Investigation of the Structure and Properties of MoS2 Coatings Obtained by Electrospark Alloying
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roik, T.A.; Gavrysh, O.A.; Vitsiuk, I.I.; Kholiavko, V.V. Wear-Resistant Composites Produced from Tool Steel Waste for Contact Joints of High-Speed Printing Machines. Powder Metall. Met. Ceram. 2023, 62, 215–224. [Google Scholar] [CrossRef]
- Hlushkova, D.B.; Bagrov, V.A.; Volchuk, V.M.; Murzakhmetova, U.A. Influence of structure and phase composition on wear resistance of sparingly alloyed alloys. Funct. Mater. 2023, 30, 74–78. [Google Scholar] [CrossRef]
- Martsinkovsky, V.; Yurko, V.; Tarelnik, V.; Filonenko, Y. Designing radial sliding bearing equipped with hydrostatically suspended pads. Procedia Eng. 2012, 39, 157–167. [Google Scholar] [CrossRef]
- Tarelnyk, V.; Konoplianchenko, I.; Martsynkovskyy, V.; Zhukov, A.; Kurp, P. Comparative tribological tests for face impulse seals sliding surfaces formed by various methods. In Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2019; pp. 382–391. [Google Scholar] [CrossRef]
- Martsinkovsky, V.; Yurko, V.; Tarelnik, V.; Filonenko, Y. Designing thrust sliding bearings of high bearing capacity. Procedia Eng. 2012, 39, 148–156. [Google Scholar] [CrossRef]
- Halchuk, T.N.; Povstyanoy, O.Y.; Bembenek, M.; Redko, R.G.; Chetverzhuk, T.I.; Polinkevych, R.M. Impact of technological system’s characteristics on the machining accuracy of bearing rings. J. Eng. Sci. 2023, 10, A22–A30. [Google Scholar] [CrossRef] [PubMed]
- Wasilczuk, M.; Wodtke, M. Experimental study on the feasibility of alternative materials for tilting pad thrust bearings operating in transition to mixed friction. Friction 2024, 12, 812–822. [Google Scholar] [CrossRef]
- Joseph, A.; Vijayan, A.S.; Shebeeb, M.C.; Akshay, K.S.; Mathew, K.P.J.; Sajith, V. A review on tailoring the corrosion and oxidation properties of MoS2-based coatings. J. Mater. Chem. A 2023, 7, 3172–3209. [Google Scholar] [CrossRef]
- Antoszewski, B.; Kurp, P. Effect of Surface Texture on the Sliding Pair Lubrication Efficiency. Lubricants 2022, 10, 80. [Google Scholar] [CrossRef]
- Bouti, S.; Antonova, M.N.; Hamouda, K.; Babichev, A.P.; Sayah, T. Structure and Mechanochemical Properties of the MoS2 Solid Lubricant Using Vibration Wave Treatment. Mater. Sci. 2018, 53, 739–749. [Google Scholar] [CrossRef]
- Martin, P.M. Handbook of Deposition Technologies for Films and Coatings; William Andrew Publishing: Boston, MA, USA, 2010; pp. 1–912. [Google Scholar]
- Seynstahl, A.; Köbrich, M.; Rosnitschek, T.; Göken, M.; Tremmel, S. Enhancing the lifetime and vacuum tribological performance of PVD-MoS2 coatings by nitrogen modification. Surf. Coat. Technol. 2024, 477, 130343. [Google Scholar] [CrossRef]
- Altuntepe, A.; Erkan, S.; Karadeniz, G. Large-scale synthesis of homogeneous WS2 films by physical vapor deposition. Eurasian J. Sci. Eng. Technol. 2023, 4, 36–41. [Google Scholar] [CrossRef]
- Bozheyev, F.; Friedrich, D.; Nie, M.; Rengachari, M.; Ellmer, K. Preparation of highly (001)-oriented photoactive tungsten diselenide (WSe2) films an amorphous solid-liquidcrystalline solid (aSLcS) rapid-crystallization process. Phys. Status Solidi A 2014, 211, 2013–2019. [Google Scholar] [CrossRef]
- Nozhenkov, M.V. The Ultra-Low Friction of Layer Structures. Mech. Eng. Res. 2013, 3, 73–89. [Google Scholar] [CrossRef]
- Poyraz, M.; Tunay, R.F. The effect of different sputtering parameters on coating thickness and hardness in MoS2 coated films with and without interlayer. Int. J. Surf. Sci. Eng. 2020, 14, 117–134. [Google Scholar] [CrossRef]
- Lu, X.; Sui, X.; Zhang, X.; Yan, Z.; Hao, J. Influence of V doping on the microstructure, chemical stability, mechanical and tribological properties of MoS2 coatings. Ind. Lubr. Tribol. 2024, 76, 29–40. [Google Scholar] [CrossRef]
- Torres, H.; Vuchkovb, T.; Slawikc, S.; Gachotd, C.; Prakashb, B.; Ripoll, M.R. Self-lubricating laser claddings for reducing friction and wear from room temperature to 600 °C. Wear 2018, 408–409, 22–33. [Google Scholar] [CrossRef]
- Yao, Y.H.; Wu, Y.C.; Zhang, Z.Y.; Zhu, H.; Hu, M.N.; Xu, K.; Liu, Y. Enhancement of frictional properties of Ni-MoS2 self-lubricating composite coatings by microgroove arrays. Appl. Surf. Sci. 2022, 605, 154635. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; An, Y.; Liu, D.; Zhou, H.; Chen, J. YSZ/MoS2 self-lubricating coating fabricated by thermal spraying and hydrothermal reaction. Ceram. Int. 2018, 44, 17864–17872. [Google Scholar] [CrossRef]
- Liu, T.X.; Guo, C.A.; Lu, F.S.; Zhang, X.Y.; Zhang, L.; Wang, Z.J.; Xu, Z.Y.; Zhu, G.L. Influence of deposition voltage on tribological properties of W-WS2 coatings deposited by electrospark deposition. Chalcogenide Lett. 2023, 20, 741–749. [Google Scholar] [CrossRef]
- Yue, M.; Zhao, W.; Wang, S.; Li, J.; Zhu, C.; Jin, H.; Guo, C. Tribological properties of electrospark depositing Ni-WS2 self-lubricating coating. Chalcogenide Lett. 2021, 18, 557–564. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Dai, S.; Zhu, L. Research Progress in Electrospark Deposition Coatings on Titanium Alloy Surfaces: A Short Review. Coatings 2023, 13, 1473. [Google Scholar] [CrossRef]
- Antoszewski, B.; Gaponova, O.P.; Tarelnyk, V.B.; Myslyvchenko, O.M.; Kurp, P.; Zhylenko, T.I.; Konoplianchenko, I. Assessment of Technological Capabilities for Forming Al-C-B System Coatings on Steel Surfaces by Electrospark Alloying Method. Materials 2021, 14, 739. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, X.M.; Chen, L.; Wang, Z.J.; Hou, G.C.; Guo, C.A.; Zhang, J. Influence of temperature on tribological behavior of AlCoCrFeNi coatings prepared by electrospark deposition. Dig. J. Nanomater. Biostructures 2023, 18, 145. [Google Scholar] [CrossRef]
- Tarelnyk, V.B.; Gaponova, O.P.; Loboda, V.B.; Konoplyanchenko, E.V.; Martsinkovskii, V.S.; Semirnenko, Y.I.; Tarelnyk, N.V.; Mikulina, M.A.; Sarzhanov, B.A. Improving Ecological Safety when Forming Wear-Resistant Coatings on the Surfaces of Rotation Body Parts of 12Kh18N10T Steel Using a Combined Technology Based on Electrospark Alloying. J. Surf. Eng. Appl. Electrochem. 2021, 2, 173–184. [Google Scholar] [CrossRef]
- Geng, C.X.; Zhang, H.X.; Li, X.J.; Geng, H.B. Elevated temperature tensile behaviour of 5183 aluminium alloy made by electrospark deposition additive manufacturing. Mater. Sci. Eng. A 2023, 868, 144746. [Google Scholar] [CrossRef]
- Myslyvchenko, O.M.; Gaponova, O.P.; Tarelnyk, V.B.; Krapivka, M.O. The Structure Formation and Hardness of High-Entropy Alloy Coatings Obtained by Electrospark Deposition. Powder Metall. Met. Ceram. 2020, 59, 201–208. [Google Scholar] [CrossRef]
- Cao, T.; Lei, S.; Zhang, M. The friction and wear behavior of Cu/Cu-MoS2 self-lubricating coating prepared by electrospark deposition. Surf. Coat. Technol. 2015, 270, 24–32. [Google Scholar] [CrossRef]
- Guo, C.; Kong, F.; Zhao, S.; Yan, X.; Yang, J.; Zhang, J. Performance of friction and wear of electrospark deposited ni-MoS2 self-lubricating coating. Chalcogenide Lett. 2019, 16, 309–315. [Google Scholar]
- Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Shvindina, N.V.; Levashov, E.A.; Shtansky, D.V. Comparative study of Ti-C-Ni-Al, Ti-C-Ni-Fe, and Ti-C-Ni-Al/Ti-C-Ni-Fe coatings produced by magnetron sputtering, electro-spark deposition, and a combined two-step process. Ceram. Int. 2018, 44, 7637–7646. [Google Scholar] [CrossRef]
- Tarelnyk, N. The Method of Eliminating Adhesion of Electrodes during Electrospark Alloying of Steel Parts of Equipment Subject to Radiation Exposure. Patent of Ukraine for Utility Model No. 155134: MPK (2006), B23P 6/00, B23H 1/00, C23C 28/00, 17 January 2024. Bull. No. 3. Available online: https://base.uipv.org/searchINV/search.php?action=viewdetails&IdClaim=287836 (accessed on 15 February 2024).
- Wang, J.M.; Liu, G.H.; Fang, Y.L.; Li, W.K. Marangoni effect in nonequilibrium multiphase system of material processing. Rev. Chem. Eng. 2016, 32, 551–585. [Google Scholar] [CrossRef]
- Tuo, Y.; Yang, Z.; Guo, Z.; Chen, Y.; Hao, J.; Zhao, Q.; Kang, Y.; Zhang, Y.; Zhao, Y. Pore structure optimization of MoS2/Al2O3 self-lubricating ceramic coating for improving corrosion resistance. Vacuum 2023, 207, 111687. [Google Scholar] [CrossRef]
- Borysov, Y.S.; Vihilianska, N.V.; Burlachenko, O.M.; Olevska, L.P.; Lopata, V.M. Analysis of modern experience in development of sealing coatings for parts of gas turbine engines (Review). Autom. Weld. 2022, 4, 41–49. (In Ukraine) [Google Scholar] [CrossRef]
- Boichyshyn, L.; Kovbuz, M.; Kulyk, Y.; Nosenko, V. Influence of the melt cooling rate of the structure on the alloyed iron based amorphous alloys with different form. Proc. Shevchenko Sci. Soc. Chem. Sci. 2015, 42, 101–108. [Google Scholar]
- Houserová, J.; Vřešťál, J.; Šob, M. Phase diagram calculations in the Co–Mo and Fe–Mo systems using first-principles results for the sigma phase. Calphad 2005, 29, 133–139. [Google Scholar] [CrossRef]
- Wang, W.; Du, M.; Zhang, X.; Luan, C.; Tian, Y. Preparation and Properties of Mo Coating on H13 Steel by Electro Spark Deposition Process. Materials 2021, 14, 3700. [Google Scholar] [CrossRef] [PubMed]
- Gaponova, O.P.; Antoszewski, B.; Tarelnyk, V.B.; Kurp, P.; Myslyvchenko, O.M.; Tarelnyk, N.V. Analysis of the Quality of Sulfomolybdenum Coatings Obtained by Electrospark Alloying Methods. Materials 2021, 14, 6332. [Google Scholar] [CrossRef] [PubMed]



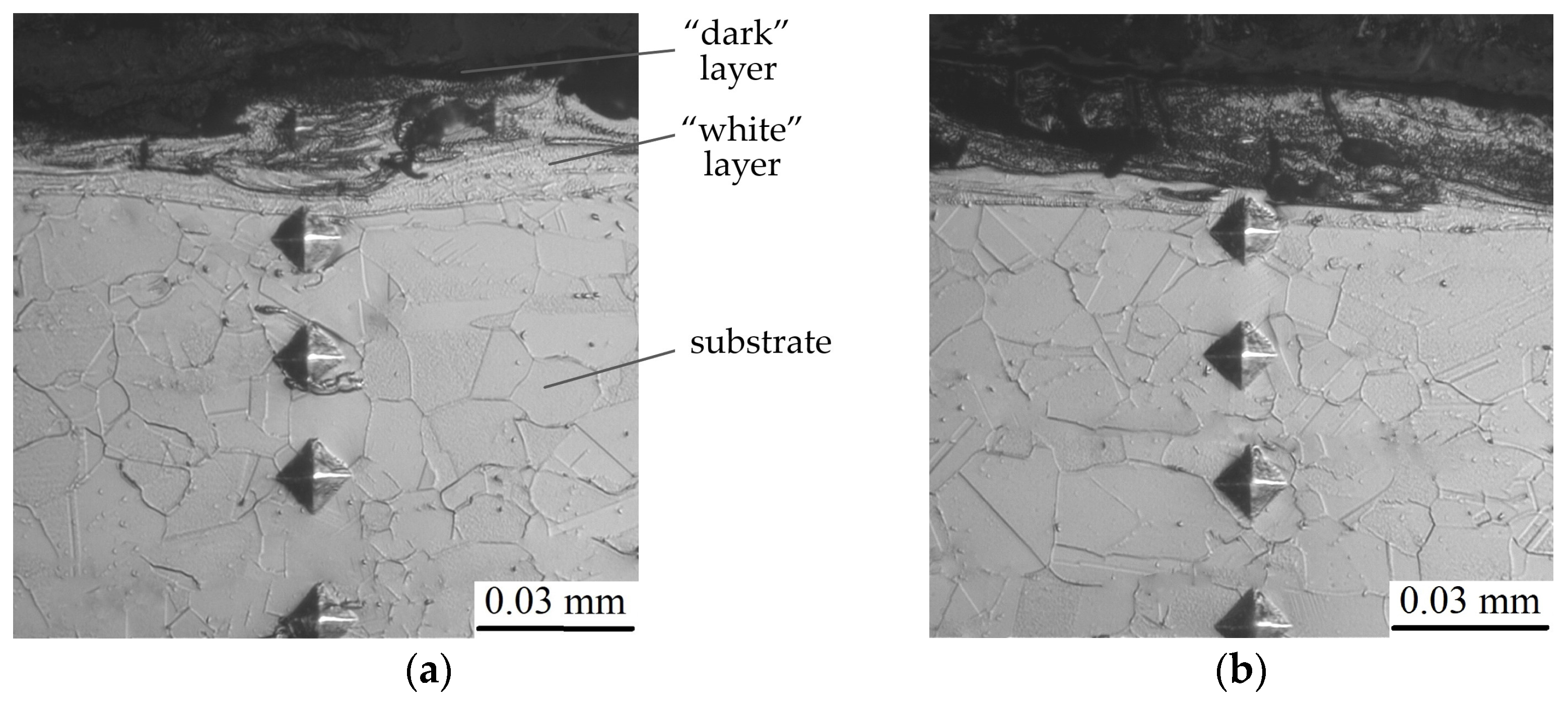
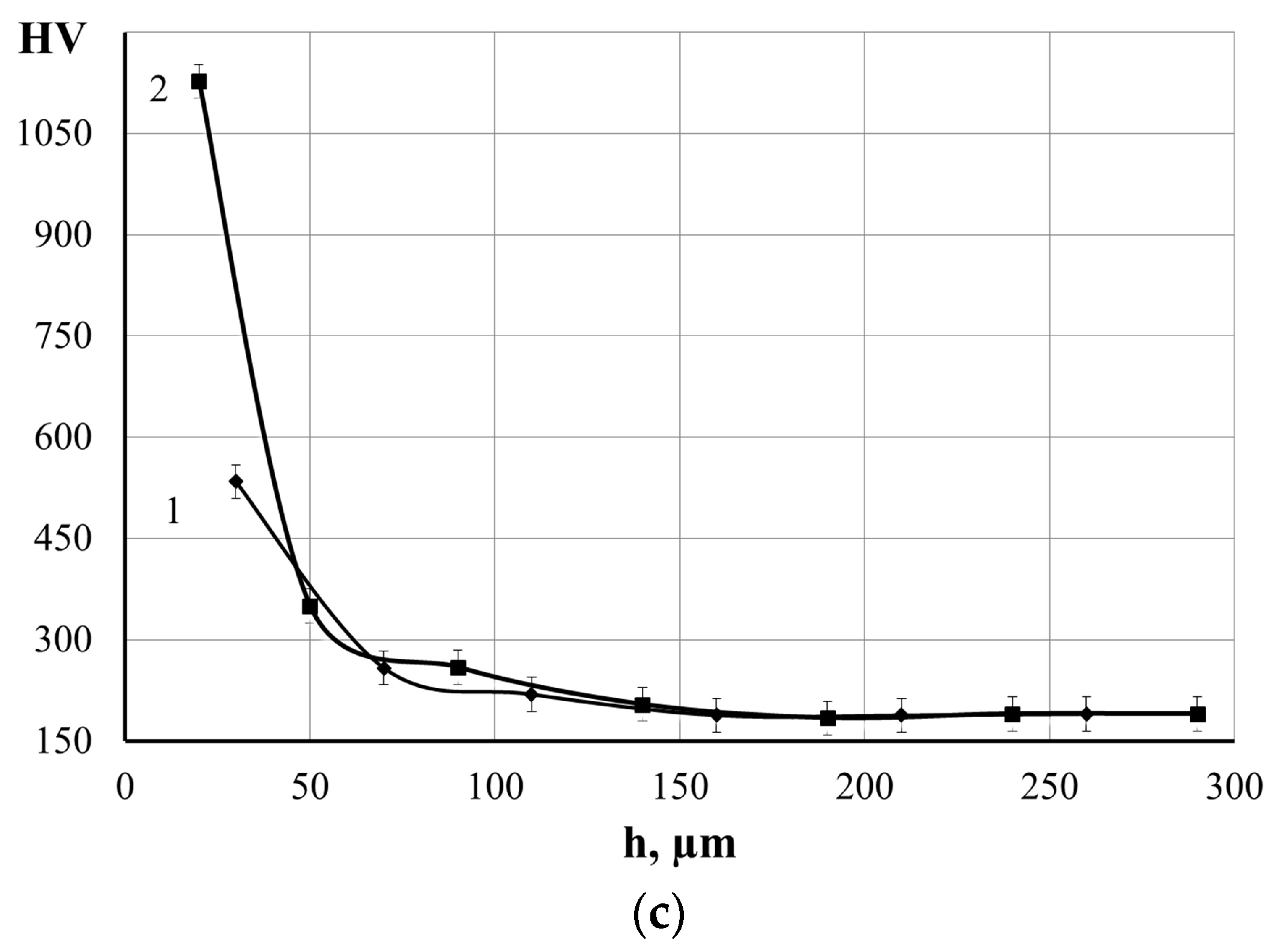
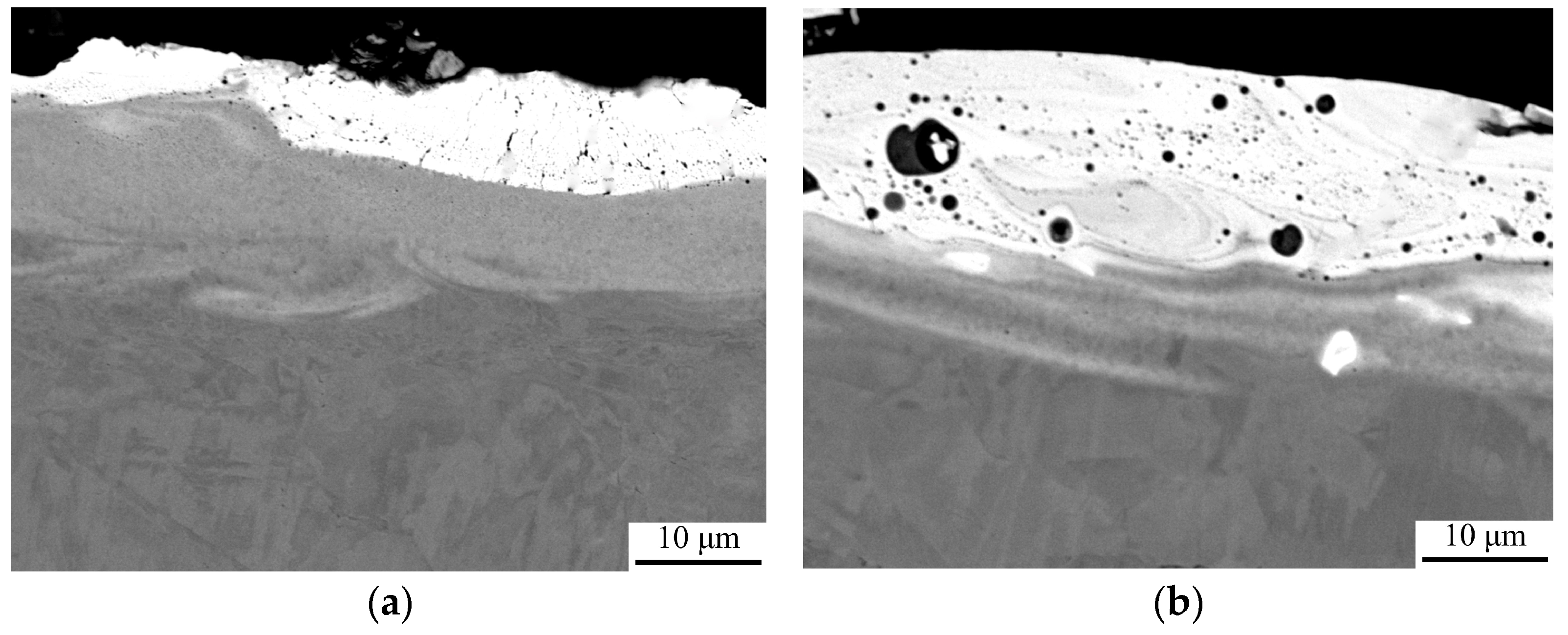
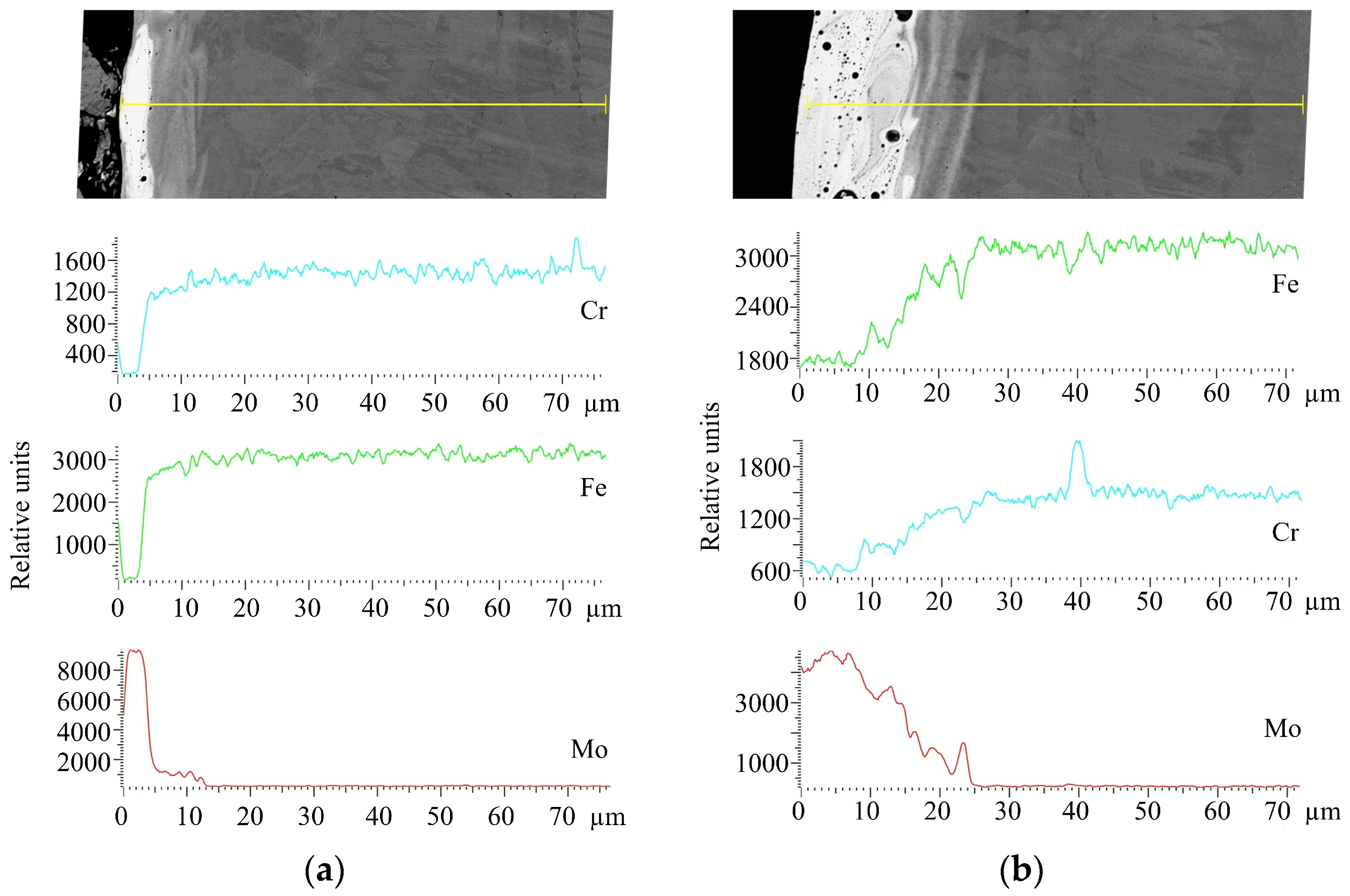
| Discharge Energy (Wp), J | Capacity of the Capacitor Battery (C), µF | Output Voltage (U), V | Vibrator Oscillation Frequency, Hz |
|---|---|---|---|
| 0.13 | 300 | 35 | 50 |
| 0.55 | 300 | 75 | 50 |
| 3.4 | 1560 | 100 | 50 |
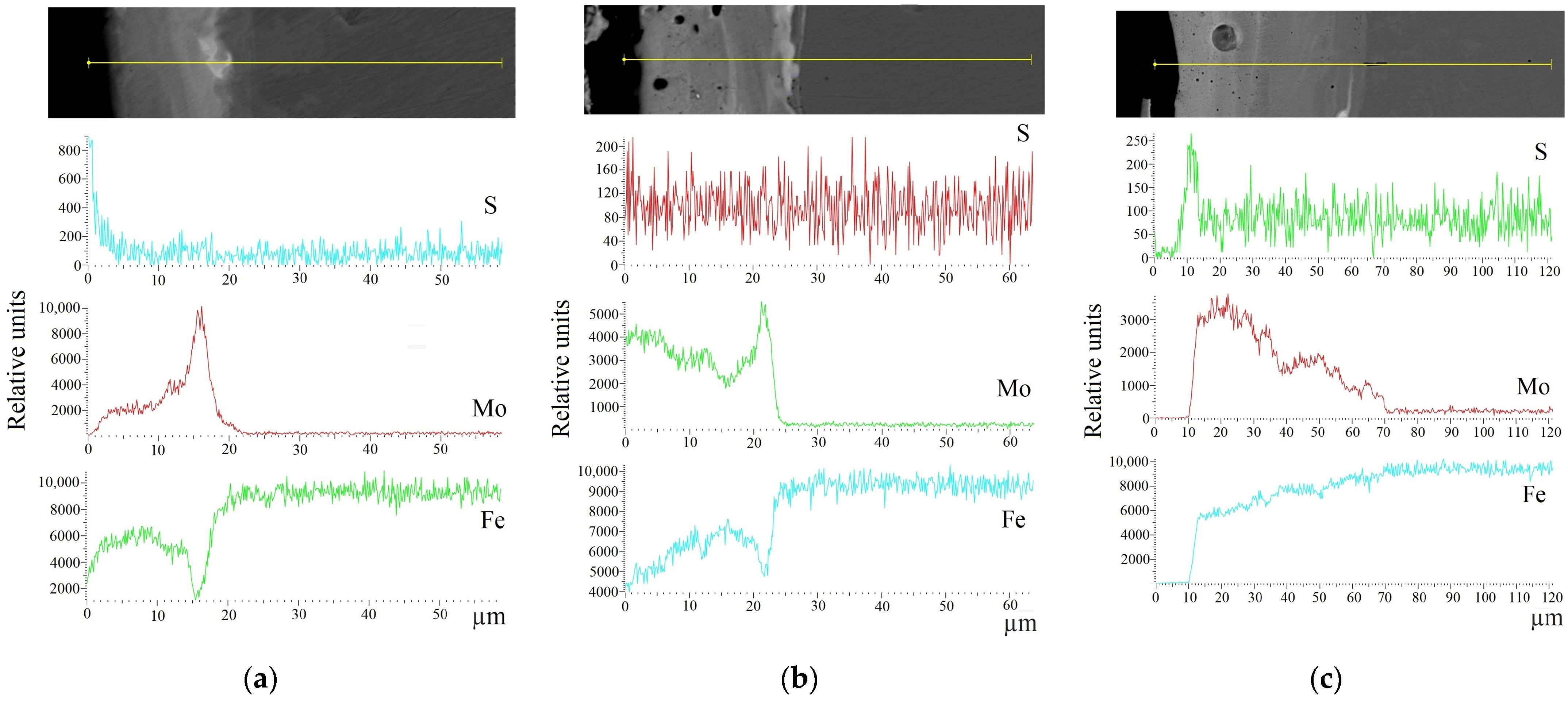
| Strategies Treatment | Substrate | Type of Processing |
|---|---|---|
| 1 | AISI 321 | ESA-processed by Mo-electrode with the use of MoS2-containing powder |
| 2 | ESA-processed by Mo-electrode with the use of S-containing paste | |
| - | ESA-processed by Mo-electrode |
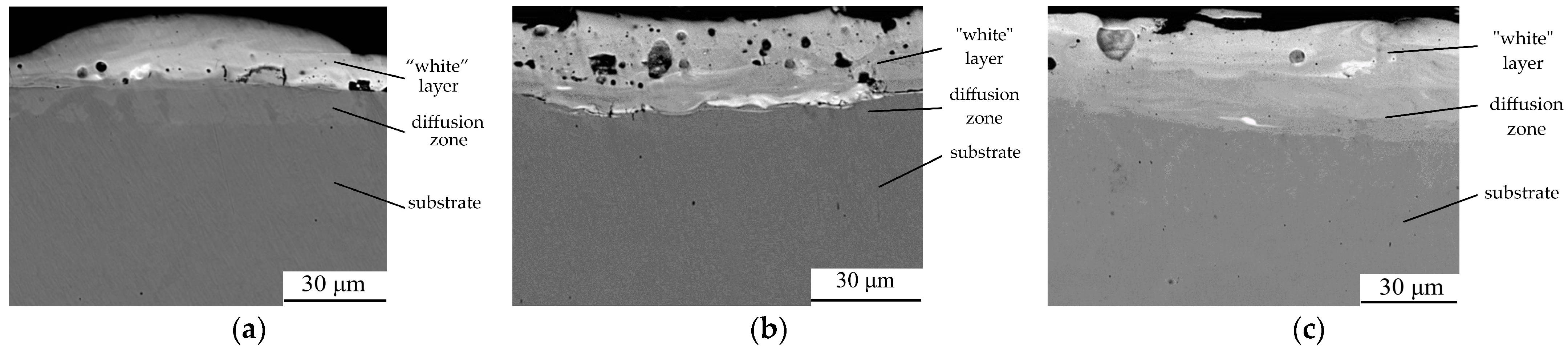
| Discharge Energy, Wp, J | Roughness, Ra, μm | Strengthened “White” Layer | ||
|---|---|---|---|---|
| HV | h, μm | Continuity, % | ||
| 0.55 | 1.2 | 534 | 20–30 | 65 |
| 3.4 | 3.2 | 1127 | 50–60 | 80 |
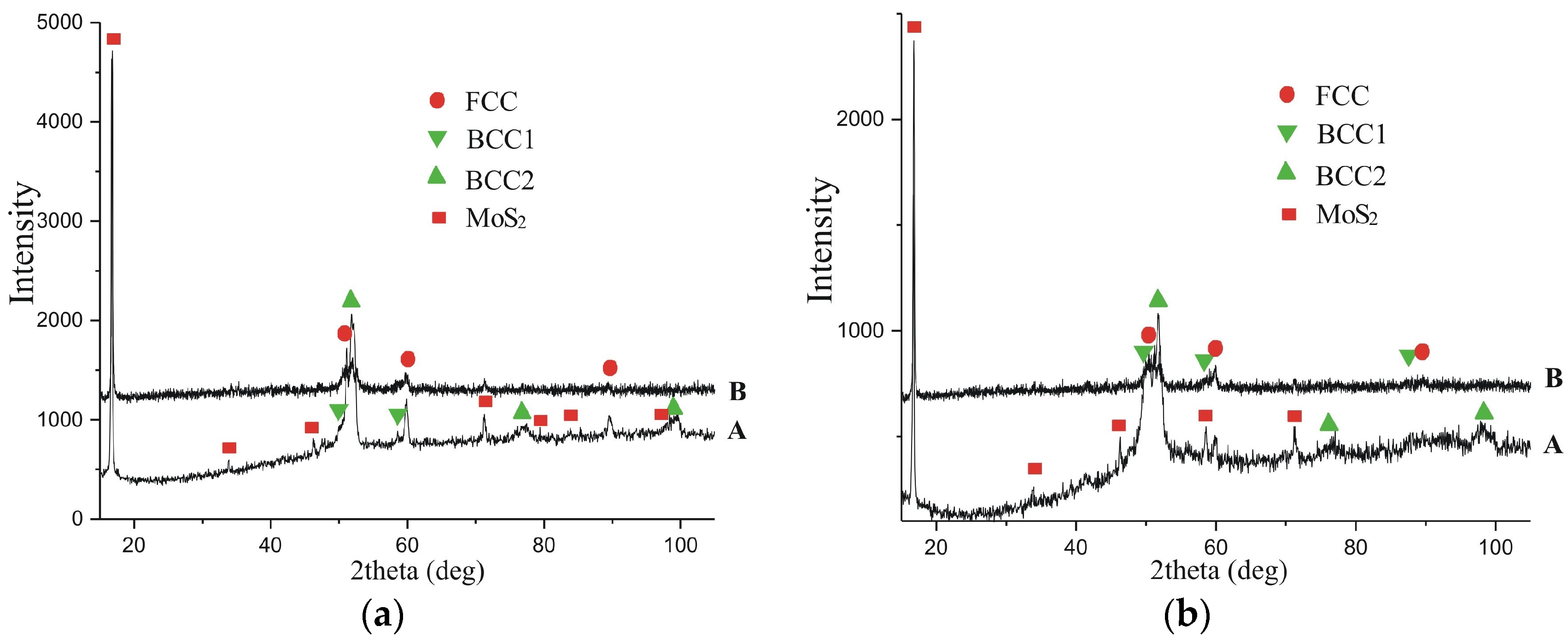
| Energy Discharge, J | Phase | Lattice Period, a, nm | Phase Content, % (wt.) |
|---|---|---|---|
| 0.55 | BCC1 solid solution on α-Fe | 2.8700 | 14.0 |
| BCC2 solid solution on α-Fe | 2.8900 | 15.0 | |
| FCC solid solution on γ-Fe | 3.5900 | 25.0 | |
| MoS2 | a = 3.1612 c = 12.2985 | 46.0 | |
| 3.4 | BCC1 solid solution on α-Fe | 2.8700 | 24.0 |
| BCC2 solid solution on α-Fe | 2.8900 | 25.0 | |
| FCC solid solution on γ-Fe | 3.6500 | 24.0 | |
| MoS2 | a = 3.1612 c = 12.2985 | 27.0 |
| Discharge Energy, Wp, J | Roughness, Ra, μm | Layer of Low Microhardness | Strengthened “White Layer” | |||
|---|---|---|---|---|---|---|
| HV | h, μm | HV | h, μm | Continuity, % | ||
| C20 Steel | ||||||
| 0.13 | 0.6 | 111.2 | 20 | 514.7 | 20 | 65 |
| 0.55 | 1.9 | 136.8 | 30 | 715.0 | 30 | 75 |
| 3.4 | 5.5 | 166.6 | 40 | 1059.6 | 50 | 90 |
| C40 Steel | ||||||
| 0.13 | 0.8 | 132.0 | 10 | 547.4 | 25 | 75 |
| 0.55 | 2.0 | 167.0 | 20 | 783.2 | 40 | 90 |
| 3.4 | 5.7 | 204.0 | 30 | 1073.1 | 70 | 95 |
| AISI 321 Steel | ||||||
| 0.13 | 0.9 | 146.4 | 10 | 651.4 | 20 | 80 |
| 0.55 | 2.2 | 173.2 | 15 | 882.7 | 30 | 90 |
| 3.4 | 6.2 | 240.3 | 20 | 1137.3 | 55 | Up to 100 |
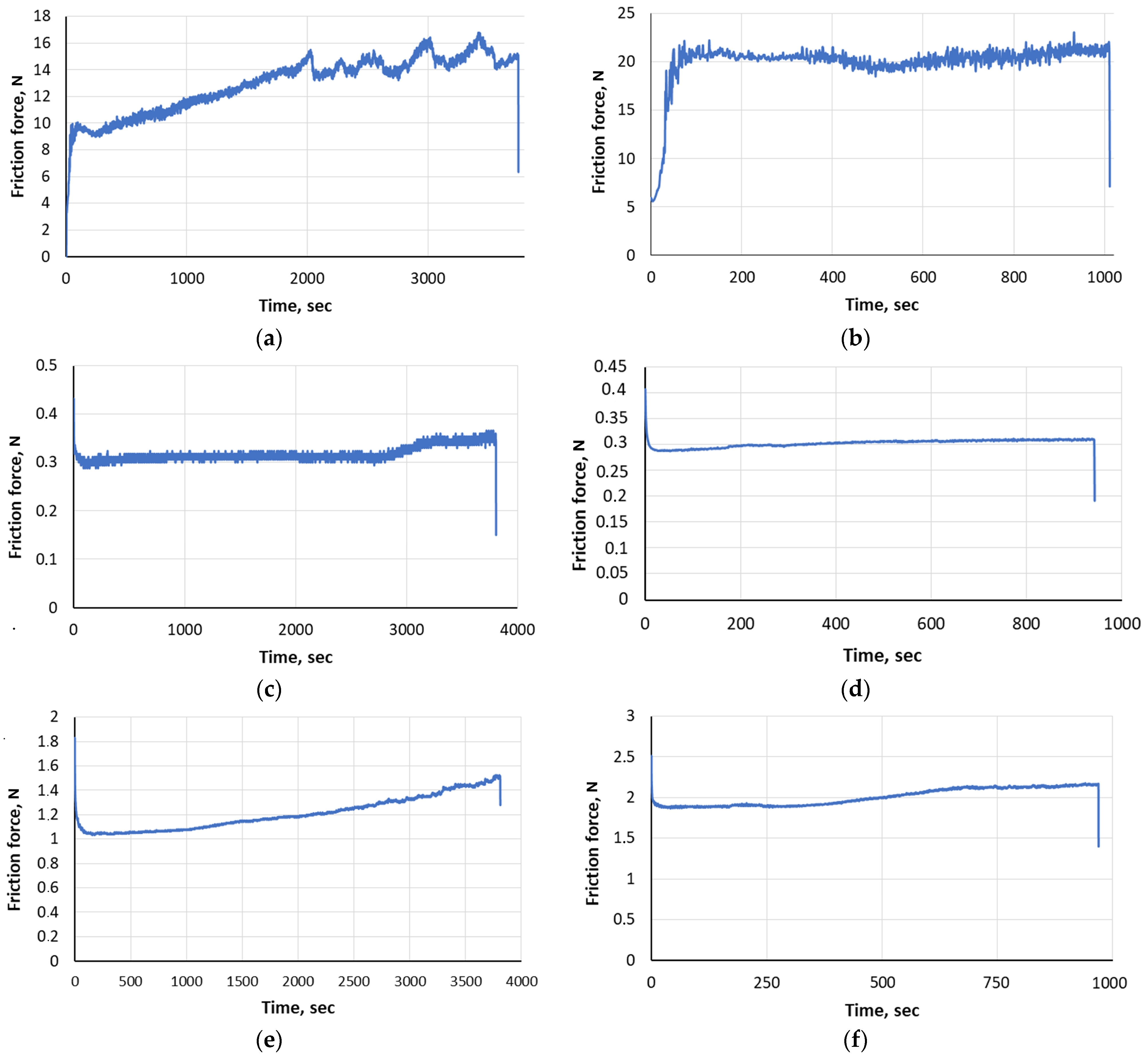
| Sample Series | Substrate | Load, P, N | Maximum Frictional Force, Fmax, N | Friction Force Average Value Faverage, N | Average Coefficient of Friction, μaverage |
|---|---|---|---|---|---|
| Mo | AISI 321 steel | 20 | 16.79 | 12.938 | 0.6469 ± 0.0257 |
| 40 | 23.06 | 19.97 | 0.4999 ± 0.0074 | ||
| Mo + S | 20 | 0.41 | 0.31 | 0.0156 ± 0.0059 | |
| 40 | 0.43 | 0.31 | 0.0078 ± 0.0001 | ||
| Mo + MoS2 | 20 | 1.83 | 1.20 | 0.0630 ± 0.0001 | |
| 40 | 2.51 | 2.00 | 0.0510 ± 0.0031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haponova, O.; Tarelnyk, V.; Mościcki, T.; Tarelnyk, N.; Półrolniczak, J.; Myslyvchenko, O.; Adamczyk-Cieślak, B.; Sulej-Chojnacka, J. Investigation of the Structure and Properties of MoS2 Coatings Obtained by Electrospark Alloying. Coatings 2024, 14, 563. https://doi.org/10.3390/coatings14050563
Haponova O, Tarelnyk V, Mościcki T, Tarelnyk N, Półrolniczak J, Myslyvchenko O, Adamczyk-Cieślak B, Sulej-Chojnacka J. Investigation of the Structure and Properties of MoS2 Coatings Obtained by Electrospark Alloying. Coatings. 2024; 14(5):563. https://doi.org/10.3390/coatings14050563
Chicago/Turabian StyleHaponova, Oksana, Viacheslav Tarelnyk, Tomasz Mościcki, Nataliia Tarelnyk, Joanna Półrolniczak, Oleksandr Myslyvchenko, Bogusława Adamczyk-Cieślak, and Joanna Sulej-Chojnacka. 2024. "Investigation of the Structure and Properties of MoS2 Coatings Obtained by Electrospark Alloying" Coatings 14, no. 5: 563. https://doi.org/10.3390/coatings14050563
APA StyleHaponova, O., Tarelnyk, V., Mościcki, T., Tarelnyk, N., Półrolniczak, J., Myslyvchenko, O., Adamczyk-Cieślak, B., & Sulej-Chojnacka, J. (2024). Investigation of the Structure and Properties of MoS2 Coatings Obtained by Electrospark Alloying. Coatings, 14(5), 563. https://doi.org/10.3390/coatings14050563








