Galvanic Corrosion Behavior of the X80 Steel Welded Joint
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Solution
2.3. Test Method
2.4. Galvanic Corrosion Characterization
3. Results and Discussion
3.1. Microstructures of the Simulated X80 Steel Welded Joint
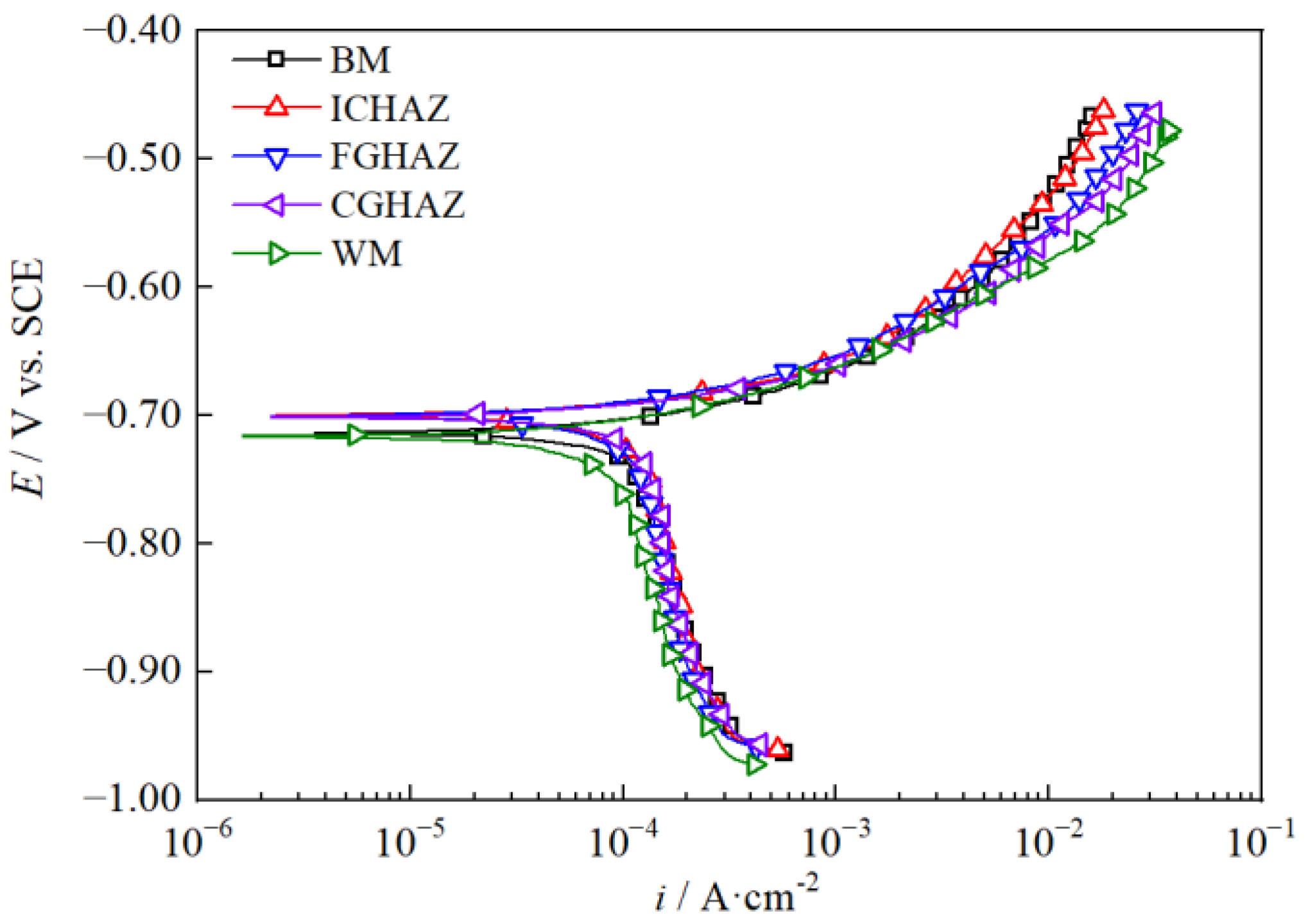
3.2. Polarization Behavior of the Simulated X80 Steel Welded Joint
3.3. Effect of Area Ratio on Potential
3.4. Effect of Area Ratio on Galvanic Current Density
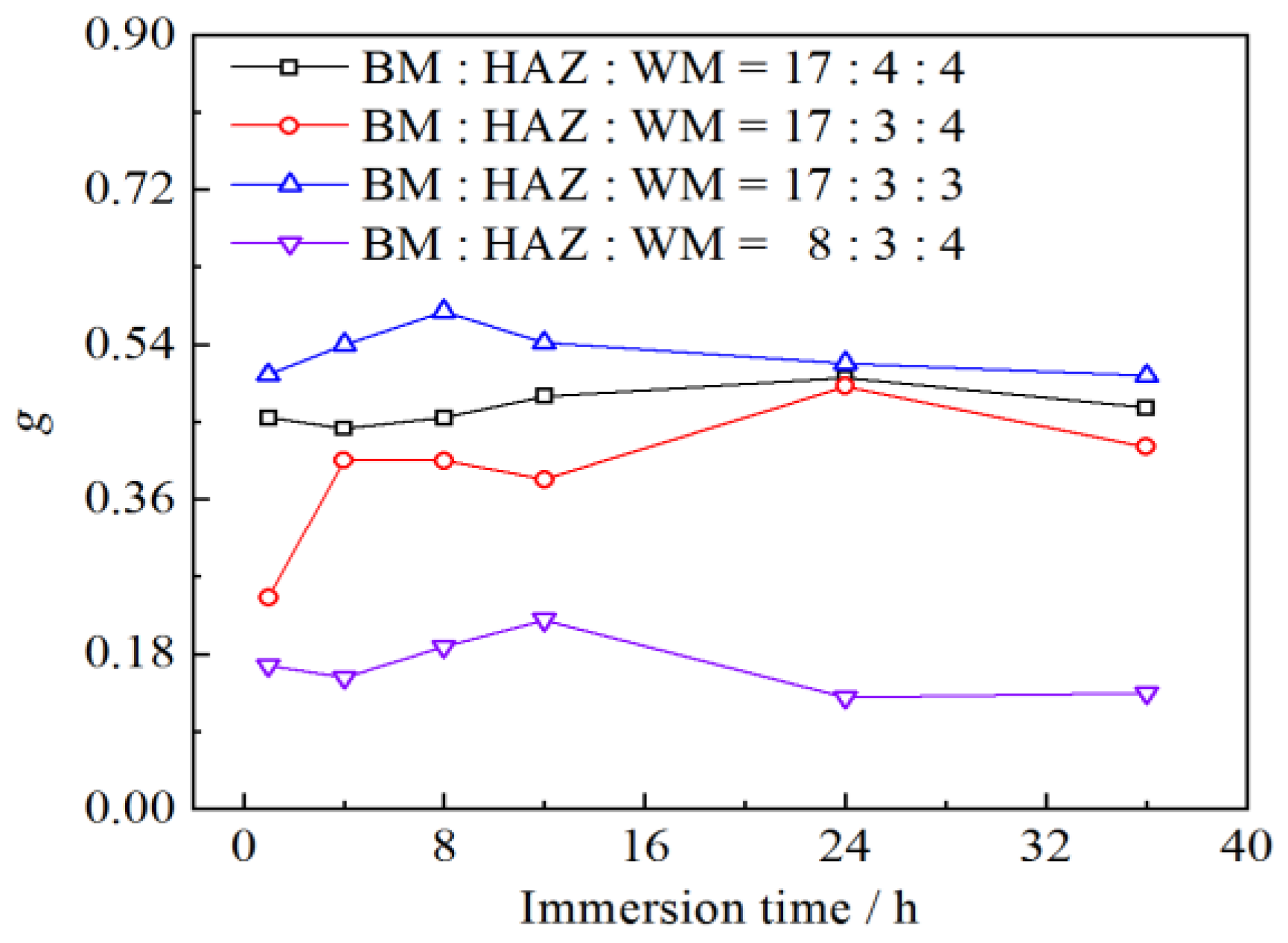
3.5. Effect of Area Ratio on Galvanic Corrosion Intensity
4. Conclusions
- (1)
- As the anode area decreased, the galvanic potential shifted positively, the maximum galvanic current density increased, the galvanic corrosion intensity increased, and the galvanic effect of the main anode decreased. The impact of the cathode area was contrary.
- (2)
- The variation of the area of the WM and the HAZ has a greater influence on the intensity of the galvanic corrosion.
- (3)
- To enhance corrosion resistance, it is advisable to choose a shape with a larger groove to increase the WM area in the welded joint. Additionally, selecting a welding method with lower heat input and a higher energy density can help reduce the HAZ area in the welded joint.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.; Zhu, M.; Wang, C.; Zhang, J.; Liu, C.; Song, Y.; Wang, Y.; Gu, S.; Li, Y. Effect of FeCO3 corrosion product scale on hydrogen adsorption and permeation of pipeline steel in gaseous hydrogen-blended natural gas transportation. Corros. Sci. 2024, 229, 111880. [Google Scholar] [CrossRef]
- Zhang, G.; Cheng, Y. Localized corrosion of carbon steel in a CO2-saturated oilfield formation water. Electrochim. Acta 2011, 56, 1676–1685. [Google Scholar] [CrossRef]
- Li, Y.; Tang, X.; Li, Y. Research progress of localized corrosion of welded joints. Mater. Rev. 2017, 31, 158–165. [Google Scholar]
- Zhang, D.; Liu, R.; Liu, Y.; Xing, S.; Yang, L.; Wei, E.; Dou, X. Multiscale characterization of seawater pipe erosion of B10 cop-per–nickel alloy welded joints. Sci. Rep. 2022, 12, 2164. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xie, F.; Li, X.; Luo, J.; Su, G.; Zhu, L.; Chen, Q. Failure analysis of the leakage in girth weld of bimet-al composite pipe. Eng. Fail. Anal. 2023, 143, 106917. [Google Scholar]
- Kosturek, R.; Wachowski, M.; Sniezek, L.; Gloc, M. The influence of the post-weld heat treatment on the microstructure of in-conel 625/carbon steel bimetal joint obtained by explosive welding. Metals 2019, 9, 246. [Google Scholar] [CrossRef]
- Pagotto, J.F.; Montemor, M.F.; Recio, F.J.; Motheo, A.J.; Simões, A.M.; Herrasti, P. Visualisation of the galvanic effects at welds on carbon steel. J. Brazil. Chem. Soc. 2015, 26, 667–675. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, L.; Feng, Z.; Frankel, G.; Lu, M.; Chang, W. Galvanic corrosion of a welded joint in 3Cr low alloy pipeline steel. Corros. Sci. 2016, 111, 391–403. [Google Scholar] [CrossRef]
- Bastos, A.C.; Simões, A.; Ferreira, M. Corrosion of electrogalvanized steel in 0.1 M NaCl studied by SVET. Port. Electrochim. Acta 2002, 21, 371–387. [Google Scholar] [CrossRef]
- Maqbool, A.; Zaman Khan, N. Microstructure and corrosion behavior of thermo-mechanically processed rare earth Mg alloy: Effect of friction stir processing. Mater. Lett. 2024, 359, 135934. [Google Scholar] [CrossRef]
- Dou, X.; He, Z.; Zhang, X.; Liu, Y.; Liu, R.; Tan, Z.; Zhang, D.; Li, Y. Corrosion behavior and mechanism of X80 pipeline steel welded joints under high shear flow fields. Colloid Surface A 2023, 665, 131225. [Google Scholar] [CrossRef]
- Li, Q.; Yao, Q.; Sun, L.; Ma, H.; Zhang, C.; Wang, N. Effect of micro-galvanic corrosion on corrosion fatigue cracking of the weld joint of high strength bridge steel. Int. J. Fatigue 2023, 170, 107568. [Google Scholar] [CrossRef]
- da Silva, R.M.P.; Izquierdo, J.; Milagre, M.X.; Betancor-Abreu, A.M.; de Oliveira, L.A.; Antunes, R.A.; Souto, R.M.; Costa, I. On the local corrosion behavior of coupled welded zones of the 2098-T351 Al-Cu-Li alloy produced by Friction Stir Welding (FSW): An amperometric and potentiometric microelectrochemical. Electrochim. Acta 2021, 373, 137910. [Google Scholar] [CrossRef]
- Fushimi, K.; Naganuma, A.; Azumi, K.; Kawahara, Y. Current distribution during galvanic corrosion of carbon steel welded with type-309 stainless steel in NaCl solution. Corros. Sci. 2008, 50, 903–911. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Zhang, D. Multiscale characterization of erosion of TA2 titanium alloy welded joints. Front. Mater. 2022, 9, 910319. [Google Scholar] [CrossRef]
- Shi, L.; Song, Y.; Zhao, P.; Wang, H.; Dong, K.; Shan, D.; Han, E. Variations of galvanic currents and corrosion forms of 2024/Q235/304 tri-metallic couple with multivariable cathode/anode area ratios: Experiments and modeling. Electrochimi. Acta 2020, 359, 136947. [Google Scholar] [CrossRef]
- Okonkwo, B.; Ming, H.; Wang, J.; Han, E.; Rahimi, E.; Davoodi, A.; Hosseinpour, S. A New method to determine the synergistic effects of area ratio and microstructure on the galvanic corrosion of LAS A508/309L/308L SS dissimilar metals weld. J. Mater. Sci. Technol. 2021, 78, 38–50. [Google Scholar] [CrossRef]
- Nakatsugawa, I.; Chino, Y. Effect of area ratio on the galvanic corrosion of AZX611 magnesium alloy/A6N01 aluminum alloy joint. J. Jpn. I. Met. 2021, 62, 1764–1770. [Google Scholar]
- Huang, Y.; Zhang, J.; Xuan, F.; Ma, Y. Modeling and prediction of galvanic corrosion for an overlaying welded structure. J. Mater. Eng. Perform. 2023. [Google Scholar] [CrossRef]
- Tang, X.; Li, J.; Wu, Y.; Hu, H. Electrochemical formation mechanism of micro-droplets on pure iron. Front. Chem. 2021, 9, 610738. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Yang, R.; Tang, X. Reconstruction of X80 pipeline steel welded joints and corrosion behavior in NACE solution. J. China Univ. Pet. Ed. Nat. Sci. 2018, 42, 153–160. [Google Scholar]
- Li, Y.; Li, Q.; Tang, X.; Li, Y. Reconstruction and characterization of galvanic corrosion behavior of X80 pipeline steel welded joints. Acta Metall. Sin. 2019, 55, 801–810. [Google Scholar]
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Wang, X.; Cui, H. Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- Wang, X.; Huang, F.; Hu, Q.; Zhang, J.; Liu, J. Galvanic corrosion behavior of welded joint in marine atmosphere environment based on capillary microcell. NPJ Mater Degrad. 2024, 8, 1–12. [Google Scholar] [CrossRef]
- Kahyarian, A.; Schumaker, A.; Brown, B.; Nesic, S. Acidic corrosion of mild steel in the presence of acetic acid: Mechanism and prediction. Electrochim. Acta 2017, 258, 639–652. [Google Scholar] [CrossRef]
- Li, Y.; Xu, N.; Guo, X.; Zhang, G. The role of acetic acid or H+ in initiating crevice corrosion of N80 carbon steel in CO2-saturated NaCl solution. Corros. Sci. 2017, 128, 9–22. [Google Scholar] [CrossRef]
- McCafferty, E. Validation of corrosion rates measured by the Tafel extrapolation method. Corros. Sci. 2005, 47, 3202–3215. [Google Scholar] [CrossRef]
- Lu, Y.; Jing, H.; Han, Y.; Feng, Z.; Xu, L. Recommend design of filler metal to minimize carbon steel weld metal preferential corrosion in CO2-saturated oilfield produced water. Appl. Surf. Sci. 2016, 389, 609–622. [Google Scholar] [CrossRef]
- Yu, K.; Feng, S.; Ding, C.; Yu, P.; Huang, M. Improving anti-corrosion properties of CoCrFeMnNi high entropy alloy by introducing Si into nonmetallic inclusions. Corros. Sci. 2022, 208, 110616. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.; Zheng, Y. Electrochemical techniques for determining corrosion rate of rusted steel in seawater. Corros. Sci. 2011, 53, 208–216. [Google Scholar] [CrossRef]
- Zeszut, R.; Landau, U. Determination of redox currents in electroless systems: Correct application of mixed potential analysis. J. Electrochem. Soc. 2019, 166, 737–741. [Google Scholar] [CrossRef]
- Jia, J.; Song, G.; Atrens, A. Influence of geometry on galvanic corrosion of AZ91D coupled to steel. Corros. Sci. 2006, 48, 2133–2153. [Google Scholar] [CrossRef]
- Bi, Z.; Wang, R.; Jing, X. Grooving corrosion of oil coiled tubes manufactured by electrical resistance welding. Corros. Sci. 2012, 57, 67–73. [Google Scholar] [CrossRef]
- Han, Y.; Zhong, S.; Tian, L.; Fei, J.; Sun, Y.; Zhao, L.; Xu, L. Welding heat input for synergistic improvement in toughness and stress corrosion resistance of X65 pipeline steel with pre-strain. Corros. Sci. 2022, 206, 110478. [Google Scholar] [CrossRef]




| Regions | C | Si | Mn | P | S | Cr | Ni | Nb | Mo | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM | 0.066 | 0.190 | 1.66 | 0.006 | 0.002 | 0.222 | 0.187 | 0.060 | 0.127 | 0.128 | balanced |
| WM | 0.066 | 0.532 | 1.29 | 0.012 | 0.009 | 0.046 | 0.034 | 0.006 | 0.012 | 0.028 | balanced |
| Regions | ba (mV·dec−1) | Ecorr (VSCE) | iL (mA·cm−2) | icorr (mA·cm−2) |
|---|---|---|---|---|
| BM | 58 | −0.703 | 0.119 | 0.136 |
| ICHAZ | 58 | −0.701 | 0.120 | 0.146 |
| FGHAZ | 61 | −0.701 | 0.124 | 0.147 |
| CGHAZ | 61 | −0.702 | 0.128 | 0.159 |
| WM | 55 | −0.717 | 0.104 | 0.107 |
| Coupled Time/h | 1 | 4 | 8 | 12 | 24 | 36 |
|---|---|---|---|---|---|---|
| BM:HAZ:WM = 17:4:4 | 2.60 | 1.94 | 1.67 | 1.69 | 1.73 | 1.87 |
| BM:HAZ:WM = 17:3:4 | 2.36 | 2.25 | 2.20 | 1.66 | 1.68 | 1.73 |
| BM:HAZ:WM = 17:3:3 | 2.88 | 2.77 | 2.34 | 2.49 | 2.07 | 1.95 |
| BM:HAZ:WM = 8:3:4 | 2.13 | 1.81 | 1.71 | 1.80 | 1.64 | 1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Sang, J.; Yang, Y.; Fang, G.; Pang, J.; Liu, F. Galvanic Corrosion Behavior of the X80 Steel Welded Joint. Coatings 2024, 14, 528. https://doi.org/10.3390/coatings14050528
Li Y, Sang J, Yang Y, Fang G, Pang J, Liu F. Galvanic Corrosion Behavior of the X80 Steel Welded Joint. Coatings. 2024; 14(5):528. https://doi.org/10.3390/coatings14050528
Chicago/Turabian StyleLi, Yadong, Jiaxu Sang, Yunzhi Yang, Guoxin Fang, Jianjun Pang, and Feng Liu. 2024. "Galvanic Corrosion Behavior of the X80 Steel Welded Joint" Coatings 14, no. 5: 528. https://doi.org/10.3390/coatings14050528
APA StyleLi, Y., Sang, J., Yang, Y., Fang, G., Pang, J., & Liu, F. (2024). Galvanic Corrosion Behavior of the X80 Steel Welded Joint. Coatings, 14(5), 528. https://doi.org/10.3390/coatings14050528





