Microstructure and Biocompatibility of Graphene Oxide/BCZT Composite Ceramics via Fast Hot-Pressed Sintering
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Preparation
2.2. Characterization of Materials
2.3. Evaluation of Vitro Cell Compatibility
2.3.1. Surface Wettability
2.3.2. CCK-8 Assay
2.3.3. DAPI Staining
2.3.4. Alkaline Phosphatase Activity
3. Results and Discussion
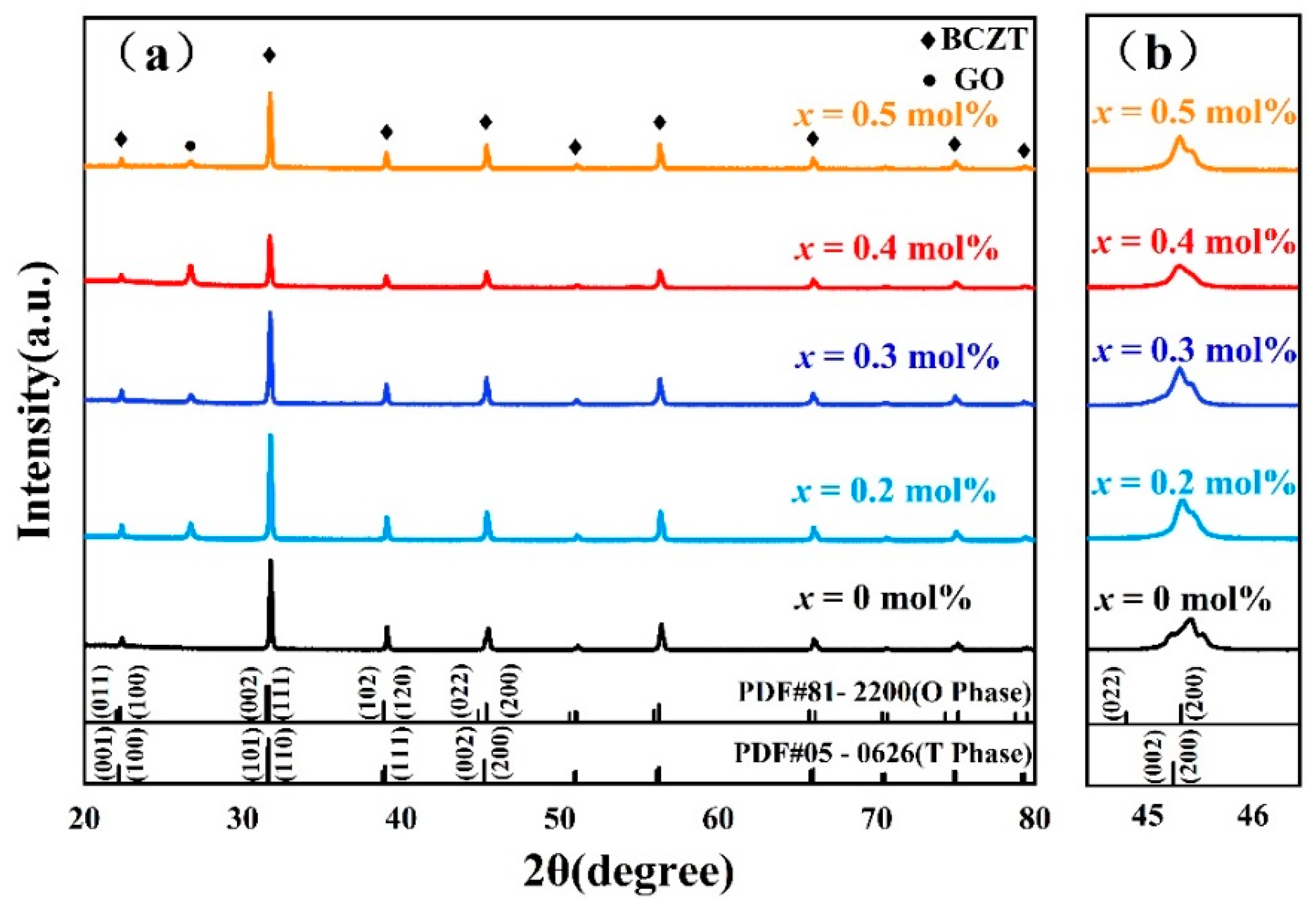
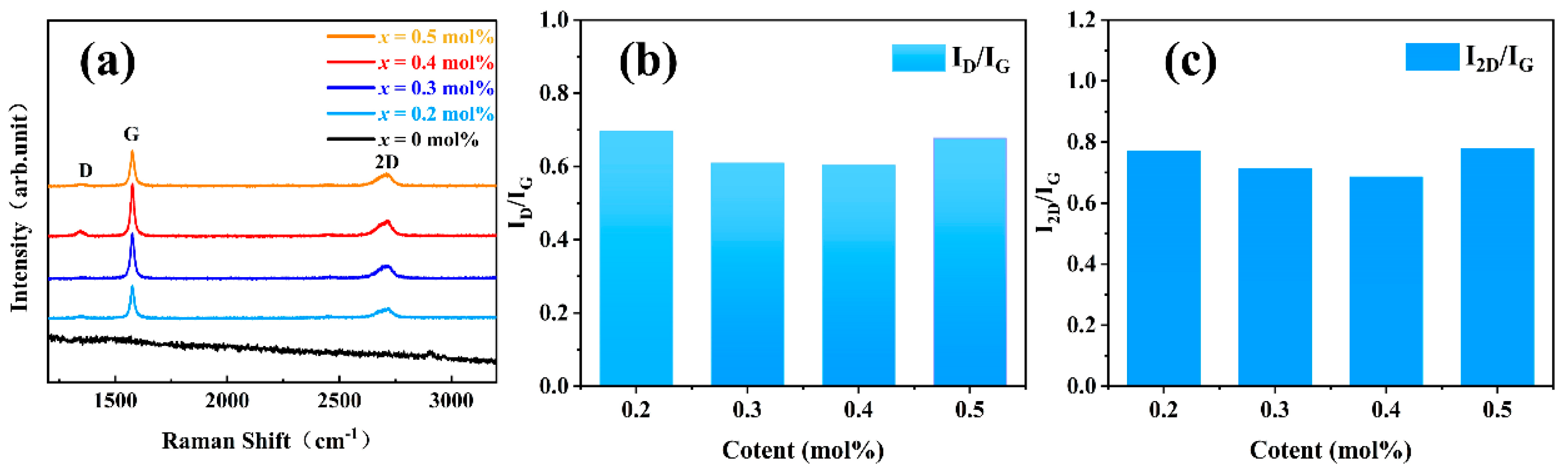
3.1. Crystal Phase Structure
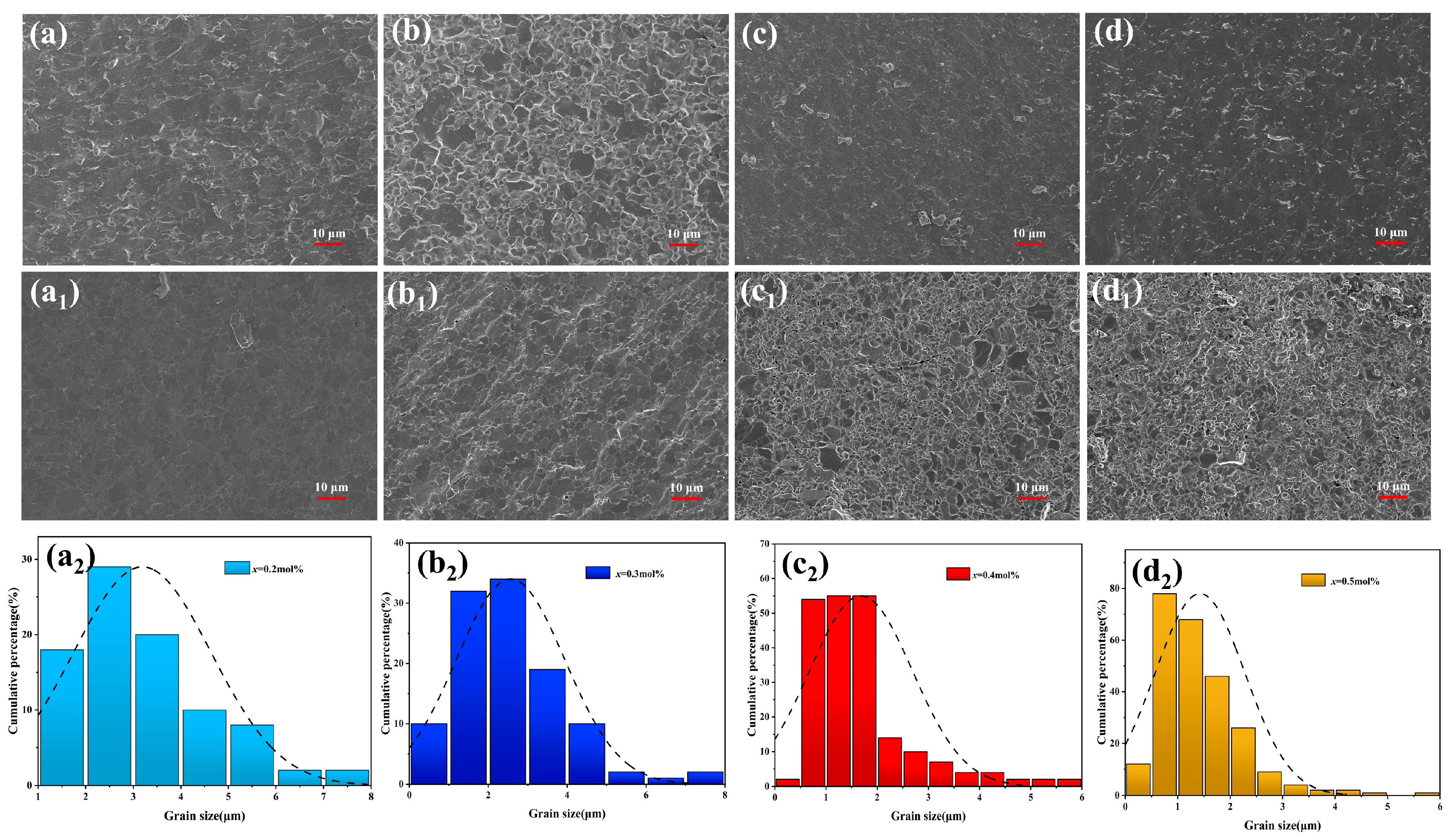
3.2. Microstructure and Fracture Morphology
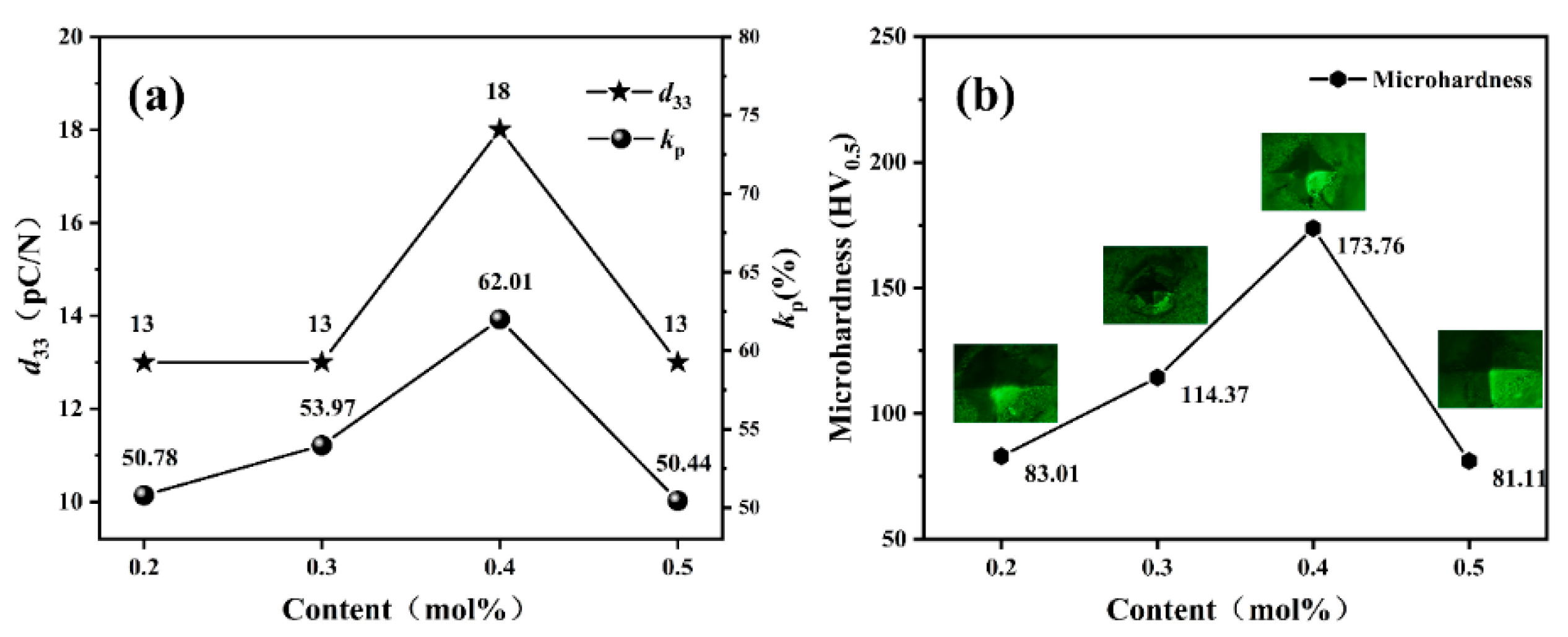
3.3. Piezoelectric Properties
3.4. Mechanical Properties
3.5. Water Contact Angle
3.6. Cell Cytotoxicity
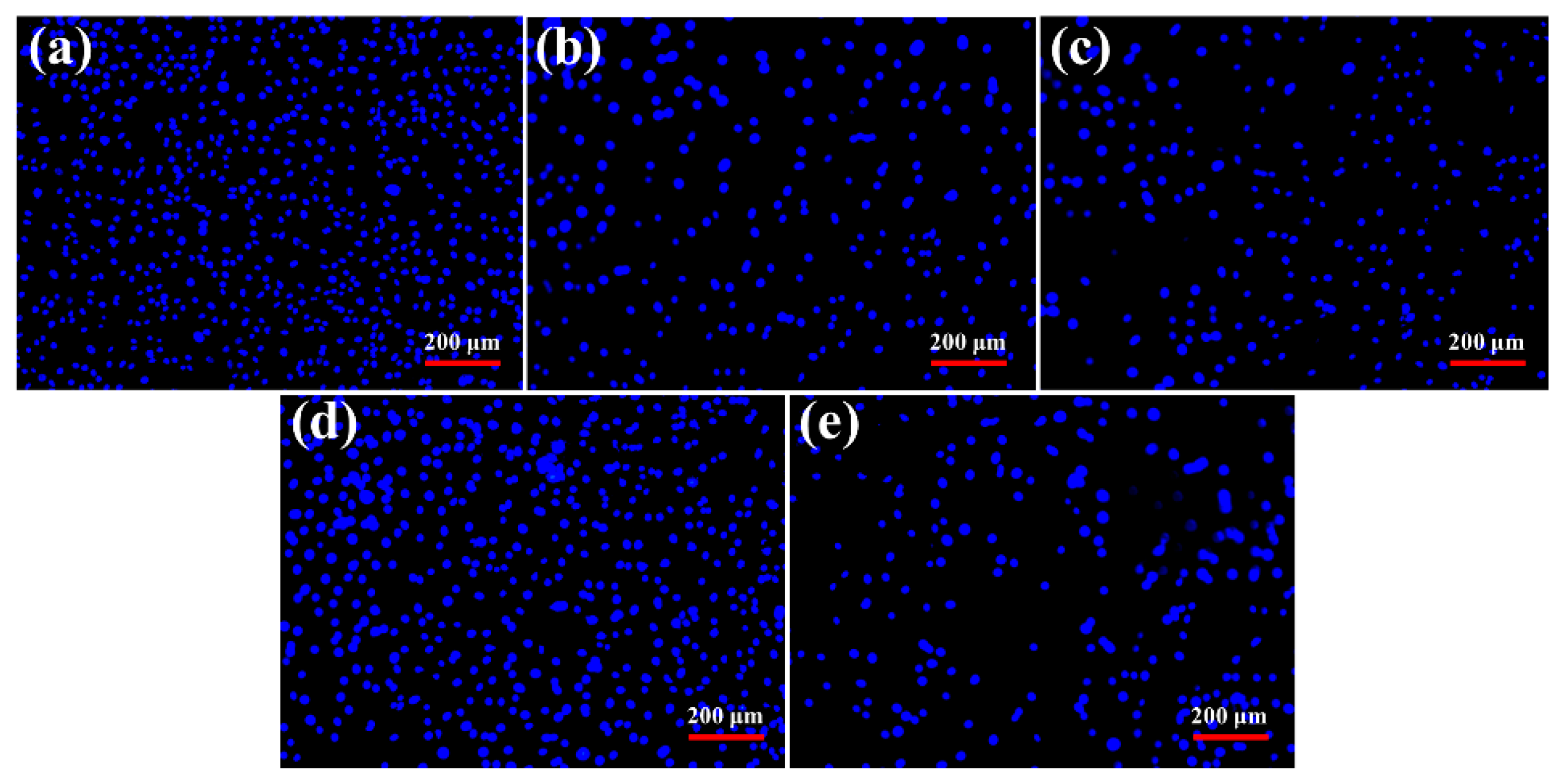
3.7. Apoptosis Evaluation
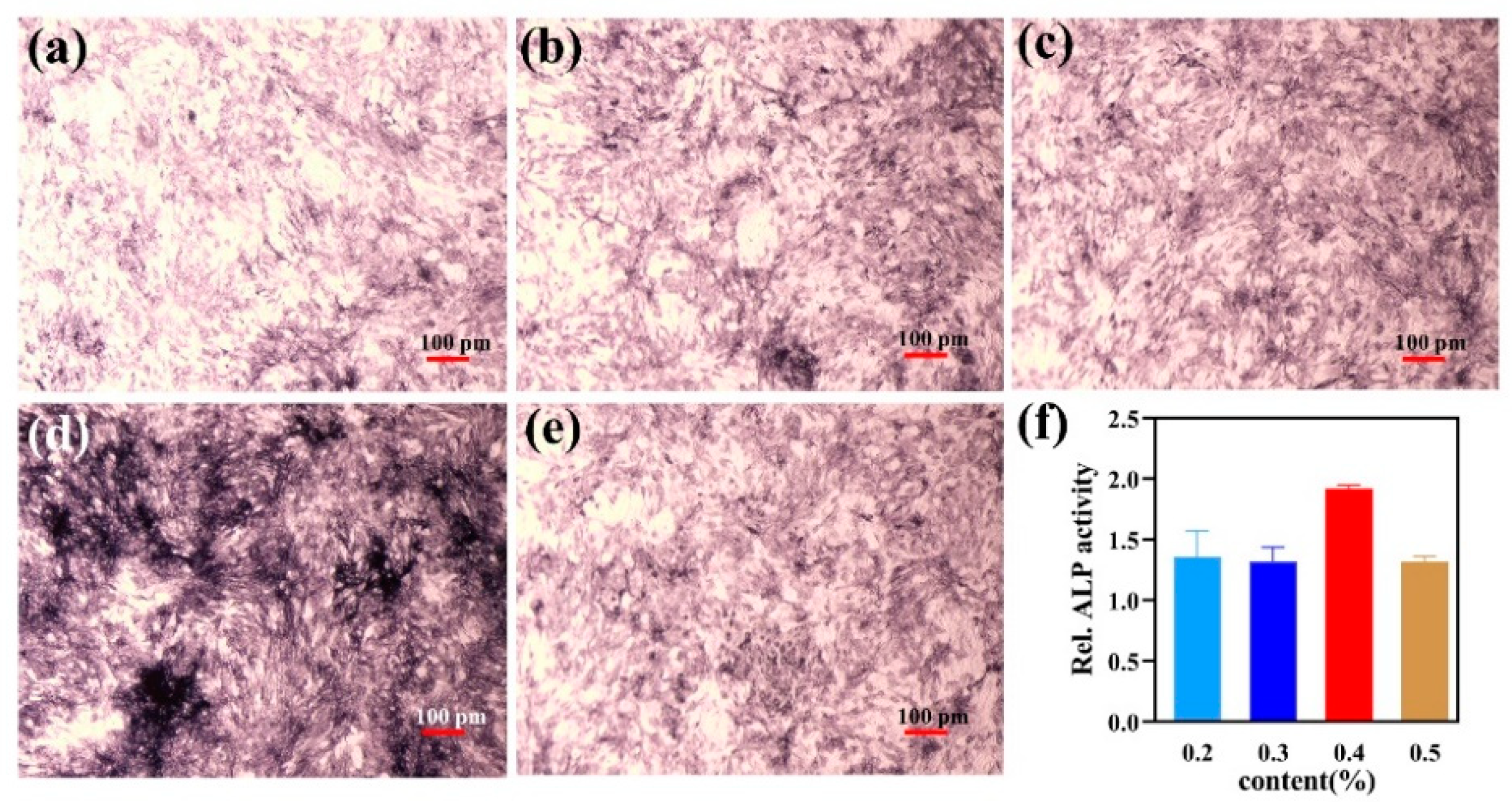
3.8. Alkaline Phosphatase Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Khan, A.F.; Saleem, M.; Afzal, A.; Ali, A.; Khan, A.; Khan, A.R. Bioactive behavior of silicon substituted calcium phosphate based bioceramics for bone regeneration. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2014, 35, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, M.D.; Altuntaş, A. Congenital vomeral bone defect. Am. J. Otolaryngol. 2005, 26, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhang, Y.; Zhou, Z.; Wang, J.; Han, D.; Sun, J.; Chen, G.; Tang, Q.; Sun, W.; Chen, L. Dysfunction of macrophages leads to diabetic bone regeneration deficiency. Front. Immunol. 2022, 13, 990457. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Harada, N.; Sato, K.; Abe, S.; Yamanaka, K.; Matushita, T. Stem cell therapy: Is there a future for reconstruction of large bone defects? Int. J. Care Inj. 2016, 47, S47–S51. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, Y.; Luo, Y.; Zhang, W.; Zhou, Y.; Tu, C. Uncemented three-dimensional-printed prosthetic reconstruction for massive bone defects of the proximal tibia. World J. Surg. Oncol. 2018, 16, 47. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; He, X.; Chen, S.; Yu, H. Advances in the use of calcium silicate-based materials in bone tissue engineering. Ceram. Int. 2023, 49, 19355–19363. [Google Scholar] [CrossRef]
- Valtanen, R.S.; Yang, Y.P.; Gurtner, G.C.; Maloney, W.J.; Lowenberg, D.W. Synthetic and Bone tissue engineering graft substitutes: What is the future? Int. J. Care Inj. 2021, 52, S72–S77. [Google Scholar] [CrossRef]
- Jung, R.E.; Glauser, R.; Schärer, P.; Hämmerle, C.H.; Sailer, H.F.; Weber, F.E. Effect of rhBMP-2 on guided bone regeneration in humans. Clin. Oral Implant. Res. 2003, 14, 556–568. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, E.D.; Battle, R.K.; Zahra, S.; Keating, J.F.; Marson, L.P.; Turner, D.M. Allosensitization Following Bone Graft. Am. J. Transplant. 2017, 17, 2207–2211. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Phuoc, H.D.; Hoang, P.N.; Yang, S.; Fraser, D.; Nguyen, V.T. Osseointegrability of 3D-printed porous titanium alloy implant on tibial shaft bone defect in rabbit model. PLoS ONE 2023, 18, e0282457. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Han, J.; Zhang, X.; Wu, Y.; Deng, X.-L. Efficacy of a mineralized collagen bone-grafting material for peri-implant bone defect reconstruction in mini pigs. Regen. Biomater. 2019, 6, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.M.; Yetisgin, A.A.; Demir, E.; Sahin, S.B.; Cetinel, S. Polysaccharide-bioceramic composites for bone tissue engineering: A review. Int. J. Biol. Macromol. 2023, 250, 126237. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Majee, P.; Dhar, S.; Mitra, P.K.; Lalzawmliana, V.; Nandi, S.K.; Basak, P.; Kundu, B. In vivo bone regeneration analysis of trilayer coated 316L stainless steel implant in rabbit model. J. Mater. Res. 2018, 33, 2106–2117. [Google Scholar] [CrossRef]
- Yu, H.; Liu, H.; Shen, Y.; Ao, Q. Synthetic biodegradable polymer materials in the repair of tumor-associated bone defects. Front. Bioeng. Biotechnol. 2023, 11, 1096525. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, W.; Bi, S.; Li, R.; Hu, H.; Lin, H.; Tuan, R.S.; Khor, K.A. Incorporating silica-coated graphene in bioceramic nanocomposites to simultaneously enhance mechanical and biological performance. J. Biomed. Mater. Res. A 2020, 108, 1016–1027. [Google Scholar] [CrossRef]
- Budharaju, H.; Suresh, S.; Sekar, M.P.; De Vega, B.; Sethuraman, S.; Sundaramurthi, D.; Kalaskar, D.M. Ceramic materials for 3D printing of biomimetic bone scaffolds—Current state-of-the-art & future perspectives. Mater. Des. 2023, 231, 112064. [Google Scholar] [CrossRef]
- Fukada, E.; Yasuda, I. On the Piezoelectric Effect of Bone. J. Phys. Soc. Jpn. 1957, 12, 1158–1162. [Google Scholar] [CrossRef]
- Tang, H.; Liao, R.; Tian, J. Application and design of piezoelectric materials for bone defect repair. Chin. J. Tissue Eng. Res. 2023, 27, 1117–1125. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. J. Artif. Organs 2019, 43, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Bhunia, S.; Karan, S.K.; Chowdhury, R.; Ghosh, I.; Saha, S.; Das, K.; Mondal, A.; Nanda, A.; Khatua, B.B.; Reddy, C.M. Mechanically flexible piezoelectric organic single crystals for electrical energy harvesting. Chem 2024. [Google Scholar] [CrossRef]
- Zheng, T.; Zhao, H.; Huang, Y.; Gao, C.; Zhang, X.; Cai, Q.; Yang, X. Piezoelectric calcium/manganese-doped barium titanate nanofibers with improved osteogenic activity. Ceram. Int. 2021, 47, 28778–28789. [Google Scholar] [CrossRef]
- Mokhtari, F.; Azimi, B.; Salehi, M.; Hashemikia, S.; Danti, S. Recent advances of polymer-based piezoelectric composites for biomedical applications. J. Mech. Behav. Biomed. Mater. 2021, 122, 104669. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Cuozzo, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Autologous Bone Grafting in Trauma and Orthopaedic Surgery: An Evidence-Based Narrative Review. J. Clin. Med. 2021, 10, 4347. [Google Scholar] [CrossRef]
- Gheno, E.; Alves, G.G.; Ghiretti, R.; Mello-Machado, R.C.; Signore, A.; Lourenço, E.S.; Leite, P.E.C.; Mourão, C.F.d.A.B.; Sohn, D.-S.; Calasans-Maia, M.D. “Sticky Bone” Preparation Device: A Pilot Study on the Release of Cytokines and Growth Factors. Materials 2022, 15, 1474. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, C.; Wu, Z.; Hu, L.; Zhang, W.; Zhao, K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci. Rep. 2017, 7, 43360. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, X.; Jiao, Y.; Wu, C.; Guo, S.; Xiao, X.; Wei, X.; Wu, J.; Gao, P.; Wang, N.; et al. Biological Effects of a Three-Dimensionally Printed Ti6Al4V Scaffold Coated with Piezoelectric BaTiO3 Nanoparticles on Bone Formation. ACS Appl. Mater. Interfaces 2020, 12, 51885–51903. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ren, X. Large piezoelectric effect in Pb-free ceramics. Phys. Rev. Lett. 2009, 103, 257602. [Google Scholar] [CrossRef]
- Poon, K.K.; Schafföner, S.; Einarsrud, M.-A.; Glaum, J. Barium titanate-based bilayer functional coatings on Ti alloy biomedical implants. J. Eur. Ceram. Soc. 2021, 41, 2918–2922. [Google Scholar] [CrossRef]
- Liu, R.; Qiao, Y.; Zhang, X.; Lu, C.-X. Effects of calcination temperatures on the morphology, architecture and dielectric properties of BCZT ceramics. Bull. Mater. Sci. 2019, 42, 140. [Google Scholar] [CrossRef]
- Poon, K.K.; Wurm, M.C.; Evans, D.M.; Einarsrud, M.-A.; Lutz, R.; Glaum, J. Biocompatibility of (Ba,Ca)(Zr,Ti)O3 piezoelectric ceramics for bone replacement materials. J. Biomed. Mater. Res. Part B 2020, 108, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, L.; Duan, Z.; Zhao, K.; Wu, Z. Enhanced compressive strengths and induced cell growth of 1-3-type BaTiO3/PMMA bio-piezoelectric composites. Mater. Sci. Eng. C-Biomimetic Supramol. Syst. 2021, 120, 111699. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kim, J.H. Synthesis, toxicity, biocompatibility, and biomedical applications of graphene and graphene-related materials. Int. J. Nanomed. 2016, 11, 1927–1945. [Google Scholar] [CrossRef] [PubMed]
- Manohar, C.S.; Kumar, B.S.; Sadhu, S.P.P.; Srimadh, S.K.; Muthukumar, V.S.; Venketesh, S.; Varma, K.B.R. Novel Lead-free biocompatible piezoelectric Hydroxyapatite (HA)—BCZT (Ba0.85Ca0.15Zr0.1Ti0.9O3) nanocrystal composites for bone regeneration. Nanotechnol. Rev. 2019, 8, 61–78. [Google Scholar] [CrossRef]
- Chiticaru, E.A.; Ionita, M. Graphene toxicity and future perspectives in healthcare and biomedicine. FlatChem 2022, 35, 100417. [Google Scholar] [CrossRef]
- Orek, C.; Bartolomei, M.; Coletti, C.; Bulut, N. Graphene as Nanocarrier for Gold(I)-Monocarbene Complexes: Strength and Nature of Physisorption. Molecules 2023, 28, 3941. [Google Scholar] [CrossRef]
- Wu, C.; Xia, L.; Han, P.; Xu, M.; Fang, B.; Wang, J.; Chang, J.; Xiao, Y. Graphene-oxide-modified β-tricalcium phosphate bioceramics stimulate in vitro and in vivo osteogenesis. Carbon 2015, 93, 116–129. [Google Scholar] [CrossRef]
- Iatsenko, A.; Sych, O.; Synytsia, A.; Zaremba, P.; Zahorodnia, S.; Nikolenko, A.; Tomila, T.; Bykov, O. Structure and properties of biogenic hydroxyapatite bioceramics modified by graphene-like structures. Appl. Nanosci. 2023, 13, 7477–7483. [Google Scholar] [CrossRef]
- Shi, L.; Chen, J.; Teng, L.; Wang, L.; Zhu, G.; Liu, S.; Luo, Z.; Shi, X.; Wang, Y.; Ren, L. The Antibacterial Applications of Graphene and Its Derivatives. Small 2016, 12, 4165–4184. [Google Scholar] [CrossRef]
- Norahan, M.H.; Amroon, M.; Ghahremanzadeh, R.; Rabiee, N.; Baheiraei, N. Reduced graphene oxide: Osteogenic potential for bone tissue engineering. IET Nanobiotechnol. 2019, 13, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cao, G.; Wei, S.; Han, Y.; Xu, P. Progress in the application of graphene and its derivatives to osteogenesis. Heliyon 2023, 9, e21872. [Google Scholar] [CrossRef]
- Palmieri, V.; Bugli, F.; Lauriola, M.C.; Cacaci, M.; Torelli, R.; Ciasca, G.; Conti, C.; Sanguinetti, M.; Papi, M.; De Spirito, M. Bacteria Meet Graphene: Modulation of Graphene Oxide Nanosheet Interaction with Human Pathogens for Effective Antimicrobial Therapy. ACS Biomater. Sci. Eng. 2017, 3, 619–627. [Google Scholar] [CrossRef]
- Ilyas, K.; Zahid, S.; Batool, M.; Chaudhry, A.A.; Jamal, A.; Iqbal, F.; Nawaz, M.H.; Goerke, O.; Gurlo, A.; Shah, A.T.; et al. In-vitro investigation of graphene oxide reinforced bioactive glass ceramics composites. J. Non-Cryst. Solids 2019, 505, 122–130. [Google Scholar] [CrossRef]
- Joy, A.; Unnikrishnan, G.; Megha, M.; Haris, M.; Thomas, J.; Kolanthai, E.; Senthilkumar, M. Hybrid gold/graphene oxide reinforced polycaprolactone nanocomposite for biomedical applications. Surf. Interfaces 2023, 40, 103000. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Li, M.; Shi, L.; Ong, J.L.T.; Jańczewski, D.; Neoh, K.G. Parallel Control over Surface Charge and Wettability Using Polyelectrolyte Architecture: Effect on Protein Adsorption and Cell Adhesion. ACS Appl. Mater. Interfaces 2016, 8, 30552–30563. [Google Scholar] [CrossRef]
- Kubiak, K.J.; Wilson, M.C.T.; Mathia, T.G.; Carval, P. Wettability versus roughness of engineering surfaces. Wear 2011, 271, 523–528. [Google Scholar] [CrossRef]
- Lasocka, I.; Szulc-Dąbrowska, L.; Skibniewski, M.; Skibniewska, E.; Gregorczyk-Zboroch, K.; Pasternak, I.; Hubalek Kalbacova, M. Cytocompatibility of Graphene Monolayer and Its Impact on Focal Cell Adhesion, Mitochondrial Morphology and Activity in BALB/3T3 Fibroblasts. Materials 2021, 14, 643. [Google Scholar] [CrossRef]
- Nakamura, T.; Nakamura-Takahashi, A.; Kasahara, M.; Yamaguchi, A.; Azuma, T. Tissue-nonspecific alkaline phosphatase promotes the osteogenic differentiation of osteoprogenitor cells. Biochem. Biophys. Res. Commun. 2020, 524, 702–709. [Google Scholar] [CrossRef]
- Cuenca-Bracamonte, Q.; Yazdani-Pedram, M.; Aguilar-Bolados, H. Electrical Properties of Polyetherimide-Based Nanocomposites Filled with Reduced Graphene Oxide and Graphene Oxide-Barium Titanate-Based Hybrid Nanoparticles. Polymers 2022, 14, 4266. [Google Scholar] [CrossRef]
- Wang, X.W.; Zhang, B.H.; Li, Y.Y.; Shi, Y.C.; Sun, L.Y.; Feng, G.; Li, C.L.; Liang, Y.F.; Zheng, Y.P.; Shang, S.Y.; et al. Structure, dielectric, and ferroelectric properties of Ba0.85Ca0.15Zr0.1Ti0.9O3 ceramics sintered at various temperatures. J. Mater. Sci.-Mater. Electron. 2020, 31, 4732–4742. [Google Scholar] [CrossRef]
- Ismail, M.F.; Islam, M.A.; Khorshidi, B.; Sadrzadeh, M. Prediction of surface charge properties on the basis of contact angle titration models. Mater. Chem. Phys. 2021, 258, 123933. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, B.; Ma, Y.; Huang, Y.; Li, N.; Zhang, F.; Chen, Y. Efficient and large-scale synthesis of few-layered graphene using an arc-discharge method and conductivity studies of the resulting films. Nano Res. 2010, 3, 661–669. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K.; Kim, K. Study of antibacterial mechanism of graphene oxide using Raman spectroscopy. Sci. Rep. 2016, 6, 28443. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-Y.; Yao, X.; Müllen, K. Polycyclic aromatic hydrocarbons in the graphene era. Sci. China Chem. 2019, 62, 1099–1144. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Das, A.; Chakraborty, B.; Sood, A.K. Raman spectroscopy of graphene on different substrates and influence of defects. Bull. Mat. Sci. 2008, 31, 579–584. [Google Scholar] [CrossRef]
- Lucchese, M.M.; Stavale, F.; Ferreira, E.H.M.; Vilani, C.; Moutinho, M.V.O.; Capaz, R.B.; Achete, C.A.; Jorio, A. Quantifying ion-induced defects and Raman relaxation length in graphene. Carbon 2010, 48, 1592–1597. [Google Scholar] [CrossRef]
- Quispe, L.T.; Menezes, J.W.; Chong, W.; Zegarra, L.B.R.; Armas, L.E.G. Influence of gold nanoholes and nanoslits arrays on Raman spectra and optical reflectance of graphene oxide. Opt. Express 2018, 26, 31253–31263. [Google Scholar] [CrossRef]
- Crowther, A.C.; Ghassaei, A.; Jung, N.; Brus, L.E. Strong Charge-Transfer Doping of 1 to 10 Layer Graphene by NO2. ACS Nano 2012, 6, 1865–1875. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Roca, R.A.; Badillo, F.A.L.; Eiras, J.A. Spark plasma sintering and electric conductivity of anatase TiO2 nanoceramics. J. Mater. Sci.-Mater. Electron. 2022, 33, 4375–4387. [Google Scholar] [CrossRef]
- Lu, N.; He, G.; Yang, Z.; Li, Y.; Li, J. Fabrication and densification mechanism of MgO/Graphene composites with LiF as additive. Scr. Mater. 2020, 174, 91–94. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, S.; Qi, F.; Zan, J.; Liu, G.; Zhao, Z.; Shuai, C. Graphene-assisted barium titanate improves piezoelectric performance of biopolymer scaffold. Mater. Sci. Eng. C-Biomim. Supramol. Syst. 2020, 116, 111195. [Google Scholar] [CrossRef] [PubMed]
- Li, S.B.; Wang, C.B.; Li, L.; Shen, Q.; Zhang, L.M. Effect of annealing temperature on structural and electrical properties of BCZT ceramics prepared by Plasma Activated Sintering. J. Alloys Compd. 2018, 730, 182–190. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Li, Q.; Gao, R.; Chen, G.; Deng, X.; Wang, Z.; Cao, X.; Fu, C. Enhanced piezoelectric response of (Ba,Ca)(Ti, Zr)O3 ceramics by super large grain size and construction of phase boundary. J. Alloys Compd. 2019, 794, 542–552. [Google Scholar] [CrossRef]
- Kurapova, O.Y.; Lomakin, I.V.; Sergeev, S.N.; Solovyeva, E.N.; Zhilyaev, A.P.; Archakov, I.Y.; Konakov, V.G. Fabrication of nickel-graphene composites with superior hardness. J. Alloys Compd. 2020, 835, 155463. [Google Scholar] [CrossRef]
- Suhaimin, N.S.; Hanifah, M.F.R.; Azhar, M.; Jaafar, J.; Aziz, M.; Ismail, A.F.; Othman, M.H.D.; Rahman, M.A.; Aziz, F.; Yusof, N.; et al. The evolution of oxygen-functional groups of graphene oxide as a function of oxidation degree. Mater. Chem. Phys. 2022, 278, 125629. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Jiang, Z.; Lan, J.; Zhao, L.; Si, P. Effect of graphene oxide on the mechanical, tribological, and biological properties of sintered 3Y–ZrO2/GO composite ceramics for dental implants. Ceram. Int. 2021, 47, 6940–6946. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, J.; Chen, F.; Hou, F.; Bai, D.; Xi, P.; Zeng, Z. Biomimetic and Cell-Mediated Mineralization of Hydroxyapatite by Carrageenan Functionalized Graphene Oxide. ACS Appl. Mater. Interfaces 2014, 6, 3132–3140. [Google Scholar] [CrossRef]
- Shin, D.S.; Kim, H.G.; Ahn, H.S.; Jeong, H.Y.; Kim, Y.-J.; Odkhuu, D.; Tsogbadrakh, N.; Lee, H.-B.-R.; Kim, B.H. Distribution of oxygen functional groups of graphene oxide obtained from low-temperature atomic layer deposition of titanium oxide. RSC Adv. 2017, 7, 13979–13984. [Google Scholar] [CrossRef]
- Li, Q.; Shen, A.; Wang, Z. Enhanced osteogenic differentiation of BMSCs and M2-phenotype polarization of macrophages on a titanium surface modified with graphene oxide for potential implant applications. RSC Adv. 2020, 10, 16537–16550. [Google Scholar] [CrossRef] [PubMed]

| C (at.%) | O (at.%) | Ca (at.%) | Ti (at.%) | Ba (at.%) | |
|---|---|---|---|---|---|
| x = 0 mol% | 10.13 | 43.08 | 4.26 | 20.62 | 21.90 |
| x = 0.2 mol% | 15.19 | 48.16 | 3.01 | 16.58 | 17.05 |
| x = 0.3 mol% | 10.32 | 45.04 | 3.77 | 20.04 | 20.82 |
| x = 0.4 mol% | 25.55 | 49.53 | 2.03 | 8.69 | 14.20 |
| x = 0.5 mol% | 23.95 | 39.84 | 2.88 | 16.35 | 16.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Liu, Q.; Tang, G.; Wang, D. Microstructure and Biocompatibility of Graphene Oxide/BCZT Composite Ceramics via Fast Hot-Pressed Sintering. Coatings 2024, 14, 689. https://doi.org/10.3390/coatings14060689
Zhao B, Liu Q, Tang G, Wang D. Microstructure and Biocompatibility of Graphene Oxide/BCZT Composite Ceramics via Fast Hot-Pressed Sintering. Coatings. 2024; 14(6):689. https://doi.org/10.3390/coatings14060689
Chicago/Turabian StyleZhao, Bingqing, Qibin Liu, Geng Tang, and Dunying Wang. 2024. "Microstructure and Biocompatibility of Graphene Oxide/BCZT Composite Ceramics via Fast Hot-Pressed Sintering" Coatings 14, no. 6: 689. https://doi.org/10.3390/coatings14060689
APA StyleZhao, B., Liu, Q., Tang, G., & Wang, D. (2024). Microstructure and Biocompatibility of Graphene Oxide/BCZT Composite Ceramics via Fast Hot-Pressed Sintering. Coatings, 14(6), 689. https://doi.org/10.3390/coatings14060689





