Preparation and Properties of Environmentally Friendly, Hydrophobic, Corrosion-Resistant, Multifunctional Composite Coatings
Abstract
:1. Introduction
2. Methods
2.1. Materials and Reagents
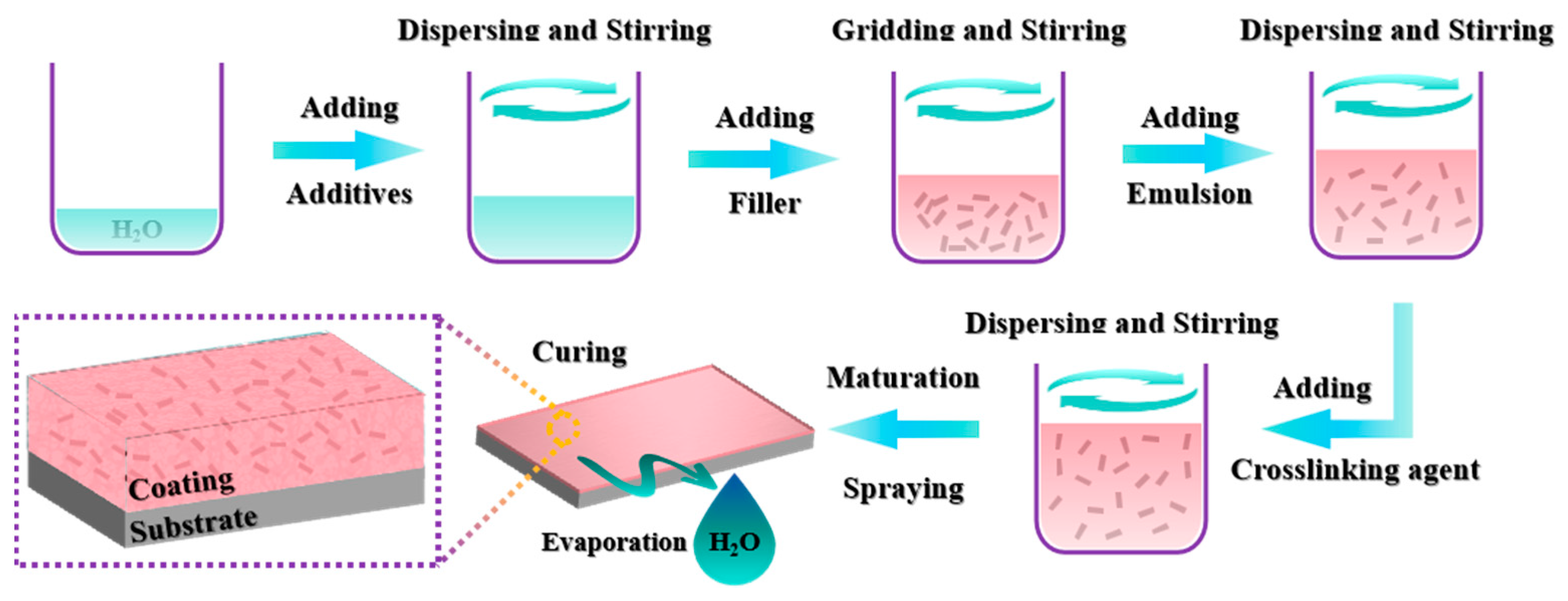
2.2. Preparation of Different Coatings
2.3. Characterization
3. Discussion and Results
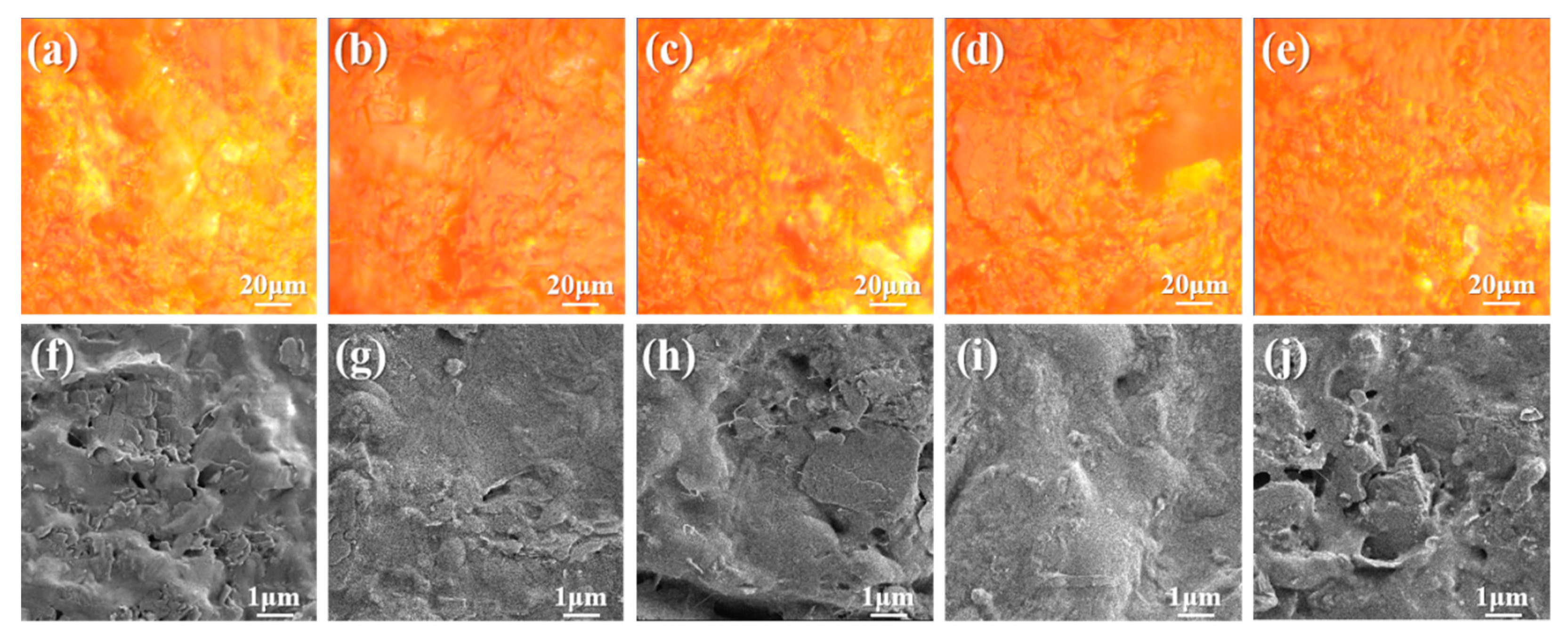
3.1. Status of Filler
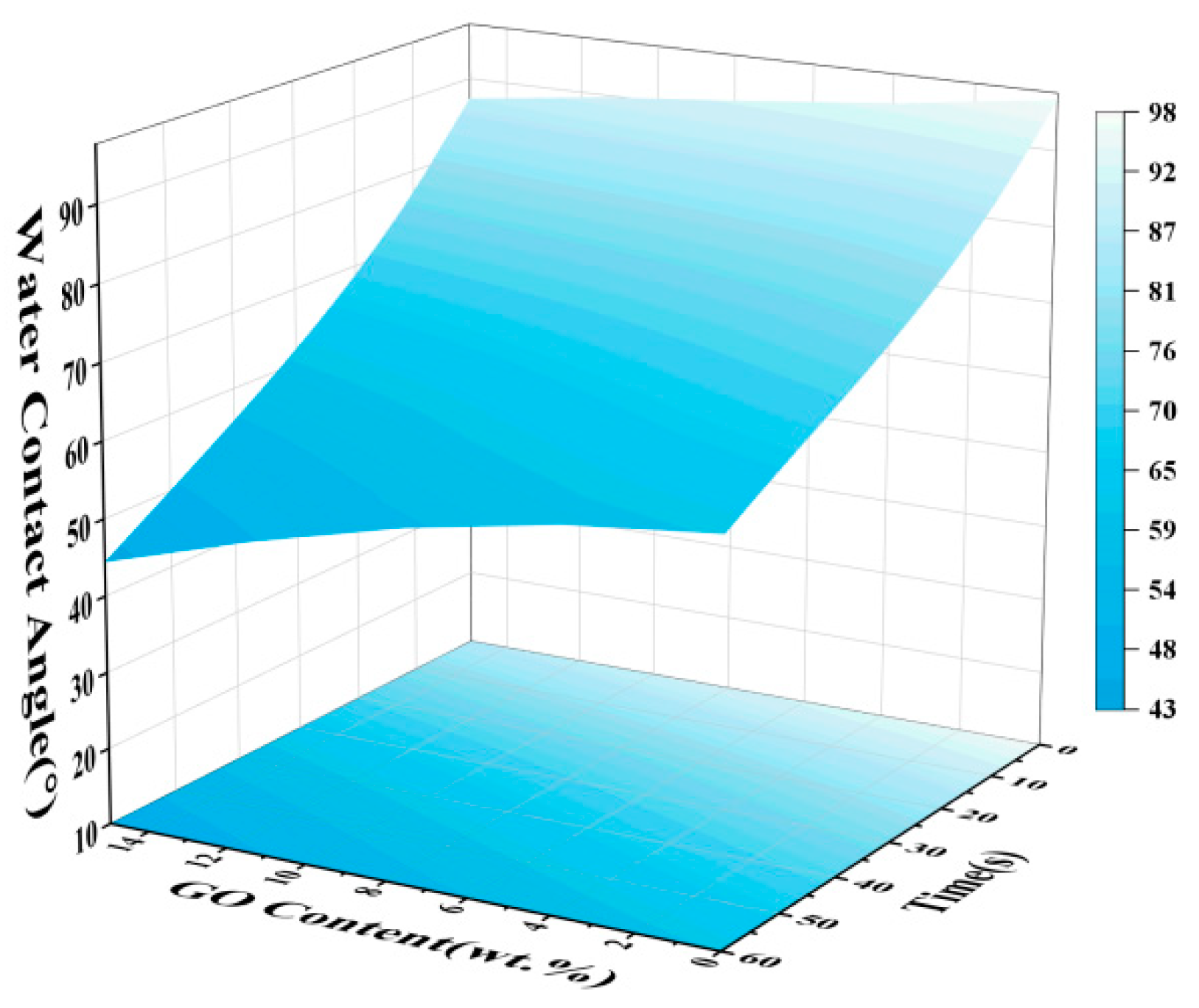
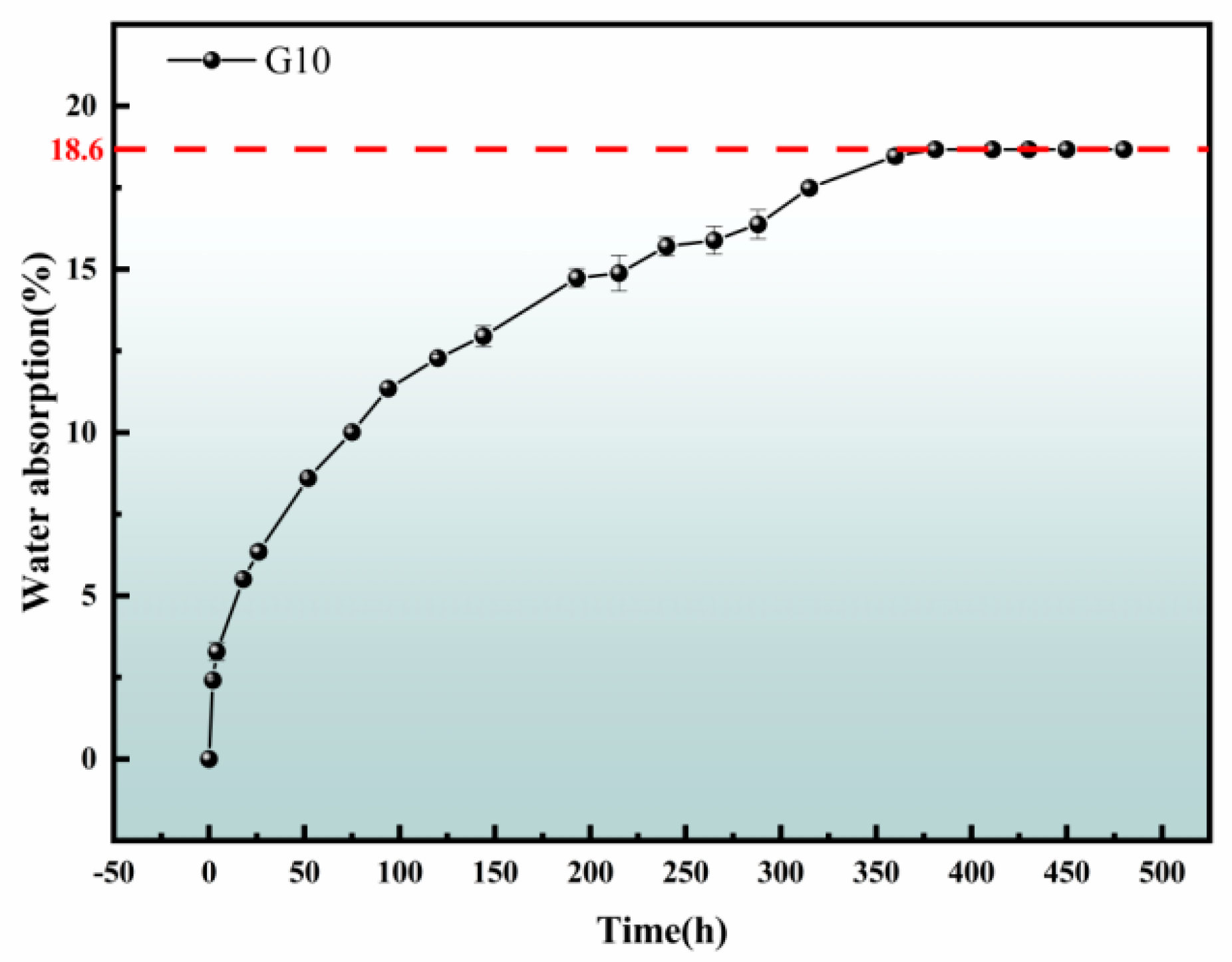
3.2. Hydrophobicity
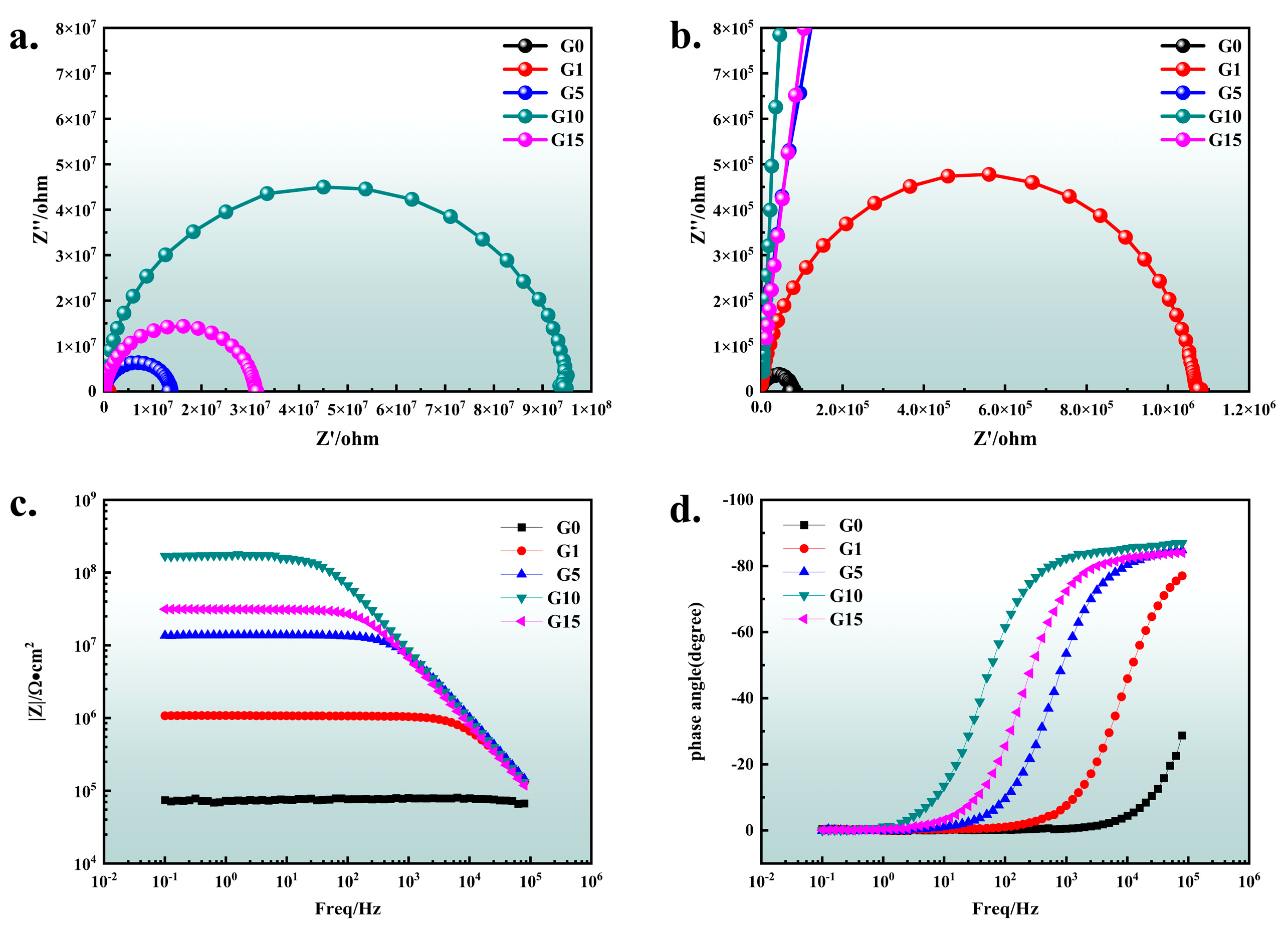
3.3. Corrosion Resistance
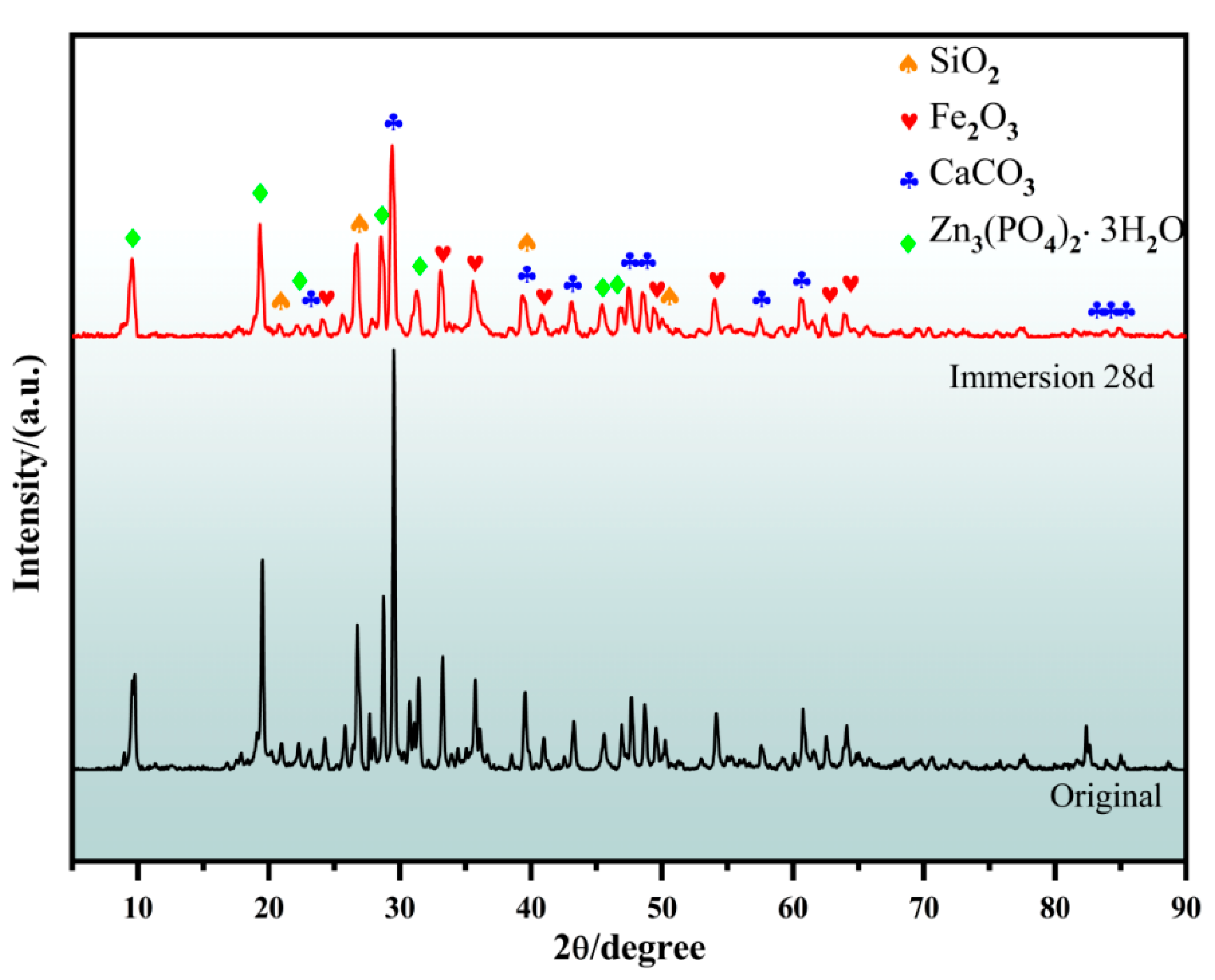
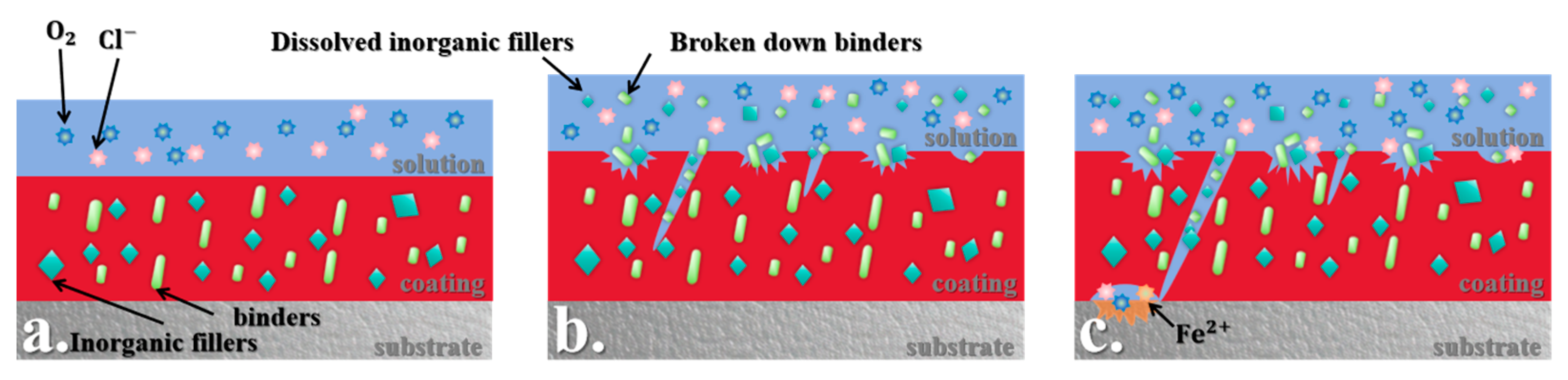
3.4. Corrosion Mechanism
3.5. Failure Mechanism Analysis of Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Cong, W.; Gui, T.; Xing, T.; Wu, L. Effect and Remediation of Biofouling on Marine Aquaculture. Mater. Rep. 2020, 34, 78–81. [Google Scholar]
- Sun, B.-K.; Fan, H.-S.; Pan, X.-L.; Lu, A.-D.; Hu, J.-K. Development of Copper-free Self-polishing Anti-fouling Paints by Using Acrylate Resin. Surf. Technol. 2022, 51, 280–286. [Google Scholar]
- Xu, F.; Wang, T.; Bohling, J.; Maurice, A.M.; Chen, H.; Wu, L.; Zhou, S. Extended hydrophobicity and self-cleaning performance of waterborne PDMS/TiO2 nanocomposite coatings under accelerated laboratory and outdoor exposure testing. J. Coat. Technol. Res. 2018, 15, 1025–1034. [Google Scholar] [CrossRef]
- Khanjani, J.; Pazokifard, S.; Zohuriaan-Mehr, M.J. Improving, dirt pickup resistance in wat erborne coatings using latex blends of acrylic/PDMS polymers. Prog. Org. Coat. 2017, 102, 151–166. [Google Scholar] [CrossRef]
- Wang, Q.; Li, C.; Li, G. Study on stability of water-based two-component, i norganic coatings based on silica sols. Electroplat. Finish. 2021, 40, 654–659. [Google Scholar]
- Shan, X.; Wang, S.; Zhang, L.; Jiang, L. Preparation of silica sol-based water-based pure in organic coatings. Appl. Chem. Ind. 2018, 47, 1195–1199. [Google Scholar]
- Wang, C.; Zhao, L.; Jia, J.; Wang, D.; Peng, Z. Effect of mixed acid functionalized carbon nanotubes doping on the electrical and thermal conductivity of epoxy resin. Trans. China Electrotech. Soc. 2019, 34, 457–464. [Google Scholar]
- Tian, Y.; Xie, Y.; Dai, F.; Huang, H.; Zhong, L.; Zhang, X. Ammonium-grafted graphene oxide for enhanced corrosion resistance of waterborne epoxy coatings. Surf. Coat. Technol. 2020, 383, 125227. [Google Scholar] [CrossRef]
- Huang, S.; Kong, G.; Yang, B.; Zhang, S.; Che, C. Effects of graphene on the corrosion evolution of zinc particles in waterborne epoxy zinc-containing coatings. Prog. Org. Coat. 2020, 140, 105531. [Google Scholar] [CrossRef]
- Li, S.; Zhang, S.; Yang, B.; Guo, H.; Li, Y.; Wen, F. Research progress of graphene anticorrosive coatings. Corros. Sci. Prot. Technol. 2019, 31, 455–461. [Google Scholar]
- Chen, S.; Brown, L.; Levendorf, M.; Cai, W.; Ju, S.Y.; Edgeworth, J.; Li, X.; Magnuson, C.W.; Velamakanni, A.; Piner, R.D.; et al. Oxidation resistance of graphene-coated Cu and Cu/Ni alloy. ACS Nano 2011, 5, 1321–1327. [Google Scholar] [CrossRef]
- Chen, C.; He, Y.; Xiao, G.; Zhong, F.; Xia, Y.; Wu, Y. Graphic C3N4-assisted dispersion of graphene to improve the corrosion resistance of waterborne epoxy coating. Prog. Org. Coat. 2020, 139, 105448. [Google Scholar] [CrossRef]
- Jiang, Y.; Jiang, H.Y.; Xiong, S.P. Effect of Reduced Graphene Oxide on Corrosion Resistance of Waterborne Inorganic Zinc-rich Coatings. Paint. Coat. Ind. 2023, 53, 68–72. [Google Scholar]
- Xiao, P.; Wang, D.-H.; Lang, J.-W. Comparison in Factors Affecting Electrochemical Properties of Thermal-Reduced Graphene Oxide for Supercapacitors. J. Electrochem. 2014, 20, 553–562. [Google Scholar]
- Darowicki, K. The application of impedance measurements for the determination of the probability of the course of corrosion processes. Corros. Sci. 1997, 39, 1087–1092. [Google Scholar] [CrossRef]
- Wang, W.J.; Guan, Z.C.; Han, J.C.; Xu, H.; Jin, X.; Di, Z.G. Application of Electrochemical Impedance Spectroscopy in the Evaluation of Protective Performance of Organic Coating. Paint. Coat. Ind. 2023, 53, 58–64. [Google Scholar]
- Gao, L.X.; Zhang, D.Q.; Zhou, G.D.; Li, H.G. Study on water absorption and corrosion protection properties of modified epoxy coatings. Chin. J. Corros. Prot. 2002, 22, 41–43. [Google Scholar]
- Van Westing, E.P.M.; Ferrari, G.M.; De Wit, J.H.W. The determination of coating performance with impedance measurements—II. Water uptake of coatings. Corros. Sci. 1994, 36, 957–977. [Google Scholar] [CrossRef]
- Cai, G.; Zhang, D.; Zhao, W.; Dong, Z. Research progress on protection performance and failure evaluation of organic coatings. Corros. Prot. 2017, 38, 657–664. [Google Scholar]
- Yuan, S.J.; Chong AM, F.; Pehkonen, S.O. The influence of the marine aerobic Pseudomonas strain on the corrosion of 70/30 Cu-Ni alloy. Corros. Sci. 2007, 49, 4352–4358. [Google Scholar] [CrossRef]
- Yang, L.H.; Liu, F.C.; Zhang, Z.Q.; He, P.; Han, E.H.; Qi, L. Research on the Anti-Diffusion Behavior of ZnO/Polyurethane Coatings. J. Peking Univ. Nat. Sci. 2006, 91–96. [Google Scholar]
- Wu, C.; Lin, Z.; Li, X.; Ma, B.; Xu, L. Electrochemical Corrosion Behavior of Zinc Powder Sherardized Carbon Steel. Corros. Sci. Prot. Technol. 2014, 26, 441–445. [Google Scholar]
- de Medeiros, A.M.; Khan, L.U.; da Silva, G.H.; Ospina, C.A.; Alves, O.L.; de Castro, V.L.; Martinez, D.S. Graphene oxide-silver nanoparticle hybrid material: An integrated nanosafety study in zebrafish embryos. Ecotoxicol. Environ. Saf. 2021, 209, 111776. [Google Scholar] [CrossRef] [PubMed]
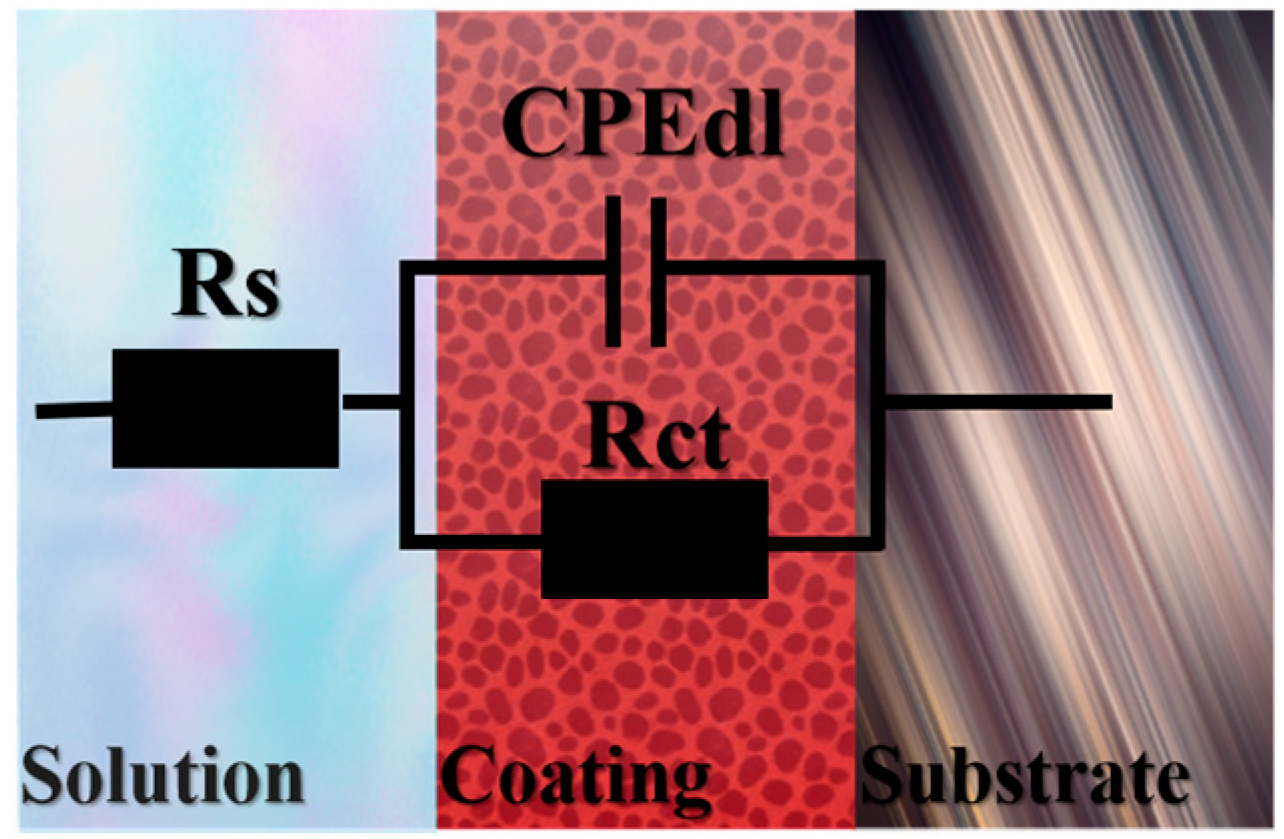
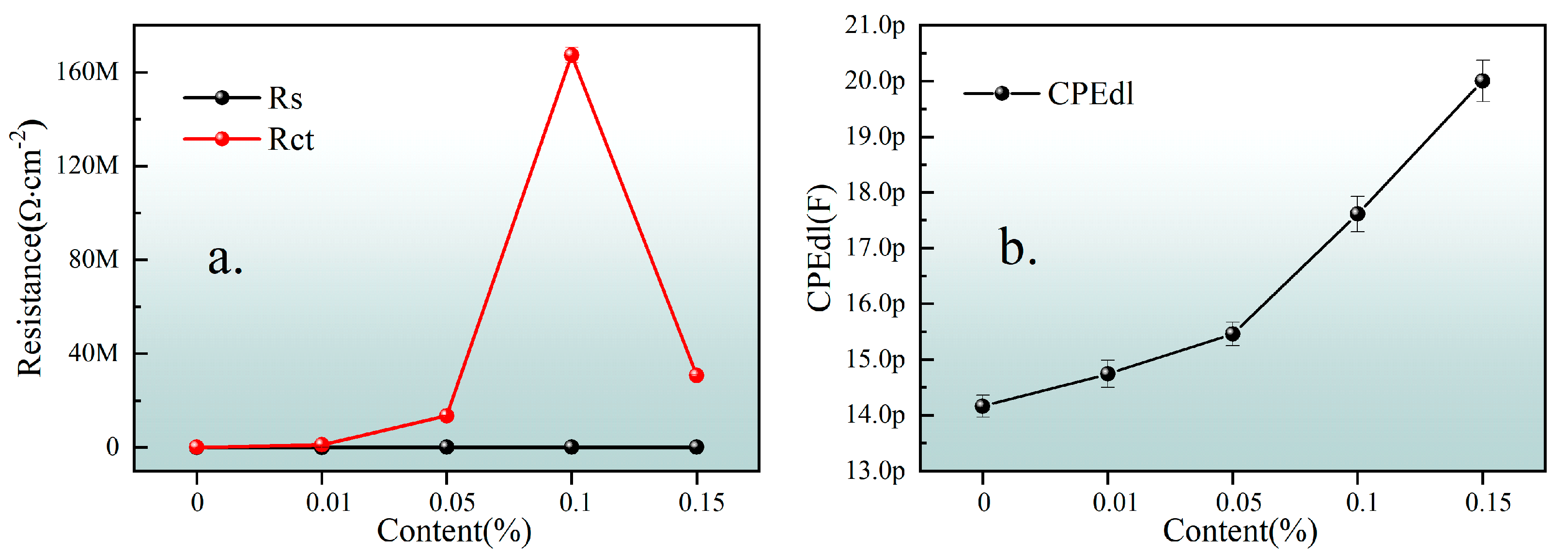



| Content (wt. %) | Rs (Ω·cm−2) | Error (%) | CPEdl (F) | Error (%) | Rct (Ω·cm−2) | Error (%) | Goodness of Fit |
|---|---|---|---|---|---|---|---|
| 0 | 0.00126 | 2.06 × 107 | 1.416 × 10−11 | 1.404 | 75,910 | 0.5475 | 0.00133 |
| 0.01 | 846.6 | 57.38 | 1.474 × 10−11 | 1.647 | 1.024 × 106 | 1.16 | 0.00547 |
| 0.05 | 16,860 | 23.76 | 1.546 × 10−11 | 1.37 | 1.351 × 107 | 0.9059 | 0.00404 |
| 0.1 | 11,140 | 51.88 | 1.761 × 10−11 | 1.823 | 1.673 × 108 | 1.987 | 0.011 |
| 0.15 | 17,080 | 27.35 | 2 × 10−11 | 1.863 | 3.064 × 107 | 1.621 | 0.00909 |
| Time (Day) | Rs (Ω·cm−2) | Error (%) | CPEdl (F) | Error (%) | Rct (Ω·cm−2) | Error (%) | Goodness of Fit |
|---|---|---|---|---|---|---|---|
| 0 | 5563 | 25.62 | 4.104 × 10−11 | 1.028 | 9.353 × 107 | 1.164 | 3.62 × 10−3 |
| 1 | 4587 | 19.99 | 4.426 × 10−11 | 1.054 | 1.266 × 106 | 0.6227 | 1.72 × 10−3 |
| 4 | 5157 | 17.16 | 4.566 × 10−11 | 1.926 | 142,000 | 0.7506 | 1.34 × 10−3 |
| 7 | 4070 | 10.42 | 4.686 × 10−11 | 1.561 | 63,960 | 0.6546 | 2.196 × 10−4 |
| 14 | 2810 | 12.09 | 4.823 × 10−11 | 1.283 | 62,420 | 0.5377 | 1.562 × 10−4 |
| 21 | 2109 | 12.94 | 5.298 × 10−11 | 2.398 | 23,670 | 1.133 | 4.756 × 10−5 |
| 28 | 3313 | 20.03 | 6.327 × 10−11 | 7.795 | 17,570 | 3.72 | 2.71 × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, Z.; Zhang, Z.; Tang, W.; Lu, J.; Xu, J. Preparation and Properties of Environmentally Friendly, Hydrophobic, Corrosion-Resistant, Multifunctional Composite Coatings. Coatings 2024, 14, 586. https://doi.org/10.3390/coatings14050586
Chu Z, Zhang Z, Tang W, Lu J, Xu J. Preparation and Properties of Environmentally Friendly, Hydrophobic, Corrosion-Resistant, Multifunctional Composite Coatings. Coatings. 2024; 14(5):586. https://doi.org/10.3390/coatings14050586
Chicago/Turabian StyleChu, Zhenhua, Zhixin Zhang, Wan Tang, Jiahao Lu, and Jingxiang Xu. 2024. "Preparation and Properties of Environmentally Friendly, Hydrophobic, Corrosion-Resistant, Multifunctional Composite Coatings" Coatings 14, no. 5: 586. https://doi.org/10.3390/coatings14050586
APA StyleChu, Z., Zhang, Z., Tang, W., Lu, J., & Xu, J. (2024). Preparation and Properties of Environmentally Friendly, Hydrophobic, Corrosion-Resistant, Multifunctional Composite Coatings. Coatings, 14(5), 586. https://doi.org/10.3390/coatings14050586








