Oxidation Resistance of Ir/HfO2 Composite Coating Prepared by Chemical Vapor Deposition: Microstructure and Elemental Migration
Abstract
:1. Introduction
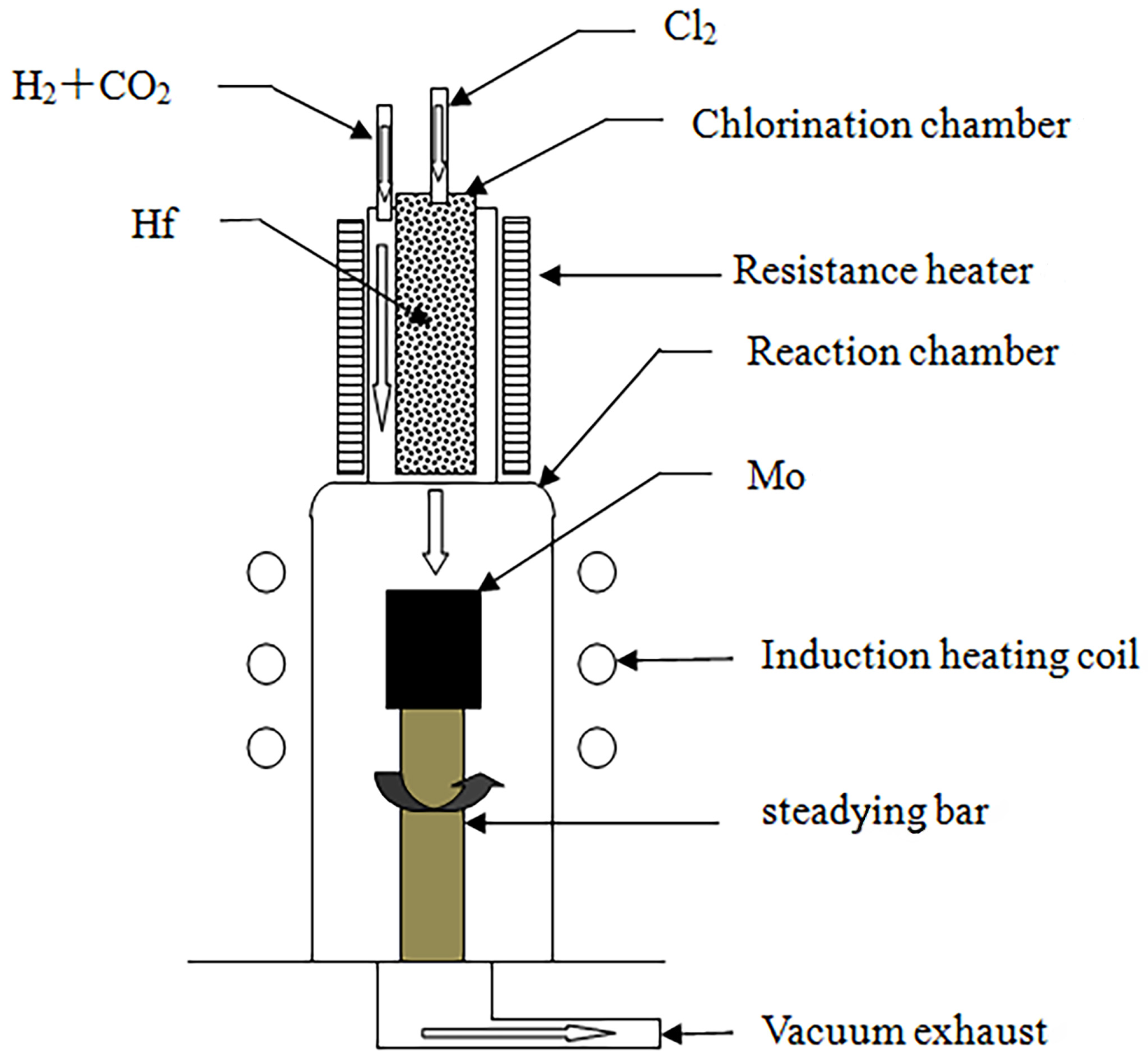
2. Materials and Methods
- (1)
- Chlorination chamber: A 65 mm diameter quartz tube was selected as the chlorination chamber, wherein Hf was heated using a self-assembled resistance furnace, and its temperature was measured with a platinum–rhodium alloy thermocouple. A CY digital display temperature controller was employed to control the temperature in the furnace.
- (2)
- Deposition chamber: A quartz tube with a diameter of 86 mm was selected as the deposition chamber, and the matrix was heated using the medium-frequency induction method. The tube’s surface temperature was measured using an infrared thermometer (WGG2-201N) (the measurement error was maintained at ±5 °C), and a self-assembled electric control cabinet was used to control the temperature.
- (3)
- Gas supply system: After drying was allowed, the reaction gas flow rate was controlled using a glass-rotor gas flow meter, which was introduced into the chlorination chamber and deposition chamber through a rubber tube.
- (4)
- Exhaust treatment system: A 2X-4 vacuum pump was used to extract the reaction residue. To prevent blockage of the pumping pipeline, a glass-filled filtration device was installed between the vacuum pump and the deposition chamber to prevent powder byproducts from being generated during the deposition process. The filtered exhaust gas was fed into a dilute NaOH solution for treatment.
3. Results and Discussion
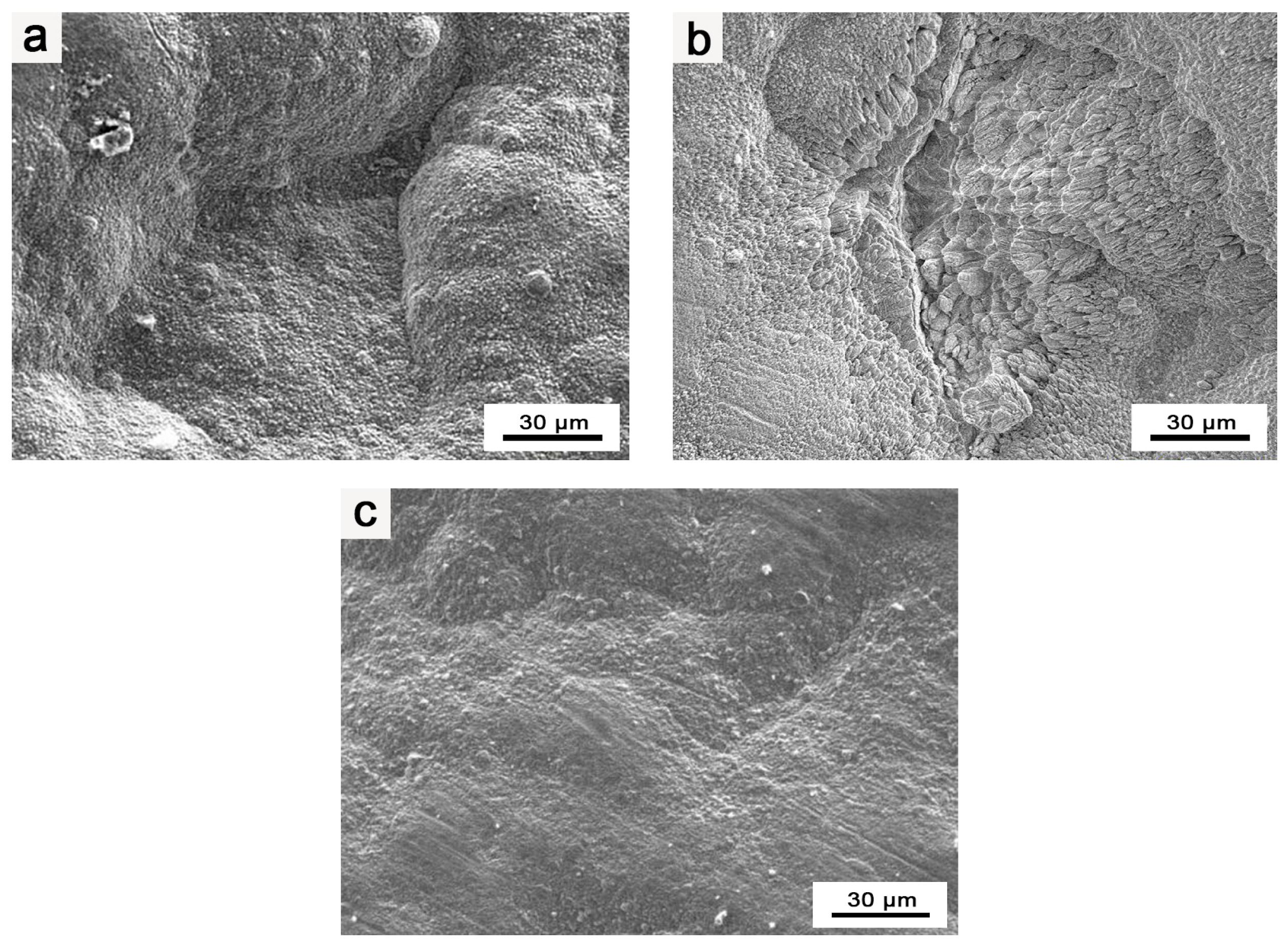
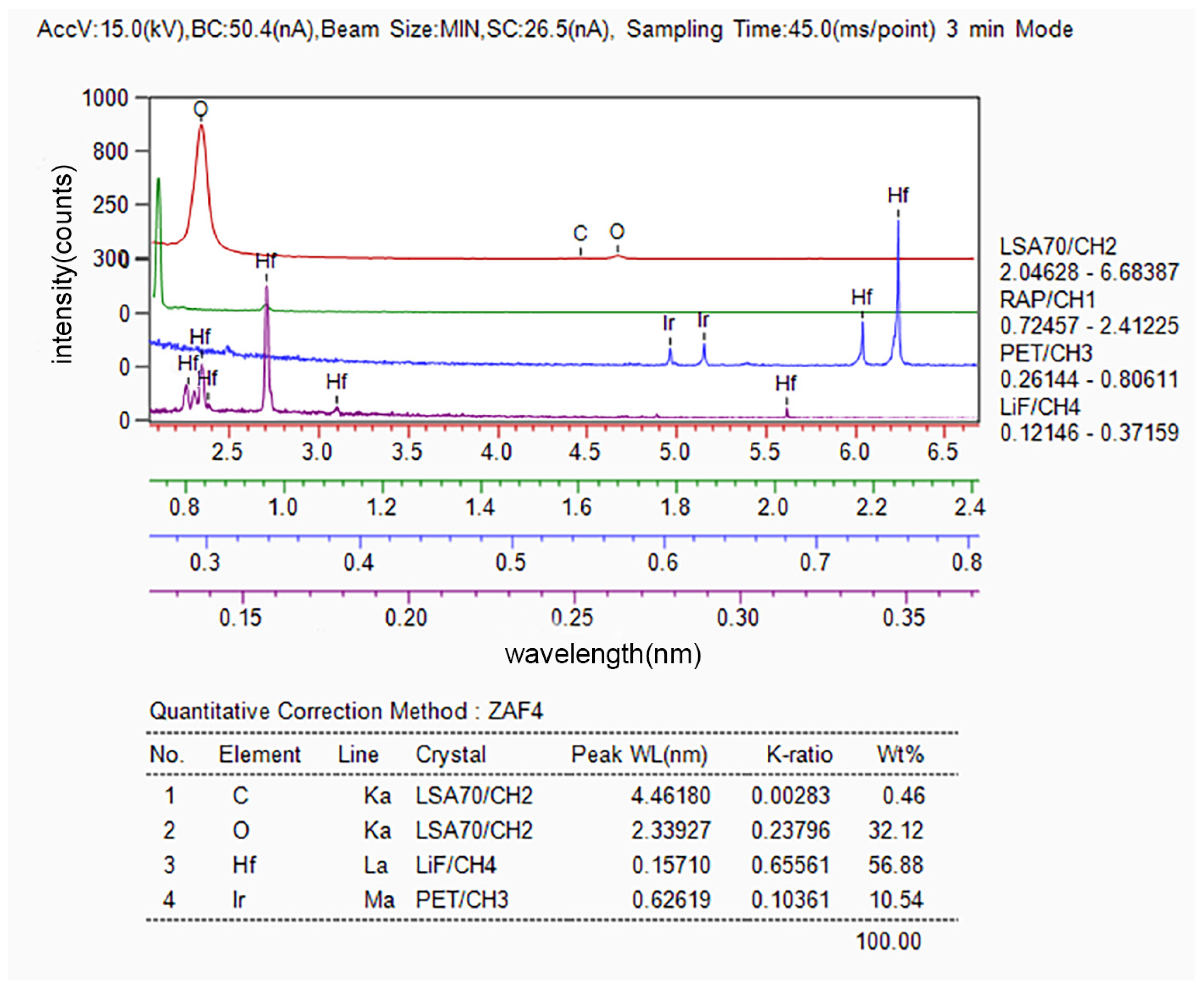
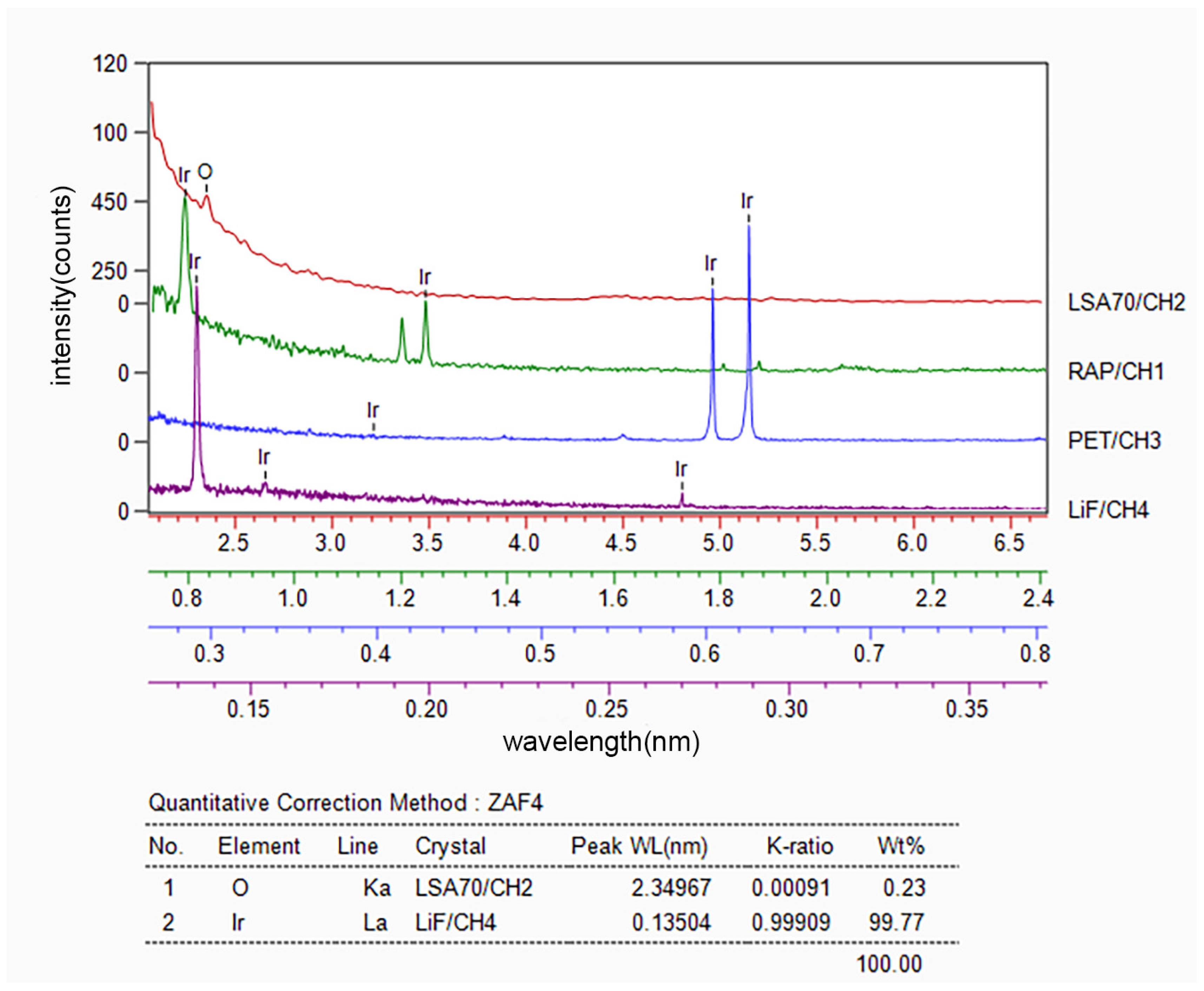
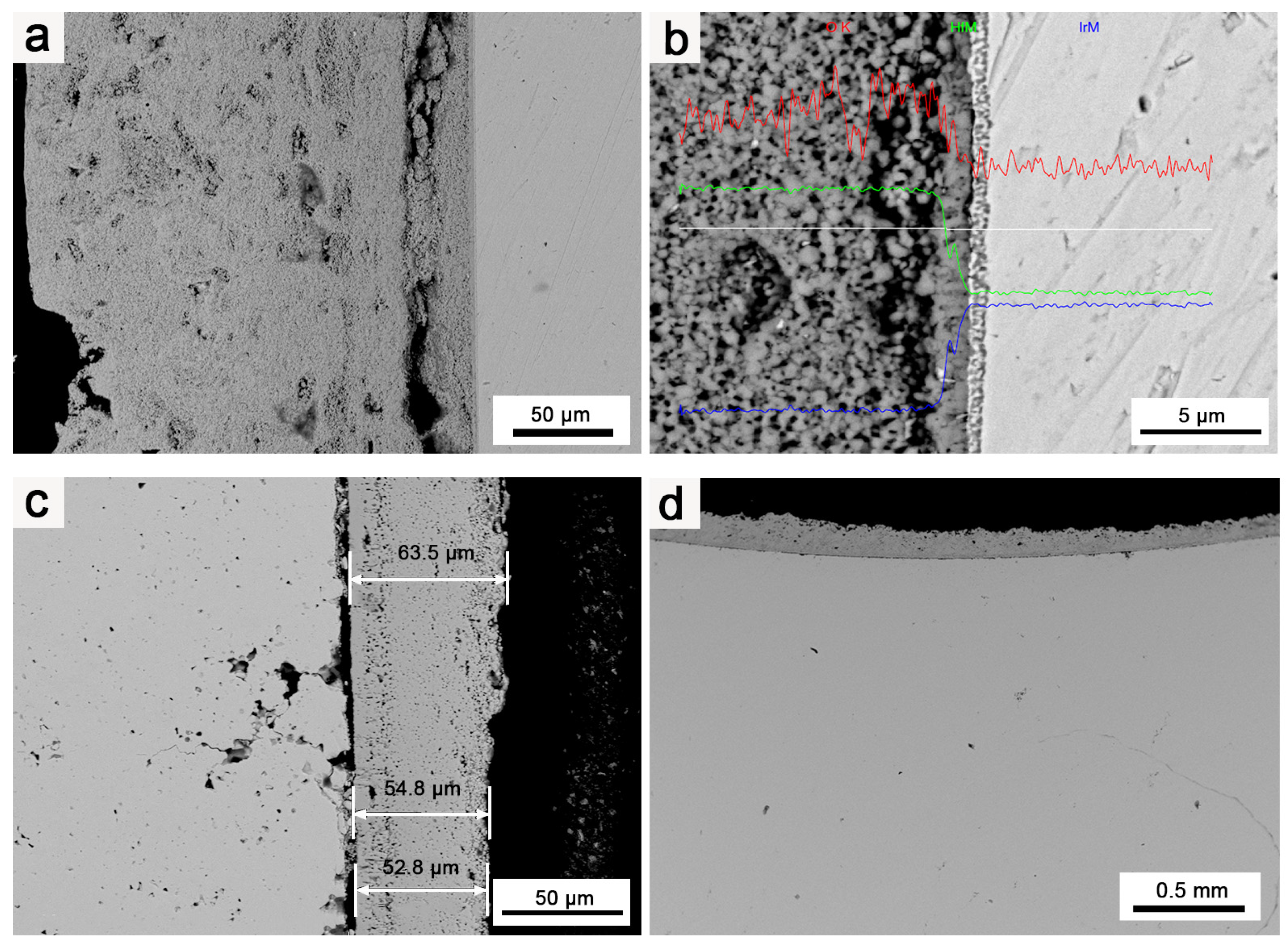
3.1. Microstructure and Composition of Ir/HfO2 Composite Coating
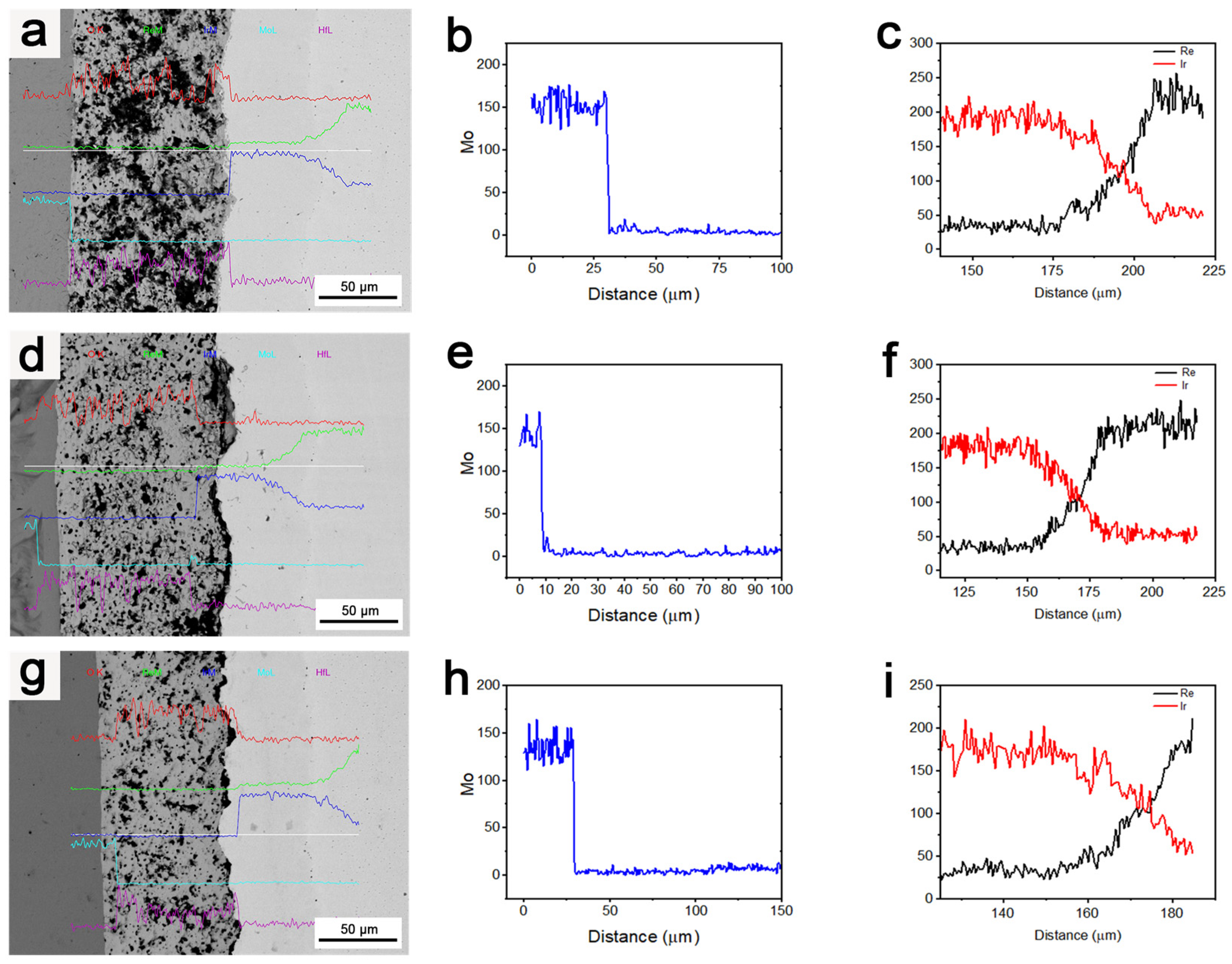
3.2. Oxidation Resistance and Structure of Ir and Ir/HfO2
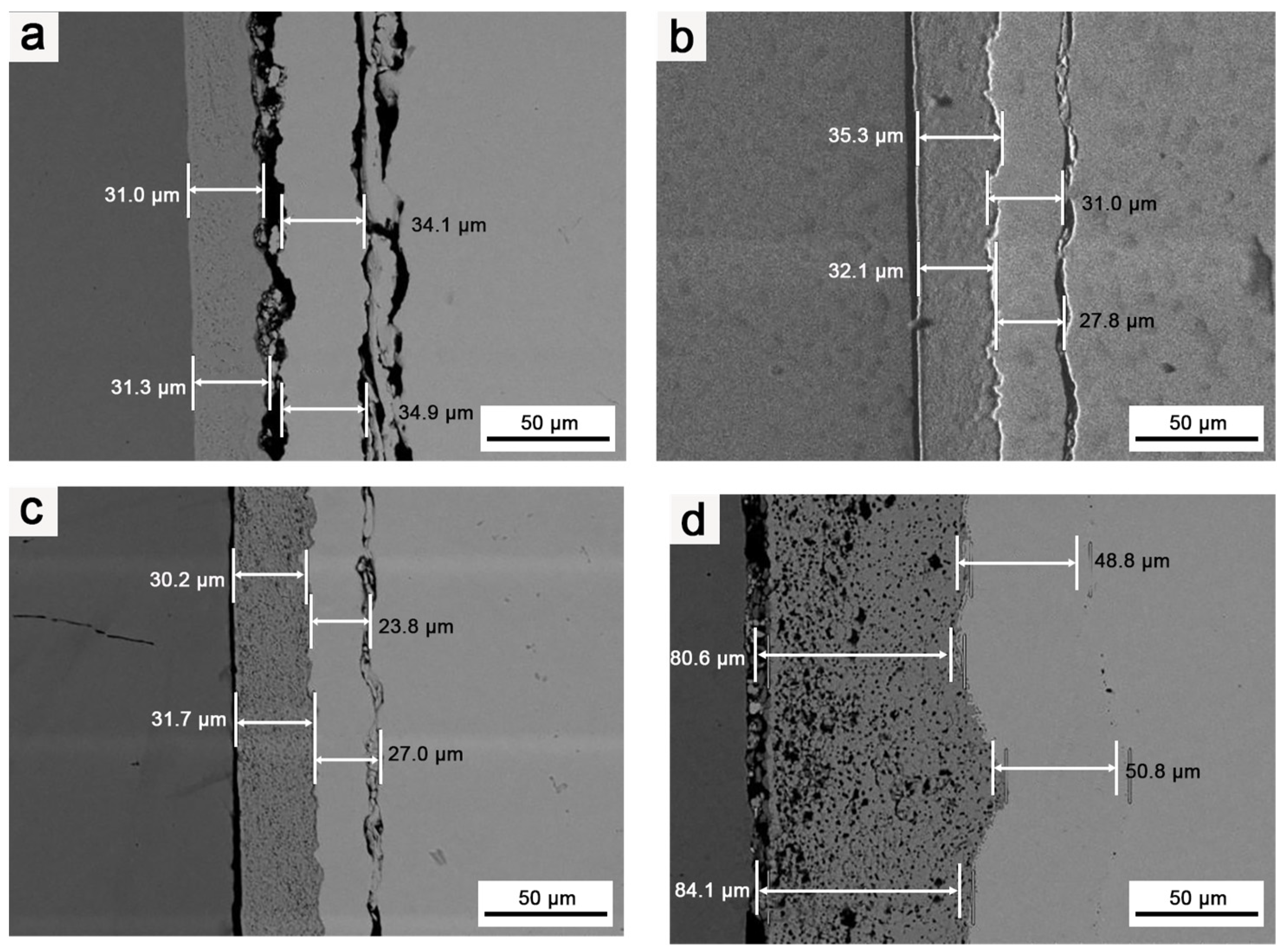
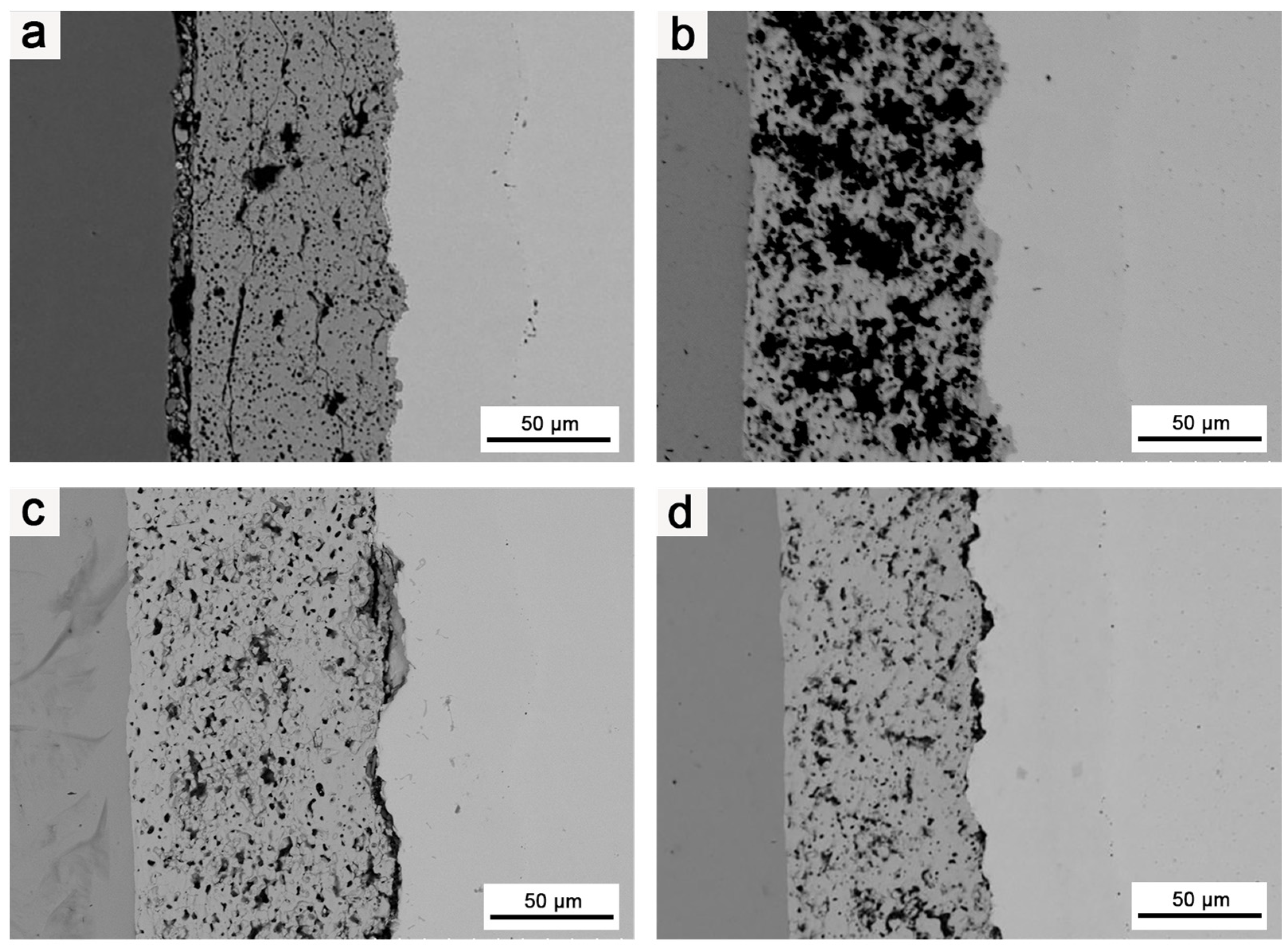
3.3. Effects of Heat Treatment Temperature on the Microstructure of the Composite Coating
3.4. Effects of Heat Treatment Duration on the Composite Coating’s Microstructure
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schütze, M. Fundamentals of High Temperature Corrosion. In Materials Science and Technology: A Comprehensive Treatment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; pp. 67–130. ISBN 978-3-527-61930-6. [Google Scholar]
- Thakare, J.G.; Pandey, C.; Mahapatra, M.M.; Mulik, R.S. Thermal Barrier Coatings—A State of the Art Review. Met. Mater. Int. 2021, 27, 1947–1968. [Google Scholar] [CrossRef]
- Biaglow, J. High Temperature Rhenium Material Properties. In 34th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit; American Institute of Aeronautics and Astronautics: Washington, DC, USA, 1998. [Google Scholar]
- Liu, C.; Chen, J.; Han, H.; Wang, Y.; Zhang, Z. A Long Duration and High Reliability Liquid Apogee Engine for Satellites. Acta Astronaut. 2004, 55, 401–408. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, H.Y.; Jiang, Z.R.; Xia, Z.X. Mechanical Properties and Thermal Shock Resistance of Rhenium Coating in Iridium/Rhenium/Carbon-Carbon Composites. Procedia Eng. 2015, 99, 1407–1414. [Google Scholar] [CrossRef]
- Jacobson, N.S.; Myers, D.L.; Zhu, D.; Humphrey, D.L. Rhenium/Oxygen Interactions at Elevated Temperatures. Oxid. Met. 2001, 55, 471–480. [Google Scholar] [CrossRef]
- Mumtaz, K.; Echigoya, J.; Taya, M. Preliminary Study of Iridium Coating on Carbon/Carbon Composites. J. Mater. Sci. 1993, 28, 5521–5527. [Google Scholar] [CrossRef]
- Davis, J.R. ASM Specialty Handbook: Heat-Resistant Materials; ASM International: Materials Park, OH, USA, 1997; ISBN 978-0-87170-596-9. [Google Scholar]
- Hu, H.B.; Chen, H.Y.; Yan, Y.; Zhang, F.; Yin, J.H.; Zheng, D. Investigation of Chemical Kinetic Model for Hypergolic Propellant of Monomethylhydrazine and Nitrogen Tetroxide. J. Energy Resour. Technol. 2020, 143, 062304. [Google Scholar] [CrossRef]
- Osmont, A.; Catoire, L.; Klapötke, T.M.; Vaghjiani, G.L.; Swihart, M.T. Thermochemistry of Species Potentially Formed During NTO/MMH Hypergolic Ignition. Propellants Explos. Pyrotech. 2008, 33, 209–212. [Google Scholar] [CrossRef]
- Chang, Y.-P.; Boyer, E.; Kuo, K.K. Combustion Behavior and Flame Structure of XM46 Liquid Propellant. J. Propuls. Power 2001, 17, 800–808. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Huang, H.; Fang, J. Design and Performance Evaluation of a Novel H2/O2 Electro-Chemical Hybrid Thruster. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2023, 237, 2594–2601. [Google Scholar] [CrossRef]
- Betti, B.; Bianchi, D.; Nasuti, F.; Martelli, E. Chemical Reaction Effects on Heat Loads of CH4/O2 and H2/O2 Rockets. AIAA J. 2016, 54, 1693–1703. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y.; Zhu, C.; Xiang, H.; Chen, H.; Sun, L.; Gao, Y.; Zhou, Y. Advances on Strategies for Searching for next Generation Thermal Barrier Coating Materials. J. Mater. Sci. Technol. 2019, 35, 833–851. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, W.; Cong, X. Oxidation Resistance Coatings of Ir–Zr and Ir by Double Glow Plasma. J. Mater. Sci. Technol. 2014, 30, 268–274. [Google Scholar] [CrossRef]
- Ai, T.; Wang, F.; Feng, X. High-Temperature Oxidation Behavior of Al2O3/TiAl Matrix Composite in Air. Sci. China Ser. E-Technol. Sci. 2009, 52, 1273–1282. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Sun, W.; Yang, Y.; Zhang, C.; Ma, Y.; Cui, Y.; Zhao, C.; Wang, L.; Dong, Y.; et al. Microstructure and Properties of Al2O3–ZrO2–Y2O3 Composite Coatings Prepared by Plasma Spraying. J. Therm. Spray Tech. 2020, 29, 967–978. [Google Scholar] [CrossRef]
- Chen, D.; Lu, J.; Sun, C.; Wang, Q.; Ning, X. Microstructure and Thermal Cycling Behavior of Ta2O5 and Y2O3 Co-Doped ZrO2 Coatings. J. Therm. Spray Tech. 2023, 32, 1327–1337. [Google Scholar] [CrossRef]
- Cverna, F.; ASM International (Eds.) ASM Ready Reference. Thermal Properties of Metals; ASM Materials Data Series; ASM International: Materials Park, OH, USA, 2002; ISBN 978-1-68015-944-8. [Google Scholar]
- Baklanova, N.I.; Lozanov, V.V.; Morozova, N.B.; Titov, A.T. The Effect of Heat Treatment on the Tensile Strength of the Iridium-Coated Carbon Fiber. Thin Solid Film. 2015, 578, 148–155. [Google Scholar] [CrossRef]
- Kisi, E.H.; Howard, C.J. Crystal Structures of Zirconia Phases and Their Inter-Relation. Key Eng. Mater. 1998, 153–154, 1–36. [Google Scholar] [CrossRef]
- Johnson, B.; Jones, J.L. Structures, Phase Equilibria, and Properties of HfO2. In Ferroelectricity in Doped Hafnium Oxide: Materials, Properties and Devices; Schroeder, U., Hwang, C.S., Funakubo, H., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK, 2019; pp. 25–45. ISBN 978-0-08-102430-0. [Google Scholar]
- Alper, A.M. High Temperature Oxides: Oxides of Rare Earths, Titanium, Zirconium, Hafnium, Niobium and Tantalum; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-1-4832-7139-2. [Google Scholar]
- Mergia, K.; Liedtke, V.; Speliotis, T.; Apostolopoulos, G.; Messoloras, S. Thermo-Mechanical Behaviour of HfO2 Coatings for Aerospace Applications. Adv. Mater. Res. 2009, 59, 87–91. [Google Scholar] [CrossRef]
- Kasajima, M.; Akashi, T.; Shimada, S. Effect of HfO2 Coating Films on Oxidation Resistance of SiC Ceramics. Key Eng. Mater. 2009, 403, 193–196. [Google Scholar] [CrossRef]
- Bi, M.; Zhu, J.; Luo, Y.; Cai, H.; Li, X.; Wang, X.; Wei, Y.; Wang, X.; Hu, C.; Hu, J.; et al. Effect of Deposition Temperature on the Surface, Structural, and Mechanical Properties of HfO2 Using Chemical Vapor Deposition (CVD). Coatings 2022, 12, 1731. [Google Scholar] [CrossRef]
- McGinn, P.J. Thin-Film Processing Routes for Combinatorial Materials Investigations—A Review. ACS Comb. Sci. 2019, 21, 501–515. [Google Scholar] [CrossRef]
- Abdul Hadi, S.; Humood, K.M.; Abi Jaoude, M.; Abunahla, H.; Shehhi, H.F.A.; Mohammad, B. Bipolar Cu/HfO2/P++ Si Memristors by Sol-Gel Spin Coating Method and Their Application to Environmental Sensing. Sci. Rep. 2019, 9, 9983. [Google Scholar] [CrossRef]
- Zhang, C.; Divitt, S.; Fan, Q.; Zhu, W.; Agrawal, A.; Lu, Y.; Xu, T.; Lezec, H.J. Low-Loss Metasurface Optics down to the Deep Ultraviolet Region. Light Sci. Appl. 2020, 9, 55. [Google Scholar] [CrossRef]
- McCormack, S.J.; Tseng, K.-P.; Weber, R.J.K.; Kapush, D.; Ushakov, S.V.; Navrotsky, A.; Kriven, W.M. In-Situ Determination of the HfO2–Ta2O5-Temperature Phase Diagram up to 3000 °C. J. Am. Ceram. Soc. 2019, 102, 4848–4861. [Google Scholar] [CrossRef]
- Chaston, B.J.C. The Oxidation of the Platinum Metals: A Descriptive Survey of the Reactions Involved. Platin. Met. Rev. 1975, 19, 135–140. [Google Scholar] [CrossRef]
- Wimber, R.T.; Kraus, H.G. Oxidation of Iridium. Metall. Trans. 1974, 5, 1565–1571. [Google Scholar] [CrossRef]
- Wimber, R.T.; Hills, S.W.; Wahl, N.K.; Tempero, C.R. Kinetics of Evaporation/Oxidation of Iridium. Metall. Trans. A 1977, 8, 193–199. [Google Scholar] [CrossRef]
- Carpenter, J.H. Equilibrium Reaction of Iridium and Oxygen at High Temperatures. J. Less Common Met. 1989, 152, 35–45. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, L.; Hua, Y.; Cheng, L. Thermal Stability of Iridium Coating Prepared by MOCVD. Int. J. Refract. Met. Hard Mater. 2009, 27, 33–36. [Google Scholar] [CrossRef]
- Mumtaz, K.; Echigoya, J.; Enoki, H.; Hirai, T.; Shindo, Y. Thermal Cycling of Iridium Coatings on Isotropic Graphite. J. Mater. Sci. 1995, 30, 465–472. [Google Scholar] [CrossRef]
- Wang, J.M.; Zhang, Z.W.; Xu, Z.H.; Lin, X.; Wu, W.P.; Chen, Z.F. Oxidation of Double Glow Plasma Discharge Coatings of Iridium on Molybdenum for Liquid Fuelled Rocket Motor Casings. Corros. Eng. Sci. Technol. 2011, 46, 732–736. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Li, W.; Cai, H.; Wang, X.; Wang, X.; Wei, Y.; Hu, C.; Zhao, X.; Zhang, X. Oxidation Resistance of Ir/HfO2 Composite Coating Prepared by Chemical Vapor Deposition: Microstructure and Elemental Migration. Coatings 2024, 14, 695. https://doi.org/10.3390/coatings14060695
Zhu J, Li W, Cai H, Wang X, Wang X, Wei Y, Hu C, Zhao X, Zhang X. Oxidation Resistance of Ir/HfO2 Composite Coating Prepared by Chemical Vapor Deposition: Microstructure and Elemental Migration. Coatings. 2024; 14(6):695. https://doi.org/10.3390/coatings14060695
Chicago/Turabian StyleZhu, Junyu, Wenting Li, Hongzhong Cai, Xian Wang, Xingqiang Wang, Yan Wei, Changyi Hu, Xingdong Zhao, and Xuxiang Zhang. 2024. "Oxidation Resistance of Ir/HfO2 Composite Coating Prepared by Chemical Vapor Deposition: Microstructure and Elemental Migration" Coatings 14, no. 6: 695. https://doi.org/10.3390/coatings14060695
APA StyleZhu, J., Li, W., Cai, H., Wang, X., Wang, X., Wei, Y., Hu, C., Zhao, X., & Zhang, X. (2024). Oxidation Resistance of Ir/HfO2 Composite Coating Prepared by Chemical Vapor Deposition: Microstructure and Elemental Migration. Coatings, 14(6), 695. https://doi.org/10.3390/coatings14060695




