Dual Strategy Based on Quantum Dot Doping and Phenylethylamine Iodide Surface Modification for High-Performance and Stable Perovskite Solar Cells
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
2.2. Synthesis of CsPbBr3 Perovskite QDs
2.3. Device Fabrication
2.4. Characterizations
3. Results and Discussion
3.1. Morphology and Optical Properties of CsPbBr3 QDs
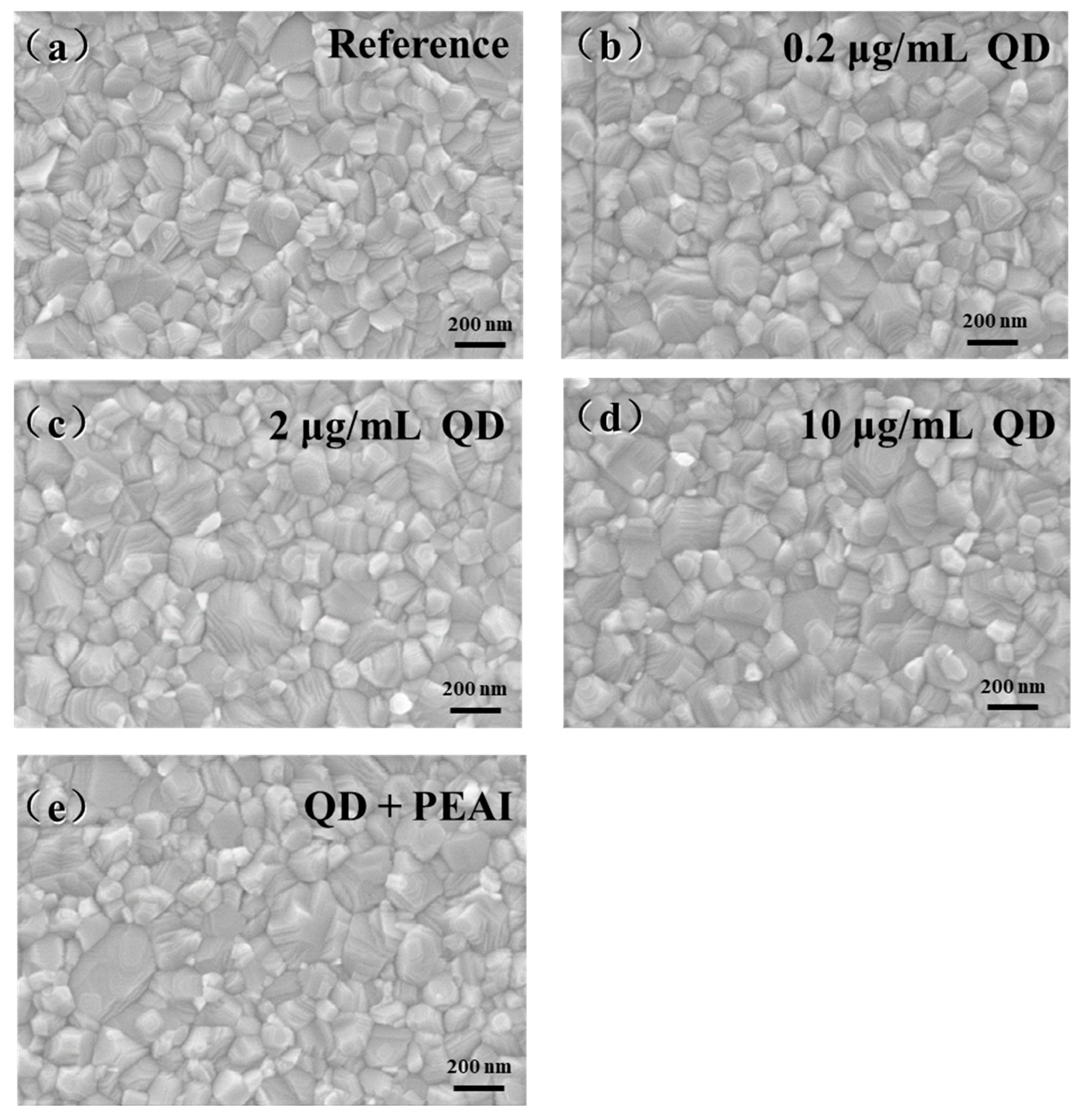
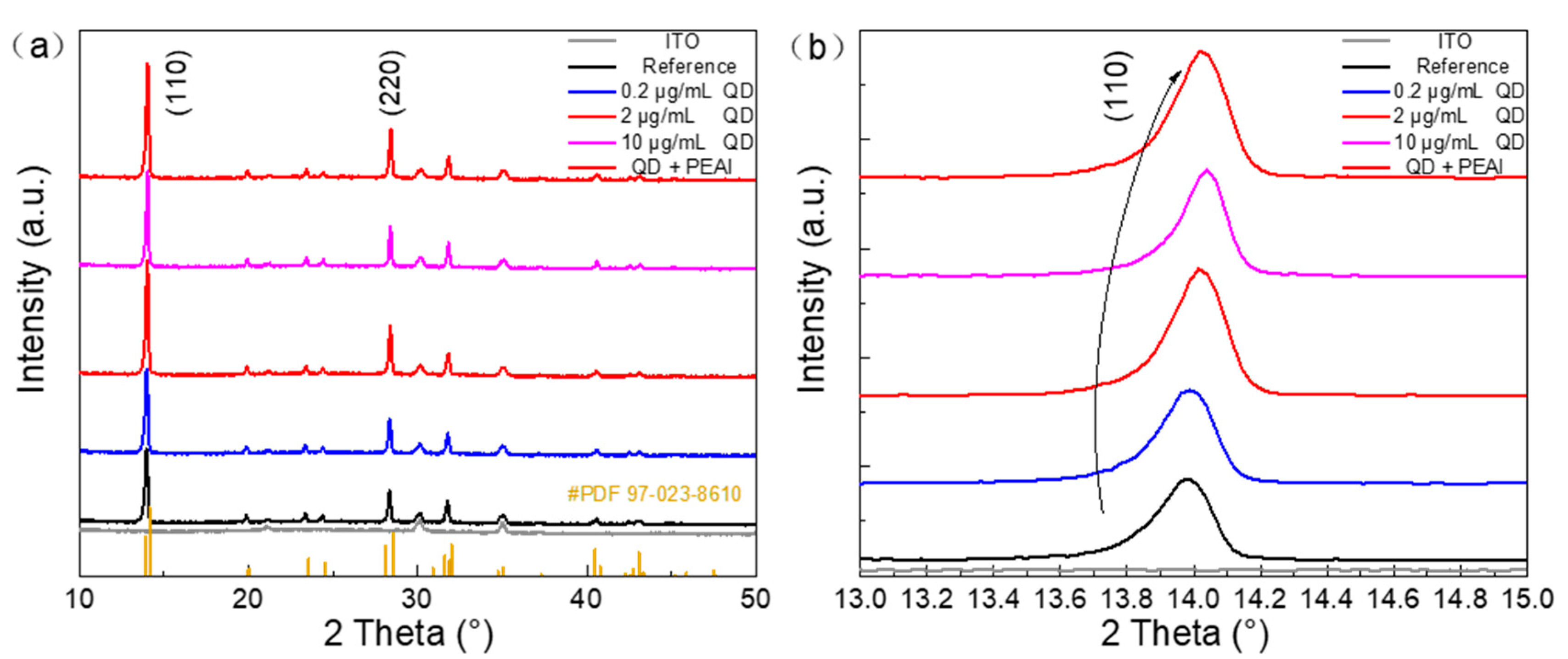
3.2. Effect of CsPbBr3 QDs Doping and PEAI Surface Modification on the Morphology and Crystallization of PFs
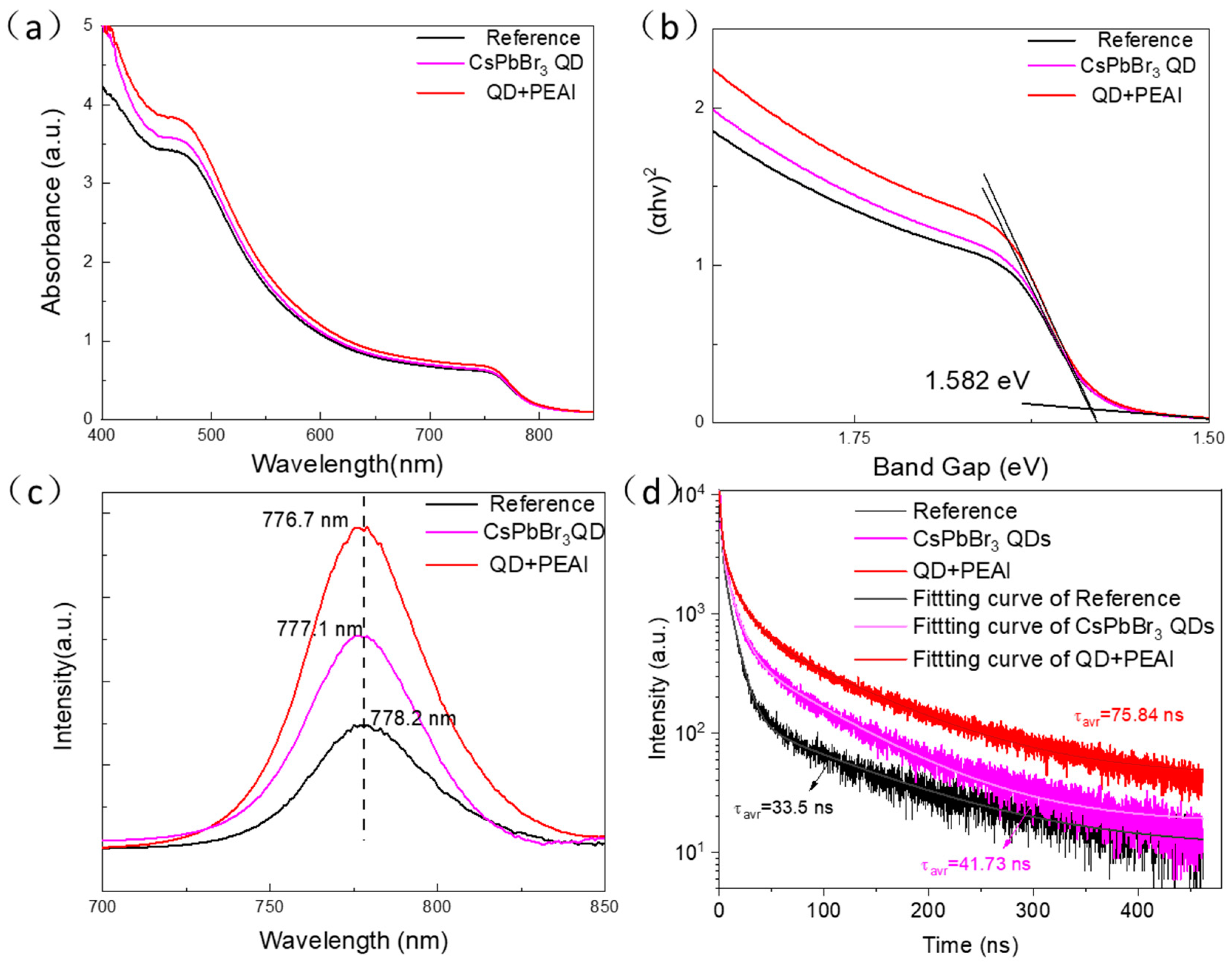
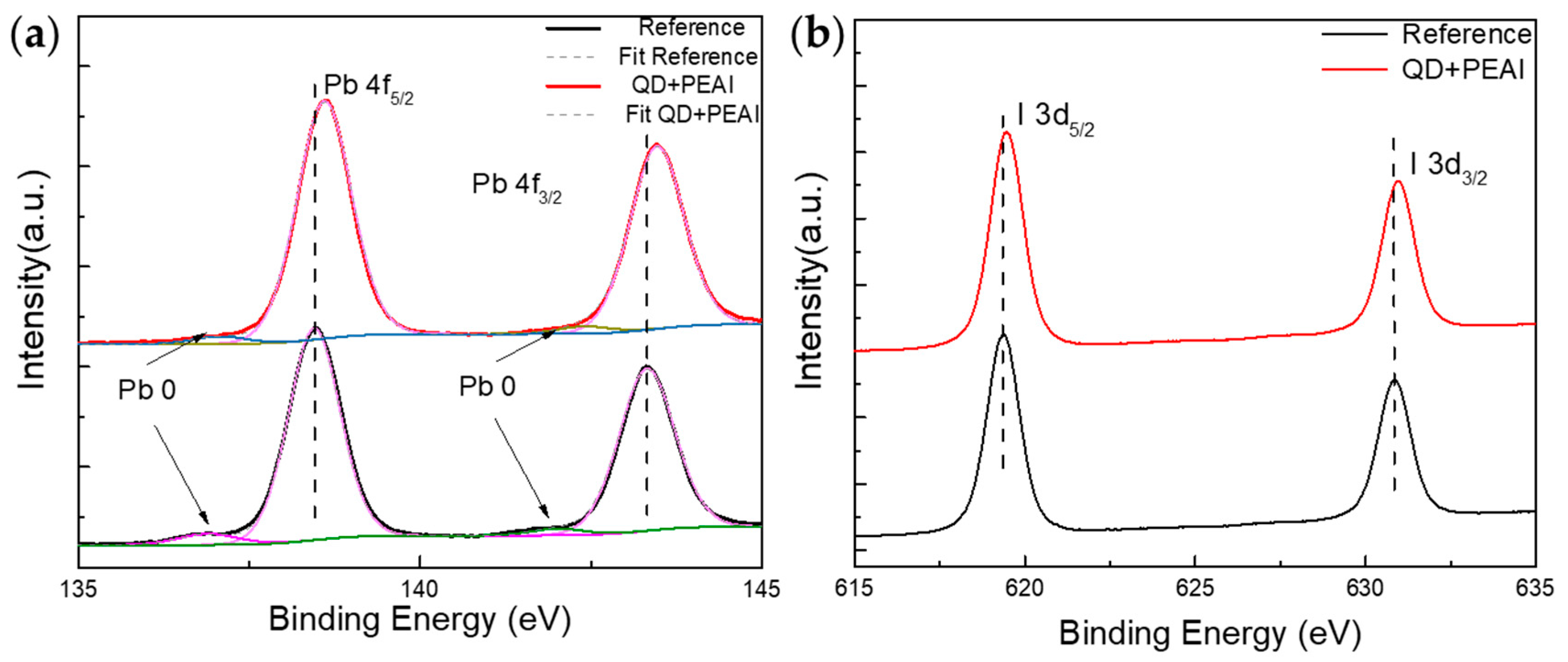
3.3. Effect of Joint Modification of CsPbBr3 QDs Combined with PEAI on Optical Properties as Well as Binding Energy of PFs
3.4. Photovoltaic Properties of PSCs Jointly Modified by CsPbBr3 QDs and PEAI
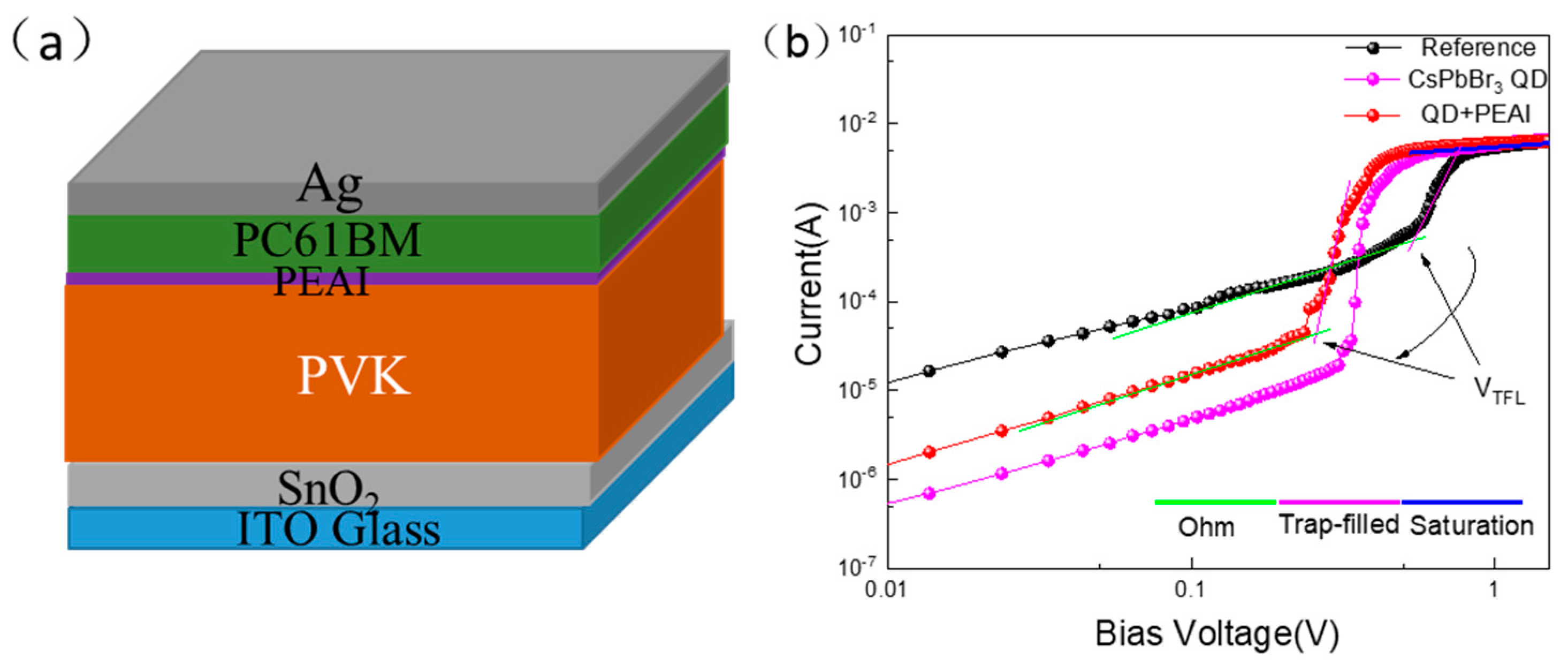
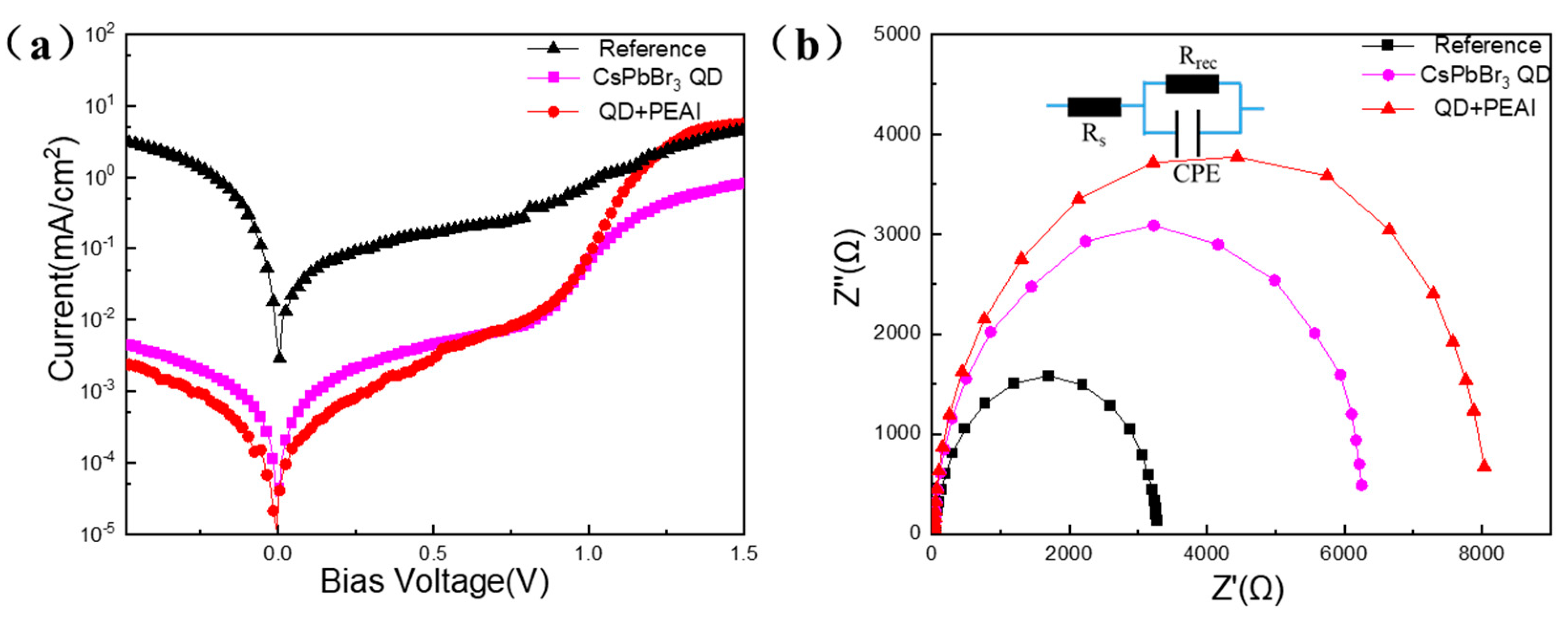
3.5. Stabilities of PSCs Jointly Modified by CsPbBr3 QDs and PEAI
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.S.; Park, B.-W.; Jung, E.H.; Jeon, N.J.; Kim, Y.C.; Lee, D.U.; Shin, S.S.; Seo, J.; Kim, E.K.; Noh, J.H.; et al. Iodide Management in Formamidinium-Lead-Halide–Based Perovskite Layers for Efficient Solar Cells. Science 2017, 356, 1376–1379. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; García De Arquer, F.P.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and Stable Solution-Processed Planar Perovskite Solar Cells via Contact Passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef] [PubMed]
- Jeon, N.J.; Noh, J.H.; Yang, W.S.; Kim, Y.C.; Ryu, S.; Seo, J.; Seok, S.I. Compositional Engineering of Perovskite Materials for High-Performance Solar Cells. Nature 2015, 517, 476–480. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Calió, L.; Kazim, S.; Grätzel, M.; Ahmad, S. Hole-Transport Materials for Perovskite Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 14522–14545. [Google Scholar] [CrossRef]
- Kim, M.; Kim, G.-H.; Lee, T.K.; Choi, I.W.; Choi, H.W.; Jo, Y.; Yoon, Y.J.; Kim, J.W.; Lee, J.; Huh, D.; et al. Methylammonium Chloride Induces Intermediate Phase Stabilization for Efficient Perovskite Solar Cells. Joule 2019, 3, 2179–2192. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient Perovskite Solar Cells via Improved Carrier Management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Li, T.; Mao, K.; Meng, H.; Zhu, Z.; Peng, W.; Yuan, S.; Xu, J.; Feng, X.; Xu, Z.; Xu, J. Understanding the Interfacial Reactions and Band Alignment for Efficient and Stable Perovskite Solar Cells Built on Metal Substrates with Reduced Upscaling Losses. Adv. Mater. 2023, 35, 2211959. [Google Scholar] [CrossRef]
- Jung, E.H.; Jeon, N.J.; Park, E.Y.; Moon, C.S.; Shin, T.J.; Yang, T.-Y.; Noh, J.H.; Seo, J. Efficient, Stable and Scalable Perovskite Solar Cells Using Poly(3-Hexylthiophene). Nature 2019, 567, 511–515. [Google Scholar] [CrossRef]
- Bu, T.; Ono, L.K.; Li, J.; Su, J.; Tong, G.; Zhang, W.; Liu, Y.; Zhang, J.; Chang, J.; Kazaoui, S.; et al. Modulating Crystal Growth of Formamidinium–Caesium Perovskites for over 200 cm2 Photovoltaic Sub-Modules. Nat. Energy 2022, 7, 528–536. [Google Scholar] [CrossRef]
- Wu, G.; Liang, R.; Ge, M.; Sun, G.; Zhang, Y.; Xing, G. Surface Passivation Using 2D Perovskites toward Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2022, 34, 2105635. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, J.; Yun, H.-S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled Growth of Perovskite Layers with Volatile Alkylammonium Chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef]
- Zhou, N.; Shen, Y.; Li, L.; Tan, S.; Liu, N.; Zheng, G.; Chen, Q.; Zhou, H. Exploration of Crystallization Kinetics in Quasi Two-Dimensional Perovskite and High Performance Solar Cells. J. Am. Chem. Soc. 2018, 140, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Xie, F.; Chen, H.; Yang, X.; Ye, F.; Bi, E.; Wu, Y.; Cai, M.; Han, L. Annealing-Free Perovskite Films by Instant Crystallization for Efficient Solar Cells. J. Mater. Chem. A 2016, 4, 8548–8553. [Google Scholar] [CrossRef]
- Ahn, N.; Son, D.-Y.; Jang, I.-H.; Kang, S.M.; Choi, M.; Park, N.-G. Highly Reproducible Perovskite Solar Cells with Average Efficiency of 18.3% and Best Efficiency of 19.7% Fabricated via Lewis Base Adduct of Lead(II) Iodide. J. Am. Chem. Soc. 2015, 137, 8696–8699. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, S.; Lei, L.; Cao, Q.; Shao, J.; Zhang, S.; Liu, Y. Ultrasmooth Perovskite Film via Mixed Anti-Solvent Strategy with Improved Efficiency. ACS Appl. Mater. Interfaces 2017, 9, 3667–3676. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Johnston, M.B.; Snaith, H.J. Efficient Planar Heterojunction Perovskite Solar Cells by Vapour Deposition. Nature 2013, 501, 395–398. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, C.; Wang, S.; Yan, Y.; Feng, Y.; Bian, J.; Shi, Y. Insight into the Interfacial Elastic Contact in Stacking Perovskite Solar Cells. Adv. Mater. Interfaces 2019, 6, 1900157. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, Y.; Lu, H.; Chen, C.; Liu, Y.; Bai, X.; Yang, L.; Yu, W.W.; Dai, Q.; Zhang, Y. CsPbBr3 Perovskite Nanoparticles as Additive for Environmentally Stable Perovskite Solar Cells with 20.46% Efficiency. Nano Energy 2019, 59, 517–526. [Google Scholar] [CrossRef]
- Tian, Q.; Ding, G.; Cai, Y.; Li, Z.; Tang, X.; Xie, R.-J.; Gao, P. Enhanced Performance of Perovskite Solar Cells Loaded with Iodine-Rich CsPbI3 Quantum Dots. ACS Appl. Energy Mater. 2021, 4, 7535–7543. [Google Scholar] [CrossRef]
- Zhang, Q.; Zheng, W.; Wan, Q.; Liu, M.; Feng, X.; Kong, L.; Li, L. Confined Synthesis of Stable and Uniform CsPbBr3 Nanocrystals with High Quantum Yield up to 90% by High Temperature Solid-State Reaction. Adv. Opt. Mater. 2021, 9, 2002130. [Google Scholar] [CrossRef]
- Yao, Y.; Hang, P.; Wang, P.; Xu, L.; Cui, C.; Xie, J.; Xiao, K.; Li, G.; Lin, P.; Liu, S.; et al. CsPbBr3 Quantum Dots Assisted Crystallization of Solution-Processed Perovskite Films with Preferential Orientation for High Performance Perovskite Solar Cells. Nanotechnology 2020, 31, 085401. [Google Scholar] [CrossRef] [PubMed]
- Xiang, W. A Review on the Stability of Inorganic Metal Halide Perovskites: Challenges and Opportunities for Stable Solar Cells. Environ. Sci. 2021, 14, 2090–2113. [Google Scholar] [CrossRef]
- Ma, X.; Chen, M.; Zheng, Z.; Bullen, D.; Wang, J.; Harrison, C.; Gratz, E.; Lin, Y.; Yang, Z.; Zhang, Y.; et al. Recycled Cathode Materials Enabled Superior Performance for Lithium-Ion Batteries. Joule 2021, 5, 2955–2970. [Google Scholar] [CrossRef]
- Li, F.; Deng, X.; Qi, F.; Li, Z.; Liu, D.; Shen, D.; Qin, M.; Wu, S.; Lin, F.; Jang, S.-H.; et al. Regulating Surface Termination for Efficient Inverted Perovskite Solar Cells with Greater Than 23% Efficiency. J. Am. Chem. Soc. 2020, 142, 20134–20142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Guo, T.; Yuan, H.; Yu, L.; Zhao, R.; Deng, Z.; Zhang, J.; Liu, X.; Hu, Z.; Zhu, Y. Reconstruction of the (EMIm)xMA1−xPb[(BF4)xI1−x]3 Interlayer for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2021, 13, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Dou, J.; Kou, S.; Dang, J.; Ji, Y.; Yang, G.; Wu, W.; Kuang, D.; Wang, M. Multifunctional Phosphorus-Containing Lewis Acid and Base Passivation Enabling Efficient and Moisture-Stable Perovskite Solar Cells. Adv. Funct. Mater. 2020, 30, 1910710. [Google Scholar] [CrossRef]
- Khan, J.; Zhang, X.; Yuan, J.; Wang, Y.; Shi, G.; Patterson, R.; Shi, J.; Ling, X.; Hu, L.; Wu, T.; et al. Tuning the Surface-Passivating Ligand Anchoring Position Enables Phase Robustness in CsPbI3 Perovskite Quantum Dot Solar Cells. ACS Energy Lett. 2020, 5, 3322–3329. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, J.; Zhang, J.; Huang, S.; Ou-Yang, W.; Bao, Q.; Sun, Z.; Chen, X. High Efficiency and Stability of Inverted Perovskite Solar Cells Using Phenethyl Ammonium Iodide-Modified Interface of NiOx and Perovskite Layers. ACS Appl. Mater. Interfaces 2020, 12, 771–779. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhao, Y.; Zhang, X.; Yang, X.; Chen, Y.; Chu, Z.; Ye, Q.; Li, X.; Yin, Z.; You, J. Surface Passivation of Perovskite Film for Efficient Solar Cells. Nat. Photonics 2019, 13, 460–466. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum Dot Light-Emitting Diodes Based on Inorganic Perovskite Cesium Lead Halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef]
- Bag, S.; Durstock, M.F. Large Perovskite Grain Growth in Low-Temperature Solution-Processed Planar p-i-n Solar Cells by Sodium Addition. ACS Appl. Mater. Interfaces 2016, 8, 5053–5057. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Xu, Y.; Wang, S.; Xia, C.; Liu, Y.; Yu, B.; Xuan, T.; Li, H. Zinc Ions Doped Cesium Lead Bromide Perovskite Nanocrystals with Enhanced Efficiency and Stability for White Light-Emitting Diodes. J. Alloys Compd. 2021, 866, 158969. [Google Scholar] [CrossRef]
- Ran, C.; Xi, J.; Gao, W.; Yuan, F.; Lei, T.; Jiao, B.; Hou, X.; Wu, Z. Bilateral Interface Engineering toward Efficient 2D–3D Bulk Heterojunction Tin Halide Lead-Free Perovskite Solar Cells. ACS Energy Lett. 2018, 3, 713–721. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, H.; Zhou, W.; Yang, D.; Shang, Y.; Shi, Z.; Li, B.; Jiang, X.; Zhang, L.; Quan, L.N.; et al. Highly Oriented Low-Dimensional Tin Halide Perovskites with Enhanced Stability and Photovoltaic Performance. J. Am. Chem. Soc. 2017, 139, 6693–6699. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhu, T.; Wu, H.; Liu, L.; Gong, X. Poly(Ethylene Glycol) Diacrylate as the Passivation Layer for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 45045–45055. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Xiao, Z.; Bi, C.; Yuan, Y.; Huang, J. Origin and Elimination of Photocurrent Hysteresis by Fullerene Passivation in CH3NH3PbI3 Planar Heterojunction Solar Cells. Nat. Commun. 2014, 5, 5784. [Google Scholar] [CrossRef]
- Pu, Y.-C.; Fan, H.-C.; Liu, T.-W.; Chen, J.-W. Methylamine Lead Bromide Perovskite/Protonated Graphitic Carbon Nitride Nanocomposites: Interfacial Charge Carrier Dynamics and Photocatalysis. J. Mater. Chem. A 2017, 5, 25438–25449. [Google Scholar] [CrossRef]
- Ren, M.; Cao, S.; Zhao, J.; Zou, B.; Zeng, R. Advances and Challenges in Two-Dimensional Organic–Inorganic Hybrid Perovskites Toward High-Performance Light-Emitting Diodes. Nano-Micro Lett. 2021, 13, 163. [Google Scholar] [CrossRef]
- Xu, L.; Liu, G.; Xiang, H.; Wang, R.; Shan, Q.; Yuan, S.; Cai, B.; Li, Z.; Li, W.; Zhang, S.; et al. Charge-Carrier Dynamics and Regulation Strategies in Perovskite Light-Emitting Diodes: From Materials to Devices. Appl. Phys. Rev. 2022, 9, 021308. [Google Scholar] [CrossRef]
- Fu, S.; Li, X.; Wan, L.; Wu, Y.; Zhang, W.; Wang, Y.; Bao, Q.; Fang, J. Efficient Passivation with Lead Pyridine-2-Carboxylic for High-Performance and Stable Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1901852. [Google Scholar] [CrossRef]
- Wieliczka, B.M.; Márquez, J.A.; Bothwell, A.M.; Zhao, Q.; Moot, T.; VanSant, K.T.; Ferguson, A.J.; Unold, T.; Kuciauskas, D.; Luther, J.M. Probing the Origin of the Open Circuit Voltage in Perovskite Quantum Dot Photovoltaics. ACS Nano 2021, 15, 19334–19344. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Gan, X.; Yu, L.; Yuan, H.; Shang, M.; Lu, C.; Hou, D.; Hu, Z.; Zhu, Y.; et al. Pb-Reduced CsPb0.9Zn0.1I2 Br Thin Films for Efficient Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1900896. [Google Scholar] [CrossRef]
- Guo, H.; Pei, Y.; Zhang, J.; Cai, C.; Zhou, K.; Zhu, Y. Doping with SnBr2 in CsPbBr3 to Enhance the Efficiency of All-Inorganic Perovskite Solar Cells. J. Mater. Chem. C 2019, 7, 11234–11243. [Google Scholar] [CrossRef]
- Chen, W.; Wu, Y.; Yue, Y.; Liu, J.; Zhang, W.; Yang, X.; Chen, H.; Bi, E.; Ashraful, I.; Grätzel, M.; et al. Efficient and Stable Large-Area Perovskite Solar Cells with Inorganic Charge Extraction Layers. Science 2015, 350, 944–948. [Google Scholar] [CrossRef]
- Varache, R.; Leendertz, C.; Gueunier-Farret, M.E.; Haschke, J.; Muñoz, D.; Korte, L. Investigation of Selective Junctions Using a Newly Developed Tunnel Current Model for Solar Cell Applications. Sol. Energy Mater. Sol. Cells 2015, 141, 14–23. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Guo, X.; Lu, C.; Wei, J.; Fang, J. Constructing Heterojunctions by Surface Sulfidation for Efficient Inverted Perovskite Solar Cells. Science 2022, 375, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wang, J.; Mak, C.H.; Du, M.; Li, F.-F.; Shen, H.-H.; Santoso, S.P.; Yu, E.T.; Yu, X.; Chu, P.K.; et al. Optimizing Black Phosphorus/Halide Perovskite Compositions by Scanning Photoelectrochemical Microscopy. J. Electrochem. Soc. 2022, 169, 096510. [Google Scholar] [CrossRef]
- Feng, Z.; Weng, C.; Hua, Y.; Chen, X.; Huang, S. Efficient and Stable Perovskite Solar Cells: The Effect of Octadecyl Ammonium Compound Side-Chain. Chem. Eng. J. 2023, 475, 146498. [Google Scholar] [CrossRef]
- Lu, R.; Liu, Y.; Zhang, J.; Zhao, D.; Guo, X.; Li, C. Highly Efficient (200) Oriented MAPbI3 Perovskite Solar Cells. Chem. Eng. J. 2022, 433, 133845. [Google Scholar] [CrossRef]
- Kim, T.W.; Uchida, S.; Kim, M.; Cho, S.G.; Kim, S.J.; Kondo, T.; Segawa, H. Phase Control of Organometal Halide Perovskites for Development of Highly Efficient Solar Cells. ACS Appl. Mater. Interfaces 2023, 15, 21974–21981. Available online: https://pubs.acs.org/doi/10.1021/acsami.2c22769 (accessed on 24 March 2024). [CrossRef] [PubMed]
- Feng, Z.; Wu, Z.; Hua, Y.; Weng, C.; Chen, X.; Huang, S. Azadipyrromethene Dye-Assisted Defect Passivation for Efficient and Stable Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2022, 14, 14388–14399. Available online: https://pubs.acs.org/doi/full/10.1021/acsami.1c20923 (accessed on 24 March 2024). [CrossRef] [PubMed]
- Nguyen, M.H.; Kim, K.-S. Controlling the Grain Formation Process with Oleylamine and 4-Dimethylaminopyridine Additives for Efficient and Stable MAPbI3 Solar Cells. Mater. Today Chem. 2023, 34, 101799. [Google Scholar] [CrossRef]
- Hua, Y.; Feng, Z.; Weng, C.; Chen, X.; Huang, S. Reinforcing the Efficiency and Stability of Perovskite Solar Cells Using a Cesium Sulfate Additive. J. Mater. Sci. Mater. Electron. 2023, 34, 571. Available online: https://link.springer.com/article/10.1007/s10854-023-10008-6 (accessed on 24 March 2024). [CrossRef]


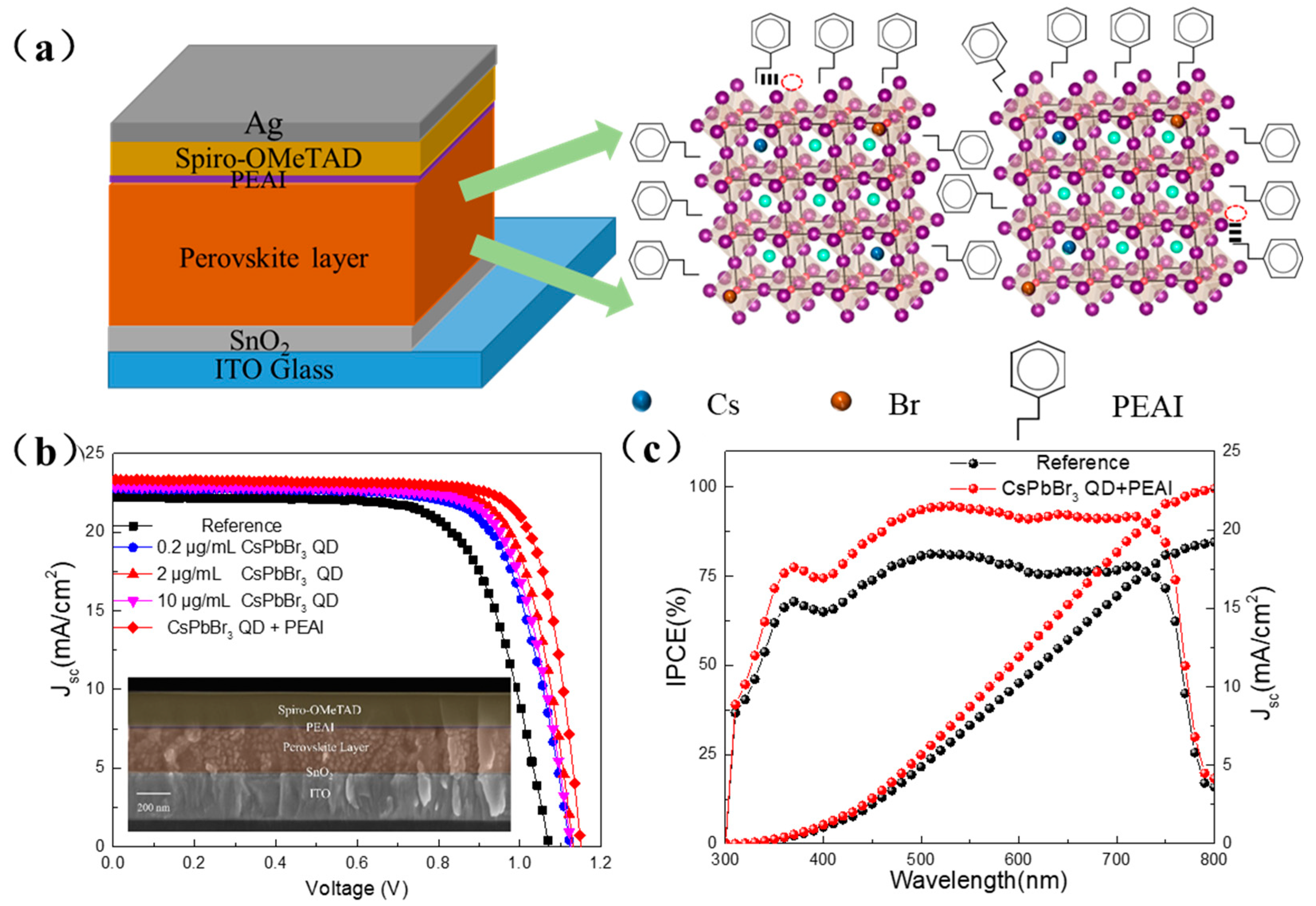

| Sample | Voc (V) | Jsc (mA/cm2) | FF (%) | PCE (%) |
|---|---|---|---|---|
| Reference | Champion 1.07 Average 1.06 ± 0.03 | 22.4 21.9 ± 0.82 | 71.6 70.1 ± 1.7 | 17.21 16.3 ± 1.1 |
| 0.2 μg/mL CsPbBr3 QDs | Champion 1.11 Average 1.09 ± 0.02 | 22.7 22.4 ± 0.52 | 72.3 70.9 ± 1.7 | 18.22 17.6 ± 0.6 |
| 2 μg/mL CsPbBr3 QDs | Champion 1.13 Average 1.11 ± 0.02 | 23.2 22.8 ± 0.55 | 76.6 75.8 ± 1.2 | 20.08 19.7 ± 0.5 |
| 10 μg/mL CsPbBr3 QDs | Champion 1.12 Average 1.11 ± 0.02 | 22.8 22.5 ± 0.47 | 73.1 72.2 ± 1.2 | 18.67 18.1 ± 0.6 |
| CsPbBr3 QDs +PEAI | Champion 1.15 Average 1.14 ± 0.01 | 23.3 22.9 ± 0.56 | 78.6 77.2 ± 1.3 | 21.04 20.3 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Chen, R.; Qu, M.; Long, B.; He, N.; Huang, S.; Chen, X.; Li, H.; Xuan, T. Dual Strategy Based on Quantum Dot Doping and Phenylethylamine Iodide Surface Modification for High-Performance and Stable Perovskite Solar Cells. Coatings 2024, 14, 409. https://doi.org/10.3390/coatings14040409
Zhang S, Chen R, Qu M, Long B, He N, Huang S, Chen X, Li H, Xuan T. Dual Strategy Based on Quantum Dot Doping and Phenylethylamine Iodide Surface Modification for High-Performance and Stable Perovskite Solar Cells. Coatings. 2024; 14(4):409. https://doi.org/10.3390/coatings14040409
Chicago/Turabian StyleZhang, Shulan, Renjie Chen, Mujing Qu, Biyu Long, Nannan He, Sumei Huang, Xiaohong Chen, Huili Li, and Tongtong Xuan. 2024. "Dual Strategy Based on Quantum Dot Doping and Phenylethylamine Iodide Surface Modification for High-Performance and Stable Perovskite Solar Cells" Coatings 14, no. 4: 409. https://doi.org/10.3390/coatings14040409
APA StyleZhang, S., Chen, R., Qu, M., Long, B., He, N., Huang, S., Chen, X., Li, H., & Xuan, T. (2024). Dual Strategy Based on Quantum Dot Doping and Phenylethylamine Iodide Surface Modification for High-Performance and Stable Perovskite Solar Cells. Coatings, 14(4), 409. https://doi.org/10.3390/coatings14040409







