Prussian Blue Encapsulated with Brush-like Polyorganosiloxane Nanospheres with Tunable Functionality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
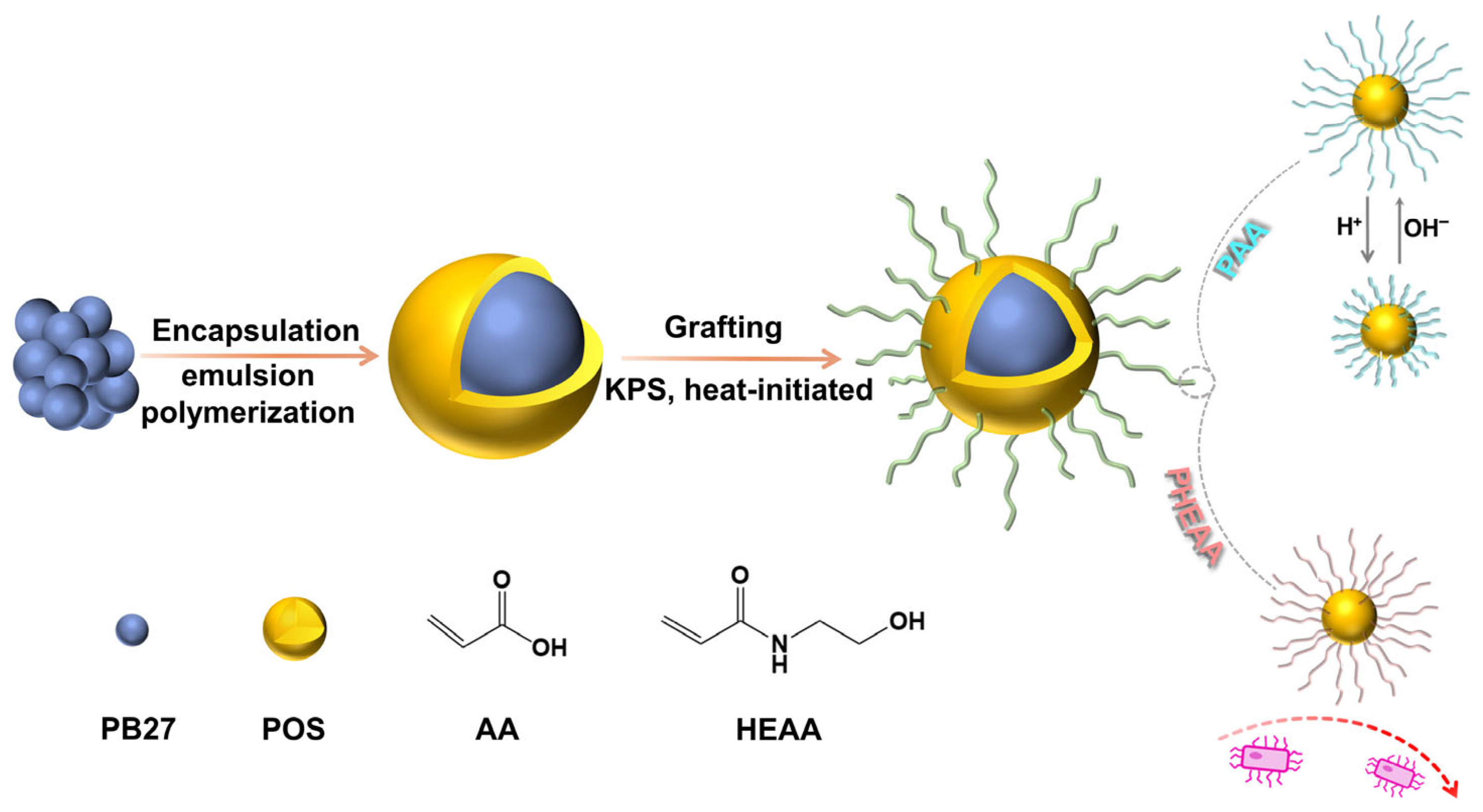
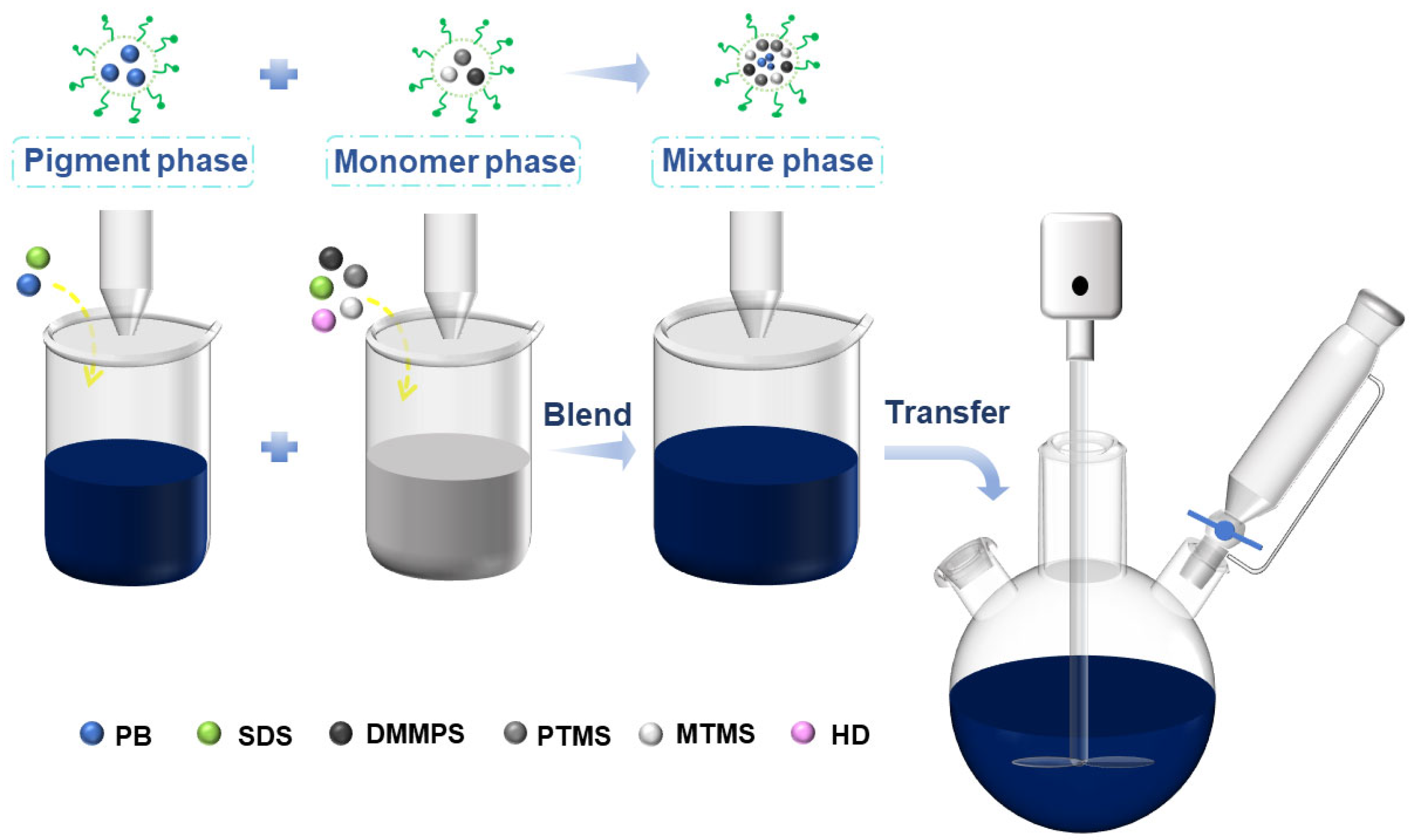
2.2. Preparation of PB27@POS
2.3. Functionality of PB27@POS
2.4. Characterization
2.4.1. General Characterizations
2.4.2. Performance Characterizations
2.4.3. Functionality Characterizations
3. Results and Discussion
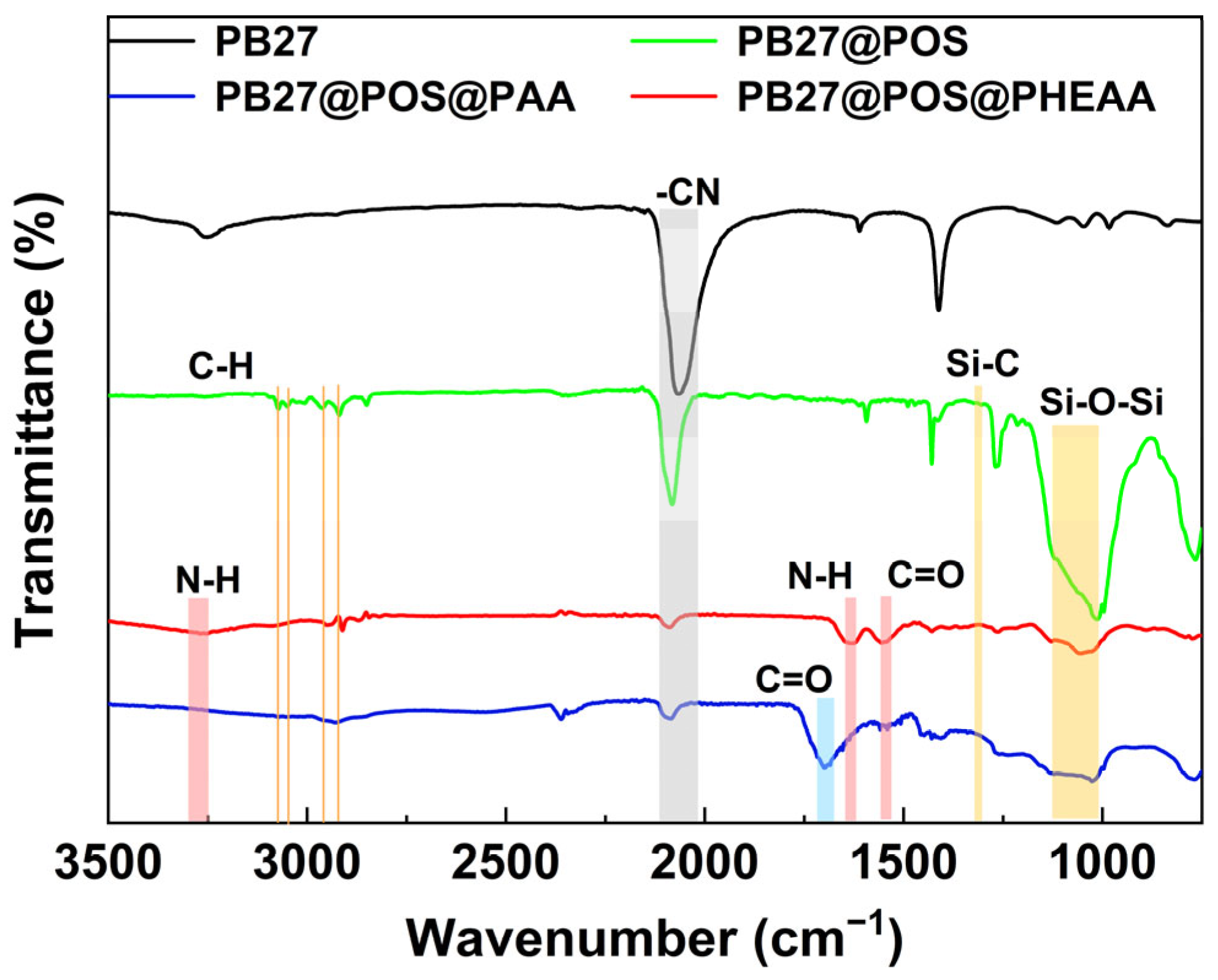
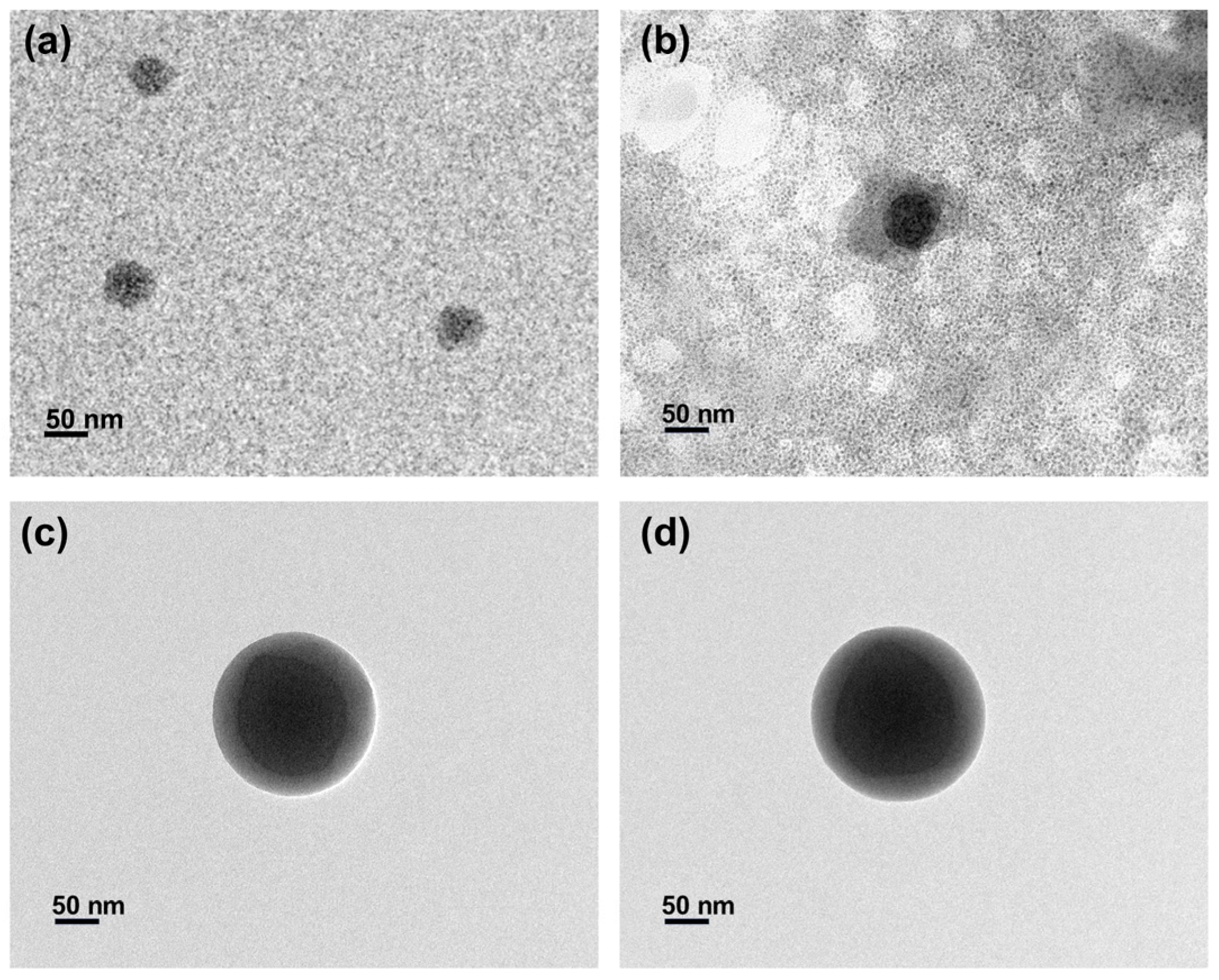
3.1. Characterization of the Composite Pigment Particles
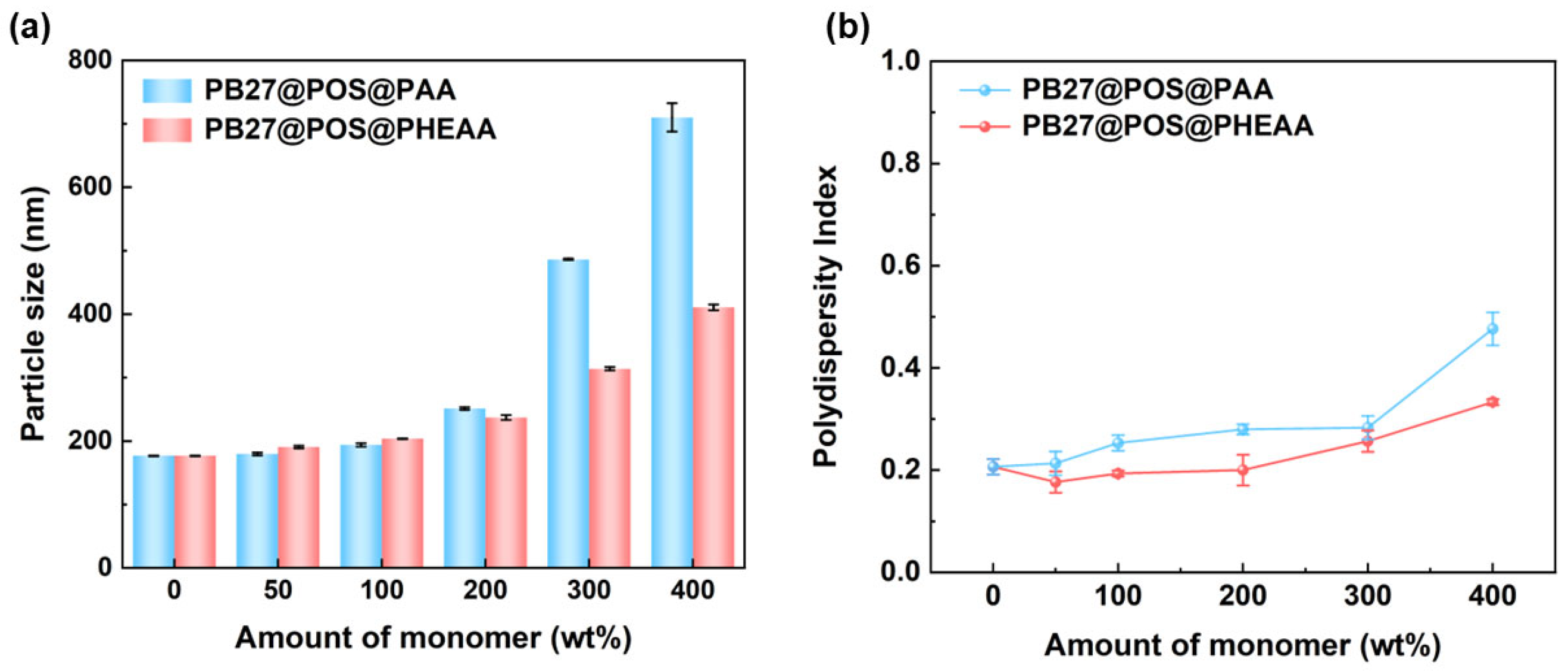
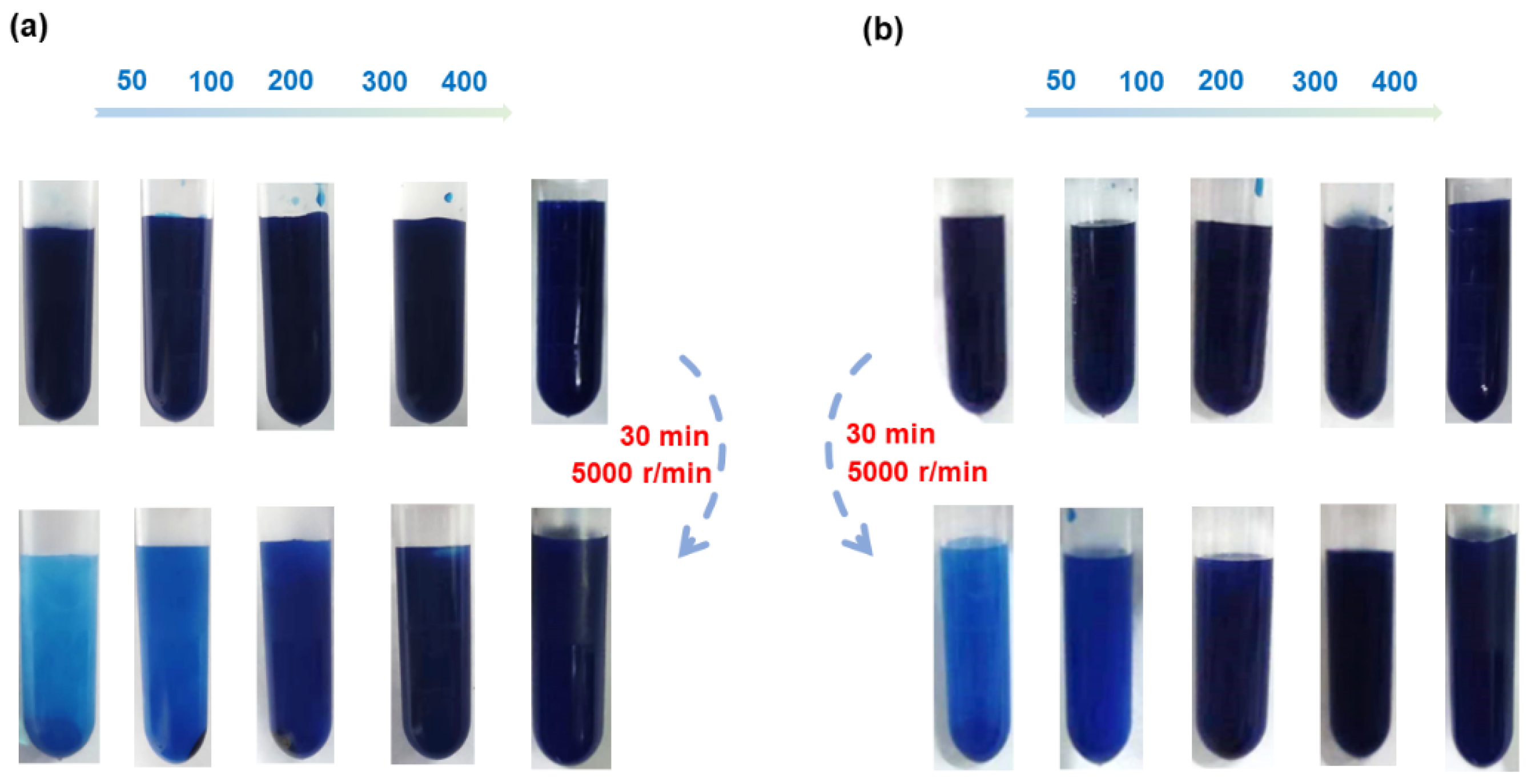
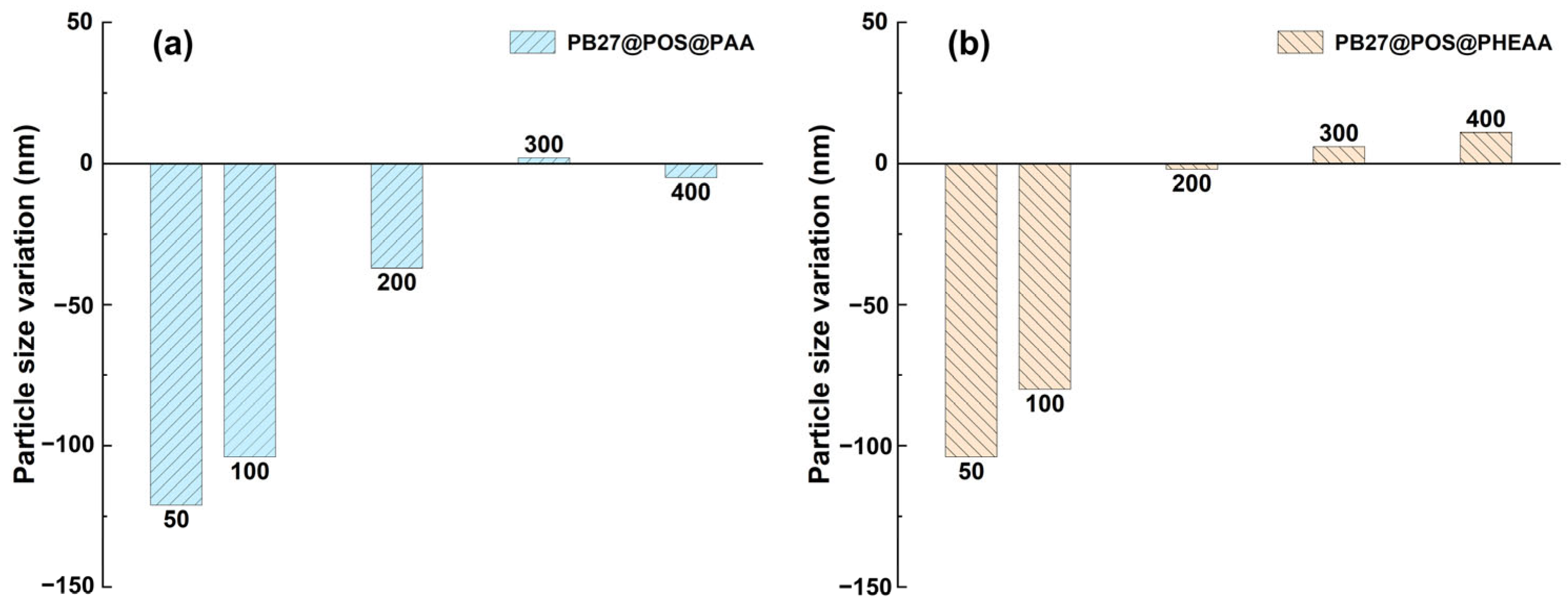
3.2. The Effect of Monomer Dosage
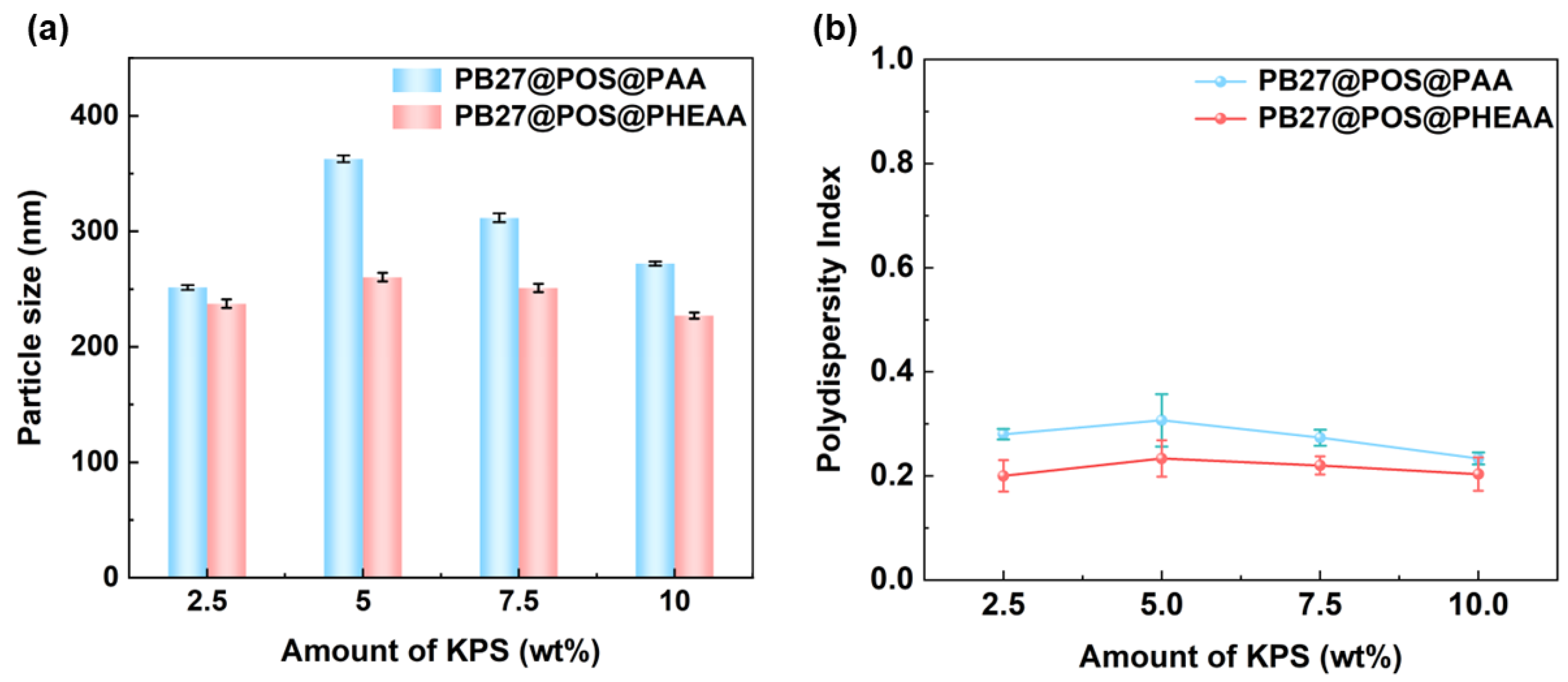
3.3. The Effect of KPS Dosage
3.4. Performance Characterizations
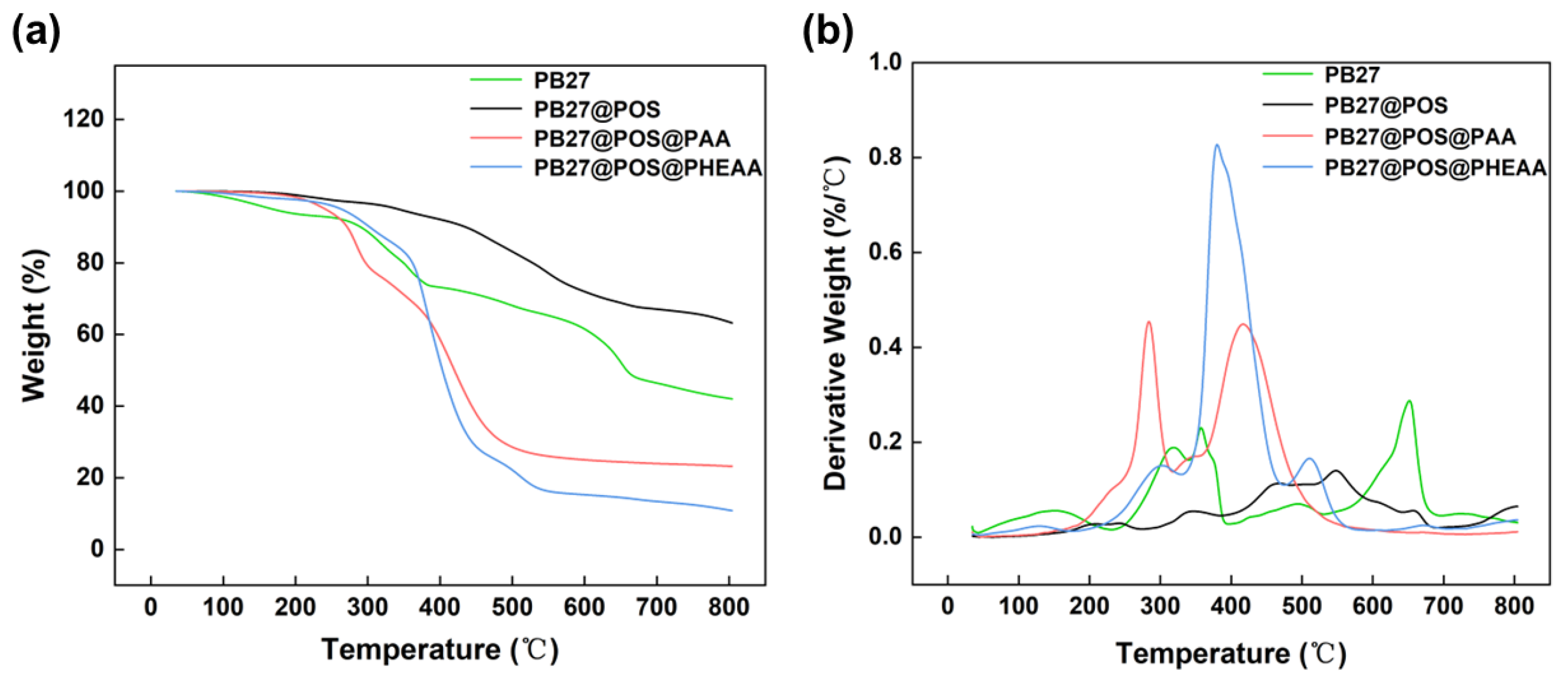
3.4.1. Thermostability
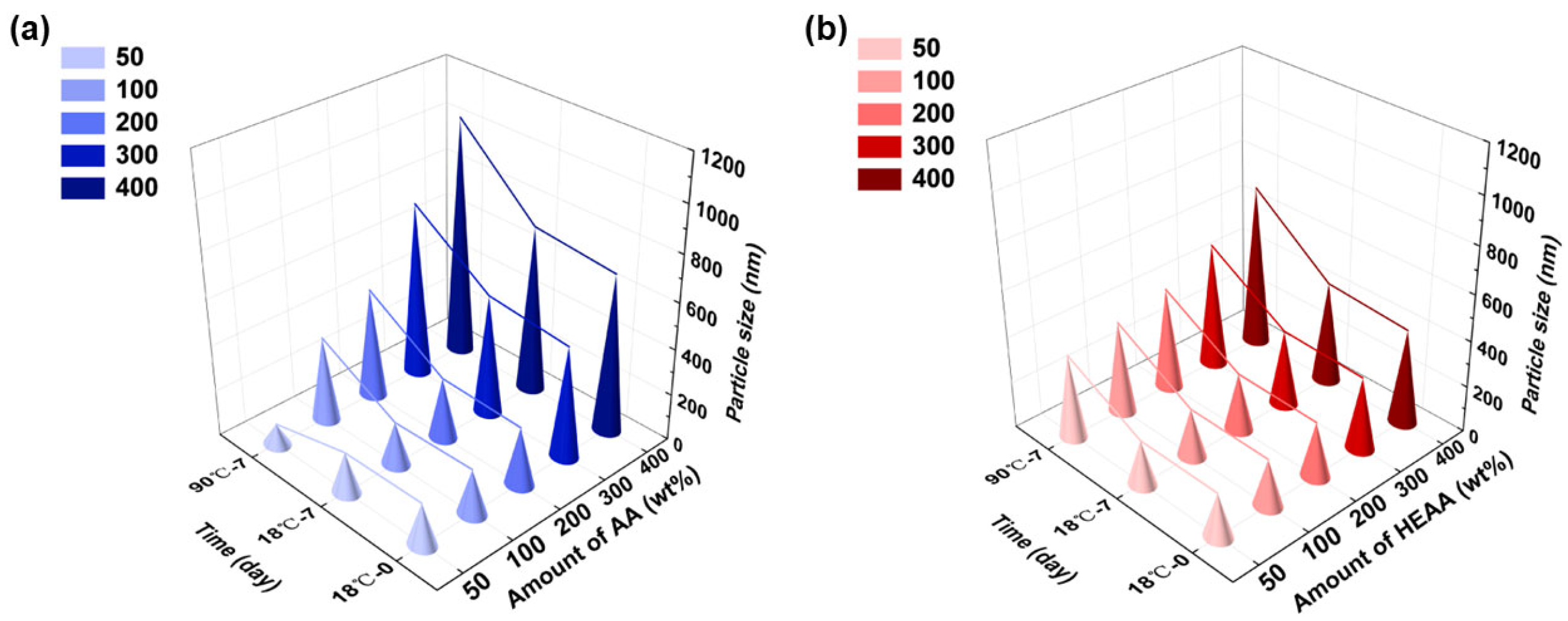
3.4.2. Mechanical Stability
3.4.3. Alkali Resistance
3.5. Functionality
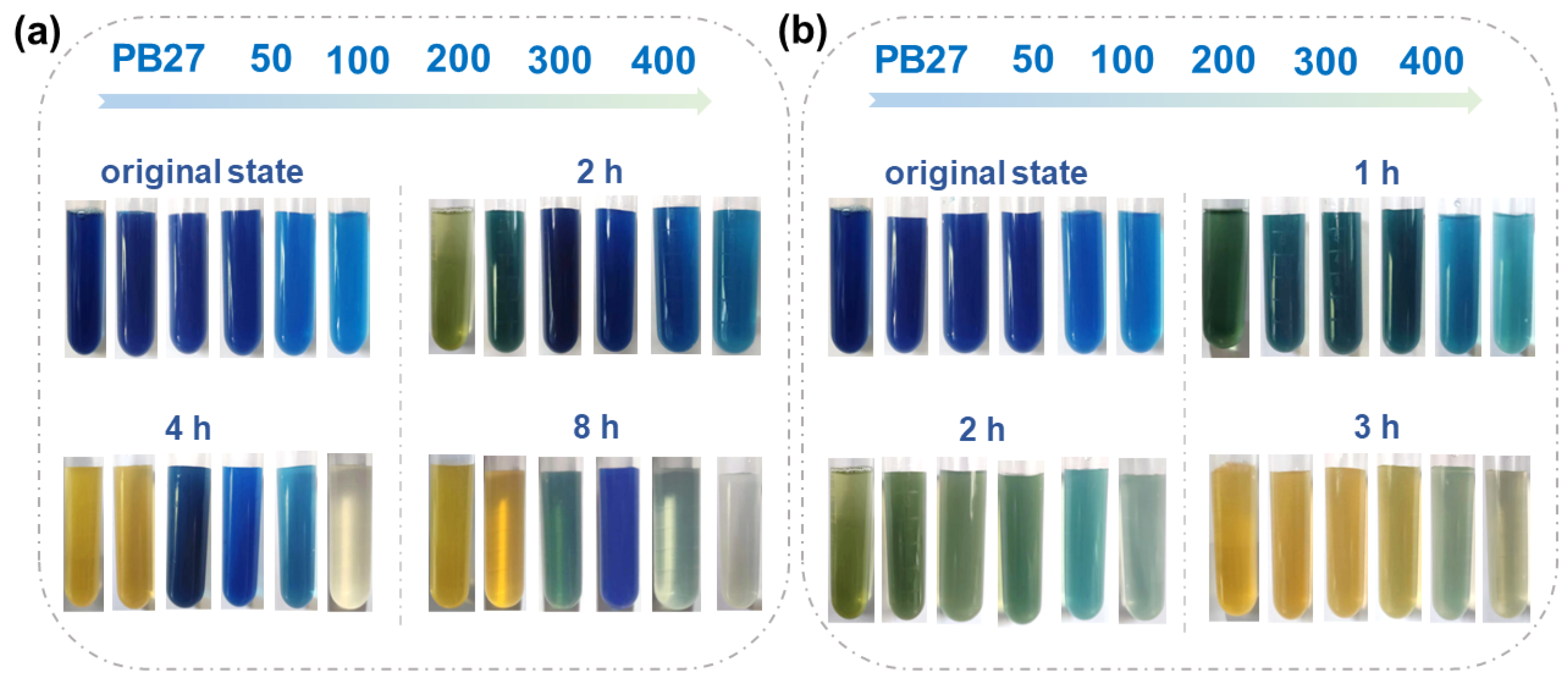
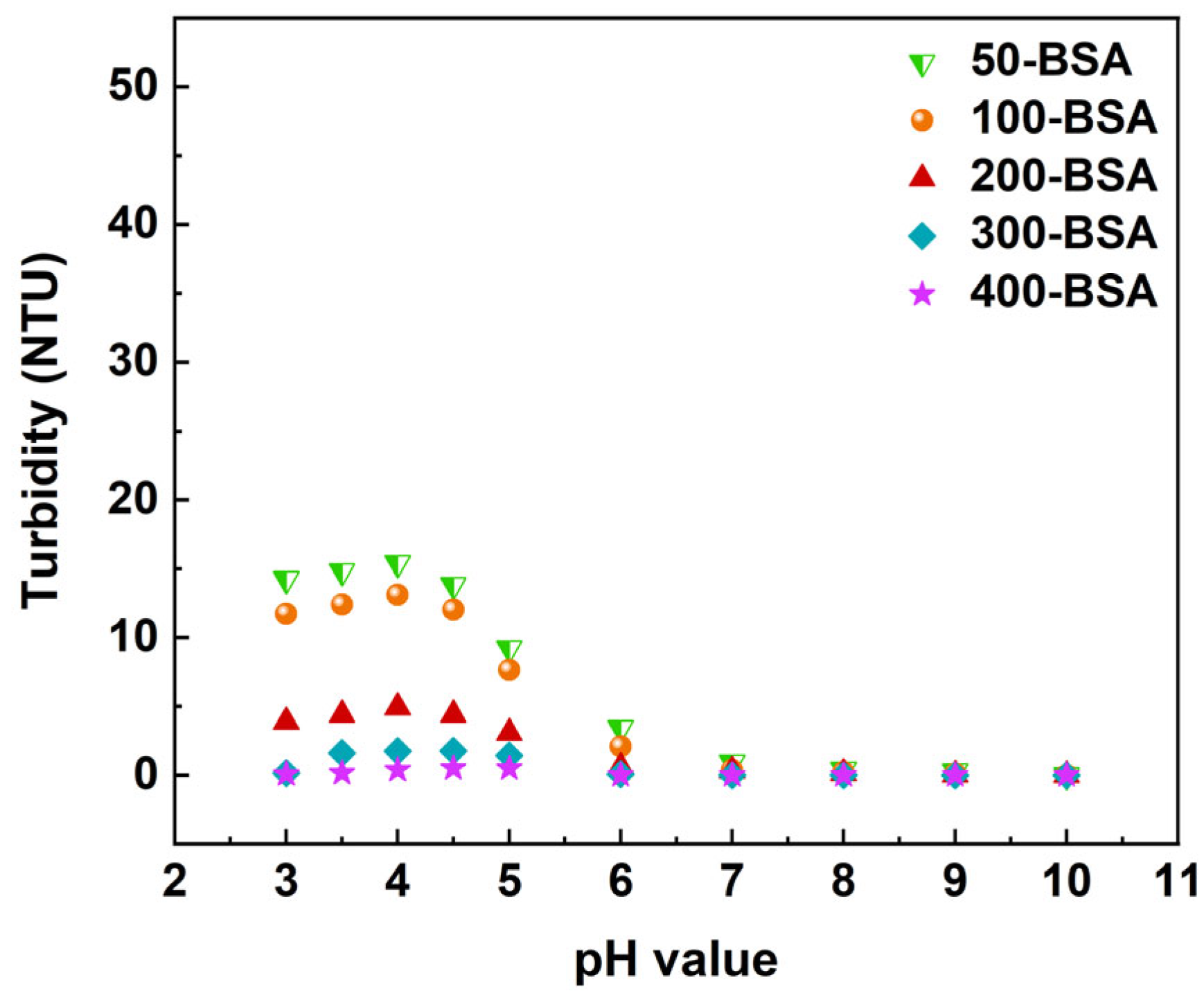
3.5.1. pH Response of PB27@POS@PAA
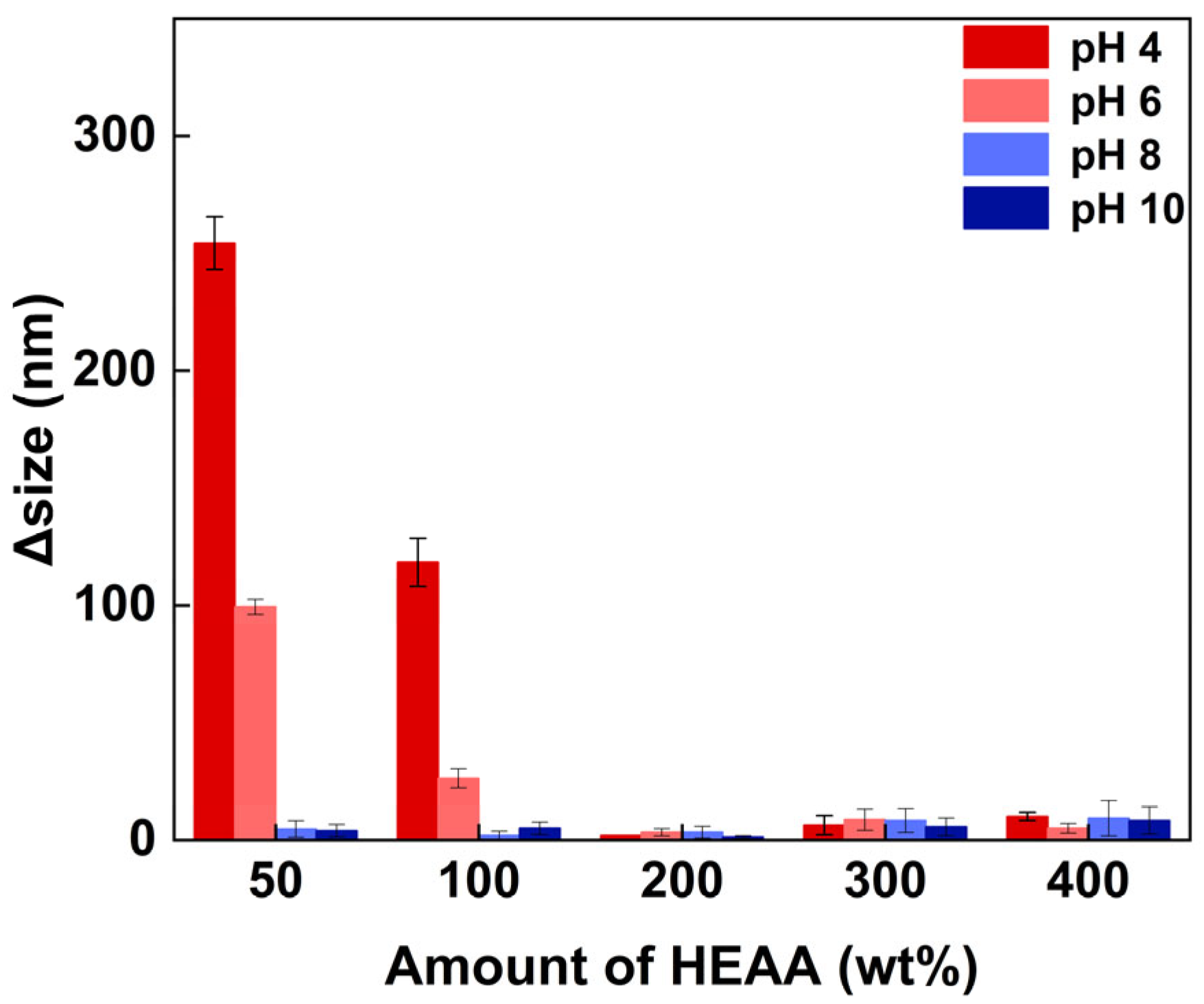
3.5.2. Antifouling Property of PB27@POS@PHEAA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahltig, B.; Zhang, J.; Wu, L.; Darko, D.; Wendt, M.; Lempa, E.; Rabe, M.; Haase, H. Effect pigments for textile coating: A review of the broad range of advantageous functionalization. J. Coat. Technol. Res. 2017, 14, 35–55. [Google Scholar] [CrossRef]
- Ali, M.; Lin, L.; Cartridge, D. High electrical conductivity waterborne inks for textile printing. J. Coat. Technol. Res. 2019, 16, 1337–1349. [Google Scholar] [CrossRef]
- Varney, D.; Toivakka, M.; Bousfield, D. Discrete element method to predict the mechanical properties of pigmented coatings. J. Coat. Technol. Res. 2019, 16, 1683–1689. [Google Scholar] [CrossRef]
- Nie, L.; Chang, G.; Li, R. Preparation and Characterization of Self-Dispersing Phthalocyanine Blue 15:4 Pigment for Dyeing of Wool Textiles. Coatings 2020, 10, 741. [Google Scholar] [CrossRef]
- Nie, L.; Dong, Y.; Chen, Y.; Chang, G.; Li, R. A study for self-dispersing pigment-based inks printing on various fabrics. Colloids Surf. A 2023, 658, 130689. [Google Scholar] [CrossRef]
- Gong, L.; Hua, X.; Yao, B.; Liang, J.; Tian, G. Novel red composite pigment with high thermostability from iron ore tailings: Synthesis and coloring mechanism. Ceram. Int. 2023, 49, 5066–5076. [Google Scholar] [CrossRef]
- Men, P.; Liang, J.; He, J.; Chen, J.; Geng, B.; Li, W. Preparation of alkali-resistant aluminum pigment encapsulated with fluoropolymer by in situ polymerization. J. Coat. Technol. Res. 2021, 18, 1227–1235. [Google Scholar] [CrossRef]
- Marzec, A.; Szadkowski, B. Improved Aging Stability of Ethylene-Norbornene Composites Filled with Lawsone-Based Hybrid Pigment. Polymers 2019, 11, 723. [Google Scholar] [CrossRef]
- Karangutkar, A.V.; Ananthanarayan, L. Evaluating the effect of additives on stability of betacyanin pigments from Basella rubra in a model beverage system during storage. J. Food Sci. Technol. Mysore 2021, 58, 1262–1273. [Google Scholar] [CrossRef]
- Lv, D.; Sung, H.-S.; Li, X.; Zhang, X.; Li, Z.; Chen, D. Effects of single layer graphene and graphene oxide modification on the properties of phthalocyanine blue pigments. Dye. Pigm. 2020, 180, 108449. [Google Scholar] [CrossRef]
- Hakeim, O.A.; Arafa, A.A.; Zahran, M.K.; Dou, L.A.W. UV-curable encapsulation of surface Modified organic pigments for inkjet printing of textiles. Colloids Surf. A 2014, 447, 172–182. [Google Scholar] [CrossRef]
- Kozlowska, M.; Lipinska, M.; Okraska, M.; Pietrasik, J. Polypropylene Color Masterbatches Containing Layered Double Hydroxide Modified with Quinacridone and Phthalocyanine Pigments-Rheological, Thermal and Application Properties. Materials 2023, 116, 6243. [Google Scholar] [CrossRef]
- Ren, F.; Fei, X.; Cui, L.; Cao, L.; Lv, D.; Zhu, S.; Han, X.; Liu, L. Preparation and properties of hydrophilic PR 57:1 with inorganic core/solid solution shell. Dye. Pigm. 2020, 183, 108699. [Google Scholar] [CrossRef]
- Chen, X.-H.; Tang, C.-H. Highly transparent antioxidant high internal phase emulsion gels stabilized solely by C-phycocyanin: Facilitated formation through subunit dissociation and refractive index matching. Colloids Surf. A 2021, 625, 126866. [Google Scholar] [CrossRef]
- Win, K.Y.; Teng, C.P.; Tee, S.Y.; Guan, G.; Loh, X.J.; Han, M.-Y. Natural polymer towards lustrous multicolored silk: Hermetical encapsulation and understanding of colorants via controlled de/recrystallization process. Polymer 2021, 233, 124163. [Google Scholar] [CrossRef]
- Chen, Y.; Li, X.; Luan, Z.; Zhu, L.; Hu, J.; Xia, X.; Ma, K.; Geng, B.; Yan, M. Fabrication of intelligent anticorrosion waterborne aluminum pigments based on encapsulation of composited zeolitic imidazole framework layers. J. Mater. Sci. 2023, 58, 8143–8156. [Google Scholar] [CrossRef]
- Nguyen, D.; Huynh, V.; Lam, M.; Serelis, A.; Davey, T.; Paravagna, O.; Such, C.; Hawkett, B. Encapsulation by Directed PISA: RAFT-Based Polymer-Vesiculated Pigment for Opacity Enhancement in Paint Films. Macromol. Rapid Commun. 2024, 42, 2100008. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Chen, K.; Gan, W.; Wen, S.; Wang, B. Structural design and preparation of pigment/resin integrated ultra-stable antibacterial composite particles. Dye. Pigm. 2022, 201, 110242. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, K.; Liu, L.; Wen, S.; Gui, T. The Design and Preparation of Antibacterial Polymer Brushes with Phthalocyanine Pigments. Coatings 2023, 13, 1114. [Google Scholar] [CrossRef]
- Zhang, L.; Shao, G.; Xu, R.; Ding, C.; Hu, D.; Zhao, H.; Huang, X. Multicovalent crosslinked double-network graphene-polyorganosiloxane hybrid aerogels toward efficient thermal insulation and water purification. Colloids Surf. A 2022, 647, 129129. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Y.; Ma, Z.; Zhang, H. Robust surface with thermally stable hydrophobicity enabled by electrosprayed fluorinated SiO2 particles. J. Coat. Technol. Res. 2022, 19, 347–353. [Google Scholar] [CrossRef]
- Yang, F.; Zhu, L.; Han, D.; Li, W.; Chen, Y.; Wang, X.; Ning, L. Effects of fluorine and silicon components on the hydrophobicity failure behavior of acrylic polyurethane coatings. J. Coat. Technol. Res. 2017, 14, 691–699. [Google Scholar] [CrossRef]
- Qian, H.; Jiang, B. Silicone Resin Applications for Heat-Resistant Coatings: A Review. Polym. Sci. Ser. C Sel. Top. 2023, 65, 143–145. [Google Scholar]
- Mathew, A.; Kurmvanshi, S.; Mohanty, S.; Nayak, S.K. Sustainable production of polyurethane from castor oil, functionalized with epoxy- and hydroxyl-terminated poly(dimethyl siloxane) for biomedical applications. J. Mater. Sci. 2018, 53, 3119–3130. [Google Scholar] [CrossRef]
- Smith, G.N.; Brok, E.; Schmiele, M.; Mortensen, K.; Bouwman, W.G.; Duif, C.P.; Hassenkam, T.; Alm, M.; Thomsen, P.; Arleth, L. The microscopic distribution of hydrophilic polymers in interpenetrating polymer networks (IPNs) of medical grade silicone. Polymer 2021, 224, 123671. [Google Scholar] [CrossRef]
- Lv, S.; Zhang, X.; Yang, X.; Xu, Q.; Chen, G.; Liu, X.; Yang, Z.; Zhai, Y. Slippery surface for enhancing surface robustness and chemical stability. J. Mater. Sci. 2023, 58, 5837–5847. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Bongiovanni, R.; Marchisio, D.L.; Fontana, D.; Egger, C. Surface modification of iron oxide (Fe2O3) pigment particles with amino-functional polysiloxane for improved dispersion stability and hydrophobicity. Pigment Resin Technol. 2014, 43, 219–227. [Google Scholar] [CrossRef]
- Xie, Z.; Yan, X.; Li, J.; Zhu, C.; Qi, D. Pigment printing of polyester fabric using a single step synthesized PDMS-modified polyurethane-acrylic/pigment hybrid emulsion. Text. Res. J. 2022, 92, 2818–2829. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Lu, L.; Yan, X.; Qi, D.; Chen, Z. One-step mini-emulsion copolymerisation of waterborne polysiloxane-modified polyacrylate/pigment hybrid latex and its application in textile pigment printing. Color. Technol. 2022, 138, 291–303. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, M. Structure of vinyl polysiloxane on properties of polyacrylates film and its pigment printing application. J. Coat. Technol. Res. 2020, 17, 937–948. [Google Scholar] [CrossRef]
- Meng, C.; Cao, Y.; Sun, L.; Liu, Y.; Kang, G.; Ma, W.; Peng, J.; Deng, K.; Ma, L.; Wei, H. Synthesis of cyclic graft polymeric prodrugs with heterogeneous grafts of hydrophilic OEG and reducibly conjugated CPT for controlled release. Biomater. Sci. 2020, 8, 4206–4215. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Chen, G. Swelling and shrinking of two opposing polyelectrolyte brushes. Phys. Rev. E 2023, 107, 024502. [Google Scholar] [CrossRef] [PubMed]
- Bauman, L.; Wen, Q.; Sameoto, D.; Yap, C.H.; Zhao, B. Durable poly(N-isopropylacrylamide) grafted PDMS micropillared surfaces for temperature-modulated wetting. Colloids Surf. A 2021, 610, 125901. [Google Scholar] [CrossRef]
- Jana, S.; Ray, J.; Mondal, B.; Pradhan, S.S.; Tripathy, T. pH responsive adsorption/desorption studies of organic dyes from their aqueous solutions by katira gum-cl-poly(acrylic acid-co-N-vinyl imidazole) hydrogel. Colloids Surf. A 2018, 553, 472–486. [Google Scholar] [CrossRef]
- Dai, X.; Xu, X.; Yu, X.; Sun, X.; Pan, J.; Zhang, X.; Min, J. Cationic core/shell polysiloxane acrylate emulsion: Synthesis, film morphology, and performance on cotton pigment coloration. Cellulose 2022, 29, 2093–2106. [Google Scholar] [CrossRef]
- Shen, J.; Hu, Y.; Li, L.-X.; Sun J-w Kan, C.-Y. Fabrication and characterization of polysiloxane/polyacrylate composite latexes with balanced water vapor permeability and mechanical properties: Effect of silane coupling agent. J. Coat. Technol. Res. 2018, 15, 165–173. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Zhou, Q.; Liang, J.; Zhang, X.; Shi, Y.; Zhu, J.; Zhong, L. Imaging application of an MMP2-sensitive tumor-targeted prussian blue fluorescent nanoprobe. J. Biomater. Appl. 2023, 38, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Tian, X.; Sun, Y.; He, X.; Li, H.; Ma, T.; Zhao, Q.; Qiu, J. Pillar effect boosting the electrochemical stability of Prussian blue-polypyrrole for potassium ion batteries. Nano Res. 2023, 16, 6326–6333. [Google Scholar] [CrossRef]
- Dwivedi, C.; Kumar, A.; Singh, K.K.; Juby, A.K.; Kumar, M.; Wattal, P.W.; Bajaj, P.N. Copper hexacyanoferrate–polymer composite beads for cesium ion removal: Synthesis, characterization, sorption, and kinetic studies. J. Appl. Polym. Sci. 2013, 129, 152–160. [Google Scholar] [CrossRef]
- Parajuli, D.; Tanaka, H.; Sakurai, K.; Hakuta, Y.; Kawamoto, T. Thermal Decomposition Behavior of Prussian Blue in Various Conditions. Materials 2021, 14, 1151. [Google Scholar] [CrossRef]
- Salatiello, S.; Spinelli, M.; Cassiano, C.; Amoresano, A.; Marini, F.; Cinti, S. Sweat urea bioassay based on degradation of Prussian Blue as the sensing architecture. Anal. Chim. Acta 2022, 1210, 339882. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, K.; Hua, C.; Guo, X. Conformation variation and tunable protein adsorption through combination of poly(acrylic acid) and antifouling poly(N-(2-hydroxyethyl) acrylamide) diblock on a particle surface. Polymers 2020, 12, 566. [Google Scholar] [CrossRef] [PubMed]
- Hua, C.; Chen, K.; Wang, Z.; Guo, X. Preparation, stability and film properties of cationic polyacrylate latex particles with various substituents on the nitrogen atom. Prog. Org. Coat. 2020, 143, 105628. [Google Scholar] [CrossRef]


| PB27 | SDS | Monomers | HD | HCl (1.0 wt%) | Deionized Water |
|---|---|---|---|---|---|
| 0.2 g | 0.3 g | 2.58 g | 0.1 g | 10.0 g | 136.7 g |
| Number | PB27@POS | KPS | Monomers |
|---|---|---|---|
| 1 | 50.0 g | 0.02 g | 0.4 g |
| 2 | 50.0 g | 0.02 g | 0.8 g |
| 3 | 50.0 g | 0.02 g | 1.6 g |
| 4 | 50.0 g | 0.02 g | 2.4 g |
| 5 | 50.0 g | 0.02 g | 3.2 g |
| 6 | 50.0 g | 0.04 g | 1.6 g |
| 7 | 50.0 g | 0.08 g | 1.6 g |
| 8 | 50.0 g | 0.10 g | 1.6 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Chen, K.; Li, Z.; Zhang, X.; Xu, C.; Wang, J.; Wen, S. Prussian Blue Encapsulated with Brush-like Polyorganosiloxane Nanospheres with Tunable Functionality. Coatings 2024, 14, 677. https://doi.org/10.3390/coatings14060677
Chang Y, Chen K, Li Z, Zhang X, Xu C, Wang J, Wen S. Prussian Blue Encapsulated with Brush-like Polyorganosiloxane Nanospheres with Tunable Functionality. Coatings. 2024; 14(6):677. https://doi.org/10.3390/coatings14060677
Chicago/Turabian StyleChang, Yue, Kaimin Chen, Ziwei Li, Xueke Zhang, Chenming Xu, Jihu Wang, and Shaoguo Wen. 2024. "Prussian Blue Encapsulated with Brush-like Polyorganosiloxane Nanospheres with Tunable Functionality" Coatings 14, no. 6: 677. https://doi.org/10.3390/coatings14060677







