Pulsed Electro Decoration of Carbon Nanotubes with FexZn1−xS
Abstract
:1. Introduction
2. Experimental Part
2.1. CNT Forest Preparation
2.2. Pulsed Electrodeposition of Fe/Zn and Sulfurization
2.3. Characterization Techniques
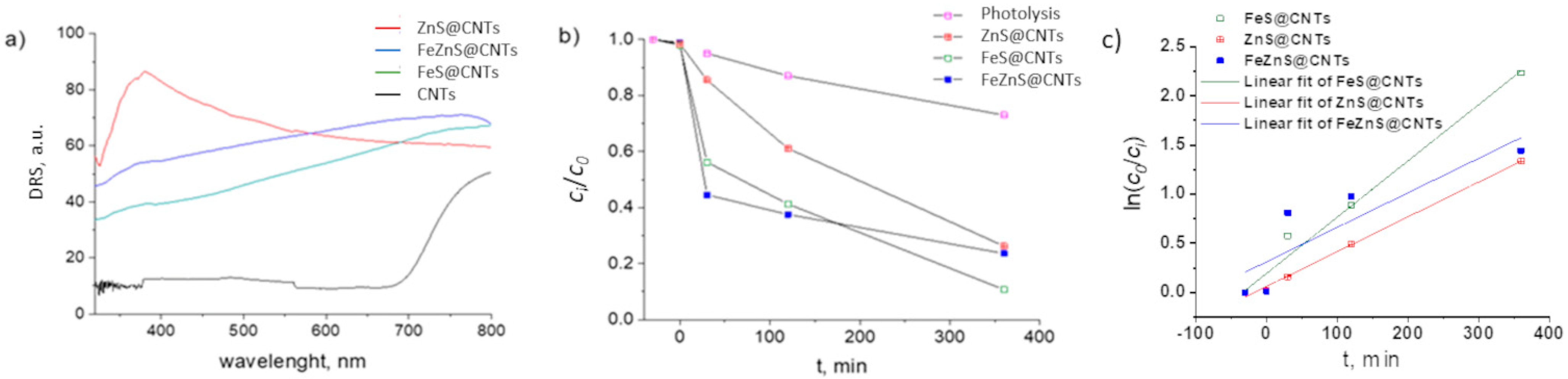
2.4. Photocatalytic Measurements
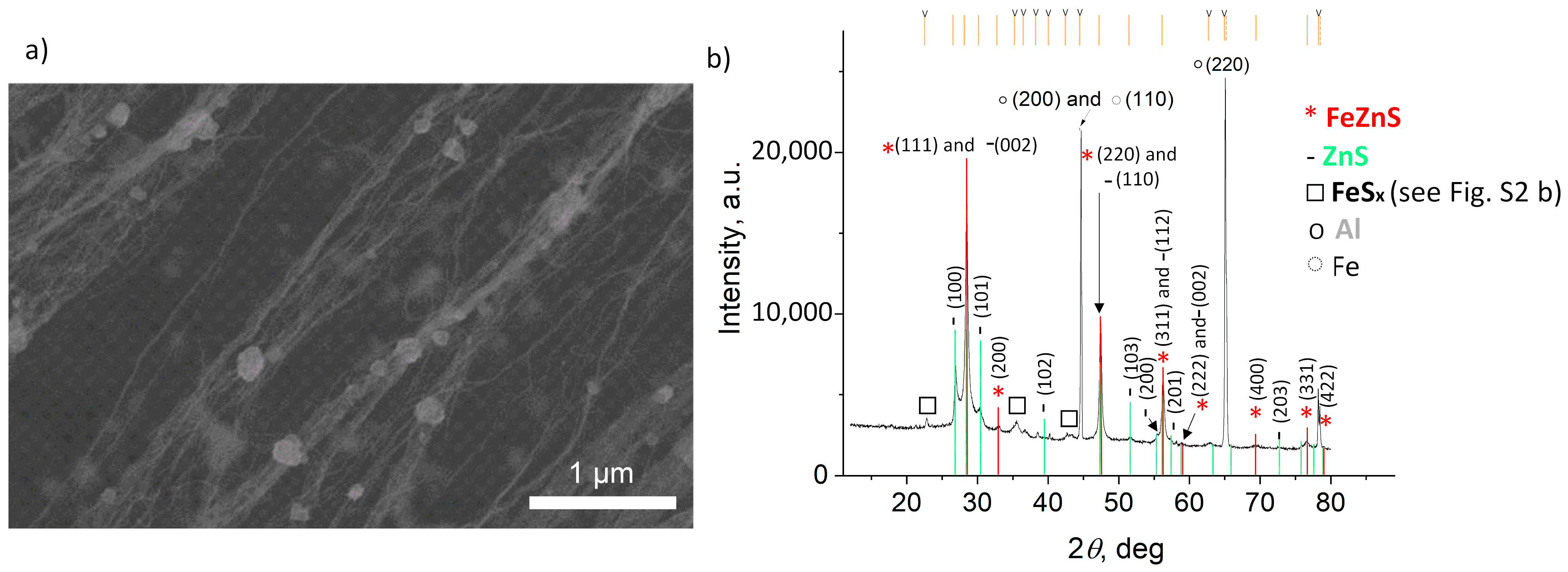
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Qin, H.; Fang, Y.; Liu, J.; Meng, L. FeS2-sensitized ZnO/ZnS nanorod arrays for the photoanodes of quantum-dot-sensitized solar cells. RSC Adv. 2015, 5, 105324–105328. [Google Scholar] [CrossRef]
- Yu, H.; Qian, C.; Ren, H.; Chen, M.; Tang, D.; Wu, H.; Lv, R. Enhanced catalytic properties of bimetallic sulfides with the assistance of graphene oxide for accelerating triiodide reduction in dye-sensitized solar cells. Sol. Energy 2020, 207, 1037–1044. [Google Scholar] [CrossRef]
- Zhu, M.; Shi, J.; Xin, X.; Wu, J.; Yao, X. Bimetallic Hexagonal Layered Ni–Co Sulfides with High Electrochemical Performance for All-Solid-State Lithium Batteries. ACS Sustain. Chem. Eng. 2021, 9, 17061–17067. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, D.; Li, X.; Sari, H.M.K.; Peng, J.; Li, Y.; Li, Y.; Li, D.; Sun, Q.; Sun, X. Recent Advances of Bimetallic Sulfide Anodes for Sodium Ion Batteries. Front. Chem. 2020, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, J.; Ma, J.; Liu, Z.; Li, Y. A bimetallic sulfide CuCo2S4 with good synergistic effect was constructed to drive high performance photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 552, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ou, X.; Xiang, Q.; Carabineiro, S.A.; Fan, J.; Lv, K. Research progress in metal sulfides for photocatalysis: From activity to stability. Chemosphere 2022, 303, 135085. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Lin, C.; Li, S.; Wang, Y.; Chen, C.; Chen, C. Solution-Processable Pyrite FeS2 Nanocrystals for the Fabrication of Heterojunction Photodiodes with Visible to NIR Photodetection. Adv. Mater. 2012, 24, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Tang, G.; Zhu, W.; Luo, Y.; Gao, X. In situ construction of Z-scheme FeS2/Fe2O3 photocatalyst via structural transformation of pyrite for photocatalytic degradation of carbamazepine and the synergistic reduction of Cr(VI). J. Environ. Sci. 2021, 101, 351–360. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lee, Z.; Wang, C.-F. Photocatalytic hydrogen production by stainless steel@ZnS core–shell wire mesh photocatalyst from saltwater. Int. J. Hydrogen Energy 2014, 39, 20754–20763. [Google Scholar] [CrossRef]
- Molland, N.-A.; Ghadyani, Z.; Karhu, E.A.; Poggio, S.; Nematollahi, M.; Kildemo, M.; Reenaas, T.W.; BelBruno, J.J.; Gibson, U.J. Band-edge modification and mid-infrared absorption of co-deposited FexZn1−xS thin films. Opt. Mater. Express 2015, 5, 1613. [Google Scholar] [CrossRef]
- Su, R.; Xue, Y.; Zhang, G.; Wang, Q.; Hu, L.; Wang, P. Optimization and Degradation Mechanism of Photocatalytic Removal of Bisphenol A Using Zn0.9Fe0.1S Synthesized by Microwave-assisted Method. Photochem. Photobiol. 2016, 92, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Kamazani, H.A.; Feizbakhsh, A.; Konoz, E.; Panahi, H.A. Zinc doped FeS2/MWCNTs nanocomposites for photo-degradation application: Preparation and structural. Mater. Res. Express 2019, 6, 105092. [Google Scholar] [CrossRef]
- Sarma, M.; Jaiswal, M.K.; Podder, S.; Bora, J.; Karmakar, S.; Choudhury, B.; Pal, A.R. A study on the applicability of thin film over powder for visible light photocatalysis. Phys. B Condens. Matter 2023, 670, 415354. [Google Scholar] [CrossRef]
- Deulkar, S.; Bhosale, C.; Sharon, M. Optical studies on non-stiochiometric (Zn, Fe)S chalcogenide bulk pellets prepared by coprecipitation. Mater. Chem. Phys. 2008, 111, 260–264. [Google Scholar] [CrossRef]
- Osadchii, E.G.; Gorbaty, Y.E. Raman spectra and unit cell parameters of sphalerite solid solutions (FexZn1−xS). Geochim. Cosmochim. Acta 2010, 74, 568–573. [Google Scholar] [CrossRef]
- Bjelajac, A.; Florea, I.; Zamfir, M.; Tusseau-Nenez, S.; Cojocaru, C.S. Photocatalytic active ZnO1-x S x @CNTs heteronanostructures. Nanotechnology 2023, 34, 495704. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Gohier, A.; Bourée, J.E.; Châtelet, M.; Cojocaru, C.-S. The role of catalytic nanoparticle pretreatment on the growth of vertically aligned carbon nanotubes by hot-filament chemical vapor deposition. Thin Solid Films 2015, 575, 84–91. [Google Scholar] [CrossRef]
- Castan, A.; Forel, S.; Catala, L.; Florea, I.; Fossard, F.; Bouanis, F.; Andrieux-Ledier, A.; Mazerat, S.; Mallah, T.; Huc, V.; et al. New method for the growth of single-walled carbon nanotubes from bimetallic nanoalloy catalysts based on Prussian blue analog precursors. Carbon 2017, 123, 583–592. [Google Scholar] [CrossRef]
- He, Z.; Maurice, J.-L.; Lee, C.; Gohier, A.; Pribat, D.; Legagneux, P.; Cojocaru, C. Etchant-induced shaping of nanoparticle catalysts during chemical vapour growth of carbon nanofibres. Carbon 2011, 49, 435–444. [Google Scholar] [CrossRef]
- Lee, S.; Peng, J.-W. Effect of plasma treatment on electrical conductivity and Raman spectra of carbon nanotubes. J. Phys. Chem. Solids 2011, 72, 1101–1103. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, L.; Liu, P.; Sheng, L.; Fan, S. Plasma etching carbon nanotube arrays and the field emission properties. Diam. Relat. Mater. 2004, 13, 1609–1613. [Google Scholar] [CrossRef]
- Hussain, S.; Amade, R.; Jover, E.; Bertran, E. Functionalization of carbon nanotubes by water plasma. Nanotechnology 2012, 23, 385604. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Pelaez, Z.; Valles, E. Electrodeposition of zinc+iron alloys I. Analysis of the initial stages of the anomalous codeposition. J. Electroanal. Chem. 1999, 469, 139–149. [Google Scholar] [CrossRef]
- Marquardt, B.; Eude, L.; Gowtham, M.; Cho, G.; Jeong, H.J.; Châtelet, M.; Cojocaru, C.S.; Kim, B.S.; Pribat, D. Density control of electrodeposited Ni nanoparticles/nanowires inside porous anodic alumina templates by an exponential anodization voltage decrease. Nanotechnology 2008, 19, 405607. [Google Scholar] [CrossRef] [PubMed]
- Gioia, D.; Casella, I.G. Pulsed electrodeposition of palladium nano-particles on coated multi-walled carbon nanotubes/nafion composite substrates: Electrocatalytic oxidation of hydrazine and propranolol in acid conditions. Sens. Actuators B Chem. 2016, 237, 400–407. [Google Scholar] [CrossRef]
- Aliofkhazraei, M.; Ahangarani, S.; Rouhaghdam, A.S. Effect of the duty cycle of pulsed current on nanocomposite layers formed by pulsed electrodeposition. Rare Met. 2010, 29, 209–213. [Google Scholar] [CrossRef]
- Ghosh, C.K. Quantum Effect on Properties of Nanomaterials. In Introduction to Nano: Basics to Nanoscience and Nanotechnology; Sengup; Springer: Berlin/Heidelberg, Germany, 2015; pp. 73–111. [Google Scholar] [CrossRef]
- Hawsawi, A.; Jo, S.-I.; Kang, J.S.; Park, K.-C.; Jeong, G.-H. Water vapor-induced structure modification of vertically-aligned carbon nanotube arrays and successive thin film coating for enhanced field emission properties. Curr. Appl. Phys. 2020, 20, 498–504. [Google Scholar] [CrossRef]
- Skinner, B.J.; Barton, J.; Kullerud, G. Effect of FeS on the unit cell edge of sphalerite, a revision. Econ. Geol. 1959, 54, 1040–1046. [Google Scholar] [CrossRef]
- Kullerud, G. The FeS-ZnS System, a geological thermometer. Nor. Geol. Tidsskr. 1953, 32, 61–147. Available online: http://books.google.ru/books?id=oM59mAEACAAJ (accessed on 1 January 2024).
- Burton, B.P.; Perrot, P. Phase Diagram of Binary Iron Alloys; Okamoto, H., Ed.; American Society for Metals: Material Park, OH, USA, 1993; p. 459. [Google Scholar]
- Shewmon, P.; Abbas, M.; Meyrick, G. Anomalously fast diffusion in the Fe-Zn System. Met. Trans. A 1986, 17, 1523–1527. [Google Scholar] [CrossRef]
- Tang, Y.; Tian, J.; Malkoske, T.; Le, W.; Chen, B. Facile ultrasonic synthesis of novel zinc sulfide/carbon nanotube coaxial nanocables for enhanced photodegradation of methyl orange. J. Mater. Sci. 2017, 52, 1581–1589. [Google Scholar] [CrossRef]
- Lonkar, S.P.; Pillai, V.V.; Alhassan, S.M. Facile and scalable production of heterostructured ZnS-ZnO/Graphene nano-photocatalysts for environmental remediation. Sci. Rep. 2018, 8, 13401. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Tao, C.; Yu, F.; Zhao, Z.; Wang, Z.; Deng, N.; Huang, X. Ball-milled layer double hydroxide as persulfate activator for efficient degradation of organic: Alkaline sites-triggered non-radical mechanism. J. Hazard. Mater. 2024, 461, 132219. [Google Scholar] [CrossRef] [PubMed]
- Weng, B.; Qi, M.-Y.; Han, C.; Tang, Z.-R.; Xu, Y.-J. Photocorrosion Inhibition of Semiconductor-Based Photocatalysts: Basic Principle, Current Development, and Future Perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Han, C.; Yang, M.-Q.; Weng, B.; Xu, Y.-J. Improving the photocatalytic activity and anti-photocorrosion of semiconductor ZnO by coupling with versatile carbon. Phys. Chem. Chem. Phys. 2014, 16, 16891–16903. [Google Scholar] [CrossRef]
- Geldasa, F.T.; Kebede, M.A.; Shura, M.W.; Hone, F.G. Experimental and computational study of metal oxide nanoparticles for the photocatalytic degradation of organic pollutants: A review. RSC Adv. 2023, 13, 18404–18442. [Google Scholar] [CrossRef]


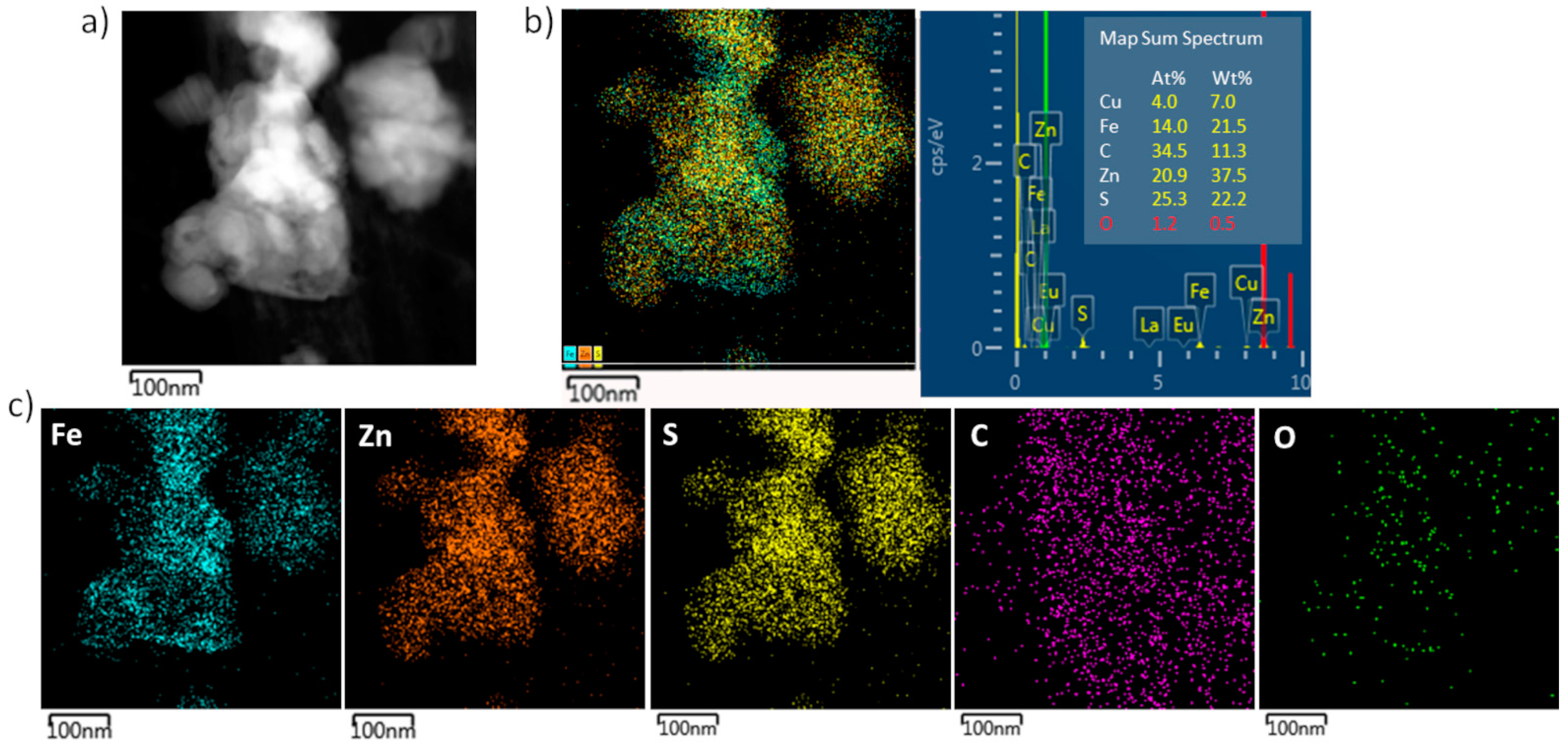
| Sample/Element, at.% | C K | O K | Al K | Fe K | Zn K | S | Total |
|---|---|---|---|---|---|---|---|
| After PED of Fe | 17.80 +/- 2.33 | 21.26 +/- 2.51 | 23.80 +/- 0.78 | 37.15 +/- 2.15 | 0.00 | 0.00 | 100.00 |
| After PED of Fe/Zn | 31.97 +/- 1.61 | 17.06 +/- 1.56 | 24.74 +/- 0.46 | 15.44 +/- 0.76 | 10.79 +/- 1.10 | 0.00 | 100.00 |
| After sulfurization | 37.04 +/- 3.05 | 3.19 +/- 1.49 | 43.40 +/- 0.82 | 3.19 +/- 0.57 | 4.20 +/- 1.15 | 8.97 +/- 0.42 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjelajac, A.; Florea, I.; Zamfir, M.; Tusseau-Nenez, S.; Cojocaru, C.S. Pulsed Electro Decoration of Carbon Nanotubes with FexZn1−xS. Coatings 2024, 14, 619. https://doi.org/10.3390/coatings14050619
Bjelajac A, Florea I, Zamfir M, Tusseau-Nenez S, Cojocaru CS. Pulsed Electro Decoration of Carbon Nanotubes with FexZn1−xS. Coatings. 2024; 14(5):619. https://doi.org/10.3390/coatings14050619
Chicago/Turabian StyleBjelajac, Andjelika, Ileana Florea, Mihai Zamfir, Sandrine Tusseau-Nenez, and Costel Sorin Cojocaru. 2024. "Pulsed Electro Decoration of Carbon Nanotubes with FexZn1−xS" Coatings 14, no. 5: 619. https://doi.org/10.3390/coatings14050619
APA StyleBjelajac, A., Florea, I., Zamfir, M., Tusseau-Nenez, S., & Cojocaru, C. S. (2024). Pulsed Electro Decoration of Carbon Nanotubes with FexZn1−xS. Coatings, 14(5), 619. https://doi.org/10.3390/coatings14050619








