Study on Stable Loose Sandstone Reservoir and Corresponding Acidizing Technology
Abstract
1. Introduction
2. Methodology
2.1. Reservoir Rock and Oil Pipe Scale
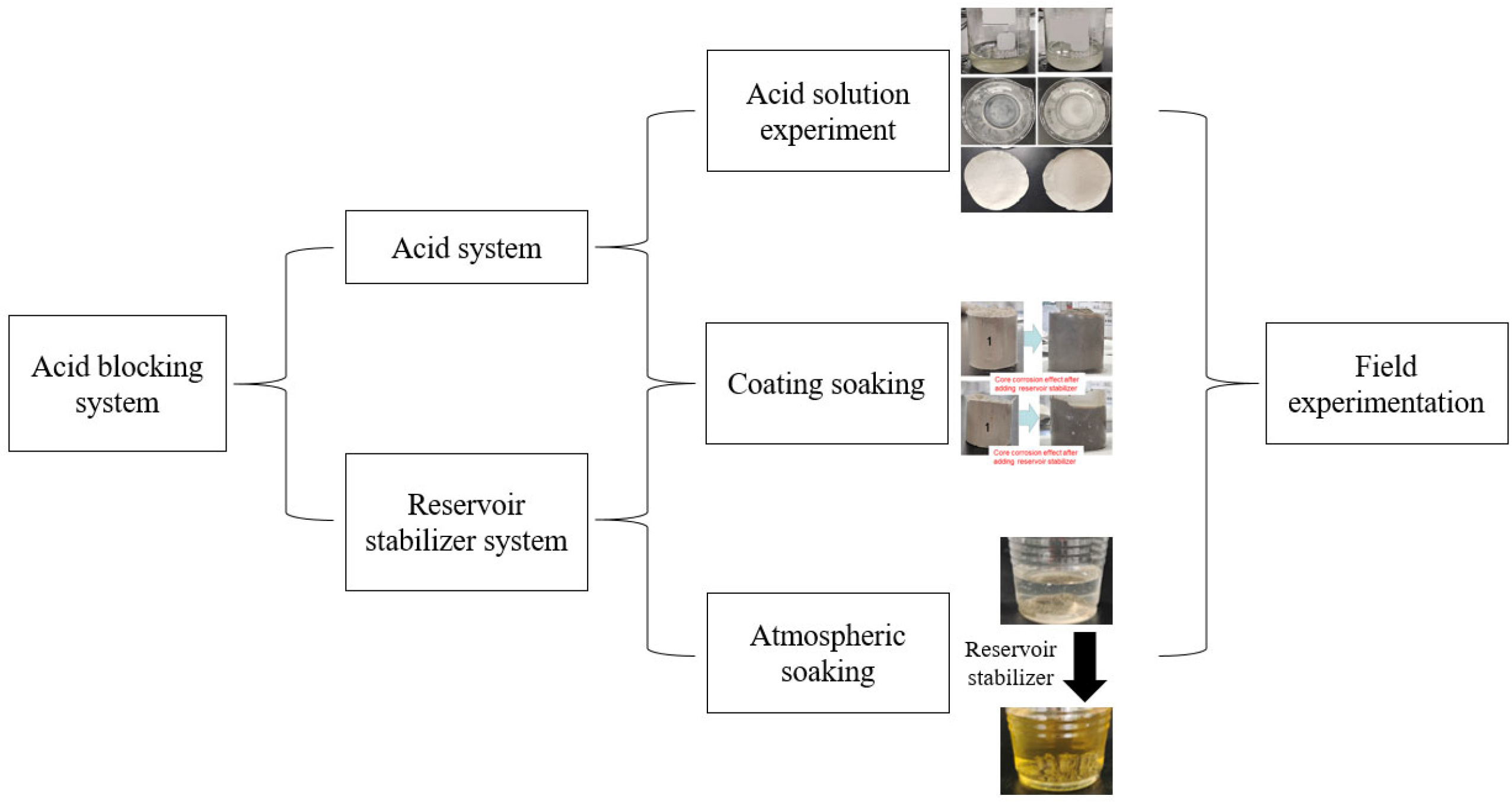
2.2. Acid Blocking System
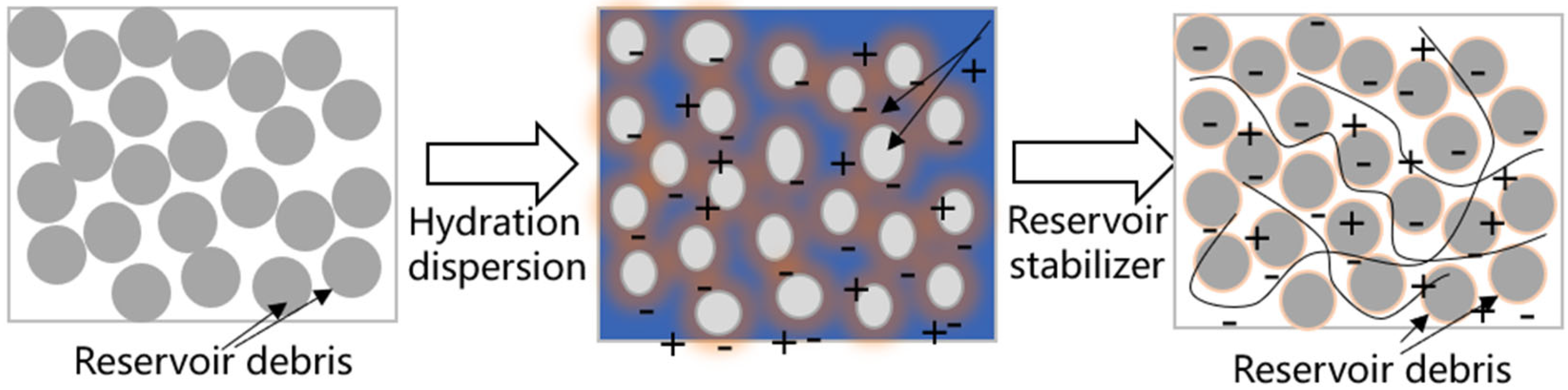
2.2.1. Reservoir Stabilizer
2.2.2. Acid System
2.3. Experimental Method
2.3.1. Blockage Analysis—Acid Corrosion Test
2.3.2. Rock Immersion Experiment at Room Temperature and Atmospheric Pressure to Evaluate Stabilizer and Acid
2.3.3. Soaking Experiment under Pressure to Evaluate Stabilizer and Acid
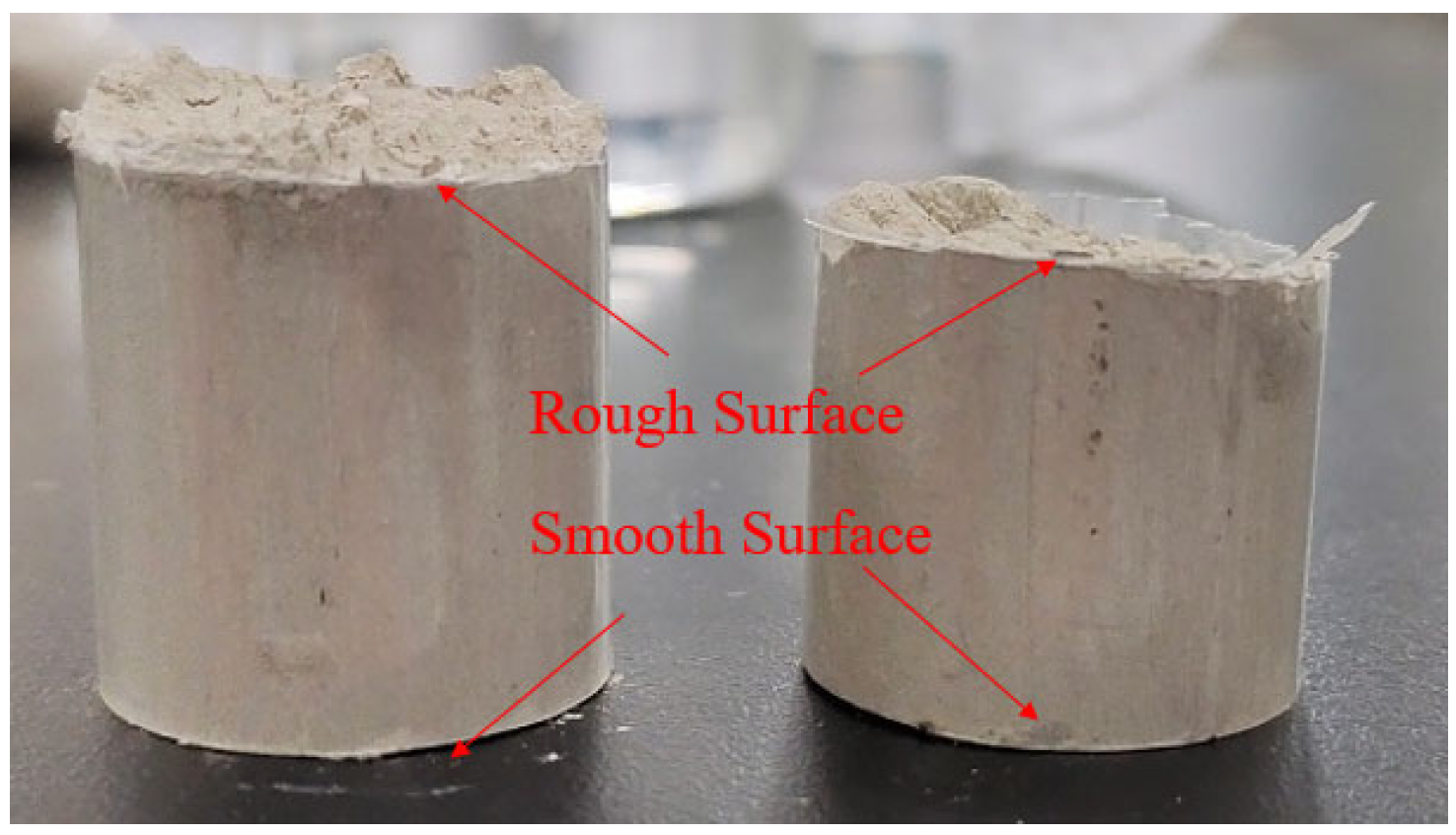
- Cut loose sandstone gas into standard core (diameter 2.5 cm, length 5 cm), and cover the surface of the core with a layer of a rubber jacket to simulate the formation conditions. The same core is broken apart and named as small core No. 1 and No. 2 to simulate the acid corrosion of a smooth surface and rough surface.
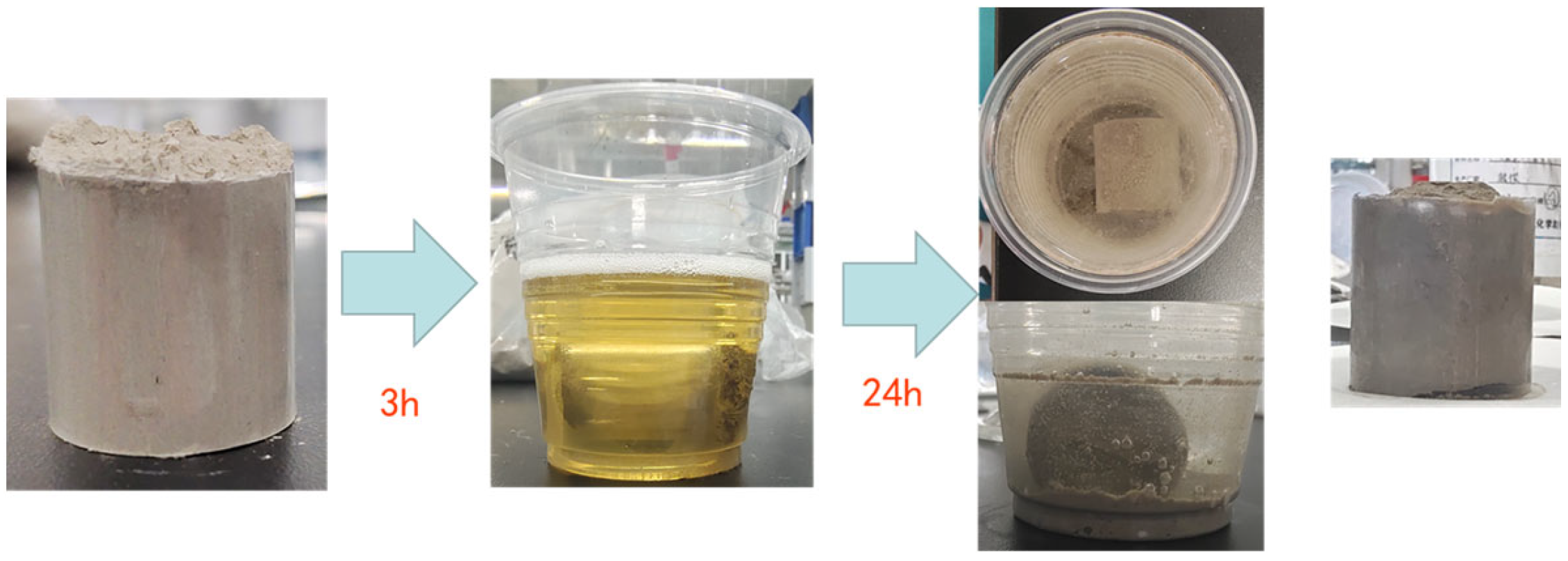
- After adding hydrochloric acid, a violent reaction occurs, resulting in rock dispersion. Therefore, our group chose several systems to carry out immersion experiments. Core No. 1 was soaked in reservoir stabilizer for 3 h and then put into an acid solution. Core No. 2 was directly put into acid solution, and the corrosion of the acid solution was observed after 24 h.
- The dissolved rock powder is dried and weighed to compare the reservoir stability effects of different systems.
3. Results and Discussion
3.1. Acid Dissolution Experiment
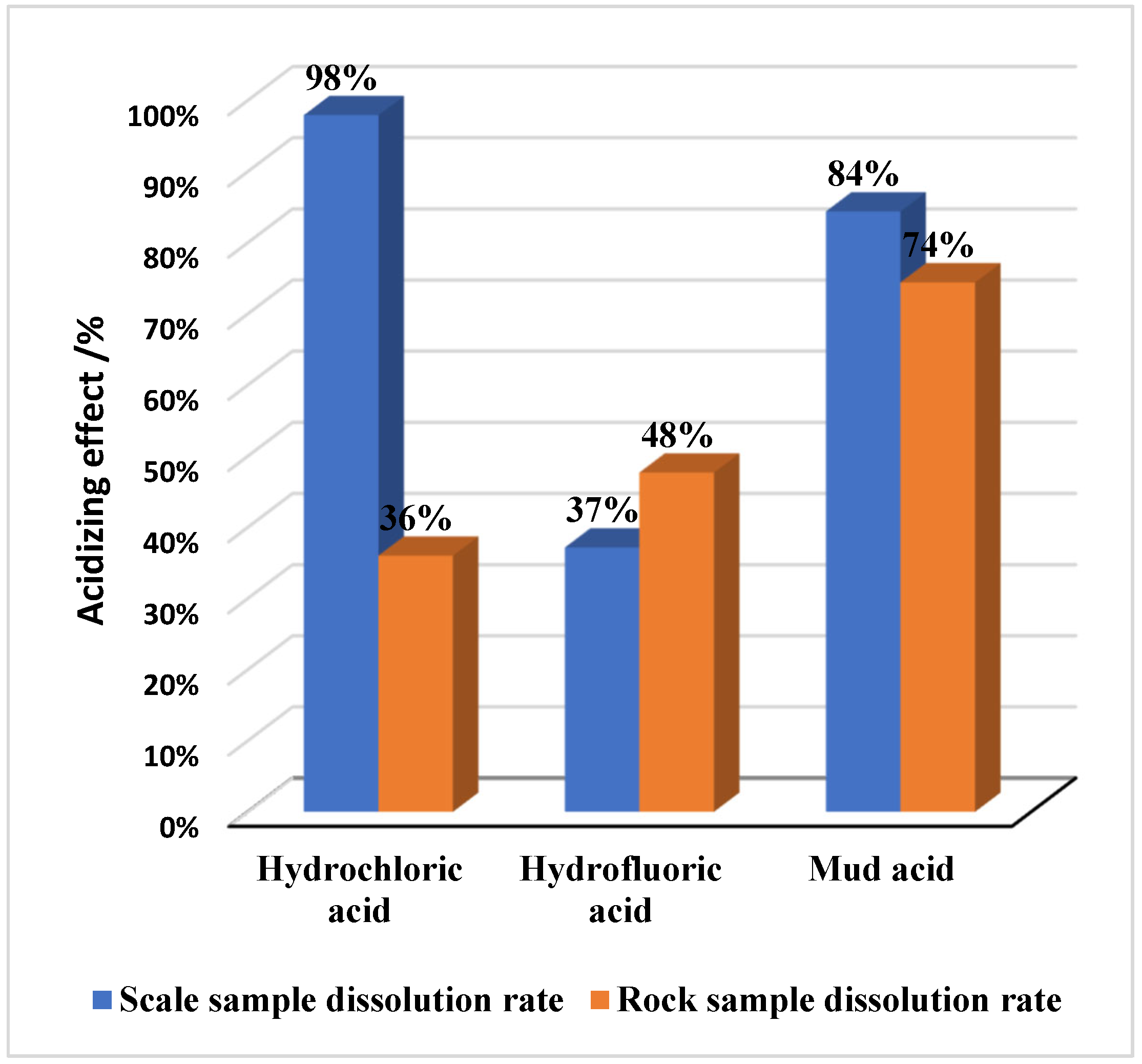
3.1.1. Conventional Acid
3.1.2. Retarded Acid
3.1.3. Compound Experiment: HCl + Solid Acid + HEDP
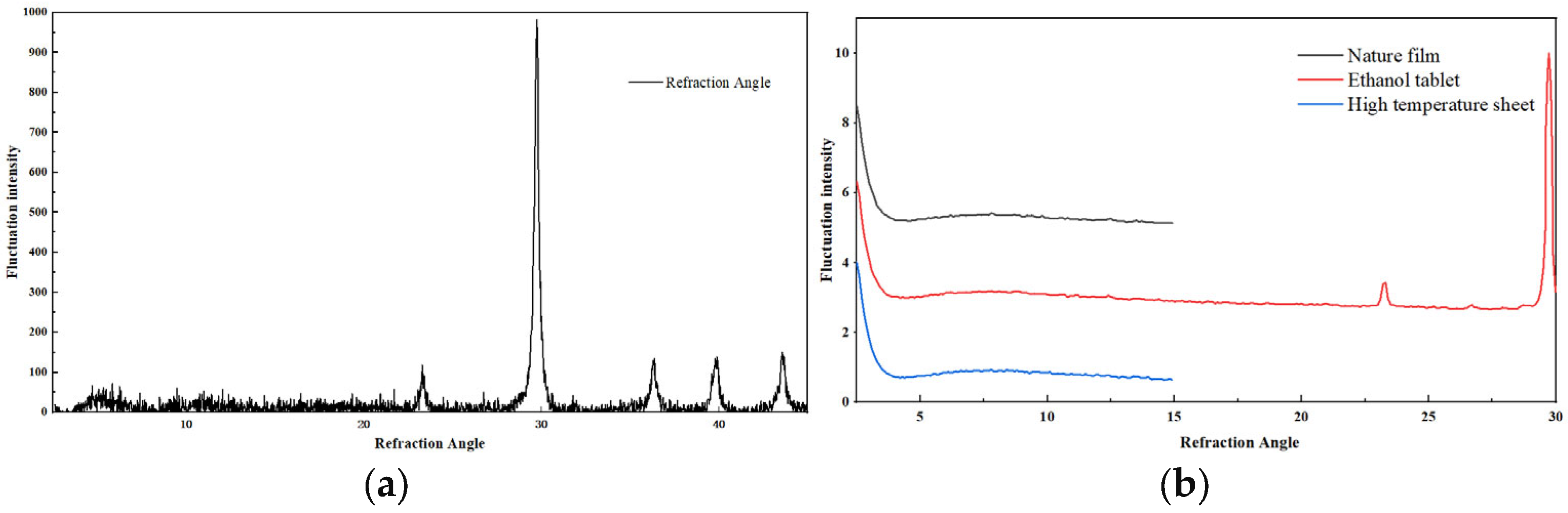
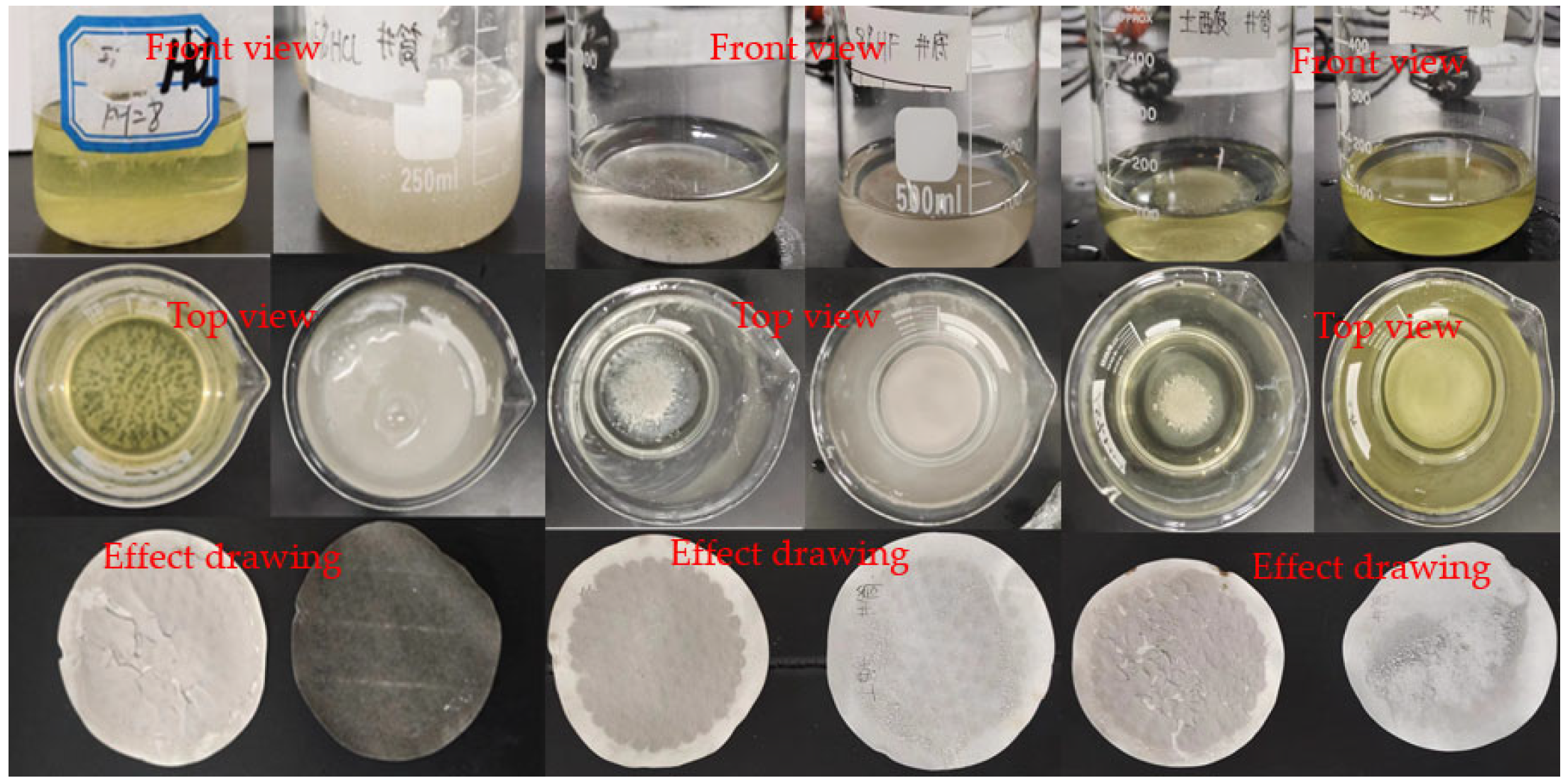
3.2. Immersion Experiment at Normal Temperature and Pressure
3.3. Immersion Test in Burden Pressure and Adaptation of Reservoir Stabilizer
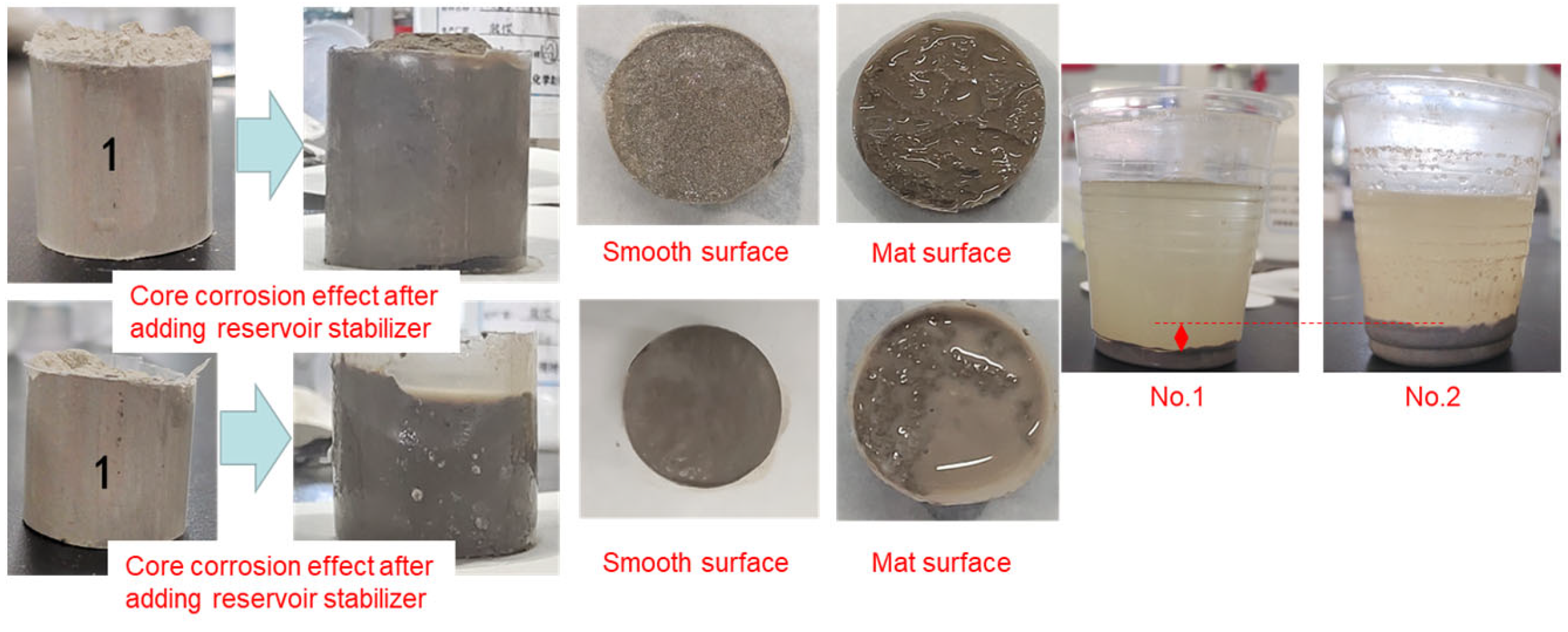
- (1)
- No. 1 core:
- (2)
- No. 2 core:
- (3)
- Comparative analysis:
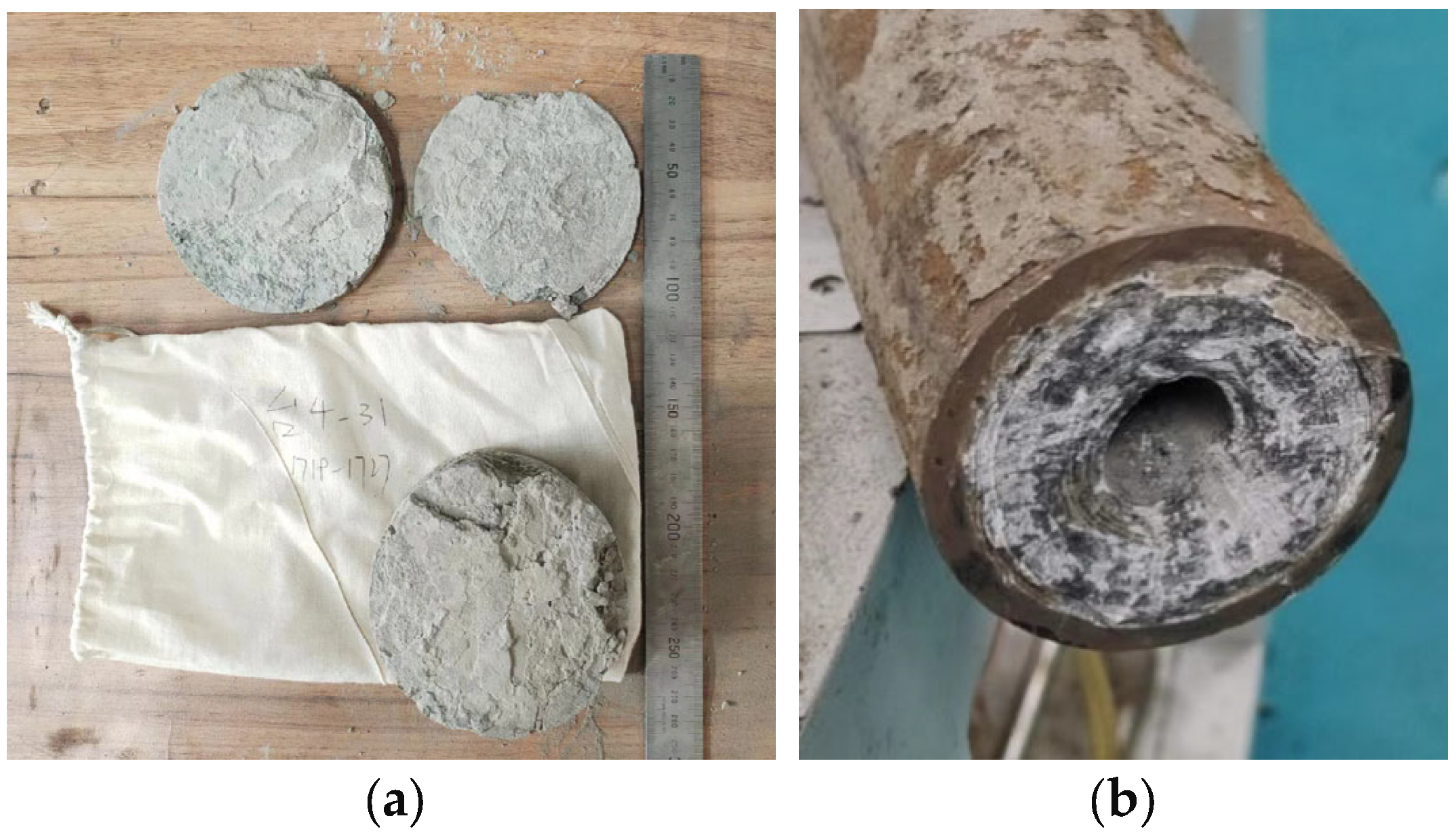
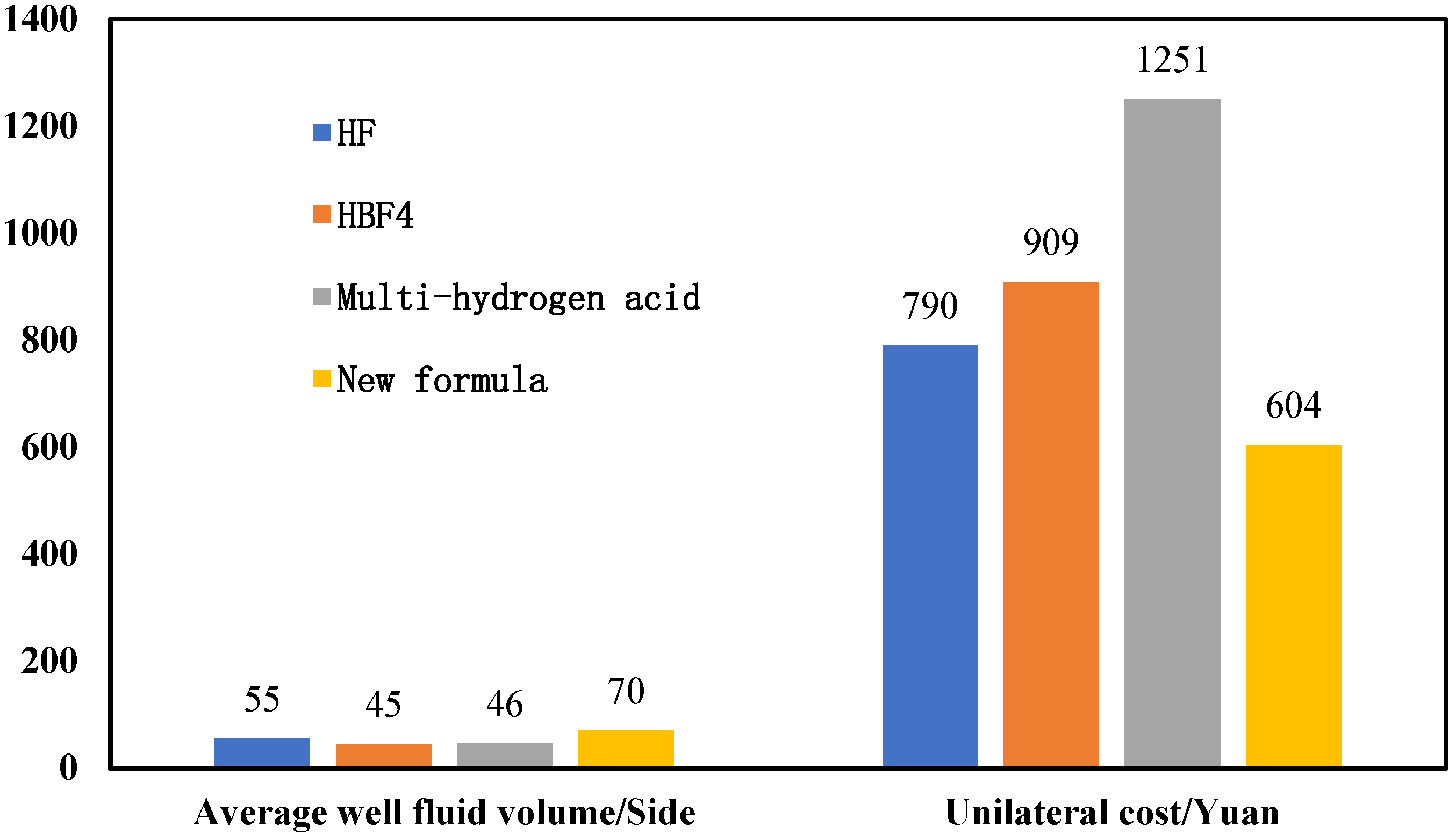
3.4. Field Test
4. Conclusions
- (1)
- Mineral analysis shows that sandstone is mainly composed of quartz (26.2%) and clay minerals (25%), while clay is mainly composed of illite (68%), which easily leads to reservoir particle migration. The main body of the scale sample is magnesium carbonate (99.8%), and it is almost completely acid-soluble. We started designing acidizing systems based on this.
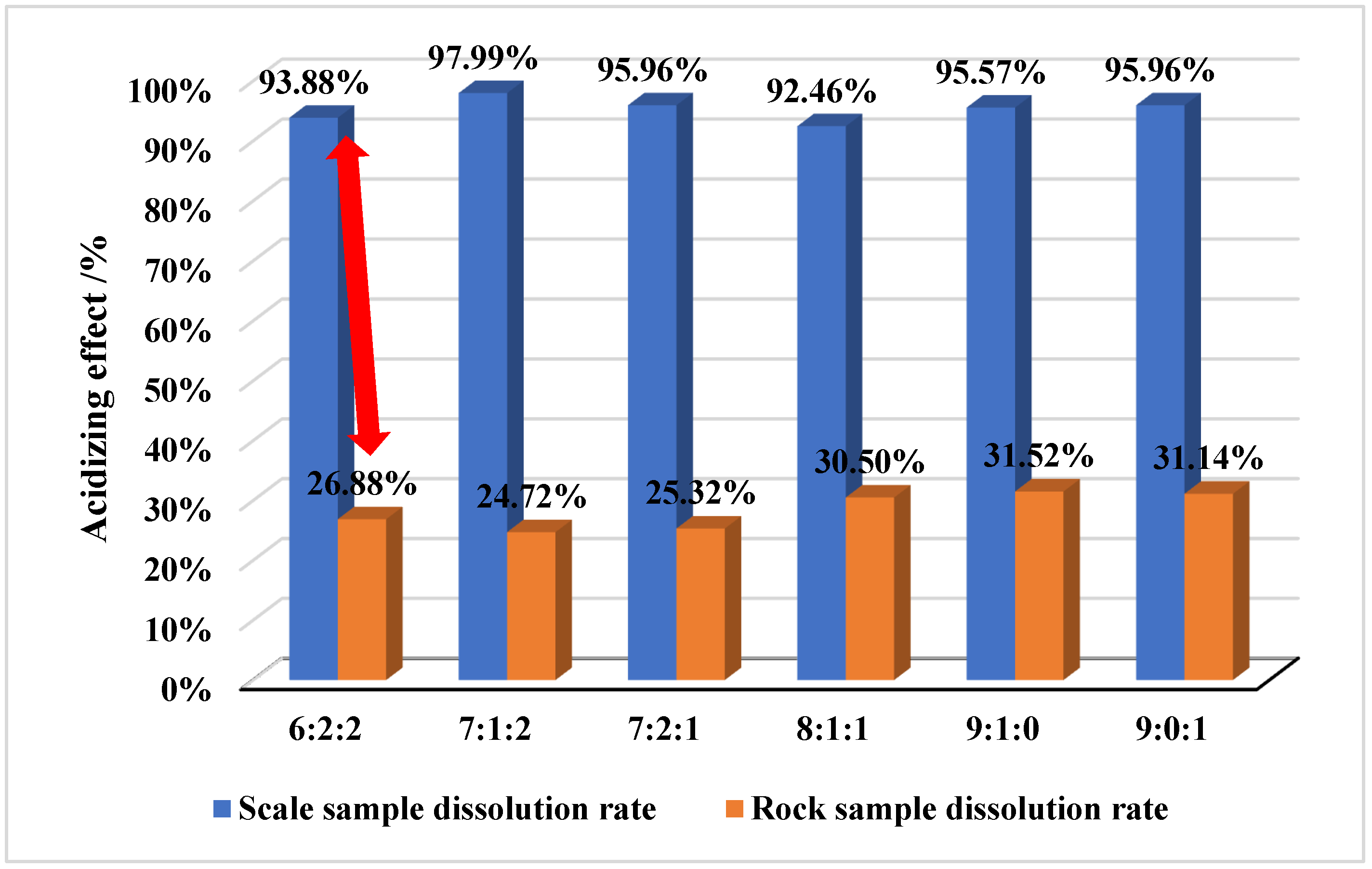
- (2)
- Hydrochloric acid (strong acid)–solid acid (polycarboxylic acid)–HEDP (organic phosphoric acid) = 7:1:2 is preferred as the plugging removal system, which can differentially dissolve rock and scale, the scale sample dissolution rate is above 95%, the rock acid corrosion can be controlled at about 25%, and the stability of the reservoir can be ensured.
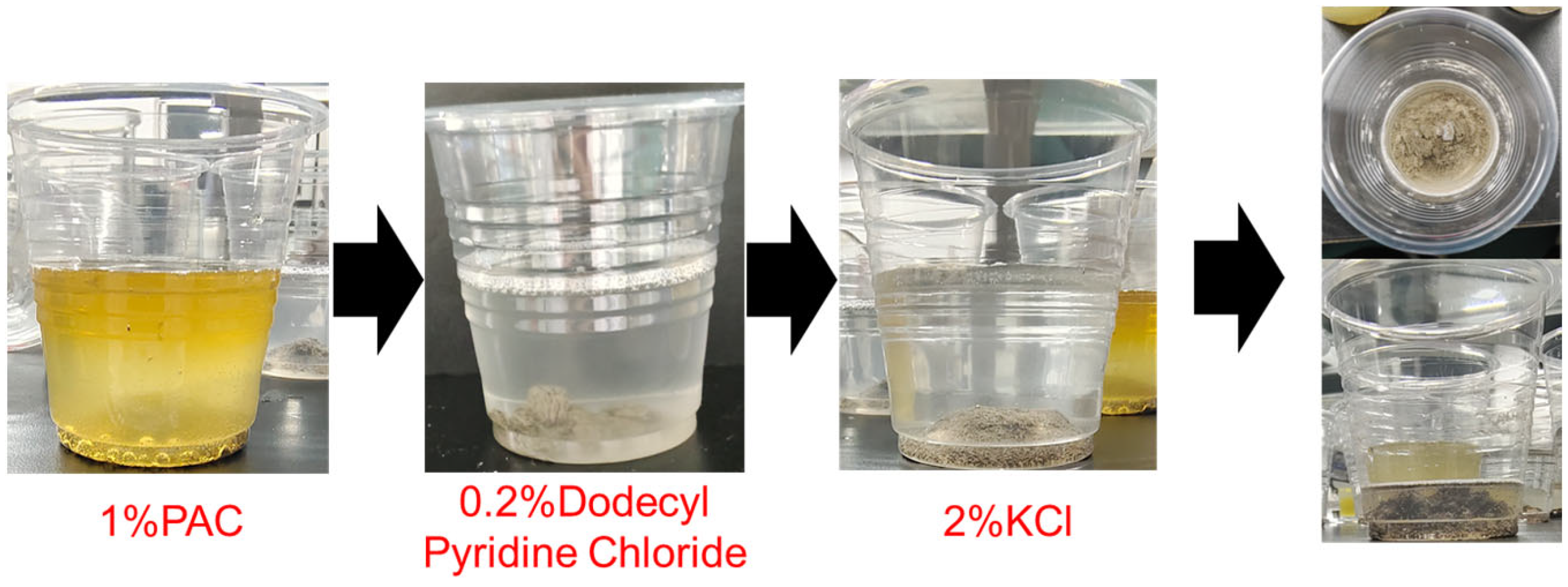
- (3)
- The preferred inhibition dispersant formulation was finally determined as 1% polyaluminum chloride + 0.2% Dodecyl pyridine chloride + 2% KCl. The addition of inhibitors can effectively reduce the acid corrosion of the core by up to 63%.
- (4)
- Field tests show that the new acid solution plugging system can increase the average fluid flow in the well while reducing the cost. The average construction fluid volume of the new formula well is 70 cubic meters, and the single cost is 29% lower than that of the conventional formula.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, N.; Jiang, W.; Jiang, Y.; Yang, J.; Cao, G.; Zheng, R.; Feng, D.; Wang, H.; Xu, Z.; Peng, B.; et al. Study into Impacts of Formation Sands on Foam Performance during Drainage Gas Production—Take Qinghai Oilfield’s Sebei Gasfield for Example. Front. Earth Sci. 2023, 11, 1180501. [Google Scholar] [CrossRef]
- Zhang, D. Accumulation Conditions and Key Technologies for Exploration and Development in Sebei Gas Field in Qaidam Basin, NW China. Pet. Res. 2019, 4, 191–211. [Google Scholar] [CrossRef]
- Li, L.; Baoming, O.U.; Jun, C.; Cheng, W.U.; Qi, J.; Yong, N.I.; Yu, Z. Plugging Mechanism and Plugging Removal Technology for Unconsolidated Sandstone Gas Reservoirs in Sebei Gas Field. Xinjiang Pet. Geol. 2023, 44, 100. [Google Scholar] [CrossRef]
- Zhou, F.; Yang, X.; Xiong, C.; Zhang, S.; Zong, Y.; Sun, L.; Guan, Z. Application and Study of Fine-Silty Sand Control Technique for Unconsolidation Quaternary Sand Gas Reservoir, Sebei Qinghai; OnePetro: Richardson, TX, USA, 2004. [Google Scholar]
- Chai, X.; Wang, Y.; Liu, J.; Chen, F.; Yang, H.; Tan, Z. Water-Gas Ratio and Early Warning of Water Invasion in Unconsolidated Sandstone Gas Reservoirs in Sebei Gas Field, Qaidam Basin. Xinjiang Pet. Geol. 2023, 44, 51. [Google Scholar]
- Taha, R.; Hill, A.D.; Sepehrnoori, K. Simulation of Sandstone-Matrix Acidizing in Heterogeneous Reservoirs. J. Pet. Technol. 1986, 38, 753–767. [Google Scholar] [CrossRef]
- Elmgerbi, A.; Thonhauser, G.; Fine, A.; Hincapie, R.E.; Borovina, A. Experimental Approach for Assessing Filter-Cake Removability Derived from Reservoir Drill-in Fluids. J. Pet. Explor. Prod. Technol. 2021, 11, 4029–4045. [Google Scholar] [CrossRef]
- Leong, V.H.; Ben Mahmud, H. A Preliminary Screening and Characterization of Suitable Acids for Sandstone Matrix Acidizing Technique: A Comprehensive Review. J. Pet. Explor. Prod. Technol. 2019, 9, 753–778. [Google Scholar] [CrossRef]
- Shafiq, M.U.; Mahmud, H.B. Sandstone Matrix Acidizing Knowledge and Future Development. J. Pet. Explor. Prod. Technol. 2017, 7, 1205–1216. [Google Scholar] [CrossRef]
- Mahmoud, M.; Gomaa, I. Sandstone Matrix Stimulation. In Fluid Chemistry, Drilling and Completion; Wang, Q., Ed.; Oil and Gas Chemistry Management Series; Gulf Professional Publishing: Houston, TX, USA, 2022; pp. 341–386. ISBN 978-0-12-822721-3. [Google Scholar]
- Herianto, T. Improving Effective Porosity of Rocks with Matrix Acidizing Stimulation on Sand Stone Formation. Int. J. Innov. Res. Dev. 2018, 7, 282–292. [Google Scholar]
- McCune, C.C.; Ault, J.W.; Dunlap, R.G. Reservoir Properties Affecting Matrix Acid Stimulation of Sandstones. J. Pet. Technol. 1975, 27, 633–640. [Google Scholar] [CrossRef]
- Liu, C.; You, Q.; Wang, T.; Zhou, B.; Peng, W.; Du, Y.; Liu, Y.; Li, Y.; Dai, C. Study of Microscopic Imbibition and Formation Plugging Mechanism of the Compact Oil Reservoir Based on SEM and NMR Analysis. Fuel 2024, 357, 129672. [Google Scholar] [CrossRef]
- Song, W.; Kui, M.; Li, J.; Wang, X.; Liu, J.; Sun, Y.; Wen, Z.; Yang, G.; Tan, X. Key Technologies and Development Countermeasures for Stable Production of Strong Water Intrusion Gas Reservoir in Sebei, Qaidam Basin. Nat. Gas Ind. 2023, 43, 37–45. [Google Scholar]
- Zeng, F.; Zhang, Q.; Guo, J.; Zeng, B.; Zhang, Y.; He, S. Mechanisms of Shale Hydration and Water Block Removal. Pet. Explor. Dev. 2021, 48, 752–761. [Google Scholar] [CrossRef]
- AL-Bazali, T. Insight on the Inhibitive Property of Potassium Ion on the Stability of Shale: A Diffuse Double-Layer Thickness (Κ−1) Perspective. J. Pet. Explor. Prod. Technol. 2021, 11, 2709–2723. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, E. Optimization and Evaluation of a Novel Stabilizer Applied to the Strong Water-Sensitive Conglomerate Reservoir; OnePetro: Richardson, TX, USA, 2023. [Google Scholar]
- Wang, T.; Zhang, Y.; Li, L.; Yang, Z.; Liu, Y.; Fang, J.; Dai, C.; You, Q. Experimental Study on Pressure-Decreasing Performance and Mechanism of Nanoparticles in Low Permeability Reservoir. J. Pet. Sci. Eng. 2018, 166, 693–703. [Google Scholar] [CrossRef]
- Wu, W.; Hou, J.; Xiao, L.; Qu, M.; Wang, W.; Stephenraj, I.R.; Wen, Y.; Wu, W.; Liang, T. Laboratory Study and Field Application of Salt-Tolerant Nano-Emulsion for Enhanced Spontaneous Imbibition Oil Recovery; OnePetro: Richardson, TX, USA, 2022. [Google Scholar]
- Reynolds, M.A. A Technical Playbook for Chemicals and Additives Used in the Hydraulic Fracturing of Shales. Energy Fuels 2020, 34, 15106–15125. [Google Scholar] [CrossRef]
- Kozikhin, R.A.; Daminov, A.M.; Fattakhov, I.G.; Kuleshova, L.S.; Gabbasov, A.K. Identifying the Efficiency Factors on the Basis of Evaluation of Acidizing of Carbonate Reservoirs. IOP Conf. Ser. Earth Environ. Sci. 2018, 194, 062013. [Google Scholar] [CrossRef]
- Shafiq, M.; Shuker, M.; Kyaw, A. Performance Comparison of New Combinations of Acids with Mud Acid in Sandstone Acidizing. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 323–328. [Google Scholar] [CrossRef]
- Alhamad, L.; Alrashed, A.; Al Munif, E.; Miskimins, J. A Review of Organic Acids Roles in Acidizing Operations for Carbonate and Sandstone Formations; OnePetro: Richardson, TX, USA, 2020. [Google Scholar]
- Chen, Y.; Yang, W. Formulation of Corrosion Inhibitors. In Water Chemistry; IntechOpen: London, UK, 2019; 22p. [Google Scholar]
- Li, N.; Yang, M.; Zhang, Q.; Zhou, H.; Zhai, C.; Feng, L. A New Multiple Chelating Acid System with Low Damage and Weak Dissolution; OnePetro: Richardson, TX, USA, 2019. [Google Scholar]
- Wilson, M.J.; Wilson, L.; Patey, I. The Influence of Individual Clay Minerals on Formation Damage of Reservoir Sandstones: A Critical Review with Some New Insights. Clay Miner. 2014, 49, 147–164. [Google Scholar] [CrossRef]
- Hjelmstad, K.E. Cationic Polymers Prevent Permeability Loss during Leaching. Min. Metall. Explor. 1990, 7, 30–35. [Google Scholar] [CrossRef]
- Karmore, V.; Madras, G. Thermal Degradation of Polystyrene by Lewis Acids in Solution. Ind. Eng. Chem. Res. 2002, 41, 657–660. [Google Scholar] [CrossRef]
- Sachin, K.M.; Karpe, S.A.; Singh, M.; Bhattarai, A. An Interaction of Anionic- and Cationic-Rich Mixed Surfactants in Aqueous Medium through Physicochemical Properties at Three Different Temperatures. J. Chem. 2018, 2018, e4594062. [Google Scholar] [CrossRef]







| Well Number | Daily Gas (Front) | Daily Gas (Rear) | Daily Gas Increase | Sweep Radius | Acidizing Radius | Preinhibitor | Body Acid | Total Acid Content | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.48 | 0.52 | 0.08 | 2 | 1.45 | 44 | 44 | 88 | Efficacious |
| 2 | 0 | 0.48 | 0.48 | 2.43 | 1.8 | 40 | 40 | 80 | Efficacious |
| 3 | 0 | 0.5 | 0.5 | 1.95 | 1.4 | 44 | 44 | 88 | Efficacious |
| 4 | 0 | 0.3 | 0.3 | 2.75 | 2 | 16 | 16 | 32 | Efficacious |
| 5 | 0 | 0.53 | 0.53 | 2.68 | 1.98 | 32 | 32 | 64 | Efficacious |
| 6 | 0 | 0.46 | 0.46 | 2.4 | 1.6 | 28 | 28 | 56 | Efficacious |
| 7 | 0 | 0.48 | 0.48 | 3.75 | 1.85 | 40 | 44 | 84 | Efficacious |
| 8 | 0 | 0.56 | 0.56 | 2.43 | 1.8 | 40 | 40 | 80 | Efficacious |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Zhang, K.; Feng, D.; Jiang, Q.; Lin, H.; Liao, L.; Kang, R.; Ou, B.; Du, J.; Wang, Y.; et al. Study on Stable Loose Sandstone Reservoir and Corresponding Acidizing Technology. Coatings 2024, 14, 667. https://doi.org/10.3390/coatings14060667
Song W, Zhang K, Feng D, Jiang Q, Lin H, Liao L, Kang R, Ou B, Du J, Wang Y, et al. Study on Stable Loose Sandstone Reservoir and Corresponding Acidizing Technology. Coatings. 2024; 14(6):667. https://doi.org/10.3390/coatings14060667
Chicago/Turabian StyleSong, Wei, Kun Zhang, Daqiang Feng, Qi Jiang, Hai Lin, Li Liao, Ruixin Kang, Baoming Ou, Jing Du, Yan Wang, and et al. 2024. "Study on Stable Loose Sandstone Reservoir and Corresponding Acidizing Technology" Coatings 14, no. 6: 667. https://doi.org/10.3390/coatings14060667
APA StyleSong, W., Zhang, K., Feng, D., Jiang, Q., Lin, H., Liao, L., Kang, R., Ou, B., Du, J., Wang, Y., & Yao, E. (2024). Study on Stable Loose Sandstone Reservoir and Corresponding Acidizing Technology. Coatings, 14(6), 667. https://doi.org/10.3390/coatings14060667







