Aging Behavior of Polyethylene and Ceramics-Coated Separators under the Simulated Lithium-Ion Battery Service Compression and Temperature Field
Abstract
:1. Introduction
2. Materials and Experimental Procedure
2.1. Materials
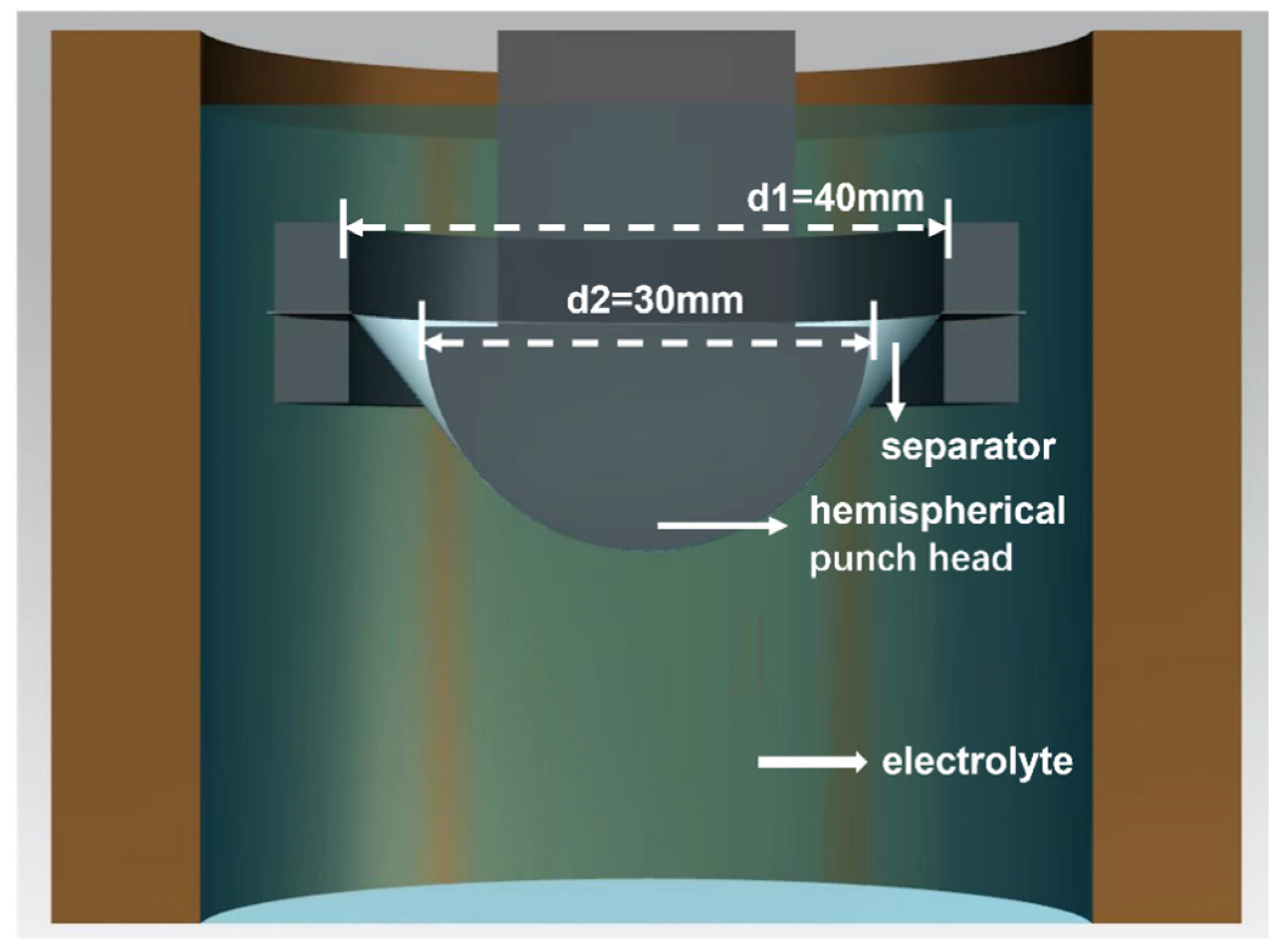
2.2. Experimental Setup and Sample Preparation
2.3. Testing and Characterization Methods
3. Results and Discussion
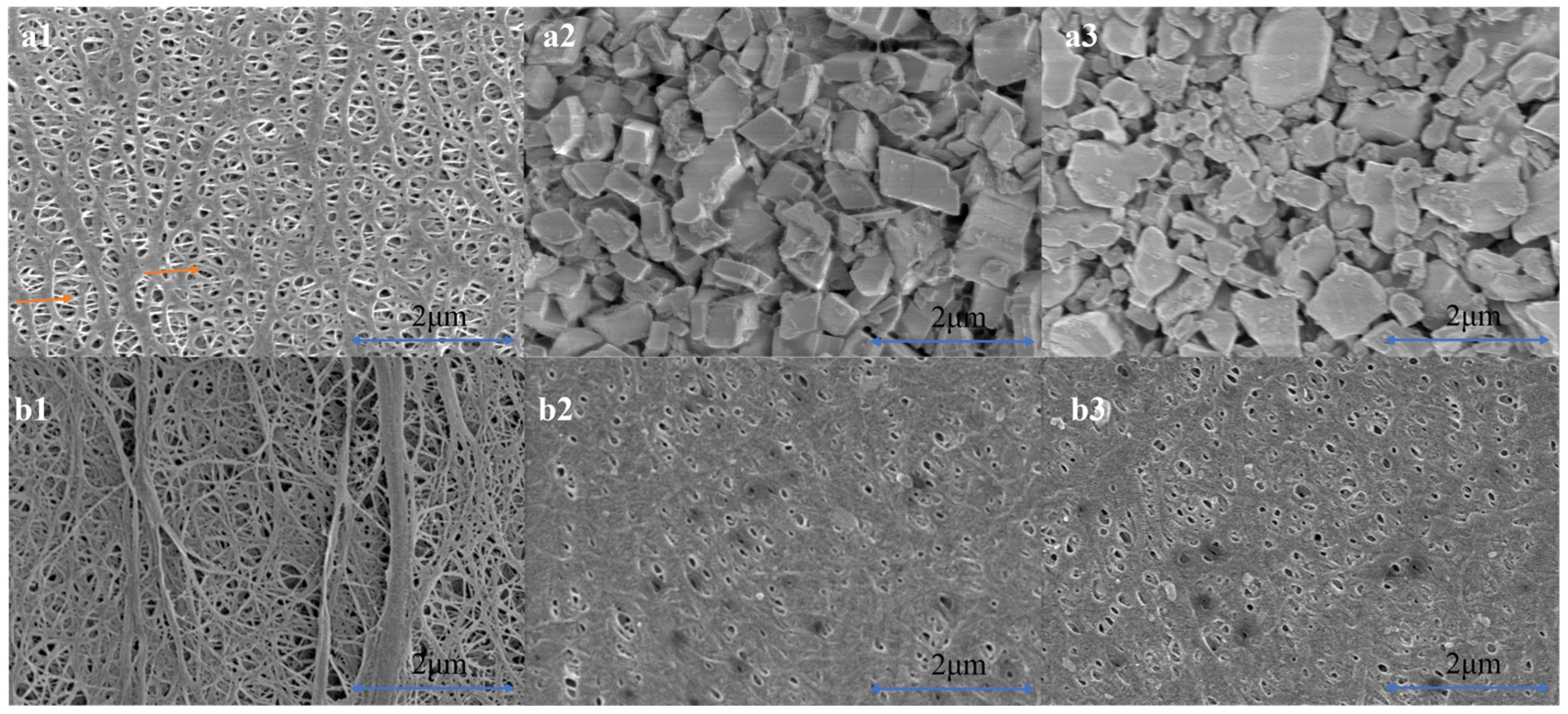
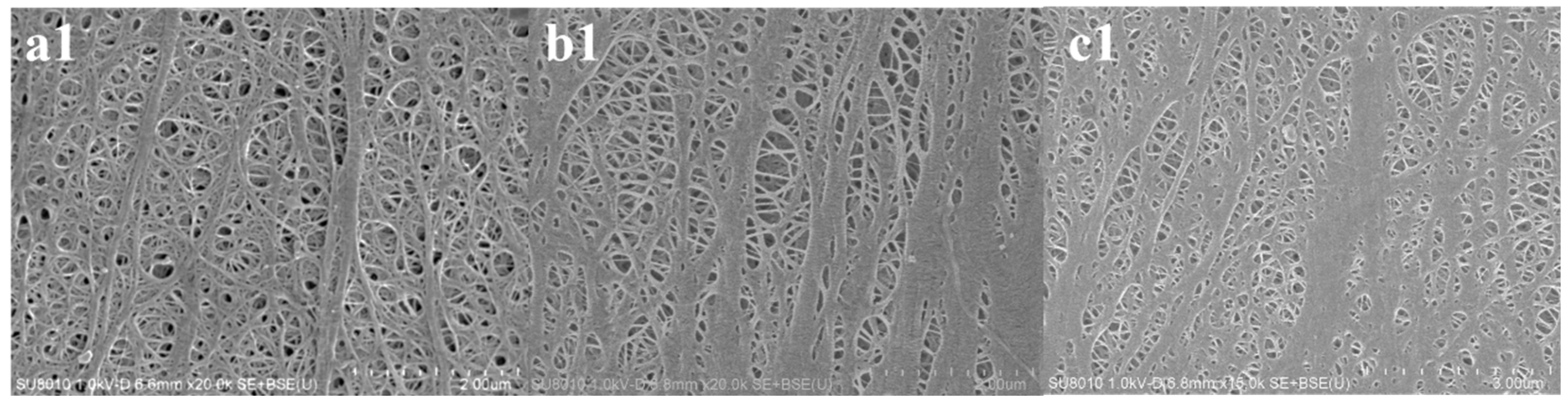
3.1. Microstructure of Three Separators before and after Cyclic Compression under 60 °C
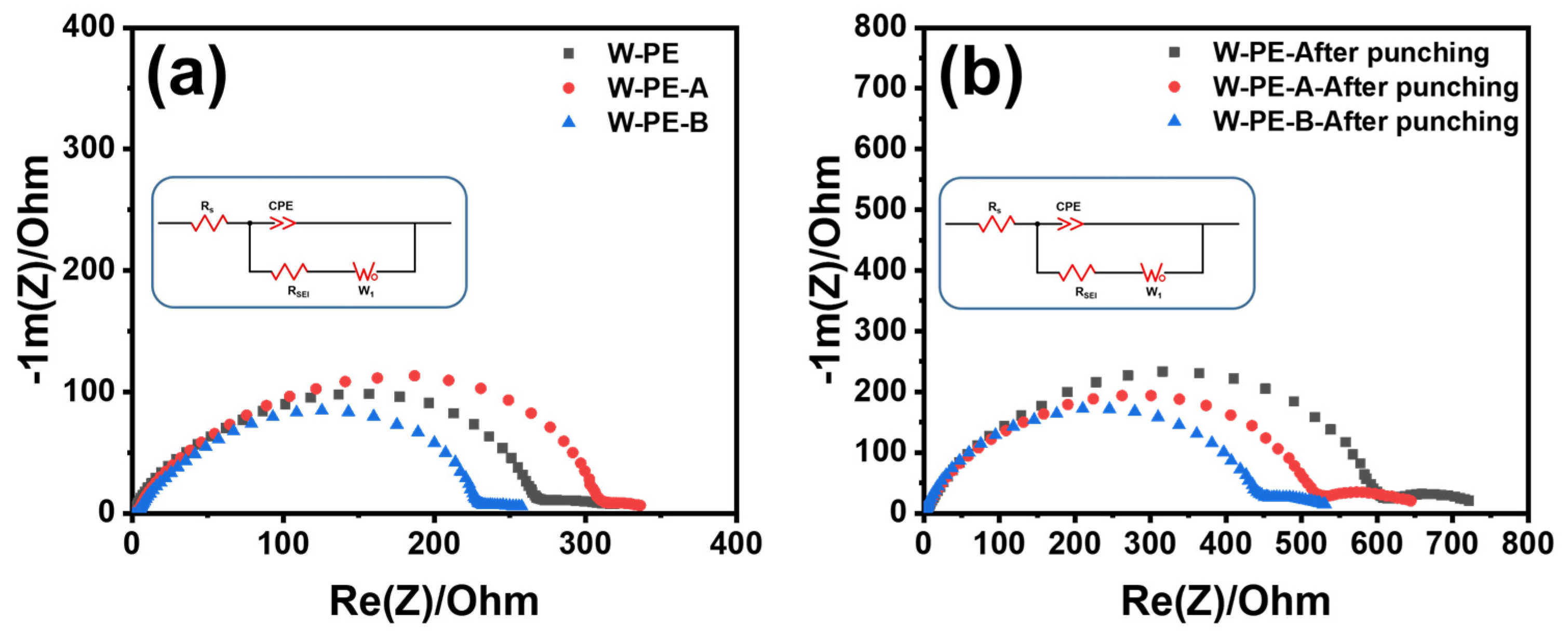
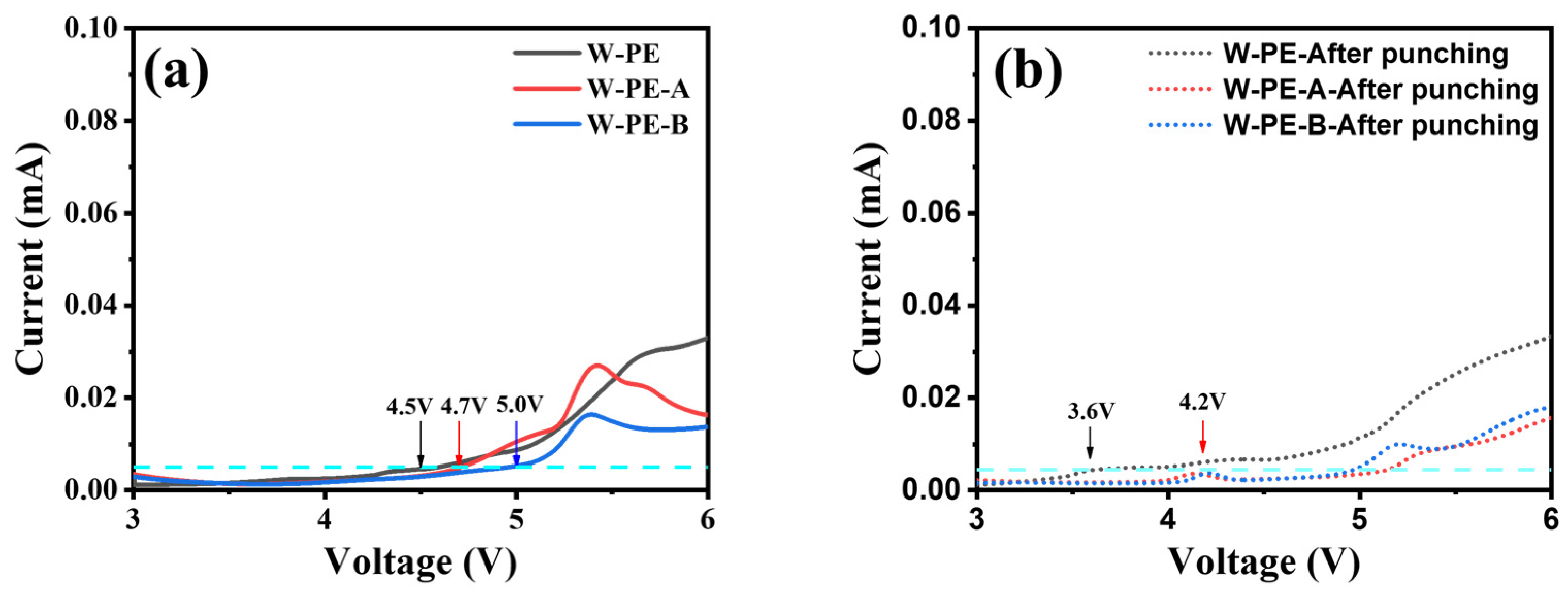
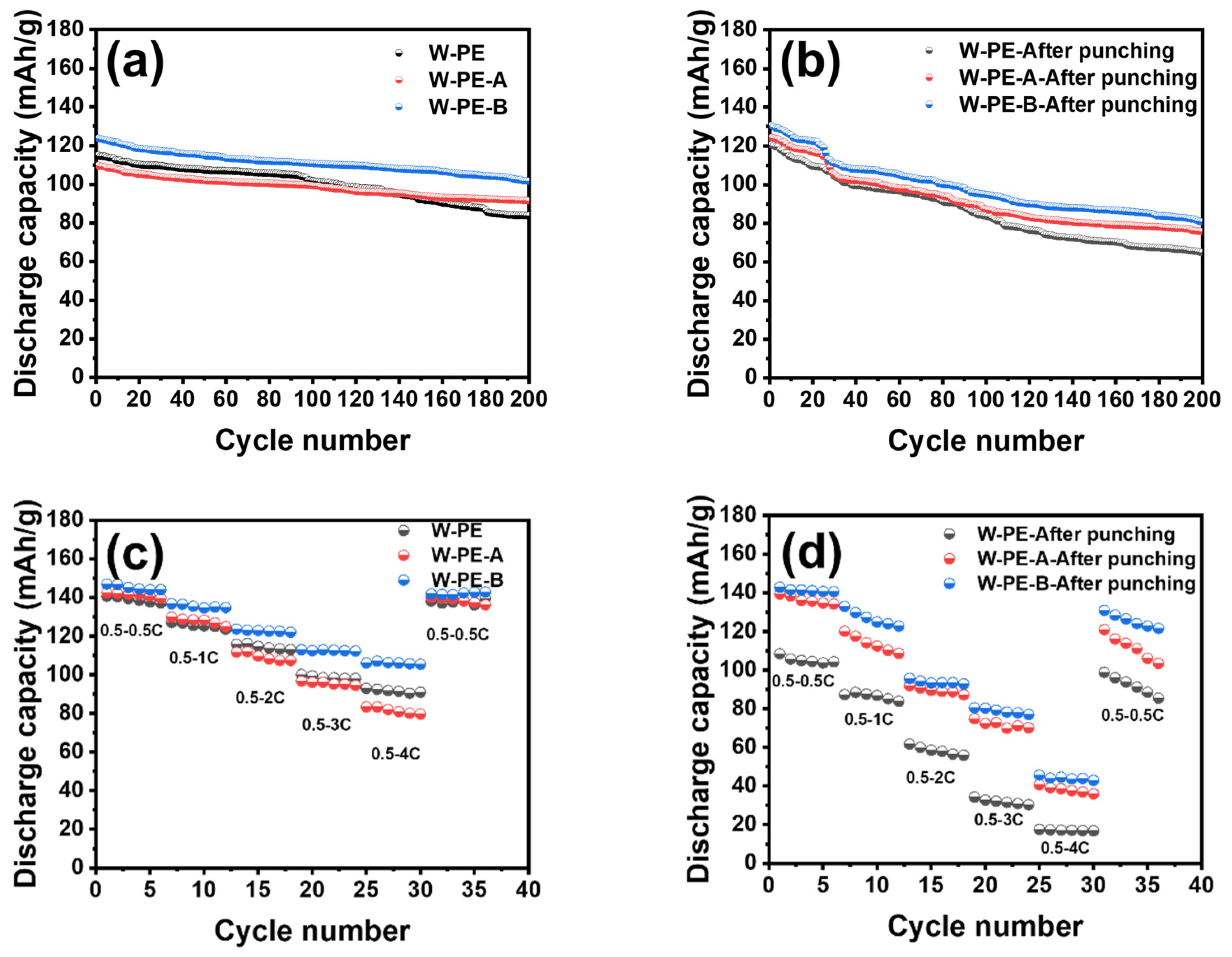
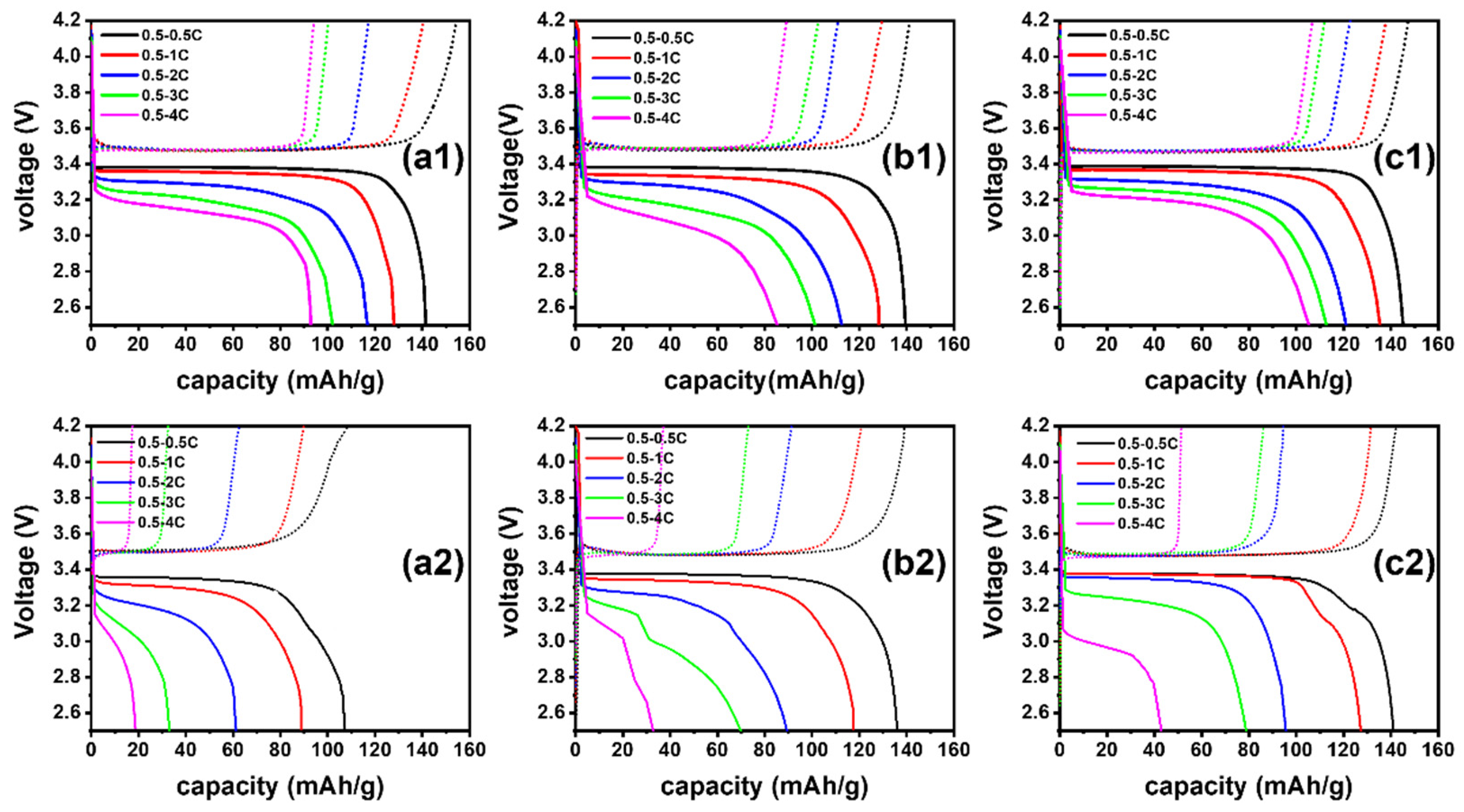
3.2. Electrochemical Performance of Three Separators before and after Cyclic Compression under 60 °C
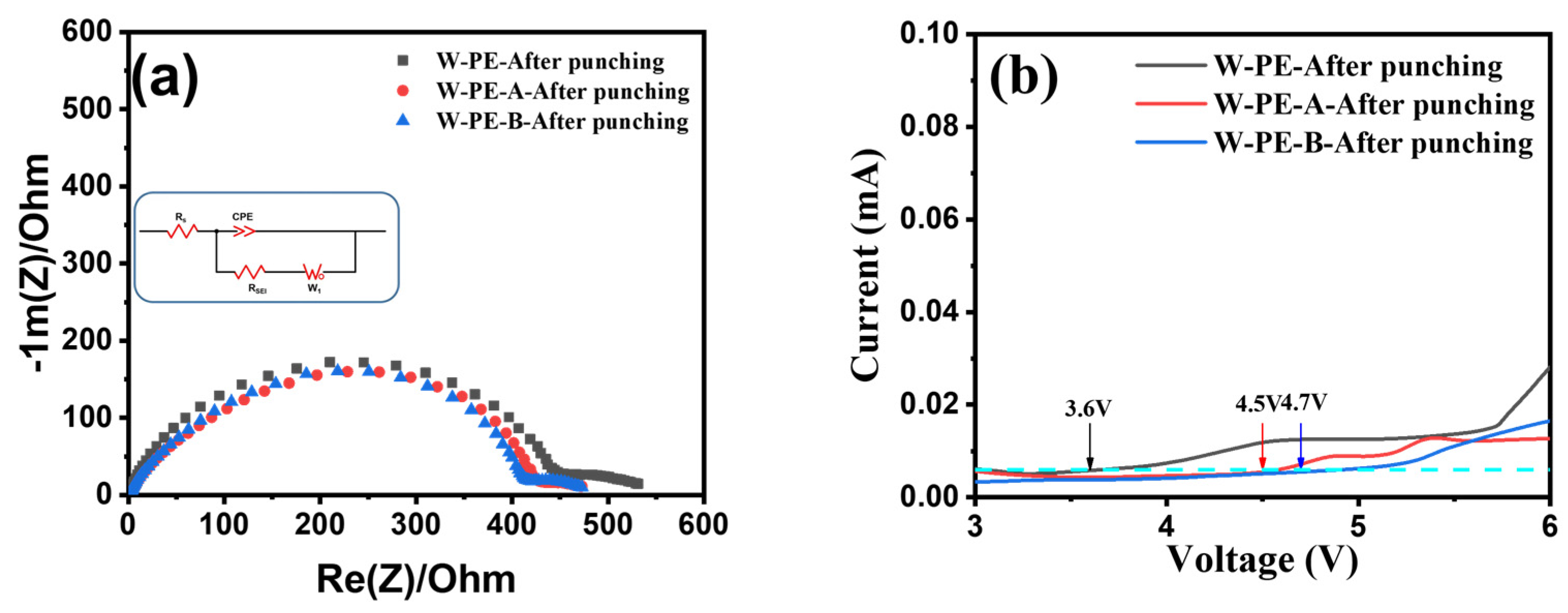
3.3. Microstructure and Electrochemical Performance of Three Separators before and after Cyclic Compression under Room Temperature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, J.; Ouyang, D. Progress of Chinese electric vehicles industrialization in 2015: A review. Appl. Energy 2017, 188, 529–546. [Google Scholar] [CrossRef]
- Zeng, X.; Li, M.; Abd El-Hady, D.; Alshitari, W.; Al-Bogami, A.S.; Lu, J.; Amine, K. Commercialization of Lithium Battery Technologies for Electric Vehicles. Adv. Energy Mater. 2019, 9, 1900161. [Google Scholar] [CrossRef]
- Wu, W.; Xiao, X.; Huang, X.; Yan, S. A multiphysics model for the in situ stress analysis of the separator in a lithium-ion battery cell. Comput. Mater. Sci. 2014, 83, 127–136. [Google Scholar] [CrossRef]
- Kriston, A.; Adanouj, I.; Ruiz, V.; Pfrang, A. Quantification and simulation of thermal decomposition reactions of Li-ion battery materials by simultaneous thermal analysis coupled with gas analysis. J. Power Sources 2019, 435, 226774. [Google Scholar] [CrossRef]
- Wang, L.; Menakath, A.; Han, F.; Wang, Y.; Zavalij, P.Y.; Gaskell, K.J.; Borodin, O.; Iuga, D.; Brown, S.P.; Wang, C.; et al. Identifying the components of the solid-electrolyte interphase in Li-ion batteries. Nat. Chem. 2019, 11, 789–796. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Zhang, F.; Lee, C.S. A Novel Aluminum–Graphite Dual–Ion Battery. Adv. Energy Mater. 2016, 6, 1502588. [Google Scholar] [CrossRef]
- Tang, C.; Bruening, M.L. Ion separations with membranes. J. Polym. Sci. 2020, 58, 2831–2856. [Google Scholar] [CrossRef]
- Costa, C.M.; Lee, Y.-H.; Kim, J.-H.; Lee, S.-Y.; Lanceros-Méndez, S. Recent advances on separator membranes for lithium-ion battery applications: From porous membranes to solid electrolytes. Energy Storage Mater. 2019, 22, 346–375. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Ion transport restriction in mechanically strained separator membranes. J. Power Sources 2013, 226, 149–155. [Google Scholar] [CrossRef]
- Martinez-Cisneros, C.; Antonelli, C.; Levenfeld, B.; Varez, A.; Sanchez, J.Y. Evaluation of polyolefin-based macroporous separators for high temperature Li-ion batteries. Electrochim. Acta 2016, 216, 68–78. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Zhang, S.; Song, X.; Tang, Y.; Cheng, H.M. Reversible calcium alloying enables a practical room-temperature rechargeable calcium-ion battery with a high discharge voltage. Nat. Chem. 2018, 10, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.H.; Tancogne-Dejean, T.; Zhu, J.; Luo, H.; Wierzbicki, T. Failure in lithium-ion batteries under transverse indentation loading. J. Power Sources 2018, 389, 148–159. [Google Scholar] [CrossRef]
- Mao, B.; Chen, H.; Cui, Z.; Wu, T.; Wang, Q. Failure mechanism of the lithium ion battery during nail penetration. Int. J. Heat Mass Transf. 2018, 122, 1103–1115. [Google Scholar] [CrossRef]
- Wang, H.; Lara-Curzio, E.; Rule, E.T.; Winchester, C.S. Mechanical abuse simulation and thermal runaway risks of large-format Li-ion batteries. J. Power Sources 2017, 342, 913–920. [Google Scholar] [CrossRef]
- Avdeev, I.; Martinsen, M.; Francis, A. Rate- and Temperature-Dependent Material Behavior of a Multilayer Polymer Battery Separator. J. Mater. Eng. Perform. 2013, 23, 315–325. [Google Scholar] [CrossRef]
- Love, C.T. Thermomechanical analysis and durability of commercial micro-porous polymer Li-ion battery separators. J. Power Sources 2011, 196, 2905–2912. [Google Scholar] [CrossRef]
- Yuan, C.; Wang, L.; Yin, S.; Xu, J. Generalized separator failure criteria for internal short circuit of lithium-ion battery. J. Power Sources 2020, 467, 228360. [Google Scholar] [CrossRef]
- Chen, J.; Kang, T.; Cui, Y.; Xue, J.; Xu, H.; Nan, J. Nonflammable and thermally stable glass fiber/polyacrylate (GFP) separator for lithium-ion batteries with enhanced safety and lifespan. J. Power Sources 2021, 496, 229862. [Google Scholar] [CrossRef]
- Yazdani, A.; Sanghadasa, M.; Botte, G.G. Integration of ceramic felt as separator / electrolyte in lithium salt thermal batteries and the prospect of rechargeability. J. Power Sources 2022, 521, 230967. [Google Scholar] [CrossRef]
- Yuan, Z.; Xue, N.; Xie, J.; Xu, R.; Lei, C. Separator Aging and Performance Degradation Caused by Battery Expansion: Cyclic Compression Test Simulation of Polypropylene Separator. J. Electrochem. Soc. 2021, 168, 030506. [Google Scholar] [CrossRef]
- Wang, E.; Wu, H.-P.; Chiu, C.-H.; Chou, P.-H. The Effect of Battery Separator Properties on Thermal Ramp, Overcharge and Short Circuiting of Rechargeable Li-Ion Batteries. J. Electrochem. Soc. 2019, 166, A125–A131. [Google Scholar] [CrossRef]
- Lagadec, M.F.; Ebner, M.; Zahn, R.; Wood, V. Communication—Technique for Visualization and Quantification of Lithium-Ion Battery Separator Microstructure. J. Electrochem. Soc. 2016, 163, A992–A994. [Google Scholar] [CrossRef]
- Cannarella, J.; Liu, X.; Leng, C.Z.; Sinko, P.D.; Gor, G.Y.; Arnold, C.B. Mechanical Properties of a Battery Separator under Compression and Tension. J. Electrochem. Soc. 2014, 161, F3117–F3122. [Google Scholar] [CrossRef]
- Cannarella, J.; Arnold, C.B. Stress evolution and capacity fade in constrained lithium-ion pouch cells. J. Power Sources 2014, 245, 745–751. [Google Scholar] [CrossRef]
- Zhang, X.; Sahraei, E.; Wang, K. Li-ion Battery Separators, Mechanical Integrity and Failure Mechanisms Leading to Soft and Hard Internal Shorts. Sci. Rep. 2016, 6, 32578. [Google Scholar] [CrossRef] [PubMed]
- Lagadec, M.F.; Zahn, R.; Wood, V. Designing Polyolefin Separators to Minimize the Impact of Local Compressive Stresses on Lithium Ion Battery Performance. J. Electrochem. Soc. 2018, 165, A1829–A1836. [Google Scholar] [CrossRef]
- Bach, T.C.; Schuster, S.F.; Fleder, E.; Müller, J.; Brand, M.J.; Lorrmann, H.; Jossen, A.; Sextl, G. Nonlinear aging of cylindrical lithium-ion cells linked to heterogeneous compression. J. Energy Storage 2016, 5, 212–223. [Google Scholar] [CrossRef]
- Perea, A.; Paolella, A.; Dubé, J.; Champagne, D.; Mauger, A.; Zaghib, K. State of charge influence on thermal reactions and abuse tests in commercial lithium-ion cells. J. Power Sources 2018, 399, 392–397. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Y.; Sun, T.; Qi, Y.; Li, X. Deformation and fracture behaviors of microporous polymer separators for lithium ion batteries. RSC Adv. 2014, 4, 14904–14914. [Google Scholar] [CrossRef]
- Kalnaus, S.; Wang, Y.; Li, J.; Kumar, A.; Turner, J.A. Temperature and strain rate dependent behavior of polymer separator for Li-ion batteries. Extrem. Mech. Lett. 2018, 20, 73–80. [Google Scholar] [CrossRef]
- Gor, G.Y.; Cannarella, J.; Leng, C.Z.; Vishnyakov, A.; Arnold, C.B. Swelling and softening of lithium-ion battery separators in electrolyte solvents. J. Power Sources 2015, 294, 167–172. [Google Scholar] [CrossRef]
- Xue, N.; Yuan, Z.; Wang, W.; Xu, R.; Xie, J.; Lei, C. Influence of flexible gelatinous coating layer on electrochemical performance and fatigue failure of lithium battery separator. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127875. [Google Scholar] [CrossRef]


| Sample | Thickness (μm) | Contact Angle (°) | Electrolyte Uptake (%) | The Gurley Value (s/100 mL) |
|---|---|---|---|---|
| W-PE | 9 | 26.3 | 226 | 178 |
| W-PE-A | 9 + 3 | 23.8 | 308 | 213 |
| W-PE-B | 9 + 3 | 19.7 | 335 | 168 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qian, W.; Wu, S.; Lei, C.; Xu, R.; Wang, Y. Aging Behavior of Polyethylene and Ceramics-Coated Separators under the Simulated Lithium-Ion Battery Service Compression and Temperature Field. Coatings 2024, 14, 419. https://doi.org/10.3390/coatings14040419
Qian W, Wu S, Lei C, Xu R, Wang Y. Aging Behavior of Polyethylene and Ceramics-Coated Separators under the Simulated Lithium-Ion Battery Service Compression and Temperature Field. Coatings. 2024; 14(4):419. https://doi.org/10.3390/coatings14040419
Chicago/Turabian StyleQian, Wang, Shuqiu Wu, Caihong Lei, Ruijie Xu, and Yanjie Wang. 2024. "Aging Behavior of Polyethylene and Ceramics-Coated Separators under the Simulated Lithium-Ion Battery Service Compression and Temperature Field" Coatings 14, no. 4: 419. https://doi.org/10.3390/coatings14040419




