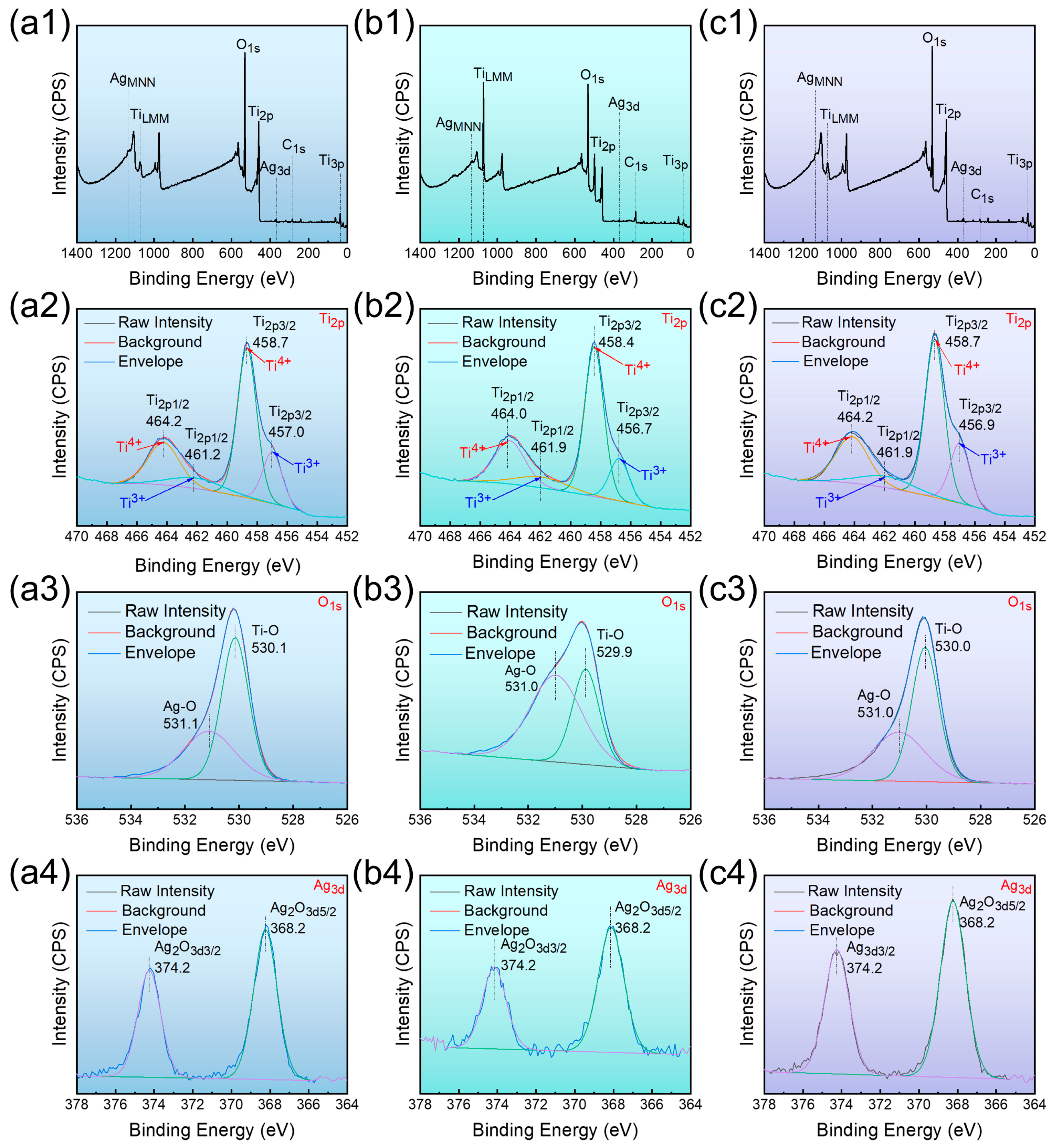
3.4. XPS
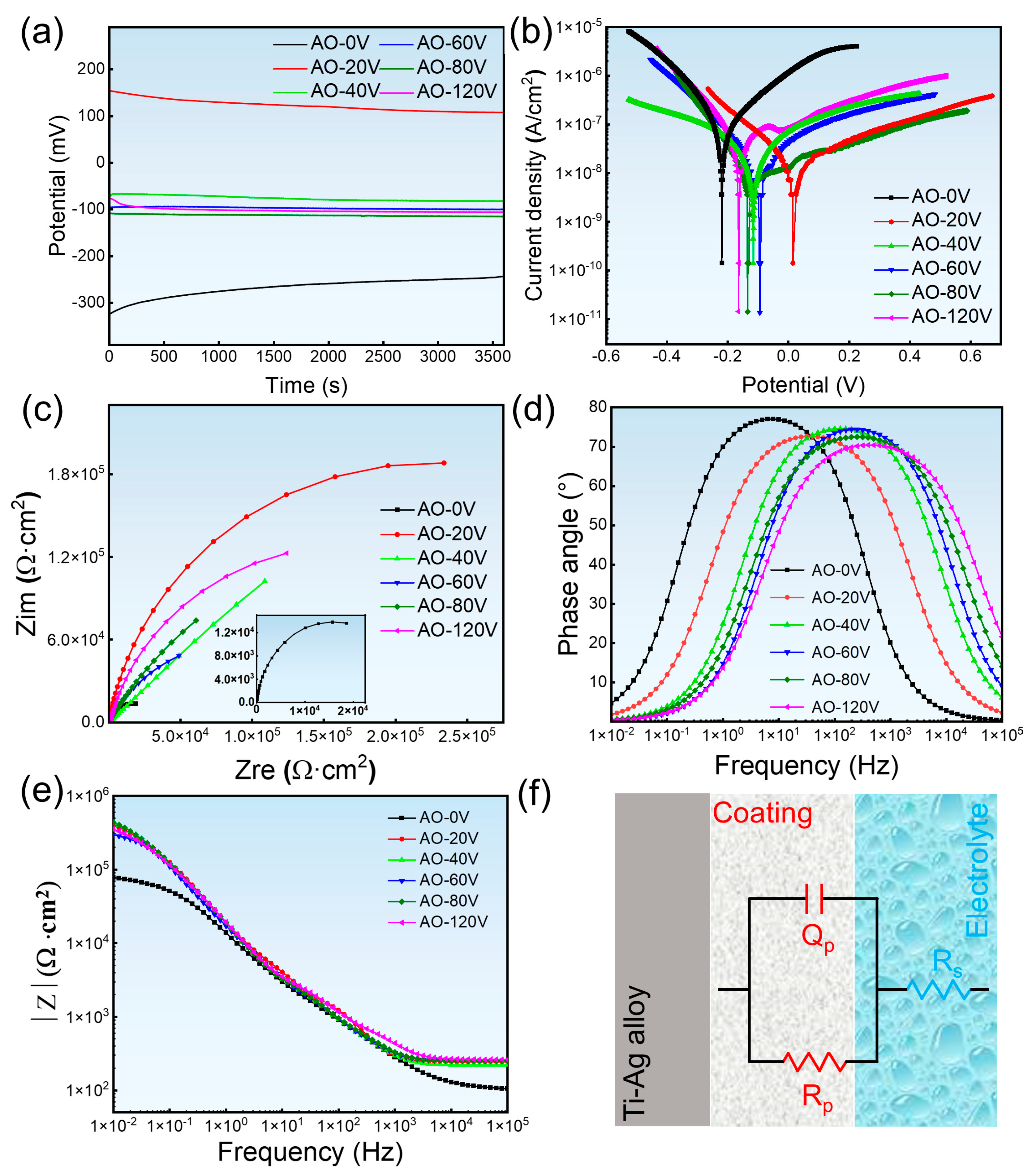
3.5. Corrosion Resisting Property
3.6. Surface Roughness and Wetting Angle
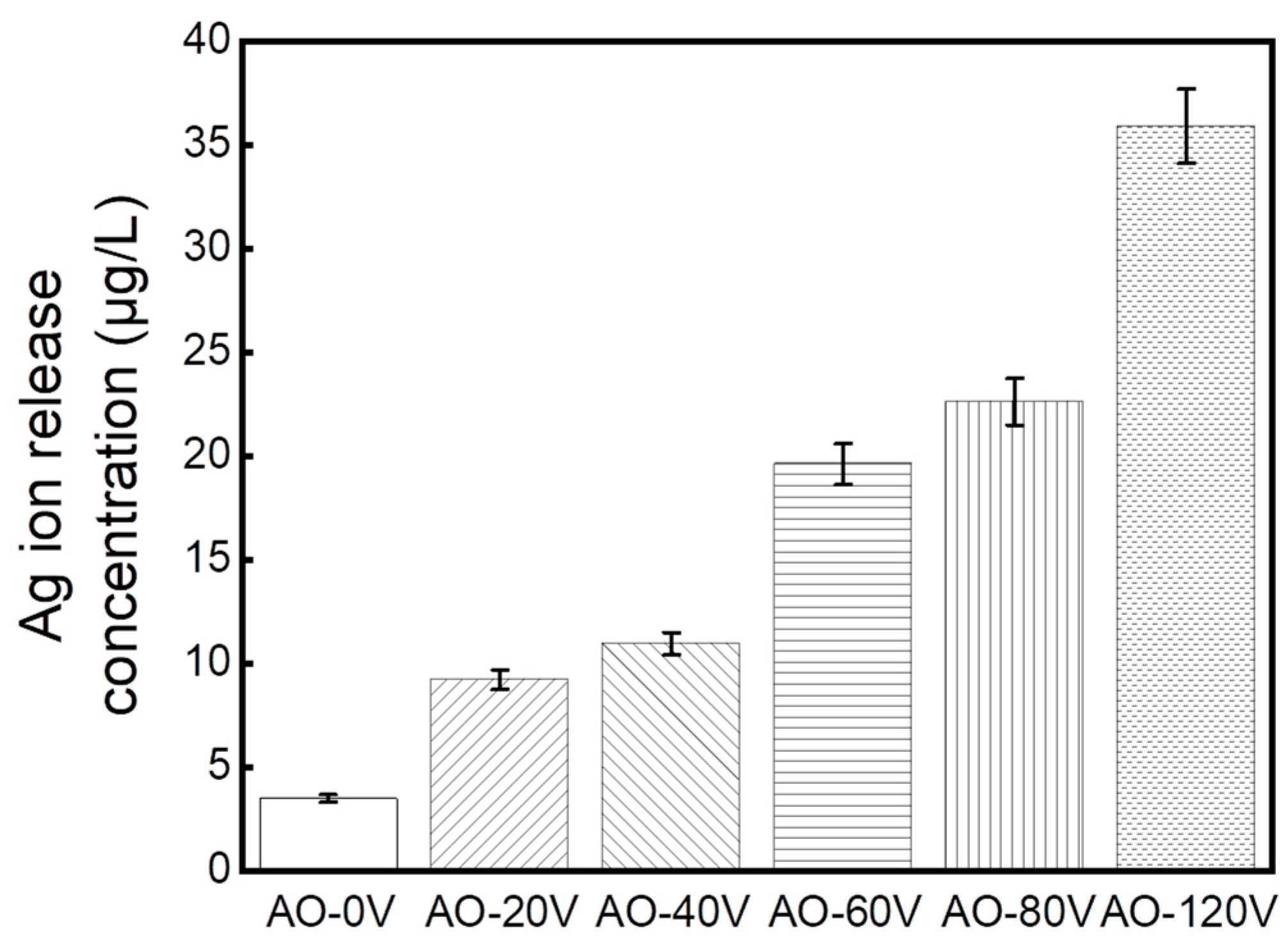
3.7. Ion Release
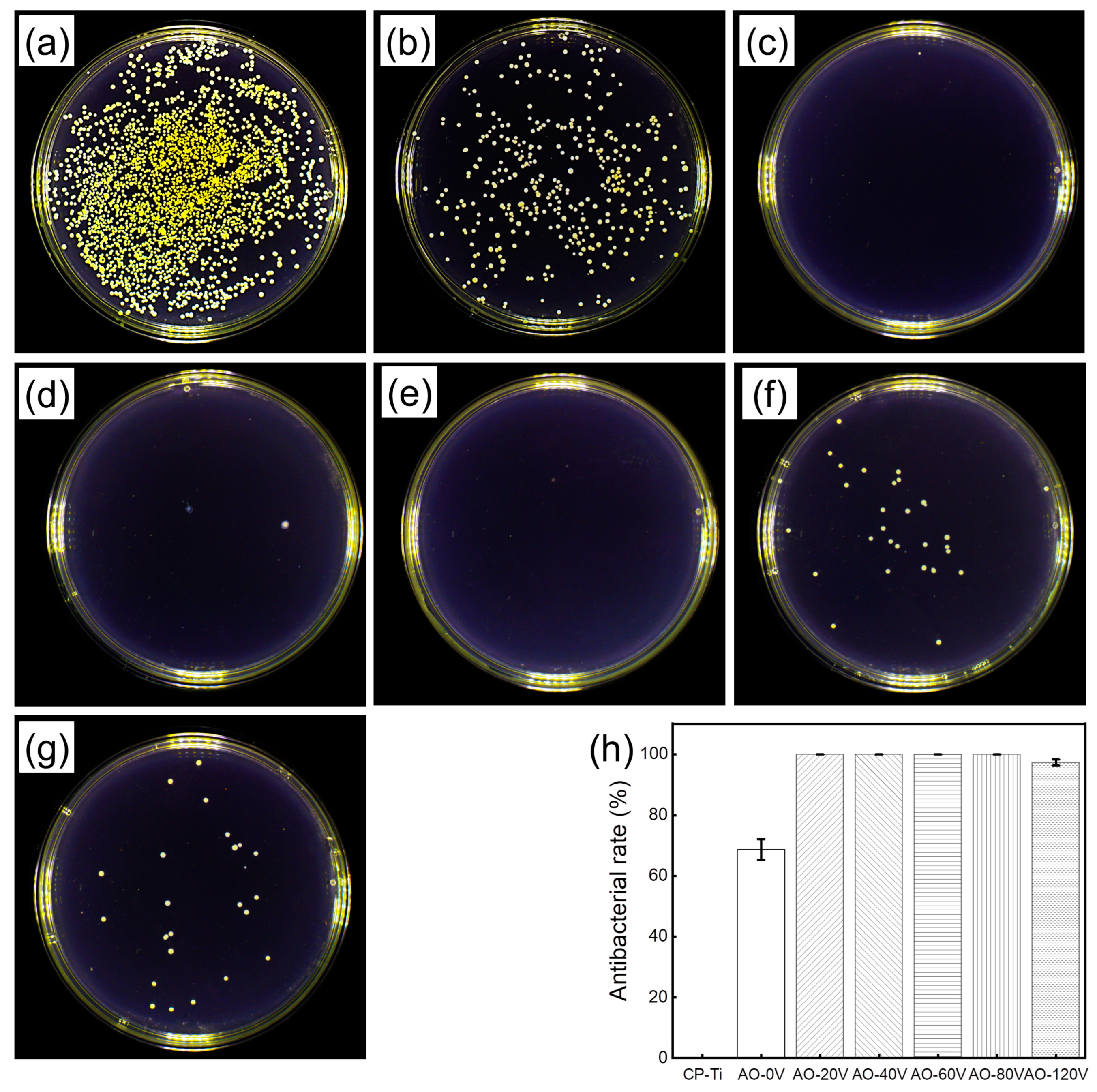
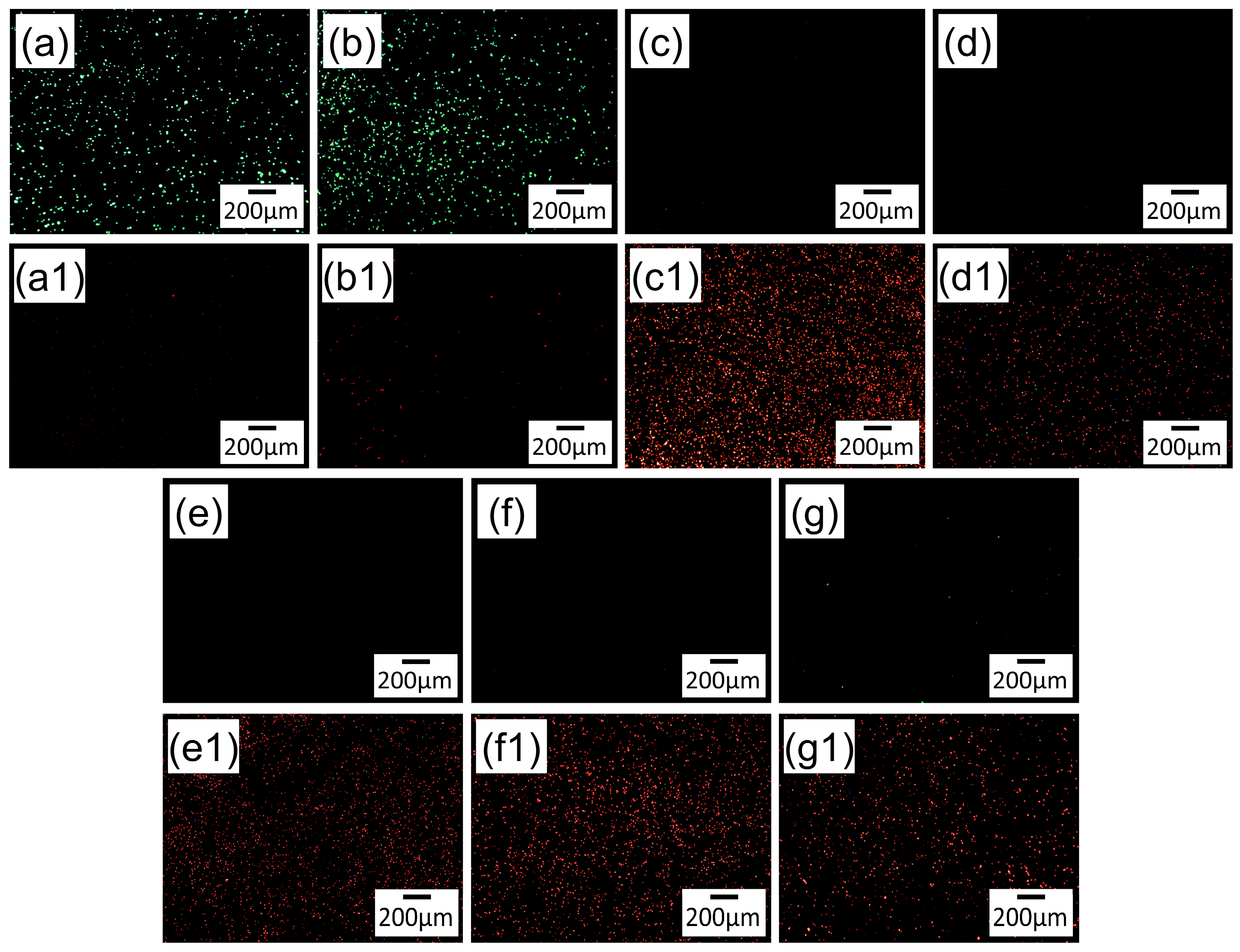
3.8. Antibacterial Property
3.8.1. Antibacterial Rate
3.8.2. Live/Dead Fluorescence Staining
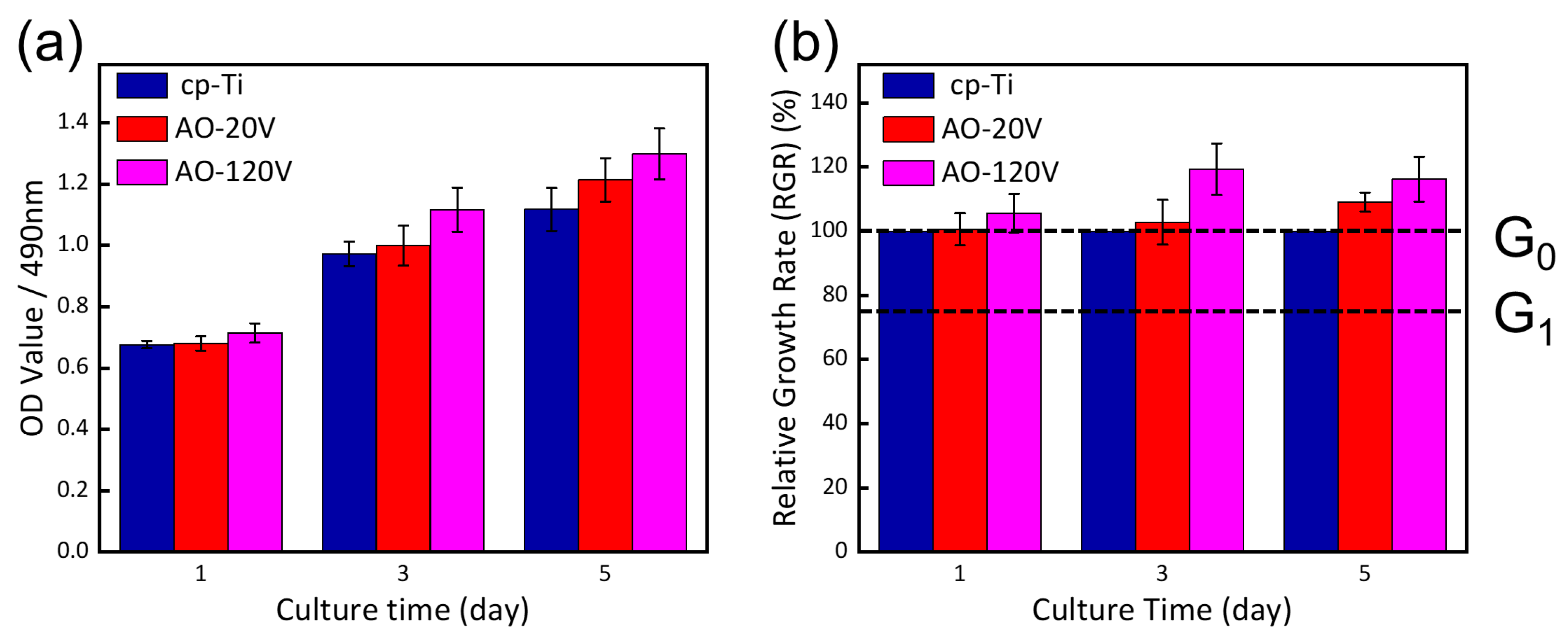
3.9. Cell Experiment
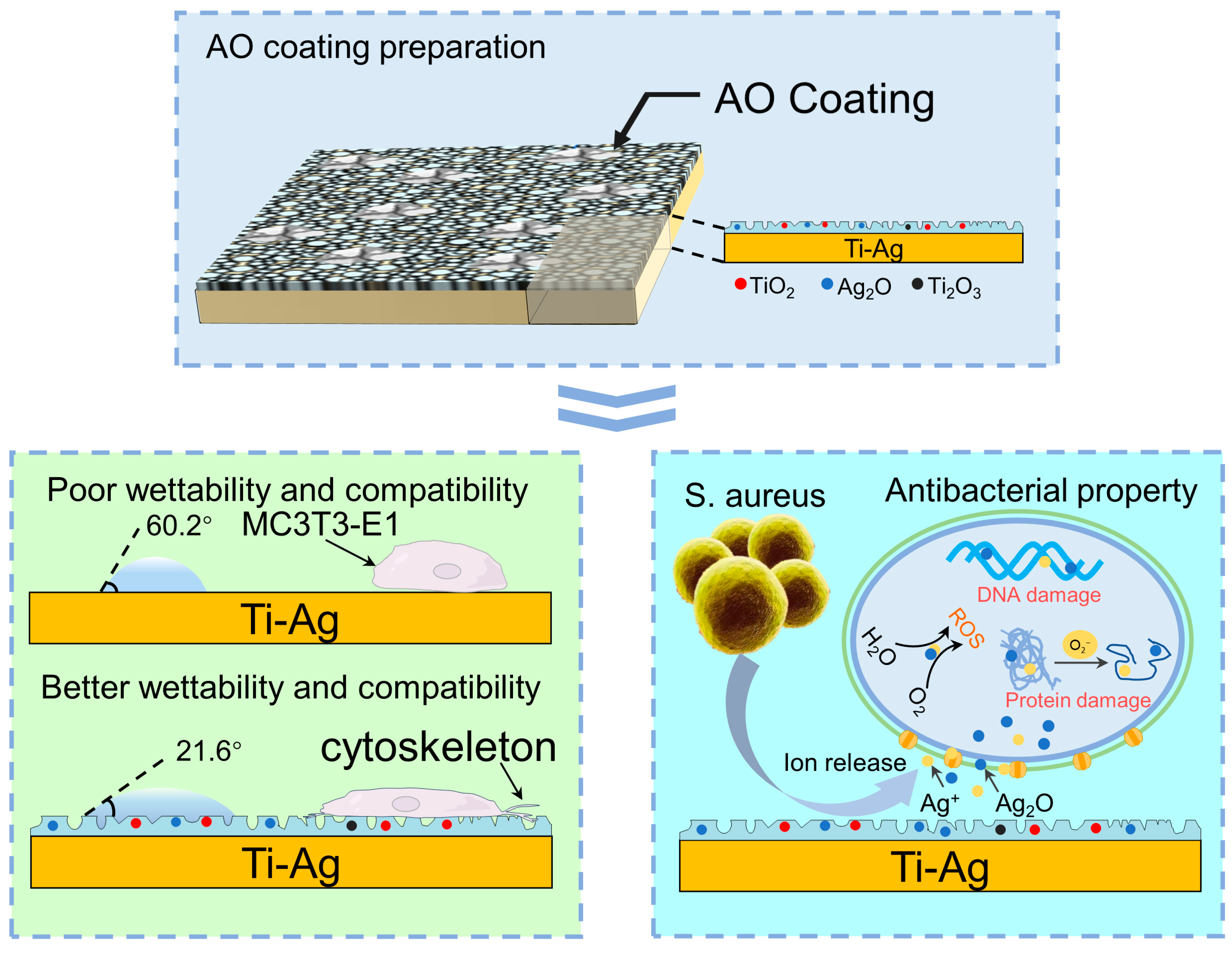
4. Discussion
4.1. Surface Physicochemical Performance
4.2. Corrosion Resistance and Ion Release
4.3. Antibacterial Property
4.4. Cytotoxicity
5. Conclusions
- (1)
- Different voltage AO treatments can change the microstructure of the Ti-Ag alloy and form a multi-functional coating with an irregular flower-like structure with a size of 1~2 μm on the surface. The coating is mainly composed of Ag2O and TiO2. The change in voltage has no obvious effect on the XRD test results of the samples, but too high a voltage will cause the burning loss of Ag.
- (2)
- After the AO treatment of the Ti-Ag alloy, the oxide film formed on the surface of the alloy improves the corrosion resistance and the corrosion resistance is higher than that of untreated samples. However, with the increase in the AO voltage, the corrosion resistance decreases. When the AO voltage is 20 V, the maximum Eocp is 107.621 mV, the maximum Ecorr is 17.035 mV, the minimum icorr is 2.241 × 10−8 A·cm−2, and the corrosion tendency and corrosion rate are the smallest.
- (3)
- AO treatment enhanced the antibacterial properties of the Ti-Ag alloy and the antibacterial rate was more than 99%. The increase in the AO voltage can promote the release of Ag+ and the improvement of the antibacterial performance is mainly attributed to the release of the Ag2O and Ag+ formed on the surface. However, the change in voltage has no obvious effect on the antibacterial performance.
- (4)
- With the increase in the AO treatment voltage, the surface roughness can be increased, the wetting angle can be reduced, and the biocompatibility of the Ti-Ag alloy can be improved. When the AO voltage is 120 V, the maximum roughness is 0.73 μm and the minimum wetting angle is 23°, showing better cell compatibility and no cytotoxicity.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alves, A.C.; Sendao, I.; Ariza, E.; Toptan, F.; Ponthiaux, P.; Pinto, P.M.A. Corrosion behaviour of porous Ti intended for biomedical applications. J. Porous Mater. 2016, 23, 1261–1268. [Google Scholar] [CrossRef]
- Huang, Y.; Song, G.; Chang, X.; Wang, Z.; Zhang, X.; Han, S.; Su, Z.; Yang, H.; Yang, D.; Zhang, X. Nanostructured Ag+-substituted fluorhydroxyapatite-TiO2 coatings for enhanced bactericidal effects and osteoinductivity of Ti for biomedical applications. Int. J. Nanomed. 2018, 13, 2665–2684. [Google Scholar] [CrossRef]
- Cai, D.G.; Zhao, X.T.; Yang, L.; Wang, R.X.; Qin, G.W.; Chen, D.F.; Zhang, E.L. A novel biomedical titanium alloy with high antibacterial property and low elastic modulus. J. Mater. Sci. Technol. 2021, 81, 13–25. [Google Scholar] [CrossRef]
- Gallo, G.; Schillaci, D. Bacterial metal nanoparticles to develop new weapons against bacterial biofilms and infections. Appl. Microbiol. Biotechnol. 2021, 105, 5357–5366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, S.Y.; Liu, H.; Ren, L.; Wang, Q.; Zhang, Y. Effects of surface roughening on antibacterial and osteogenic properties of Ti-Cu alloys with different Cu contents. J. Mater. Sci. Technol. 2019, 48, 278–284. [Google Scholar] [CrossRef]
- Campos, P.A.D.; Sabrina, R.; Batistao, D.W.D.F.; Bruuna, F.A.; Queiroz, L.L.; Brito, S.S.; Paulo, P.G.F.; Ribas, R.M. Multidrug resistance related to biofilm formation in Acinetobacter baumannii and klebsiella pneumoniae clinical strains from different pulsotypes. Curr. Microbiol 2016, 72, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Shi, M.S.; Xu, F.Y.; Qiu, Y.; Zhang, P.; Shen, K.L.; Zhao, Q.; Yu, J.G.; Zhang, Y.F. Graphdiyne-modified TiO2 nanofibers with osteoinductive and enhanced photocatalytic antibacterial activities to prevent implant infection. Nat. Commun. 2020, 11, 4465. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zhang, Z.-M.; Zhang, J.Q.; Wang, R.X.; Yang, L.; Chen, D.F.; Qin, G.W.; Zhang, E.L. Improvement in antibacterial ability and cell cytotoxicity of Ti-Cu alloy by anodic oxidation. Rare Met. 2022, 41, 594–609. [Google Scholar] [CrossRef]
- Maharubin, S.; Hu, Y.B.; Sooriyaarachchi, D.; Cong, W.L.; Tan, G.Z. Laser engineered net shaping of antimicrobial and biocompatible titanium-silver alloys. Mater. Sci. Eng. C 2019, 105, 110059. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Z.M.; Zhang, J.Q.; Qin, G.W.; Zhang, E.L. Construction of a TiO2/Cu2O multifunctional coating on Ti-Cu alloy and its influence on the cell compatibility and antibacterial properties. Surf. Coat. Technol. 2021, 421, 127438. [Google Scholar] [CrossRef]
- Sadowska, J.M.; Genoud, K.J.; Kelly, D.J.; Brien, F.J. Bone biomaterials for overcoming antimicrobial resistance: Advances in non-antibiotic antimicrobial approaches for regeneration of infected osseous tissue. Mater. Today 2021, 46, 136–154. [Google Scholar] [CrossRef]
- Ren, L.; Memarzadeh, K.; Zhang, S.Y.; Sun, Z.Q.; Yang, C.G.; Ren, G.G.; Robert, P.A.; Yang, K. A novel coping metal material CoCrCu alloy fabricated by selective laser melting with antimicrobial and antibiofilm properties. Mater. Sci. Eng. C 2016, 67, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Alejandra, R.-C.; Diego, T.; Belal, R.; Monica, O.H.; Maria, P.G.; Jose, A.C.; Jose, M.M.; Elisa, R. Bioactivity and antibacterial properties of calcium and silver-doped coatings on 3D printed titanium scaffolds. Surf. Coat. Technol. 2021, 421, 127476. [Google Scholar] [CrossRef]
- Feng, J.; Cheng, L.; Zhou, X.D.; Xu, H.H.K.; Michael, D.W.; Markus, M.; Hans, M.; Li, Q.; Hannig, M.; Rupf, S. In situ antibiofilm effect of glass-ionomer cement containing dimethyllaminododecyl methacrylate. Dent. Mater. 2015, 31, 992–1002. [Google Scholar] [CrossRef]
- Liang, Y.; Song, Y.Y.; Wang, L.; Wei, C.; Zhou, X.; Feng, Y.H. Research progress on antibacterial activity of medical titanium alloy implant materials. Odontology 2023, 111, 813–829. [Google Scholar] [CrossRef] [PubMed]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Jeannine, H. Rational approaches towards inorganic and organometallic antibacterials. Biol. Chem. 2022, 403, 363–375. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Mohammad, B.J.; Mohammad, H.Z.; Khosro, A. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C 2014, 44, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.D.; Yun, H.; Kim, G.C.; Lee, C.W.; Choi, H.C. Antibacterial activity and reusability of CNT-Ag and GO-Ag nanocomposites. Appl. Surf. Sci. 2013, 283, 227–233. [Google Scholar] [CrossRef]
- Saidin, S.; Jumat, A.M.; Amin, M.A.A.N.; Abdullah, S.S.A.H. Organic and inorganic antibacterial approaches in combating bacterial infection for biomedical application. Mater. Sci. Eng. C 2021, 118, 111382. [Google Scholar] [CrossRef]
- Luo, J.H.; Mamat, B.; Yue, Z.H.; Zhang, N.Y.; Xu, X.J.; Li, Y.L.; Su, Z.; Ma, C.; Zang, F.Z.; Wang, Y.B. Multi-metal ions doped hydroxyapatite coatings via electrochemical methods for antibacterial and osteogenesis. Colloid Interface Sci. Commun. 2021, 43, 100435. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, E.L.; Zhang, L. Microstructure, mechanical properties, bio-corrosion properties and antibacterial properties of Ti-Ag sintered alloys. Biol. Adv. 2016, 62, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Daudre, C.F. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Hadidi, M.; Bigham, A.; Saebnoori, E.; Tabrizi, S.A.H.; Rahmati, S.; Alizadeh, Z.M.; Nasirian, V.; Rafienia, M. Electrophoretic-deposited hydroxyapatite-copper nanocomposite as an antibacterial coating for biomedical applications. Surf. Coat. Technol. 2017, 321, 171–179. [Google Scholar] [CrossRef]
- Hou, X.G.; Mao, D.; Ma, H.Y.; Ai, Y.K.; Zhao, X.L.; Deng, J.H.; Li, D.J.; Liao, B. Antibacterial ability of Ag-TiO2 nanotubes prepared by ion implantation and anodic oxidation. Mater. Lett. 2015, 161, 309–312. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Jurczyk, K.; Purwin, D.; Koper, J.K.; Romaniuk, A.; Lipinska, N.; Jakubowicz, J.; Jurczyk, M.U. Molecular analysis of biocompatibility of anodized titanium with deposited silver nanodendrites. Biol. Adv. 2018, 93, 437–444. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, J.W.; Shi, Y.F.; Tang, J.C.; Huang, D.N.; Yan, M.; Dargusch, M.S. Surface modification of biomedical Ti and Ti alloys: A review on current advances journal of metals. Materials 2022, 15, 1749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.L.; Zhao, X.T.; Hu, J.L.; Wang, R.X.; Fu, S.; Qin, G.W. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 18012115. [Google Scholar] [CrossRef]
- Zhang, E.L.; Ren, J.; Li, S.Y.; Yang, L.; Qin, G.W. Optimization of mechanical properties, biocorrosion properties and antibacterial properties of as-cast Ti-Cu alloys. Biomed. Mater. 2016, 11, 065001. [Google Scholar] [CrossRef] [PubMed]
- Andrii, L.; Cecilia, F.; Oleksii, S.; Fabrizia, G.; Dario, B.; Raymond, J.T. Comparison of Antimicrobial and Antibiofilm Activity of Proflavine Co-crystallized with Silver, Copper, Zinc, and Gallium Salts. ACS Appl. Bio Mater. 2022, 5, 4203–4212. [Google Scholar] [CrossRef]
- Wang, H.; Gao, W.W.; Zhang, X.Y.; LI, Y.; Zhang, S.Y.; Ren, L.; Yang, K. Enhanced Corrosion Resistance and Biological Properties of Ultrafine-Grained Ti15Zr5Cu Alloy. Metals 2022, 12, 1144. [Google Scholar] [CrossRef]
- Hu, J.L.; Li, H.X.; Wang, X.Y.; Yang, L.; Chen, M.; Wang, R.X.; Qin, G.W.; Chen, D.F.; Zhang, E.L. Effect of ultrasonic micro-arc oxidation on the antibacterial properties and cell biocompatibility of Ti-Cu alloy for biomedical application. Biomater. Adv. 2020, 115, 110921. [Google Scholar] [CrossRef]
- Schwartz, A.; Kossenko, A.; Zinigrad, M.; Danchuk, V.; Sobolev, A. Cleaning Strategies of Synthesized Bioactive Coatings by PEO on Ti-6Al-4V Alloys of Organic Contaminations. Materials 2023, 16, 4624. [Google Scholar] [CrossRef]
- Wang, N.; Li, H.Y.; Lv, W.L.; Li, J.H.; Wang, J.S.; Zhang, Z.T.; Liu, Y.R. Effects of TiO2 nanotubes with different diameters on gene expression and osseointegration of implants in minipigs. Biomaterials 2011, 32, 6900–6911. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, Y.; Qin, G.W.; Zhang, E.L. Antibacterial effect of Ti-Ag alloy motivated by Ag-containing phases. Biomater. Adv. 2021, 128, 112266. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Jarosz, M.; Zaraska, L.; Koziel, M.; Simka, W.; Sulka, G. Electrochemical Oxidation of Ti15Mo Alloy-The Impact of Anodization Parameters on Surface Morphology of Nanostructured Oxide Layers. Nanomaterials 2020, 11, 68. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.-T.; Johansson, C.B.; Jeong, Y.; Tomas, A. The electrochemical oxide growth behavior on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Chan, C.W.; Carson, L.; Smith, G.C.; Alessio, M.; Lee, S. Enhancing the antibacterial performance of orthopaedic implant materials by fibre laser surface engineering. Appl. Surf. Sci. 2017, 404, 67–81. [Google Scholar] [CrossRef]
- Han, M.-K.; Im, J.-B.; Hwang, M.-J.; Kim, B.J.; Kim, H.Y.; Park, Y.J. Effect of indium content on the microstructure, mechanical properties and corrosion behavior of titanium alloys. Metals 2015, 5, 850–862. [Google Scholar] [CrossRef]
- Mato, S.; Thompson, G.E.; Skeldon, P.; Shimizu, K.; Habazaki, H.; Masheder, D. Enrichment of alloying elements beneath anodic oxides: Investigation of Ta-1.5 at.% Cu alloy. Corros. Sci. 2001, 43, 993–1002. [Google Scholar] [CrossRef]
- Yahalom, J.; Zahavi, J. Electrolytic breakdown crystallization of anodic oxide films on A1, Ta and Ti. Electrochim. Acta 1970, 15, 1429–1435. [Google Scholar] [CrossRef]
- Zheng, Q.C.; Mao, L.L.; Shi, Y.T.; Fu, W.H.; Hu, Y.H. Biocompatibility of Ti-6Al-4V titanium alloy implants with laser microgrooved surfaces. Mater. Technol. 2022, 37, 2039–2048. [Google Scholar] [CrossRef]
- Deligianni, D.D.; Katsala, N.D.; Koutsoukos, P.G.; Missirlis, Y.F. Effect of surface roughness of hydroxyapatite on human bone marrow cell adhesion, proliferation, differentiation and detachment strength. Biomaterials 2001, 22, 87–96. [Google Scholar] [CrossRef]
- Bernard, S.A.; Balla, V.K.; Davies, N.M.; Bose, S.; Bandyopadhyay, A. Bone cell-materials interactions and Ni ion release of anodized equiatomic NiTi alloy. Acta Biomater. 2011, 7, 1902–1912. [Google Scholar] [CrossRef] [PubMed]
- Prando, D.; Brenna, A.; Bolzoni, F.M.; Diamanti, M.V.; Pedeferri, M.; Ormellesw, P.M. Electrochemical anodizing treatment to enhance localized corrosion resistance of pure titanium. J. Appl. Biomater. Func. 2017, 15, 19–24. [Google Scholar] [CrossRef]
- Benea, L.; Ravoiu, A.; Celis, J.-P. Anticorrosion Performance of the Electrochemically Grown Mixed Porous Oxide Films on Titanium Alloy in Biological Solution. ACS Biomater. Sci. Eng. 2019, 5, 5925–5934. [Google Scholar] [CrossRef]
- Kumar, S.; Chattopadhyay, K.; Singh, V. Effect of surface nanostructuring on corrosion behavior of Ti-6Al-4V alloy. Mater. Charact. 2016, 121, 23–30. [Google Scholar] [CrossRef]
- Wan, Y.Q.; Wang, Y.; Liu, Z.M.; Qu, X.; Han, B.X.; Bei, J.Z.; Wang, S.G. Adhesion and proliferation of OCT-1 osteoblast-like cells on micro- and nano-scale topography structured poly(L-lactide). Biomaterials 2005, 26, 4453–4459. [Google Scholar] [CrossRef] [PubMed]
- Shi, A.Q.; Zhu, C.S.; Fu, S.; Wang, R.X.; Qin, G.W.; Chen, D.W.; Zhang, E.L. What controls the antibacterial activity of Ti-Ag alloy, Ag ion or Ti2Ag particles? Biomater. Adv. 2020, 109, 110548. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.K.; Hou, H.M.; Wang, S.L.; Yan, C.L.; Liu, Y.F. Exploring the interaction of silver nanoparticles with lysozyme: Binding behaviors and kinetics. Colloids Surf. B 2017, 157, 138–145. [Google Scholar] [CrossRef]
- Shang, L.; Nienhaus, K.; Nienhaus, G.U. Engineered nanoparticles interacting with cells: Size matters. J. Nanobiotechnol. 2014, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Avalos, A.; Haza, A.I.; Mateo, D.; Paloma, M. Cytotoxicity and ROS production of manufactured silver nanoparticles of different sizes in hepatoma and leukemia cells. J. Appl. Toxicol. 2014, 34, 413–423. [Google Scholar] [CrossRef] [PubMed]
- Prasher, P.; Singh, M.; Mudila, H. Oligodynamic Effect of Silver Nanoparticles: A Review. BioNanoScience 2018, 8, 951–962. [Google Scholar] [CrossRef]
- Loo, C.-Y.; MRohanizadeh, R.; Young, P.M.; Traini, D.; Cavaliere, R.; Cynthia, B.W.; Lee, W.H. Combination of Silver Nanoparticles and Curcumin Nanoparticles for Enhanced Anti-biofilm Activities. J. Agric. Food Chem. 2016, 64, 2513–2522. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Tripathi, A.; Shweta; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; et al. Uptake, Accumulation and Toxicity of Silver Nanoparticle in Autotrophic Plants, and Heterotrophic Microbes: A Concentric Review. Front. Microbiol. 2017, 8, 243339. [Google Scholar] [CrossRef]
- Rahisuddin; Thabaiti, S.A.; Khan, Z.; Manzoor, N. Biosynthesis of silver nanoparticles and its antibacterial and antifungal activities towards Gram-positive, Gram-negative bacterial strains and different species of Candida fungus. Bioproc. Biosyst. Eng. 2015, 38, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Phanjom, P.; Ahmed, G. Effect of different physico-chemical conditions on the synthesis of silver nanoparticles using fungal cell filtrate of Aspergillus oryzae (MTCC No. 1846) and their antibacterial effect. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 045016. [Google Scholar] [CrossRef]
- Hao, Y.; Winans, S.C.; Glick, B.R.; Charles, T.C. Identification and characterization of new LuxR/LuxI-type quorum sensing systems from metagenomic libraries. Environ. Microbiol. 2010, 12, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Trevino, E.N.G.; Turrent, L.L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shu, K.H.; Zhang, L.; Si, Y.B. Effects of Silver Nanoparticles on Soil Microbial Communities and Bacterial Nitrification in Suburban Vegetable Soils. Pedosphere 2017, 27, 482–490. [Google Scholar] [CrossRef]
- Pramanik, S.; Chatterjee, S.; Saha, A.; Devi, P.S.; Kumar, G.S. Unraveling the Interaction of Silver Nanoparticles with Mammalian and Bacterial DNA. J. Phys. Chem. B 2016, 120, 5313–5324. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.S.; Liu, S.; Nie, J.J.; Chen, D.F.; Wu, X.B.; Qin, G.W.; Zhang, E.L. Design and preparation of a biomedical titanium alloy with low elastic modulus and high antibacterial property based on Ti-Mo-Ag system. J. Alloys Compd. 2022, 908, 164639. [Google Scholar] [CrossRef]
- Patel, H.; Joshi, J. Green and chemical approach for synthesis of Ag2O nanoparticles and their antimicrobial activity. J. Sol-Gel Sci. Technol. 2023, 105, 814–826. [Google Scholar] [CrossRef]
- Fayyadh, A.A.; Alzubaidy, M.H.J. Green-synthesis of Ag2O nanoparticles for antimicrobial assays. J. Mech. Behav. Mater. 2021, 30, 228–236. [Google Scholar] [CrossRef]
- Heidenau, F.; Mittelmeier, W.; Detsch, R.; Haenle, M.; Stenzel, F.; Ziegler, G.; Gollwitzer, H. A novel antibacterial titania coating: Metal ion toxicity and in vitro surface colonization. J. Mater. Sci. Mater. Med. 2005, 16, 883–888. [Google Scholar] [CrossRef]
- Hardes, J.; Streitburger, A.; Ahrens, H.; Nusselt, T.; Gebert, C.; Winkelmann, W.; Battmann, A.; Gosheger, G. The influence of elementary silver versus titanium on oste-oblasts behavior in vitro using human osteosarcoma cell lines. Sarcoma 2007, 2007, 26539. [Google Scholar] [CrossRef]
- Qin, H.; Cao, H.L.; Zhao, Y.C.; Zhu, C.; Cheng, T.; Wang, Q.J.; Peng, X.C.; Cheng, M.Q.; Wang, J.X.; Jin, G.D.; et al. In vitro and in vivo anti-biofilm effects of silver nanoparticles immobilized on titanium. Biomaterials 2021, 269, 120315. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Manfred, K. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef]
- Hamad, A.; Khashan, K.S.; Hadi, A. Silver Nanoparticles and Silver Ions as Potential Antibacterial Agents. J. Inorg. Organomet. Polym. 2020, 30, 4811–4828. [Google Scholar] [CrossRef]
- Liao, C.Z.; Li, Y.C.; Tiong, S. Bactericidal and Cytotoxic Properties of Silver Nanoparticles. Int. J. Mol. Sci. 2019, 20, 449. [Google Scholar] [CrossRef]
- Xing, J.H.; Xia, Z.B.; Hu, J.F.; Zhang, Y.H.; Zhong, L. Time dependence of growth and crystallization of anodic titanium oxide films in potentiostatic mode. Corros. Sci. 2013, 75, 212–219. [Google Scholar] [CrossRef]





| Sample | α-Ti/wt% | Ti2Ag/wt% | Rwp/% |
|---|---|---|---|
| AO-0 V | 96.6 ± 1.2 | 3.4 ± 0.2 | 8.26 |
| AO-20 V | 97.3 ± 0.9 | 2.7 ± 0.4 | 9.12 |
| AO-40 V | 96.1 ± 1.4 | 3.9 ± 0.3 | 9.36 |
| AO-60 V | 96.3 ± 1.1 | 3.7 ± 0.6 | 10.26 |
| AO-80 V | 97.6 ± 0.8 | 2.4 ± 0.4 | 12.42 |
| AO-120 V | 98.5 ± 0.6 | 1.5 ± 0.5 | 8.37 |
| Oxidation Potential | Point | Element (wt%) | σ | ||||
|---|---|---|---|---|---|---|---|
| Ti | O | Ag | Ti | O | Ag | ||
| AO-20 V | A | 79.0 ± 0.1 | 16.4 ± 0.1 | 4.6 ± 0.2 | 0.5 | 0.5 | 0.2 |
| B | 73.4 ± 0.2 | 22.4 ± 0.3 | 4.2 ± 0.1 | 0.2 | 0.2 | 0.1 | |
| AO-40 V | C | 75.5 ± 0.1 | 19.9 ± 0.1 | 4.6 ± 0.3 | 0.4 | 0.4 | 0.2 |
| D | 68.8 ± 0.3 | 22.0 ± 0.1 | 9.1 ± 0.1 | 0.4 | 0.5 | 0.2 | |
| AO-60 V | E | 73.0 ± 0.2 | 22.7 ± 0.2 | 4.3 ± 0.2 | 0.4 | 0.4 | 0.1 |
| F | 63.9 ± 0.3 | 28.3 ± 0.1 | 7.8 ± 0.1 | 0.3 | 0.4 | 0.3 | |
| AO-80 V | G | 64.9 ± 0.2 | 26.5 ± 0.3 | 4.1 ± 0.1 | 0.4 | 0.2 | 0.1 |
| H | 64.9 ± 0.1 | 27.2 ± 0.1 | 7.9 ± 0.3 | 0.4 | 0.4 | 0.2 | |
| AO-120 V | I | 64.0 ± 0.2 | 32.1 ± 0.2 | 3.8 ± 0.1 | 0.4 | 0.4 | 0.1 |
| J | 52.4 ± 0.1 | 45.0 ± 0.1 | 2.6 ± 0.1 | 0.3 | 0.3 | 0.1 | |
| Samples | Eocp/mV (vs. SCE) | Ecorr/mV (vs. SCE) | icorr/(10−8 A·cm−2) | Rp0/104 Ω·cm2 |
|---|---|---|---|---|
| AO-0 V | −244.138 | −220.091 ± 0.012 | 21.477 ± 0.031 | 1.55 ± 0.23 |
| AO-20 V | 107.621 | 17.035 ± 0.016 | 2.241 ± 0.028 | 33.3 ± 0.16 |
| AO-40 V | −82.112 | −115.281 ± 0.014 | 3.661 ± 0.015 | 19.8 ± 0.19 |
| AO-60 V | −99.991 | −94.963 ± 0.019 | 2.969 ± 0.032 | 18.2 ± 0.13 |
| AO-80 V | −115.687 | −136.163 ± 0.013 | 2.378 ± 0.016 | 12.6 ± 0.24 |
| AO-120 V | −106.042 | −164.884 ± 0.017 | 9.411 ± 0.022 | 20.2 ± 0.15 |
| Samples | RS/Ω·cm2 | QP/10−5 F·cm−2 | n | RP/104 Ω·cm2 |
|---|---|---|---|---|
| AO-0 V | 105.7 ± 0.6 | 32.4 ± 0.12 | 0.915 ± 0.005 | 7.81 ± 0.12 |
| AO-20 V | 158.2 ± 0.9 | 2.48 ± 0.07 | 0.889 ± 0.002 | 44.82 ± 0.09 |
| AO-40 V | 112.3 ± 0.5 | 2.59 ± 0.03 | 0.828 ± 0.003 | 30.12 ± 0.17 |
| AO-60 V | 145.4 ± 0.8 | 7.95 ± 0.03 | 0.700 ± 0.006 | 29.28 ± 0.15 |
| AO-80 V | 115.6 ± 0.9 | 5.91 ± 0.05 | 0.669 ± 0.004 | 28.73 ± 0.17 |
| AO-120 V | 149.9 ± 0.7 | 7.71 ± 0.06 | 0.649 ± 0.005 | 32.67 ± 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Z.; Yan, Y.; Shi, C.; Di, K.; Xu, J.; Liu, Q.; Mu, L.; Zheng, J.; Hu, J.; Zhang, E. Effect of Anodic Oxidation Pulse Voltage on Antibacterial Properties and Biocompatibility of Ti-Ag Alloy. Coatings 2024, 14, 405. https://doi.org/10.3390/coatings14040405
Ma Z, Yan Y, Shi C, Di K, Xu J, Liu Q, Mu L, Zheng J, Hu J, Zhang E. Effect of Anodic Oxidation Pulse Voltage on Antibacterial Properties and Biocompatibility of Ti-Ag Alloy. Coatings. 2024; 14(4):405. https://doi.org/10.3390/coatings14040405
Chicago/Turabian StyleMa, Zhen, Yudong Yan, Chang Shi, Kexin Di, Jianwei Xu, Qicong Liu, Liting Mu, Jianming Zheng, Jiali Hu, and Erlin Zhang. 2024. "Effect of Anodic Oxidation Pulse Voltage on Antibacterial Properties and Biocompatibility of Ti-Ag Alloy" Coatings 14, no. 4: 405. https://doi.org/10.3390/coatings14040405
APA StyleMa, Z., Yan, Y., Shi, C., Di, K., Xu, J., Liu, Q., Mu, L., Zheng, J., Hu, J., & Zhang, E. (2024). Effect of Anodic Oxidation Pulse Voltage on Antibacterial Properties and Biocompatibility of Ti-Ag Alloy. Coatings, 14(4), 405. https://doi.org/10.3390/coatings14040405





