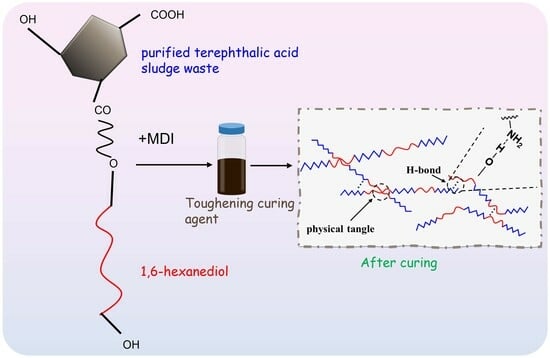
Synthesis of an Epoxy Toughening Curing Agent through Modification of Terephthalic Acid Sludge Waste
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Toughening Curing Agent
2.3. Characterization
3. Results and Discussion
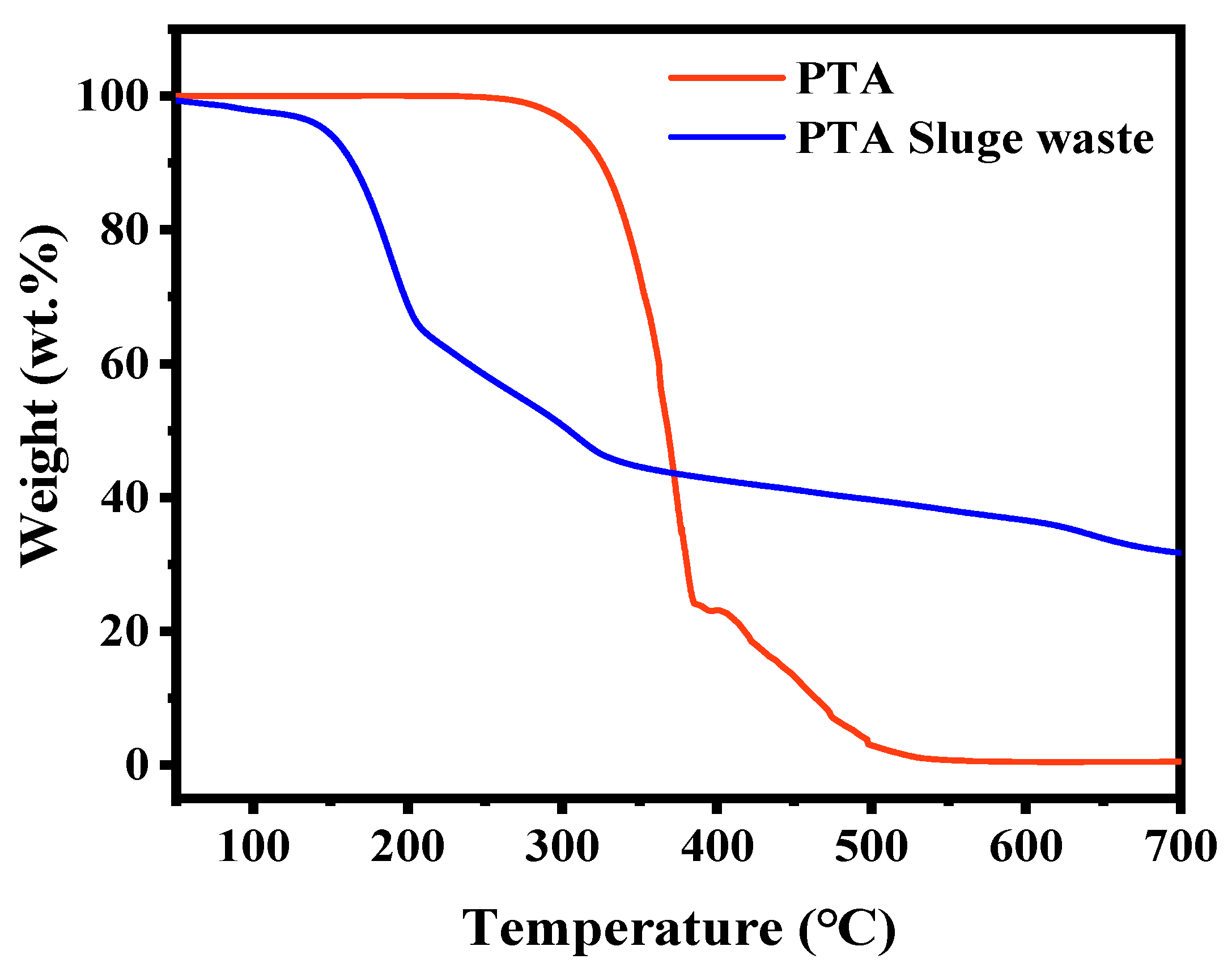
3.1. Component Analysis of PTA Sludge Waste
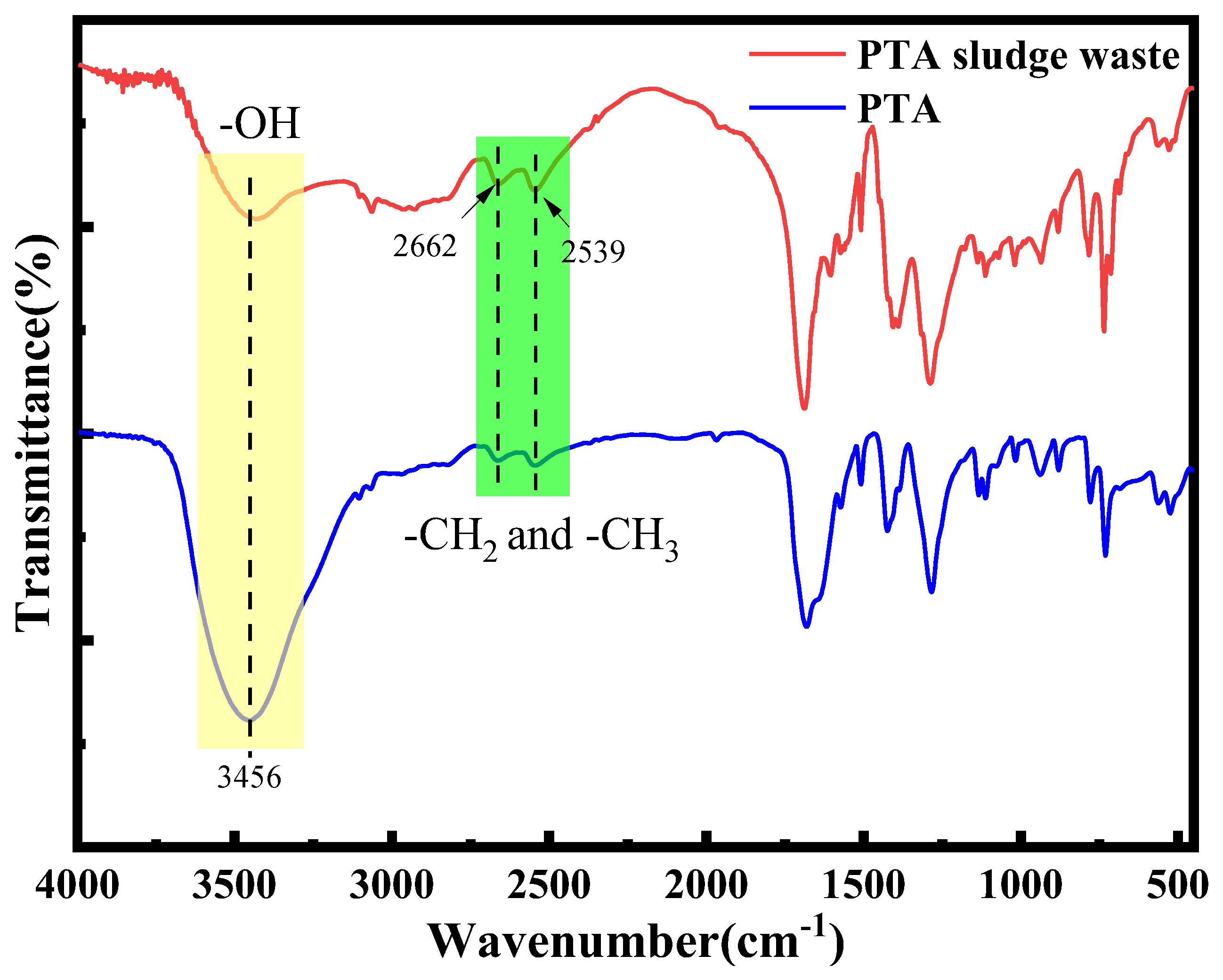
3.2. Chemical Modification of PTA Sludge Waste
3.3. Mechanical Properties of Materials
3.3.1. Hardness
3.3.2. Impact and Tensile Strength
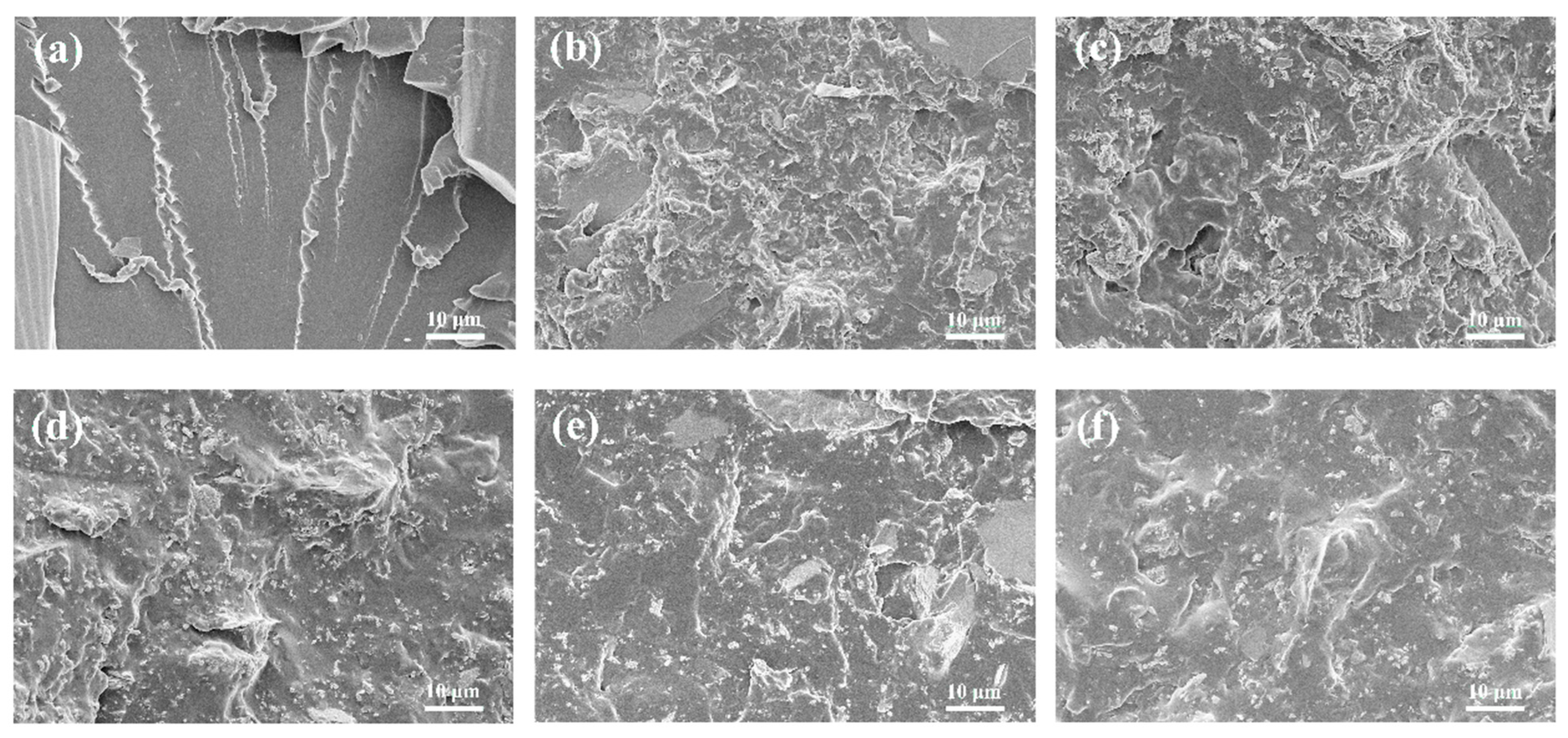
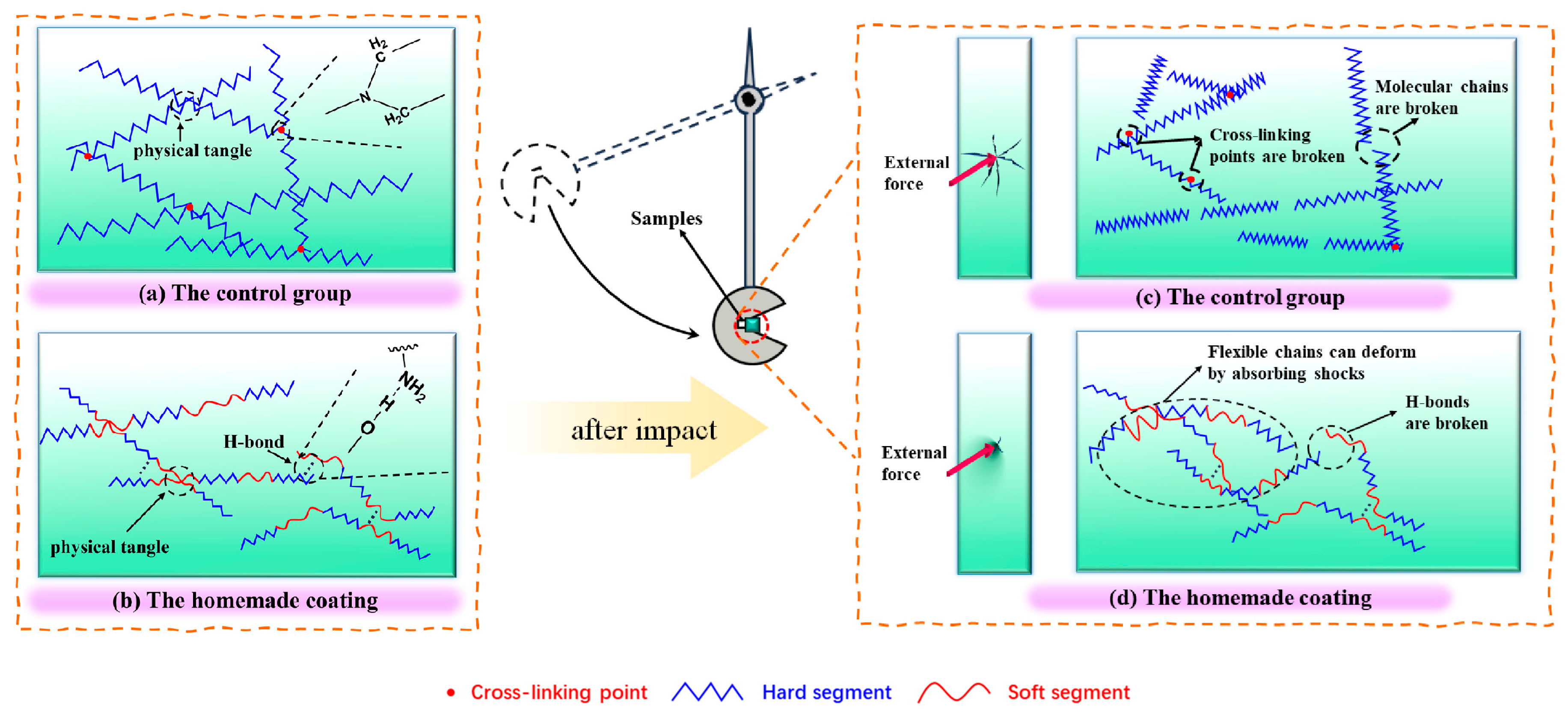
3.4. Toughening Mechanisms
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pophali, G.R.; Khan, R.; Dhodapkar, R.S.; Nandy, T.; Devotta, S. Anaerobic–Aerobic Treatment of Purified Terephthalic Acid (PTA) Effluent; a Techno-Economic Alternative to Two-Stage Aerobic Process. J. Environ. Manag. 2007, 85, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.K.; Prasad, B. Treatment of Toxic Pollutants of Purified Terephthalic Acid Waste Water: A Review. Environ. Technol. Innov. 2017, 8, 191–217. [Google Scholar] [CrossRef]
- Karthik, M.; Dafale, N.; Pathe, P.; Nandy, T. Biodegradability Enhancement of Purified Terephthalic Acid Wastewater by Coagulation–Flocculation Process as Pretreatment. J. Hazard. Mater. 2008, 154, 721–730. [Google Scholar] [CrossRef] [PubMed]
- Kudisi, D.; Lu, X.; Zheng, C.; Wang, Y.; Cai, T.; Li, W.; Hu, L.; Zhang, R.; Zhang, Y.; Zhen, G. Long-Term Performance, Membrane Fouling Behaviors and Microbial Community in a Hollow Fiber Anaerobic Membrane Bioreactor (HF-AnMBR) Treating Synthetic Terephthalic Acid-Containing Wastewater. J. Hazard. Mater. 2022, 424, 127458. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, H.; Pu, S.; Ke, J.; Wang, D.; Liu, Y.; Chen, J.; Guo, R. Development of Autotrophic and Heterotrophic Consortia via Immobilized Microbial Beads for Chemical Wastewater Treatment, Using PTA Wastewater as an Approach. Chemosphere 2021, 281, 131001. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Huang, Z.-H.; Ji, Z.-Y.; Guo, X.-F.; Zhao, L.-M.; Yuan, J.-S. Efficient Treatment of Pure Terephthalic Acid Wastewater with Na2S2O8 Based on Thermal Activation. Environ. Technol. Innov. 2020, 19, 100897. [Google Scholar] [CrossRef]
- Ordaz-Cortés, A.; Thalasso, F.; Salgado-Manjarrez, E.; Garibay-Orijel, C. Treatment of Wastewater Containing High Concentrations of Terephthalic Acid by Comamonas Sp. and Rhodococcus Sp.: Kinetic and Stoichiometric Characterization. Water Environ. J. 2014, 28, 393–400. [Google Scholar] [CrossRef]
- Vermorel, N.; San-Valero, P.; Izquierdo, M.; Gabaldón, C.; Penya-roja, J.M. Anaerobic Degradation of 2-Propanol: Laboratory and Pilot-Scale Studies. Chem. Eng. Sci. 2017, 172, 42–51. [Google Scholar] [CrossRef]
- Siddique, M.N.I.; Munaim, M.S.A.; Wahid, Z.B.A. The Combined Effect of Ultrasonic and Microwave Pre-Treatment on Bio-Methane Generation from Co-Digestion of Petrochemical Wastewater. J. Clean. Prod. 2017, 145, 303–309. [Google Scholar] [CrossRef]
- Liang, J.; Mai, W.; Tang, J.; Wei, Y. Highly Effective Treatment of Petrochemical Wastewater by a Super-Sized Industrial Scale Plant with Expanded Granular Sludge Bed Bioreactor and Aerobic Activated Sludge. Chem. Eng. J. 2019, 360, 15–23. [Google Scholar] [CrossRef]
- Cai, Z.; Ren, Y.; Li, X.; Shi, J.; Tong, B.; Dong, Y. Functional Isocyanide-Based Polymers. Acc. Chem. Res. 2020, 53, 2879–2891. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hao, T.; Xie, Q.; Tian, Y.; Zhang, J.; Guo, H. Icephobic Coating Based on Epoxy/Polydimethylsiloxane Interpenetrating Polymer Network Gel. Coatings 2024, 14, 76. [Google Scholar] [CrossRef]
- Meng, K.; Wei, W.; Wei, K.; Alexandrov, I.V.; An, X.; Wang, D.; Liu, X. Corrosion Performance of Epoxy/Sulfur–Selenium Coating on Q235 Steel. Coatings 2024, 14, 245. [Google Scholar] [CrossRef]
- Zhang, W.; Xia, W.; Chen, Z.; Zhang, G.; Qian, S.; Lin, Z. Comparison of the Cathodic Protection of Epoxy Resin Coating/Zinc-Rich Coatings on Defective Areas under Atmospheric and Immersion Conditions: The Secondary Activation of Zinc Particles. Coatings 2024, 14, 336. [Google Scholar] [CrossRef]
- Behera, R.K.; Parida, S.K.; Das, R.R. Effect of Using Fibre Reinforced Epoxy Adhesive on the Strength of the Adhesively Bonded Single Lap Joints. Compos. Part B Eng. 2023, 248, 110358. [Google Scholar] [CrossRef]
- Sadowski, Ł.; Kampa, Ł.; Chowaniec, A.; Królicka, A.; Żak, A.; Abdoulpour, H.; Vantadori, S. Enhanced Adhesive Performance of Epoxy Resin Coating by a Novel Bonding Agent. Constr. Build. Mater. 2021, 301, 124078. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, C.; Ye, Y.; Xue, Z.; Liu, H.; Zhou, X.; Zhang, Y.; Li, D.; Xie, X.; Mai, Y.-W. Advances on Thermally Conductive Epoxy-Based Composites as Electronic Packaging Underfill Materials—A Review. Adv. Mater. 2022, 34, 2201023. [Google Scholar] [CrossRef]
- Gu, H.; Ma, C.; Gu, J.; Guo, J.; Yan, X.; Huang, J.; Zhang, Q.; Guo, Z. An Overview of Multifunctional Epoxy Nanocomposites. J. Mater. Chem. C 2016, 4, 5890–5906. [Google Scholar] [CrossRef]
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of Reactions and Network Structures of Epoxy Thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef]
- Ricciardi, M.R.; Papa, I.; Langella, A.; Langella, T.; Lopresto, V.; Antonucci, V. Mechanical Properties of Glass Fibre Composites Based on Nitrile Rubber Toughened Modified Epoxy Resin. Compos. Part B Eng. 2018, 139, 259–267. [Google Scholar] [CrossRef]
- Imanaka, M.; Narita, I.; Nakamura, Y.; Hisaka, S.; Fujiwara, K.; Yoshida, S.; Hara, K. Fracture Properties of Epoxy Polymers Modified with Cross-Linked and Core–Shell Rubber Particles. J. Mater. Sci. 2021, 56, 1842–1854. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, H.; Wang, X.; Guan, J.; Li, M.; Chen, Y. Rubber-Composite-Nanoparticle-Modified Epoxy Powder Coatings with Low Curing Temperature and High Toughness. Polymers 2023, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Ma, C.; Liang, C.; Meng, X.; Gu, J.; Guo, Z. A Low Loading of Grafted Thermoplastic Polystyrene Strengthens and Toughens Transparent Epoxy Composites. J. Mater. Chem. C 2017, 5, 4275–4285. [Google Scholar] [CrossRef]
- Bahrami, A.; Cordenier, F.; Van Velthem, P.; Ballout, W.; Pardoen, T.; Nysten, B.; Bailly, C. Synergistic Local Toughening of High Performance Epoxy-Matrix Composites Using Blended Block Copolymer-Thermoplastic Thin Films. Compos. Part Appl. Sci. Manuf. 2016, 91, 398–405. [Google Scholar] [CrossRef]
- Ma, H.; Geng, P.; Xu, T.; Kumar Bandaru, A.; Aravand, A.; Falzon, B.G. Analytical Fracture Toughness Model for Multiphase Epoxy Matrices Modified by Thermoplastic and Carbon Nanotube/Thermoplastic. Compos. Part Appl. Sci. Manuf. 2024, 177, 107948. [Google Scholar] [CrossRef]
- Javanshour, F.; Prapavesis, A.; Pärnänen, T.; Orell, O.; Lessa Belone, M.C.; Layek, R.K.; Kanerva, M.; Kallio, P.; Van Vuure, A.W.; Sarlin, E. Modulating Impact Resistance of Flax Epoxy Composites with Thermoplastic Interfacial Toughening. Compos. Part Appl. Sci. Manuf. 2021, 150, 106628. [Google Scholar] [CrossRef]
- Das, A.; Chatham, C.A.; Fallon, J.J.; Zawaski, C.E.; Gilmer, E.L.; Williams, C.B.; Bortner, M.J. Current Understanding and Challenges in High Temperature Additive Manufacturing of Engineering Thermoplastic Polymers. Addit. Manuf. 2020, 34, 101218. [Google Scholar] [CrossRef]
- Ning, N.; Liu, W.; Hu, Q.; Zhang, L.; Jiang, Q.; Qiu, Y.; Wei, Y. Impressive Epoxy Toughening by a Structure-Engineered Core/Shell Polymer Nanoparticle. Compos. Sci. Technol. 2020, 199, 108364. [Google Scholar] [CrossRef]
- Thirunavukkarasu, N.; Bhuvaneswari Gunasekaran, H.; Peng, S.; Laroui, A.; Wu, L.; Weng, Z. Study on the Interface Toughening of Particle/Fibre Reinforced Epoxy Composites with Molecularly Designed Core–Shell Particles and Various Interface 3D Models. Mater. Des. 2023, 225, 111510. [Google Scholar] [CrossRef]
- Wang, J.; Xue, Z.; Li, Y.; Li, G.; Wang, Y.; Zhong, W.-H.; Yang, X. Synergistically Effects of Copolymer and Core-Shell Particles for Toughening Epoxy. Polymer 2018, 140, 39–46. [Google Scholar] [CrossRef]
- Choi, J.; Yee, A.F.; Laine, R.M. Toughening of Cubic Silsesquioxane Epoxy Nanocomposites Using Core−Shell Rubber Particles: A Three-Component Hybrid System. Macromolecules 2004, 37, 3267–3276. [Google Scholar] [CrossRef]
- Su, W.; Han, X.; Gong, J.; Xi, Z.; Zhang, J.; Wang, Q.; Xie, H. Toughening Epoxy Asphalt Binder Using Core-Shell Rubber Nanoparticles. Constr. Build. Mater. 2020, 258, 119716. [Google Scholar] [CrossRef]
- Picu, C.R.; Krawczyk, K.K.; Wang, Z.; Pishvazadeh-Moghaddam, H.; Sieberer, M.; Lassnig, A.; Kern, W.; Hadar, A.; Constantinescu, D.M. Toughening in Nanosilica-Reinforced Epoxy with Tunable Filler-Matrix Interface Properties. Compos. Sci. Technol. 2019, 183, 107799. [Google Scholar] [CrossRef]
- Goyat, M.S.; Hooda, A.; Gupta, T.K.; Kumar, K.; Halder, S.; Ghosh, P.K.; Dehiya, B.S. Role of Non-Functionalized Oxide Nanoparticles on Mechanical Properties and Toughening Mechanisms of Epoxy Nanocomposites. Ceram. Int. 2021, 47, 22316–22344. [Google Scholar] [CrossRef]
- Ravindran, A.R.; Ladani, R.B.; Wu, S.; Kinloch, A.J.; Wang, C.H.; Mouritz, A.P. Multi-Scale Toughening of Epoxy Composites via Electric Field Alignment of Carbon Nanofibres and Short Carbon Fibres. Compos. Sci. Technol. 2018, 167, 115–125. [Google Scholar] [CrossRef]
- Tangthana-umrung, K.; Zhang, X.; Gresil, M. Synergistic Toughening on Hybrid Epoxy Nanocomposites by Introducing Engineering Thermoplastic and Carbon-Based Nanomaterials. Polymer 2022, 245, 124703. [Google Scholar] [CrossRef]
- Ravindran, A.R.; Ladani, R.B.; Kinloch, A.J.; Wang, C.-H.; Mouritz, A.P. Improving the Delamination Resistance and Impact Damage Tolerance of Carbon Fibre-Epoxy Composites Using Multi-Scale Fibre Toughening. Compos. Part Appl. Sci. Manuf. 2021, 150, 106624. [Google Scholar] [CrossRef]
- Pruksawan, S.; Samitsu, S.; Fujii, Y.; Torikai, N.; Naito, M. Toughening Effect of Rodlike Cellulose Nanocrystals in Epoxy Adhesive. ACS Appl. Polym. Mater. 2020, 2, 1234–1243. [Google Scholar] [CrossRef]
- Ovari, T.-R.; Toth, T.; Katona, G.; Szabó, G.S.; Muresan, L.M. Epoxy Coatings Doped with (3-Aminopropyl)Triethoxysilane-Modified Silica Nanoparticles for Anti-Corrosion Protection of Zinc. Coatings 2023, 13, 1844. [Google Scholar] [CrossRef]
- Xia, M.; Yang, H.; Ling, J.; Yao, Q.; Li, G.; Luo, Y. The Mechanical Behaviors of Epoxy-Terminated Hyperbranched Polyester (E-HBP) as Toughener in Different Epoxy Resins. Adv. Compos. Hybrid Mater. 2018, 1, 310–319. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Zhang, D. Synthesis and Application of Epoxy-Ended Hyperbranched Polymers. Chem. Eng. J. 2018, 343, 283–302. [Google Scholar] [CrossRef]
- Zotti, A.; Zuppolini, S.; Borriello, A.; Trinchillo, L.; Vinti, V.; Zarrelli, M. Hierarchical Aerospace Epoxy Composites of Carbon Fiber and Hyperbranched Filler: Toughening Behavior from Nanocomposites to Composites. Compos. Struct. 2024, 327, 117719. [Google Scholar] [CrossRef]
- Sun, J.; Bai, J.; Li, J. The Synergistic Toughening and Strengthening Effects of Cork Particles and Nanocellulose on Rosin-Based Epoxy Resin. Polymers 2022, 14, 5064. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cui, Y.; Li, X.; Gu, Y.; Fei, X.; Luo, J.; Liu, X. Reactive Particles from in Situ Silane-Polycondensation-Induced Self-Assembly of Poly(Styrene-Alt-Maleic Anhydride) as Toughener for Epoxy Resins. J. Appl. Polym. Sci. 2019, 136, 47565. [Google Scholar] [CrossRef]
- Lou, C.; Liu, X. Functional Dendritic Curing Agent for Epoxy Resin: Processing, Mechanical Performance and Curing/Toughening Mechanism. Compos. Part B Eng. 2018, 136, 20–27. [Google Scholar] [CrossRef]
- Ishizu, K.; Makino, M.; Hatoyama, N.; Uchida, S. Emulsion-Induced Ordered Microporous Films Using Amphiphilic Poly(Ethylene Oxide)-Block-Poly(n-Butyl Isocyanate) Block Copolymers. J. Appl. Polym. Sci. 2008, 108, 3753–3759. [Google Scholar] [CrossRef]



| Water (wt.%) | TA (wt.%) | BA (wt.%) | p-Tol (wt.%) | Metal Ions (wt.%) |
|---|---|---|---|---|
| 43.10 | 11.95 | 7.79 | 33.57 | 3.59 |
| Sample Name | Curing Agent Dosage | Control-Group | ||||
|---|---|---|---|---|---|---|
| 20% | 25% | 30% | 35% | 40% | ||
| Shore hardness/HA | 85 ± 2 | 60 ± 1 | 56 ± 0.5 | 42 ± 2 | 30 ± 4 | 96 ± 0.2 |
| Tensile strength/MPa | 38.18 ± 0.16 | 31.15 ± 0.24 | 20.46 ± 1.01 | 10.13 ± 0.51 | 0.34 ± 1.12 | 35.21 ± 0.13 |
| Elongation at break/% | 2.95 ± 0.36 | 4.56 ± 0.21 | 6.90 ± 0.33 | 11.76 ± 0.27 | 75.44 ± 0.51 | 3.01 ± 0.17 |
| Impact strength/kJ m−2 | 17.14 ± 0.32 | 22.36 ± 0.46 | 28.57 ± 0.42 | 42.86 ± 0.53 | 40 ± 0.36 | 21.43 ± 0.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Kong, H.; Yu, R.; Tu, J.; Wu, Q.; Wang, M.; Niu, L.; Zhang, K. Synthesis of an Epoxy Toughening Curing Agent through Modification of Terephthalic Acid Sludge Waste. Coatings 2024, 14, 503. https://doi.org/10.3390/coatings14040503
Fu J, Kong H, Yu R, Tu J, Wu Q, Wang M, Niu L, Zhang K. Synthesis of an Epoxy Toughening Curing Agent through Modification of Terephthalic Acid Sludge Waste. Coatings. 2024; 14(4):503. https://doi.org/10.3390/coatings14040503
Chicago/Turabian StyleFu, Jinhui, Huixian Kong, Rentong Yu, Jinchun Tu, Qiang Wu, Mingyu Wang, Lina Niu, and Kexi Zhang. 2024. "Synthesis of an Epoxy Toughening Curing Agent through Modification of Terephthalic Acid Sludge Waste" Coatings 14, no. 4: 503. https://doi.org/10.3390/coatings14040503
APA StyleFu, J., Kong, H., Yu, R., Tu, J., Wu, Q., Wang, M., Niu, L., & Zhang, K. (2024). Synthesis of an Epoxy Toughening Curing Agent through Modification of Terephthalic Acid Sludge Waste. Coatings, 14(4), 503. https://doi.org/10.3390/coatings14040503