Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications
Abstract
1. Introduction
2. Three-Dimensionally (3D)-Printed Dental Implant Technology and Surface Modification
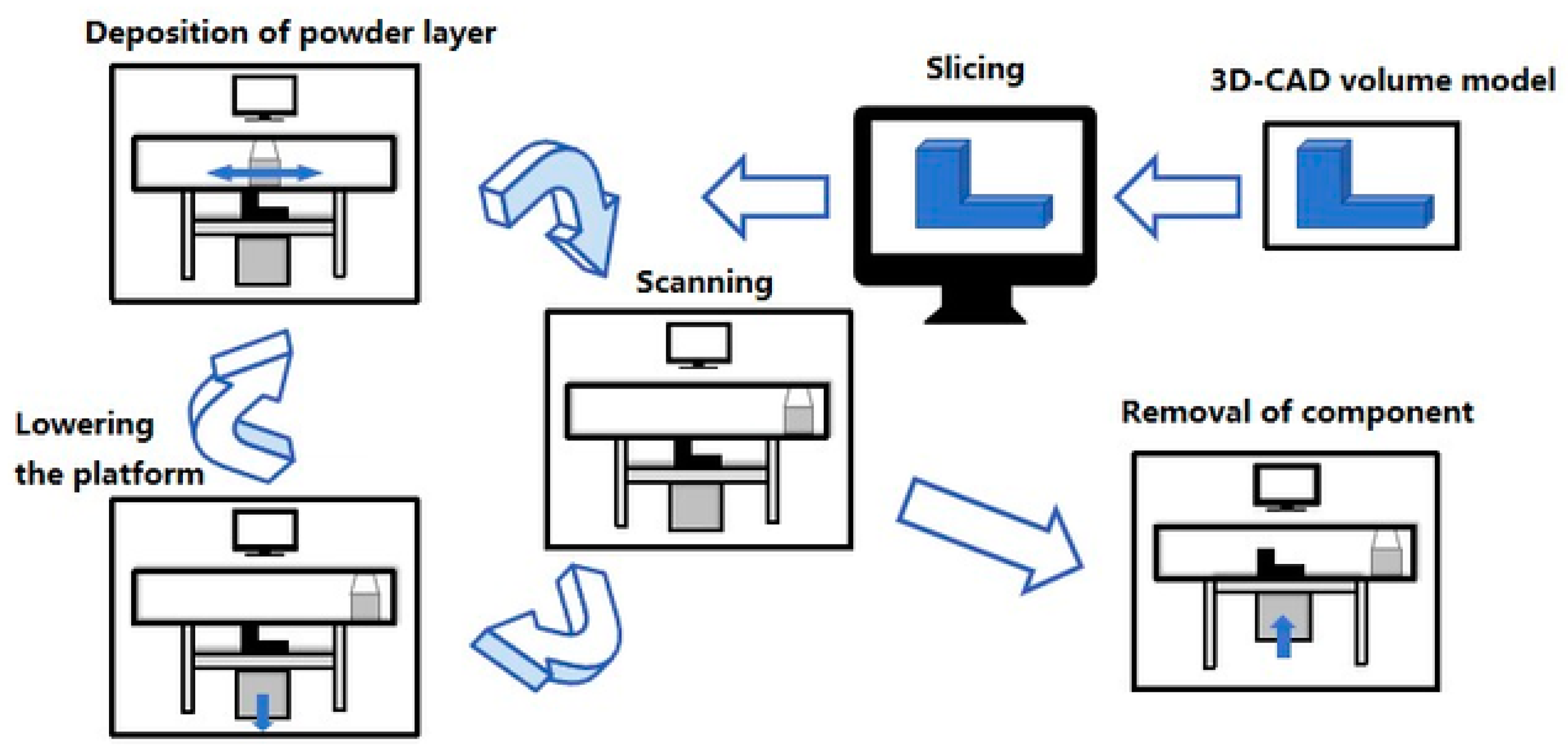
2.1. The Process Methods of 3D-Printed Dental Implants
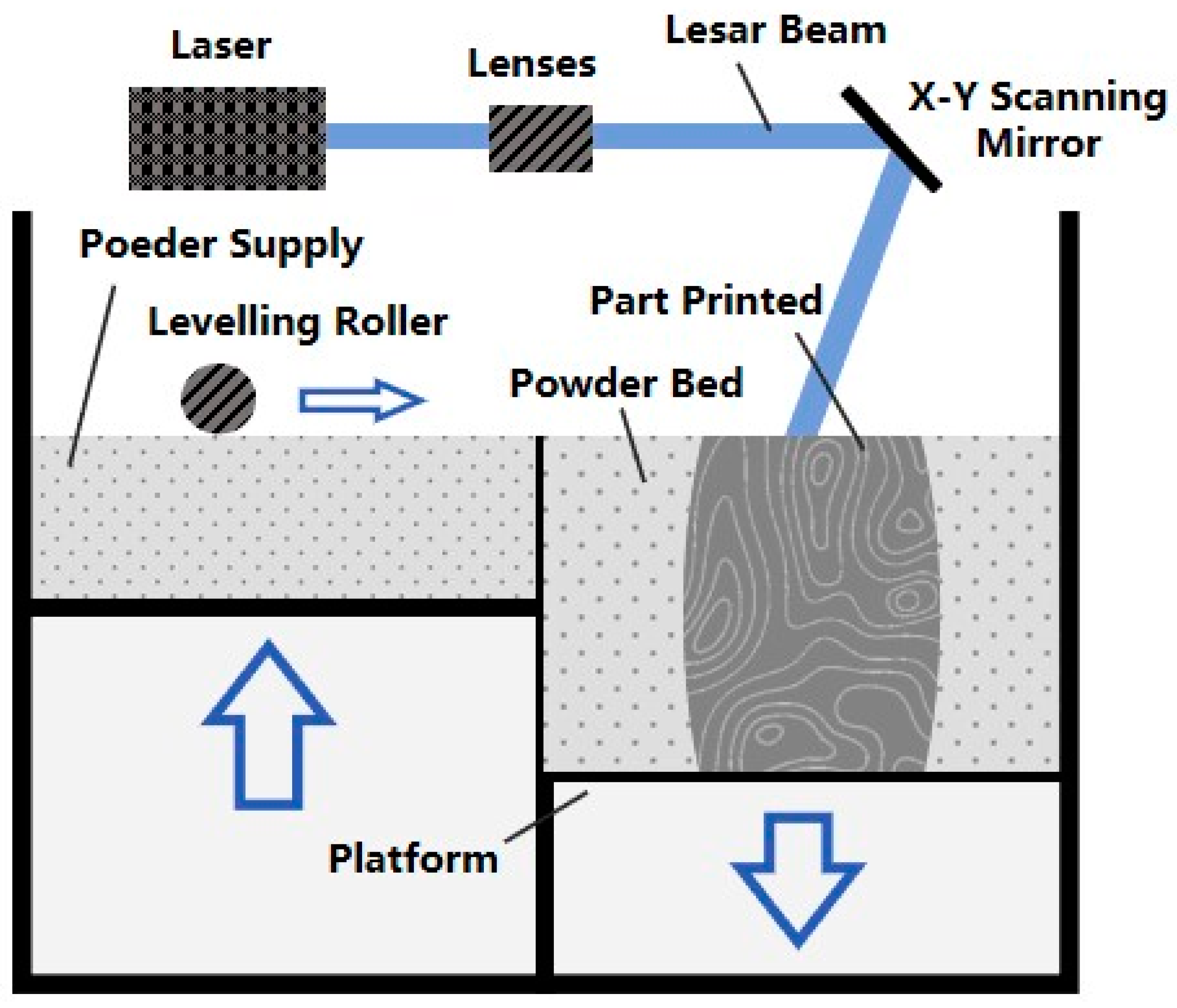
2.1.1. Selective Laser Melting
2.1.2. Electron Beam Melting
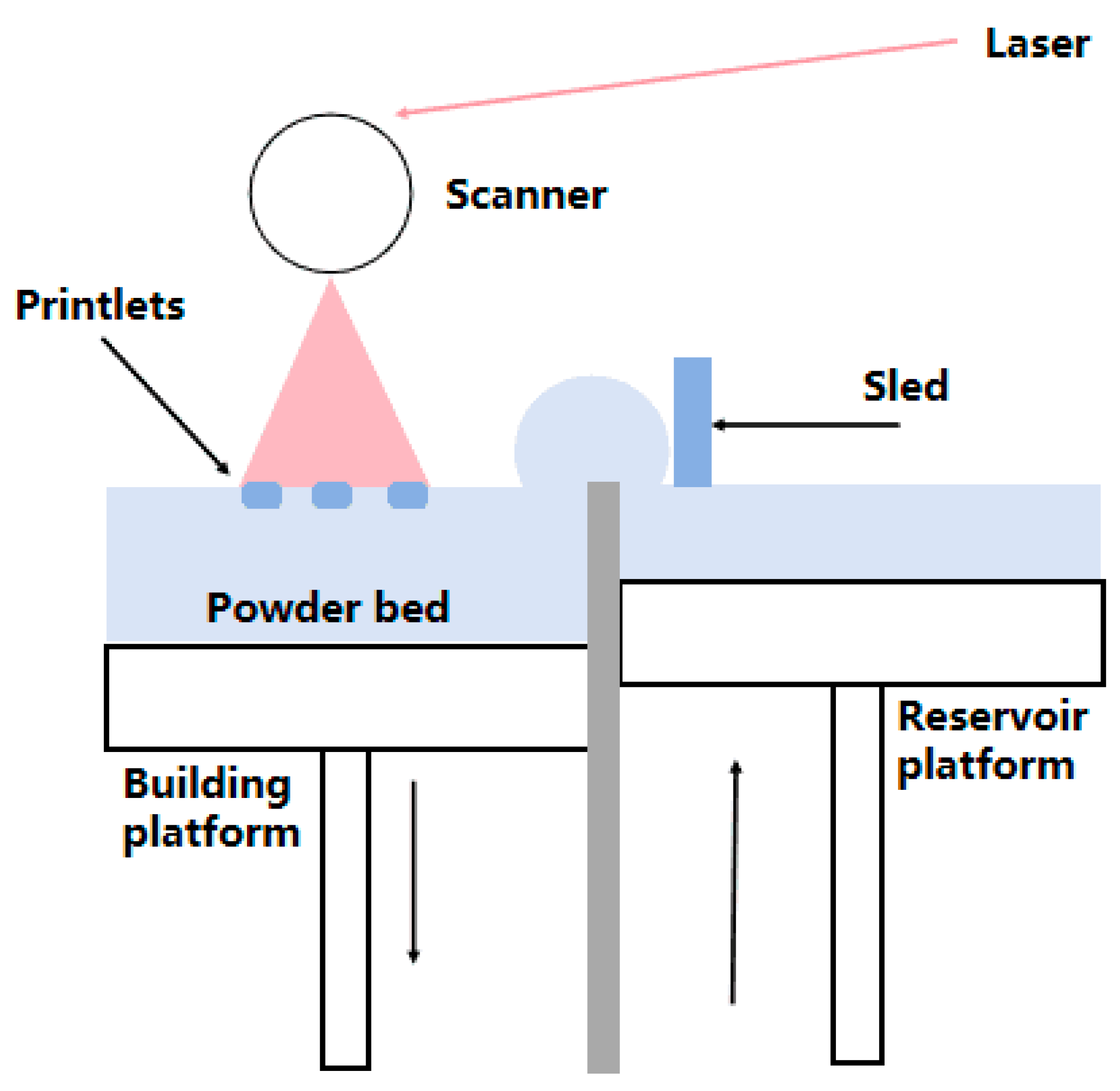
2.1.3. Selective Laser Sintering
2.2. Implant Surface Modification Methods
2.2.1. Physical Modification
2.2.2. Chemical Modification
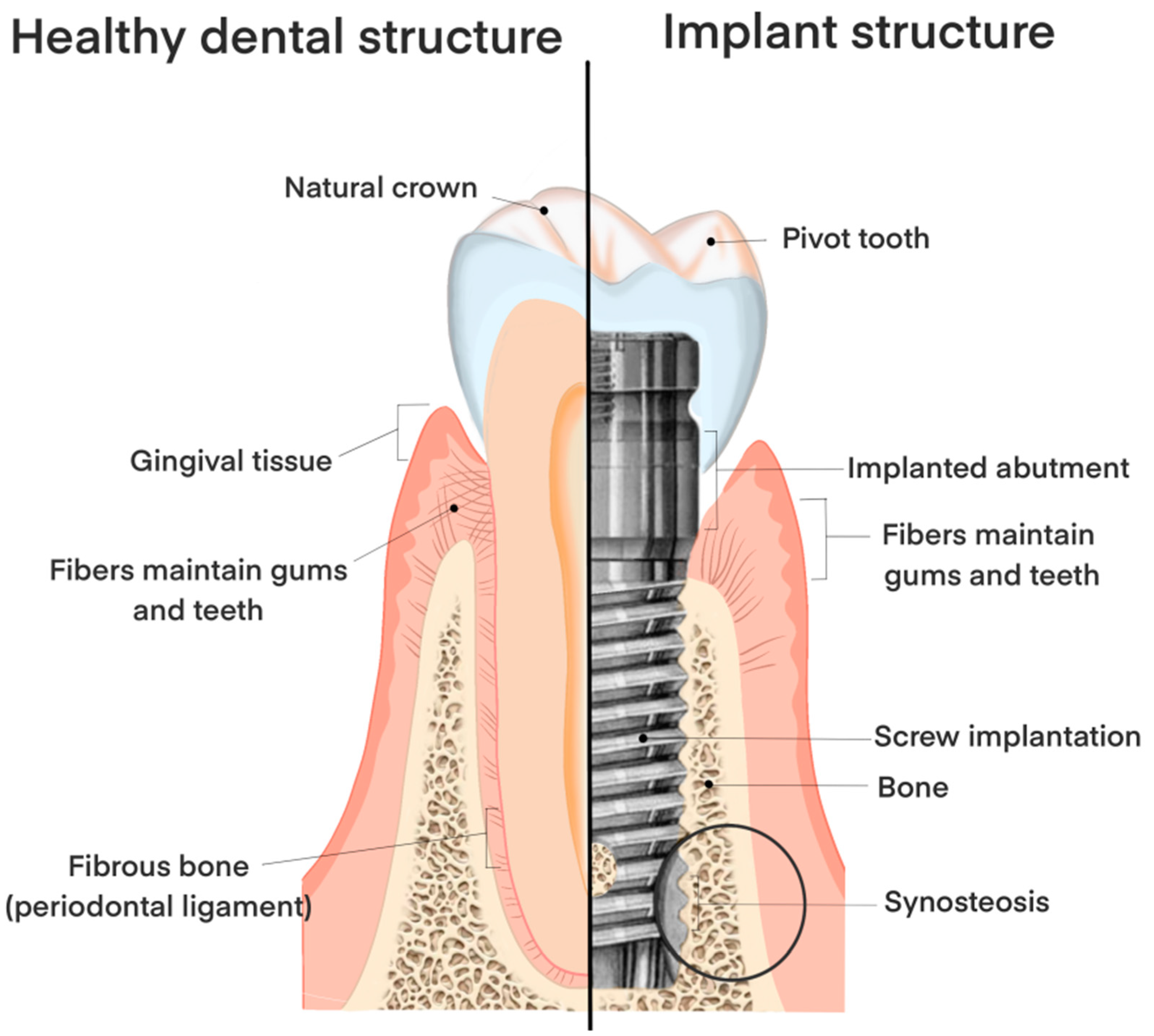
3. The Structure, Material, and Shape of the Implant
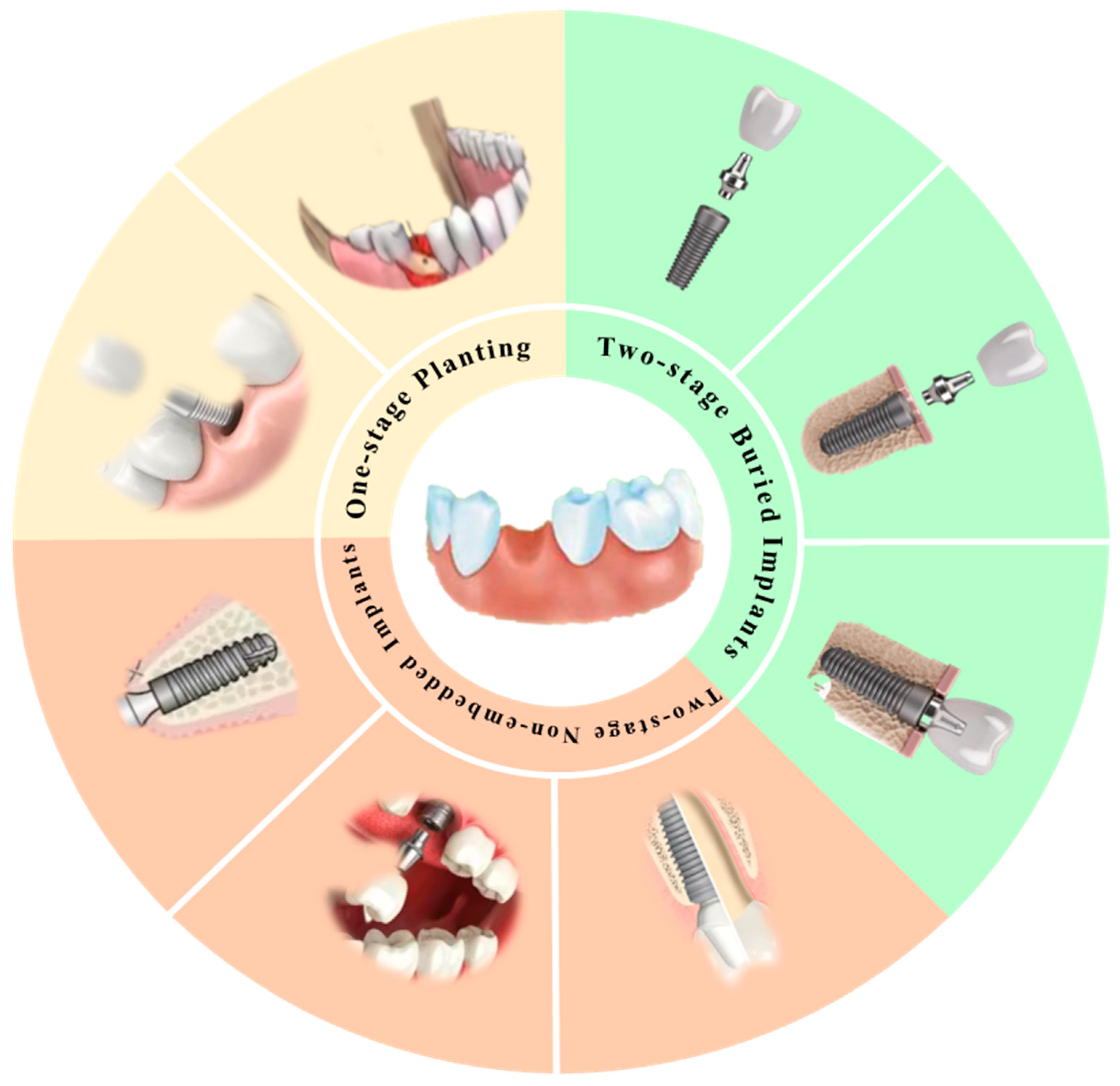
3.1. Classification of the Implant Structure
3.1.1. One-Stage Implants
3.1.2. Two-Stage Non-Embedded Implants
3.1.3. Two-Stage Embedded Implants
3.2. Classification of Implant Length
3.3. Implant Diameter Classification
3.4. New Dental Implant Materials
3.4.1. PMMA-ZrO Biomimetic Nanocomposites
3.4.2. Zirconia–Glass Ceramic Composite Material
3.4.3. Titanium Alloy Dental Implant Material
3.4.4. Glass and Ceramic Materials
3.5. Different Structures of the Tooth Root
4. Progress in the Clinical and Molecular Mechanisms of Dental Implants
4.1. Development of Dental Implant Surgery
4.2. Postoperative Evaluation and Patient Expectations of Implants
4.3. Mechanisms of Implant Osseointegration
5. Conclusions
Funding
Conflicts of Interest
References
- Zhao, X.; Qiao, S.C.; Shi, J.Y. Evaluation of the clinical and aesthetic outcomes of Straumann Standard Plus implant supported single crowns placed in nonaugmented healed sites in the anterior maxilla: A 5–8-year retrospective study. Clin. Oral Implant. Res. 2016, 27, 106–112. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses: I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Peng, T.Y.; Lin, D.J.; Mine, Y.; Tasi, C.Y.; Li, P.J.; Shih, Y.H.; Chiu, K.C.; Wang, T.H.; Hsia, S.M.; Shieh, T.M. Biofilm Formation on the Surface of (Poly)Ether-Ether-Ketone and In Vitro Antimicrobial Efficacy of Photodynamic Therapy on Peri-Implant Mucositis. Polymers 2021, 13, 940. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Zhou, Q.; Gong, Y.; Li, R.; Li, C.; Klämpfl, F.; Freund, S.; Wu, X.; Sun, Y.; et al. Laser beam melting 3D printing of Ti6Al4V based porous structured dental implant: Fabrication, biocompatibility analysis and photoelastic study. Sci. Rep. 2017, 7, 45360. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, Y. Application of 3D Printing in Implantable Medical Devices. BioMed Res. Int. 2021, 2021, 6653967. [Google Scholar] [CrossRef]
- Cao, J.; Lu, Y.; Chen, H.; Zhang, L.; Xiong, C. Preparation, properties and in vitro cellular response of multi-walled carbon nanotubes/bioactive glass/poly(etheretherketone) biocomposite for bone tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 433–441. [Google Scholar] [CrossRef]
- Baqain, Z.H.; Moqbel, W.Y.; Sawair, F.A. Early dental implant failure: Risk factors. Br. J. Oral Maxillofac. Surg. 2012, 50, 239–243. [Google Scholar] [CrossRef]
- Nchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Palladino, A.; Inchingolo, A.M.; Dipalma, G. Oral piercing and oral diseases: A short time retrospective study. Int. J. Med. Sci. 2011, 8, 649–652. [Google Scholar]
- Setzer, F.C.; Kim, S. Comparison of long-term survival of implant and endodontically treated teeth. Dent. Res. 2014, 93, 19–26. [Google Scholar] [CrossRef]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implant. requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of bone metabolism around dental implant during osseointegration and peri-implant bone loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- Gao, B.; Zhao, H.; Peng, L.; Sun, Z. A Review of Research Progress in Selective Laser Melting (SLM). Micromachines 2023, 14, 57. [Google Scholar] [CrossRef]
- Bremen, S.; Meiners, W.; Diatlov, A. Selective laser melting: A manufacturing technology for the future. Laser Tech. J. 2012, 9, 33–38. [Google Scholar] [CrossRef]
- Qin, Z.G.; He, Y.; Gao, J.J.; Dong, Z.H.; Long, S.; Cheng, L.J.; Shi, Z. Surface modification improving the biological activity and osteogenic ability of 3D printing porous dental implants. Front. Mater. 2023, 10, 2296–8016. [Google Scholar] [CrossRef]
- Zhang, J.; Song, B.; Wei, Q.; Bourell, D.L.; Shi, Y. A review of selective laser melting of aluminum alloys: Processing, microstructure, property and developing trends. J. Mater. Sci. Technol. 2019, 35, 270–284. [Google Scholar] [CrossRef]
- Vandenbroucke, B.; Kruth, J.P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Malda, J. Additive manufacturing of biomaterials, tissues, and organs. Ann. Biomed. Eng. 2017, 45, 1–11. [Google Scholar] [CrossRef]
- Tamayo, J.A.; Riascos, M.; Vargas, C.A.; Baena, L.M. Additive manufacturing of Ti6Al4V alloy via electron beam melting for the development of implant for the biomedical industry. Heliyon 2021, 7, e06892. [Google Scholar] [CrossRef]
- Galati, M.; Luca, I. A literature review of powder-based electron beam melting focusing on numerical simulations. Addit. Manuf. 2018, 19, 1–20. [Google Scholar] [CrossRef]
- Suska, F.; Kjeller, G.; Tarnow, P.; Hryha, E.; Nyborg, L.; Snis, A.; Palmquist, A. Electron Beam Melting Manufacturing Technology for Individually Manufactured Jaw Prosthesis: A Case Report. J. Oral Maxillofac. Surg. 2016, 74, 1706.e1–1706.e15. [Google Scholar] [CrossRef]
- Cheong, V.S.; Fromme, P.; Mumith, A.; Coathup, M.J.; Blunn, G.W. Novel adaptive finite element algorithms to predict bone ingrowth in additive manufactured porous implants. J. Mech. Behav. Biomed. Mater. 2018, 87, 230–239. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.F.; Wang, C.T.; Li, G.C.; Lei, W.; Zhang, Z.Y.; Wang, L. Evaluation of biological properties of electron beam melted Ti6Al4V implant with biomimetic coating in vitro and in vivo. PLoS ONE 2012, 7, e52049. [Google Scholar] [CrossRef]
- Lan, L.; Jin, X.; Gao, S.; He, B.; Rong, Y. Microstructural evolution and stress state related to mechanical properties of electron beam melted Ti-6Al-4V alloy modified by laser shock peening. J. Mater. Sci. Technol. 2020, 50, 153–161. [Google Scholar] [CrossRef]
- Fina, F.; Madla, C.M.; Goyanes, A.; Zhang, J.; Gaisford, S.; Basit, A.W. Fabricating 3D printed orally disintegrating printlets using selective laser sintering. Int. J. Pharm. 2018, 541, 101–107. [Google Scholar] [CrossRef]
- Ou, P.; Liu, J.; Hao, C.; He, R.; Chang, L.; Ruan, J. Cytocompatibility, stability and osteogenic activity of powder metallurgy Ta-xZr alloys as dental implant materials. J. Biomater. Appl. 2021, 35, 790–798. [Google Scholar] [CrossRef]
- Ran, Q.C.; Yang, W.H.; Hu, Y.; Shen, X.K.; Yu, Y.L.; Xiang, Y.; Cai, K.Y. Osteogenesis of 3D printed porous Ti6Al4V implant with different pore sizes. J. Mech. Behav. Biomed. Mater. 2018, 84, 1–11. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, C.Z.; Deng, J.L.; Xu, R.G.; Yang, Y.; Deng, F.L. Micro/Nano Topography of Selective Laser Melting Titanium Inhibits Osteoclastogenesis via Mediation of Macrophage Polarization. Biochem. Biophys. Res. Commun. 2021, 581, 53–59. [Google Scholar] [CrossRef]
- Xu, R.; Hu, X.; Yu, X.; Wan, S.; Wu, F.; Ouyang, J.; Deng, F. Micro-/nano-topography of selective laser melting titanium enhances adhesion and proliferation and regulates adhesion-related gene expressions of human gingival fibroblasts and human gingival epithelial cells. Int. J. Nanomed. 2018, 13, 5045–5057. [Google Scholar] [CrossRef]
- Fukuda, A.; Takemoto, M.; Saito, T.; Fujibayashi, S.; Neo, M.; Pattanayak, D.K.; Matsushita, T.; Sasaki, K.; Nishida, N.; Kokubo, T.; et al. Osteoinduction of porous Ti implant with a channel structure fabricated by selective laser melting. Acta Biomater. 2011, 7, 2327–2336. [Google Scholar] [CrossRef]
- Sun, X.T.; Lin, H.S.; Zhang, C.Y.; Liu, Y.; Jin, J.; Di, S. A biomimetic hierarchical structure on selective laser melting titanium with enhanced hydrophilic/hydrophobic surface. J. Alloys Compd. 2022, 895, 162585. [Google Scholar] [CrossRef]
- Ren, B.; Wan, Y.; Liu, C.; Wang, H.; Yu, M.; Zhang, X.; Huang, Y. Improved osseointegration of 3D printed Ti-6Al-4V implant with a hierarchical micro/nano surface topography: An in vitro and in vivo study. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111505. [Google Scholar] [CrossRef] [PubMed]
- Goto, M.; Matsumine, A.; Yamaguchi, S.; Takahashi, H.; Akeda, K.; Nakamura, T.; Asanuma, K.; Matsushita, T.; Kokubo, T.; Sudo, A. Osteoconductivity of bioactive Ti-6Al-4V implant with lattice-shaped interconnected large pores fabricated by electron beam melting. J. Biomater. Appl. 2021, 35, 1153–1167. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, P.; Malmström, J.; Emanuelsson, L.; René, M.; Snis, A. Electron beam-melted, free-form-fabricated titanium alloy implant: Material surface characterization and early bone response in rabbits. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Harada, Y.; Ishida, Y.; Miura, D.; Watanabe, S.; Aoki, H.; Miyasaka, T.; Shinya, A. Mechanical Properties of Selective Laser Sintering Pure Titanium and Ti-6Al-4V, and Its Anisotropy. Materials 2020, 13, 5081. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Z.; Shi, Y.; Wang, H.; Li, R.; Tu, J.; Jin, G. In vitro and in vivo comparisons of the porous Ti6Al4V alloys fabricated by the selective laser melting technique and a new sintering technique. J. Mech. Behav. Biomed. Mater. 2019, 91, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Chang Tu, C.; Tsai, P.I.; Chen, S.Y.; Kuo, M.Y.; Sun, J.S.; Chang, J.Z. 3D laser-printed porous Ti6Al4V dental implants for compromised bone support. J. Formos. Med. Assoc. 2020, 119, 420–429. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, P.; Liu, S.; Attarilar, S.; Ma, R.L.; Zhong, Y.; Wang, L. Multi-Scale Surface Treatments of Titanium Implants for Rapid Osseointegration: A Review. Nanomaterials 2020, 10, 1244. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef] [PubMed]
- Gil, F.J.; Pérez, R.A.; Olmos, J.; Herraez-Galindo, C.; Gutierrez-Pérez, J.L.; Torres-Lagares, D. The effect of using Al2O3 and TiO2 in sandblasting of titanium dental implant. J. Mater. Res. 2022, 37, 2604–2613. [Google Scholar] [CrossRef]
- Robles-Ruíz, J.J.; Ciamponi, A.L.; Medeiros, I.S.; Kanashiro, L.K. Effect of lingual enamel sandblasting with aluminum oxide of different particle sizes in combination with phosphoric acid etching on indirect bonding of lingual brackets. Angle Orthod. 2014, 84, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Galli, S.; Albrektsson, T. Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin. Cosmet. Investig. Dent. 2011, 3, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.W.; Wang, Y.B.; Shuai, K.G.; Gao, F.; Bai, Y.J.; Cheng, Y.; Xiong, X.L.; Zheng, F.; Wei, S.C. In vitro and in vivo evaluation of SLA titanium surfaces with further alkali or hydrogen peroxide and heat treatment. Biomed. Mater. 2011, 6, 025001. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.R.; Xu, F.F.; Li, J.; You, Y.H.; Liu, C.; Yin, L.H. Surface Morphology and Surface Properties of Ti and TiZr Implant Materials. Zhonghua Kou Qiang Yi Xue Za Zhi 2019, 54, 118–123. [Google Scholar] [PubMed]
- Buser, D.; Janner, S.F.M.; Wittneben, J.G.; Brägger, U.; Ramseier, C.A.; Salvi, G.E. 10-Year survival and success rates of 511 titanium implant with a sandblasted and acid-etched surface: A retrospective study in 303 partially edentulous patients. Clin. Implant. Dent. Relat. Res. 2012, 14, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Jeon, H.J.; Jung, A.; Kim, J.; Kim, J.Y.; Lee, S.H.; Kim, H.; Yeom, M.S.; Choe, W.; Gweon, B.; et al. Improvement of osseointegration efficacy of titanium implant through plasma surface treatment. Biomed. Eng. Lett. 2022, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Schafer, S.; Swain, T.; Parra, M.; Slavin, B.V.; Mirsky, N.A.; Nayak, V.V.; Witek, L.; Coelho, P.G. Nonthermal Atmospheric Pressure Plasma Treatment of Endosteal Implant for Osseointegration and Antimicrobial Efficacy: A Comprehensive Review. Bioengineering 2024, 11, 320. [Google Scholar] [CrossRef] [PubMed]
- Hameed, P.; Gopal, V.; Bjorklund, S.; Ganvir, A.; Sen, D.; Markocsan, N.; Manivasagam, G. Axial Suspension Plasma Spraying: An ultimate technique to tailor Ti6Al4V surface with HAp for orthopaedic applications. Colloids Surf. B Biointerfaces 2019, 173, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Groot, K.; Geesink, R.; Klein, C.P.; Serekian, P. Plasma sprayed coatings of hydroxylapatite. J. Biomed. Mater. Res. 1987, 21, 1375–1381. [Google Scholar] [CrossRef] [PubMed]
- Filiaggi, M.J.; Pilliar, R.M.; Coombs, N.A. Post-plasma-spraying heat treatment of the HA coating/Ti-6A1-4V implant system. J. Biomed. Mater. Res. 1993, 27, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Unabia, R.B.; Candidato, R.T., Jr.; Pawłowski, L.; Salvatori, R.; Bellucci, D.; Cannillo, V. In vitro studies of solution precursor plasma-sprayed copper-doped hydroxyapatite coatings with increasing copper content. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2579–2589. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.J.; Wang, X.; He, H.X.; E, L.L.; Li, Y.; Zhang, G.L.; Li, C.J.; Ning, C.Y.; Liu, H.C. Tantalum-incorporated hydroxyapatite coating on titanium implant: Its mechanical and in vitro osteogenic properties. J. Mater. Sci. Mater. Med. 2019, 30, 111. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Shuobo, F.; Zhong, Q.; Qi, S. Influence of Dental Implant Surface Modifications on Osseointegration and Biofilm Attachment. Coatings 2022, 12, 1654. [Google Scholar] [CrossRef]
- Sotova, C.; Yanushevich, O.; Kriheli, N.; Grigoriev, S.; Evdokimov, V.; Kramar, O.; Nozdrina, M.; Peretyagin, N.; Undritsova, N.; Popelyshkin, E. Dental Implant: Modern Materials and Methods of Their Surface Modification. Materials 2023, 16, 7383. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Qin, H.; Cao, H.; Qian, S.; Zhao, Y.; Peng, X.; Zhang, X.; Liu, X.; Chu, P.K. Synergistic effects of dual Zn/Ag ion implantation in osteogenic activity and antibacterial ability of titanium. Biomaterials 2014, 35, 7699–7713. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Z.; Raman, S.; He, F.; Huang, Y. Surface modification of medical metals by ion implantation of silver and copper. Vacuum 2007, 81, 1114–1118. [Google Scholar] [CrossRef]
- Wang, C.; Hu, H.; Li, Z.; Shen, Y.; Xu, Y.; Zhang, G.; Zeng, X.; Deng, J.; Zhao, S.; Ren, T.; et al. Enhanced Osseointegration of Titanium Alloy Implants with Laser Microgrooved Surfaces and Graphene Oxide Coating. ACS Appl. Mater. Interfaces 2019, 11, 39470–39483. [Google Scholar] [CrossRef] [PubMed]
- Tzanakakis, E.-G.C.; Skoulas, E.; Pepelassi, E.; Koidis, P.; Tzoutzas, I.G. The Use of Lasers in Dental Materials: A Review. Materials 2021, 14, 3370. [Google Scholar] [CrossRef] [PubMed]
- Doll, K.; Fadeeva, E.; Stumpp, N.S.; Grade, S.; Chichkov, B.N.; Stiesch, M. Reduced bacterial adhesion on titanium surfaces micro-structured by ultra-short pulsed laser ablation. BioNanoMaterials 2016, 17, 53–57. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.H.; Perillo, M.A.; Chang, Y.C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Koodaryan, R.; Hafezeqoran, A. Effect of laser-microtexturing on bone and soft tissue attachments to dental implants: A systematic review and meta-analysis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Botos, S.; Yousef, H.; Zweig, B.; Flinton, R.; Weiner, S. The effects of laser microtexturing of the dental implant collar on crestal bone levels and peri-implant health. Int. J. Oral Maxillofac. Implant. 2011, 26, 492–498. [Google Scholar]
- Nammour, S.; El Mobadder, M.; Namour, M.; Namour, A.; Rompen, E.; Maalouf, E.; Brugnera Junior, A.; Brugnera, A.P.; Vescovi, P.; Zeinoun, T. A Randomized Comparative Clinical Study to Evaluate the Longevity of Esthetic Results of Gingival Melanin Depigmentation Treatment Using Different Laser Wavelengths (Diode, CO2, and Er: YAG). Photobiomodul Photomed. Laser Surg. 2020, 38, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sul, Y.T.; Byon, E.; Wennerberg, A. Surface characteristics of electrochemically oxidized implant and acid-etched implant: Surface chemistry, morphology, pore configurations, oxide thickness, crystal structure, and roughness. Int. J. Oral Maxillofac. Implant. 2008, 23, 631–640. [Google Scholar]
- Marin, C.; Granato, R.; Suzuki, M.; Gil, J.N.; Janal, M.N.; Coelho, P.G. Histomorphologic and histomorphometric evaluation of various endosseous implant healing chamber configurations at early implantation times: A study in dogs. Clin. Oral Implant. Res. 2010, 21, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implant: Current knowledge and open questions. Periodontology 2000 2017, 73, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Jemat, A.; Ghazali, M.J.; Razali, M.; Otsuka, Y. Surface Modifications and Their Effects on Titanium Dental Implant. BioMed Res. Int. 2015, 2015, 791725. [Google Scholar] [CrossRef] [PubMed]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface characteristics of dental implant: A review. Dent. Mater. Off. Publ. Acad. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Li, Y.; Ma, J.; Zhang, X.; Li, B.; Liu, S.; Zhang, K. Improvement of Biological and Mechanical Properties of Titanium Surface by Anodic Oxidation. Bio-Med. Mater. Eng. 2016, 27, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Husain, F.; Gupta, S.; Sood, S.; Bhaskar, N.; Jain, A. To evaluate the effect of anodized dental implant surface on cumulative implant survival and success. A systematic review and meta-analysis. J. Indian Soc. Periodontol. 2022, 26, 525–532. [Google Scholar] [PubMed]
- Villaça-Carvalho, M.F.L.; de Araújo, J.C.R.; Beraldo, J.M.; Prado, R.F.D.; Moraes, M.E.L.; Manhães Junior, L.R.C.; Codaro, E.N.; Acciari, H.A.; Machado, J.P.B.; Regone, N.N.; et al. Bioactivity of an Experimental Dental Implant with Anodized Surface. J. Funct. Biomater. 2021, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S. Reality of dental implant surface modification: A short literature review. Open Biomed. Eng. J. 2014, 8, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramaswamy, N. Electrochemical surface modification of titanium in dentistry. Dent. Mater. J. 2009, 28, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Roguska, A.; Belcarz, A.; Zalewska, J.; Hołdyński, M.; Andrzejczuk, M.; Pisarek, M.; Ginalska, G. Metal TiO Nanotube Layers for the Treatment of Dental Implant Infections. ACS Appl. Mater. Interfaces 2018, 10, 17089–17099. [Google Scholar] [CrossRef] [PubMed]
- Alves-Rezende, M.C.R.; Capalbo, L.C.; De Oliveira Limírio, J.P.J.; Capalbo, B.C.; Limírio, P.H.J.O.; Rosa, J.L. The role of TiO2 nanotube surface on osseointegration of titanium implant: Biomechanical and histological study in rats. Microsc. Res. Tech. 2020, 83, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qian, S.; Liu, X.; Xu, L.; Miao, X.; Xu, Z.; Cao, L.; Wang, H.; Jiang, X. M2 macrophages contribute to osteogenesis and angiogenesis on nanotubular TiO2 surfaces. J. Mater. Chem. B 2017, 5, 3364–3376. [Google Scholar] [CrossRef] [PubMed]
- Mussano, F.; Genova, T.; Serra, F.G.; Carossa, M.; Munaron, L.; Carossa, S. Nano-Pore Size of Alumina Affects Osteoblastic Response. Int. J. Mol. Sci. 2018, 19, 528. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhou, L.; Wang, J.; Zhao, Q.; Lin, X.; Gao, Y.; Li, S.; Wu, J.; Rong, M.; Guo, Z.; et al. The effects of hierarchical micro/nanosurfaces decorated with TiO2 nanotubes on the bioactivity of titanium implant in vitro and in vivo. Int. J. Nanomed. 2015, 10, 6955–6973. [Google Scholar]
- Wagenberg, B.; Froum, S.J. Long-Term Bone Stability around 312 Rough-Surfaced Immediately Placed Implant with 2–12-Year Follow-Up. Clin. Implant. Dent. Relat. Res. 2015, 17, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.; Oliveira, F.; Boldrini, L.; Leite, P.; Falagan-Lotsch, P.; Linhares, A.; Zambuzzi, W.; Fragneaud, B.; Campos, A.; Gouvêa, C. Micro-arc oxidation as a tool to develop multifunctional calcium-rich surfaces for dental implant applications. Mater. Sci. Eng. C 2015, 54, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Visai, L.; Pedeferri, M.P.; Chiesa, R.; Cigada, A. Antibacterial treatments on titanium for implantology. Biomed. Pharmacother. 2006, 60, 472. [Google Scholar] [CrossRef]
- Ding, M.; Shi, J.; Wang, W.; Li, D.; Tian, L. Early osseointegration of micro-arc oxidation coated titanium alloy implant containing Ag: A histomorphometric study. BMC Oral Health 2022, 22, 628. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M. Antibacterial Property and Biocompatibility of Silver, Copper, and Zinc in Titanium Dioxide Layers Incorporated by One-Step Micro-Arc Oxidation: A Review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Cai, B.; Huang, Y.; Wang, J.; Zhu, C.; Shi, K.; Song, Y.; Feng, G.; Liu, L.; Zhang, L. Comparative Study on 3D Printed Ti6Al4V Scaffolds with Surface Modifications Using Hydrothermal Treatment and Microarc Oxidation to Enhance Osteogenic Activity. ACS Omega 2021, 6, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, H.; Wang, X.; Yang, L.; Chen, M.; Wang, R.; Qin, G.; Chen, D.-F.; Zhang, E. Effect of Ultrasonic Micro-Arc Oxidation on the Antibacterial Properties and Cell Biocompatibility of Ti-Cu Alloy for Biomedical Application. Mater. Sci. Eng. C 2020, 115, 110921. [Google Scholar] [CrossRef] [PubMed]
- Djošić, M.; Janković, A.; Mišković-Stanković, V. Electrophoretic deposition of biocompatible and bioactive hydroxyapatite-based coatings on titanium. Materials 2021, 14, 5391. [Google Scholar] [CrossRef] [PubMed]
- Seuss, S.; Boccaccini, A. Alternating Current Electrophoretic Deposition of Antibacterial Bioactive Glass-Chitosan Composite Coatings. Int. J. Mol. Sci. 2014, 15, 12231–12242. [Google Scholar] [CrossRef]
- Nuswantoro, N.F.; Manjas, M.; Suharti, N.; Juliadmi, D.; Fajri, H.; Tjong, D.H.; Affi, J.; Niinomi, M. Gunawarman. Hydroxyapatite Coating on Titanium Alloy TNTZ for Increasing Osseointegration and Reducing Inflammatory Response In Vivo on Rattus Norvegicus Wistar Rats. Ceram. Int. 2021, 47, 16094–16100. [Google Scholar] [CrossRef]
- Gaafar, M.S.; Yakout, S.M.; Barakat, Y.F.; Sharmoukh, W. Electrophoretic Deposition of Hydroxyapatite/Chitosan Nanocomposites: The Effect of Dispersing Agents on the Coating Properties. RSC Adv. 2022, 12, 27564–27581. [Google Scholar] [CrossRef]
- Fiołek, A.; Zimowski, S.; Kopia, A.; Łukaszczyk, A.; Moskalewicz, T. Electrophoretic Co-Deposition of Polyetheretherketone and Graphite Particles: Microstructure, Electrochemical Corrosion Resistance, and Coating Adhesion to a Titanium Alloy. Materials 2020, 13, 3251. [Google Scholar] [CrossRef] [PubMed]
- Pipattanachat, S.; Qin, J.; Rokaya, D.; Thanyasrisung, P.; Srimaneepong, V. Biofilm Inhibition and Bactericidal Activity of NiTi Alloy Coated with Graphene Oxide/Silver Nanoparticles via Electrophoretic Deposition. Sci. Rep. 2021, 11, 14008. [Google Scholar] [CrossRef]
- Juliadmi, D.; Nuswantoro, N.F.; Fajri, H.; Indriyani, I.Y.; Affi, J.; Manjas, M.; Suharti, N.; Tjong, D.H.; Niinomi, M.; Gunawarman, G. The Coating of Bovine-Source Hydroxyapatite on Titanium Alloy (Ti-6Al-4V ELI) Using Electrophoretic Deposition for Biomedical Application. Mater. Sci. Forum 2020, 1000, 97–106. [Google Scholar] [CrossRef]
- Danlei, Z.; Haoran, D.; Yuting, N.; Wenjie, F.; Muqi, J.; Ke, L.; Qingsong, W.; William, M.P.; Zhen, Z. Electrophoretic Deposition of Novel Semi-Permeable Coatings on 3D-Printed Ti-Nb Alloy Meshes for Guided Alveolar Bone Regeneration. Dent. Mater. 2022, 38, 431–443. [Google Scholar]
- Nicoli, L.G.; Oliveira, G.J.P.L.; Lopes, B.M.V.; Marcantonio, C.; Zandim-Barcelos, D.L.; Marcantonio, E., Jr. Survival/Success of Dental Implant with Acid-Etched Surfaces: A Retrospective Evaluation after 8 to 10 Years. Braz. Dent. J. 2017, 28, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, I.M.; Enan, E.T.; Al-Wakeel, E.E.; Yousef, M.K. Alkali and heat treatment of titanium implant material for bioactivity. Int. J. Oral Maxillofac. Implant. 2012, 27, 776–784. [Google Scholar]
- Cho, S. The Removal Torque of Titanium Screw Inserted in Rabbit Tibia Treated by Dual Acid Etching. Biomaterials 2003, 24, 3611–3617. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Poon, R.W.Y.; Kwok, S.C.H.; Chu, P.K.; Ding, C. Plasma Surface Modification of Titanium for Hard Tissue Replacements. Surf. Coat. Technol. 2004, 186, 227–233. [Google Scholar] [CrossRef]
- Chang, E.; Chang, W.J.; Wang, B.C.; Yang, C.Y. Plasma Spraying of Zirconia-Reinforced Hydroxyapatite Composite Coatings on Titanium: Part I: Phase, Microstructure and Bonding Strength. J. Mater. Sci. Mater. Med. 1997, 8, 193–200. [Google Scholar] [CrossRef]
- Yan, J.; Huang, W.; Kuang, H.; Wang, Q.; Li, B. The Effect of Etched 3D Printed Cu-Bearing Titanium Alloy on the Polarization of Macrophage. Front. Mater. 2022, 9, 941311. [Google Scholar] [CrossRef]
- Xie, H.; Shen, S.; Qian, M.; Zhang, F.; Chen, C.; Tay, F.R. Effects of Acid Treatment on Dental Zirconia: An In Vitro Study. PLoS ONE 2015, 10, e0136263. [Google Scholar] [CrossRef] [PubMed]
- Chosa, N.; Taira, M.; Saitoh, S.; Sato, N.; Araki, Y. Characterization of apatite formed on alkaline-heat-treated Ti. J. Dent. Res. 2004, 83, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Nishio, K.; Neo, M.; Akiyama, H.; Nishiguchi, S.; Kim, H.M.; Kokubo, T.; Nakamura, T. The effect of alkali and heat-treated titanium and apatite-formed titanium on osteoblastic differentiation of bone marrow cells. J. Biomed. Mater. Res. 2000, 52, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Kou, H.C.; Yang, P.; Wang, Y.; Lu, T. Enhanced bone healing in porous Ti implanted rabbit combining bioactive modification and mechanical stimulation. J. Mech. Behav. Biomed. Mater. 2018, 86, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Wittneben, J.G.; Joda, T.; Weber, H.P.; Brägger, U. Screw retained vs. cement retained implant-supported fixed dental prosthesis. Periodontology 2000 2017, 73, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Hafezeqoran, A.; Koodaryan, R. Effect of Zirconia Dental Implant Surfaces on Bone Integration: A Systematic Review and Meta-Analysis. Biomed. Res. Int. 2017, 2017, 9246721. [Google Scholar] [CrossRef] [PubMed]
- Sailer, I.; Karasan, D.; Todorovic, A.; Ligoutsikou, M.; Pjetursson, B.E. Prosthetic failures in dental implant therapy. Periodontology 2000 2022, 88, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Barros Lucena, G.A.; Molon, R.S.; Moretti, A.J.; Shibli, J.A.; Rêgo, D.M. Evaluation of Microbial Contamination in the Inner Surface of Titanium Implant Before Healing Abutment Connection: A Prospective Clinical Trial. Int. J. Oral Maxillofac. Implant. 2018, 33, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, E.; Wang, Z.; Kato, T.; Inaba, T.; Kasai, Y. Basic research on a cylindrical implant made of shape-memory alloy for the treatment of long bone fracture. Open Orthop. J. 2012, 6, 239–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Planinić, D.; Dubravica, I.; Šarac, Z.; Poljak-Guberina, R.; Celebic, A.; Bago, I.; Cabov, T.; Peric, B. Comparison of different surgical procedures on the stability of dental implant in posterior maxilla: A randomized clinical study. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.E. Two-stage implant systems. Adv. Dent. Res. 1999, 13, 162–169. [Google Scholar] [CrossRef]
- Tavelli, L.; Barootchi, S.; Avila-Ortiz, G.; Urban, I.A.; Giannobile, W.V.; Wang, H.L. Peri-implant soft tissue phenotype modification and its impact on peri-implant health: A systematic review and network meta-analysis. J. Periodontol. 2021, 92, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, F. Perioperative care experience of 10 cases of two-stage non-embedded implanted dentures. Guangxi Med. 2002, 8, 1314–1315. [Google Scholar]
- Mously, H.A. Effect of Two Implant-supported Partial Overdenture Attachment Design on the Periodontal Health. J. Contemp. Dent. Pract. 2020, 21, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Al-Johany, S.S.; Al Amri, M.D.; Alsaeed, S.; Alalola, B. Dental Implant Length and Diameter: A Proposed Classification Scheme. J. Prosthodont. 2016, 26, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Raszewski, Z.; Nowakowska-Toporowska, A.; Nowakowska, D.; Więckiewicz, W. Update on Acrylic Resins Used in Dentistry. Mini Rev. Med. Chem. 2021, 21, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Gad, M.M.; Al-Thobity, A.M. The impact of nanoparticles-modified repair resin on denture repairs: A systematic review. Jpn. Dent. Sci. Rev. 2021, 57, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Ghayebloo, M.; Alizadeh, P. Effect of zirconia nanoparticles on ZrO2-Bearing Lithium-Silicate glass-ceramic composite obtained by spark plasma sintering. J. Mech. Behav. Biomed. Mater. 2020, 110, 103880. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, A.; Payne, A.G.T.; De Silva, R.K.; Duncan, W.J. Titanium allergy: Could it affect dental implant integration? Clin. Oral Implant. Res. 2011, 22, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of titanium surface modification techniques and coatings for antibacterial applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health. 2017, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, J.; Shi, F.D.; Liu, T.; Lu, Q. Some important issues in the development of glass-ceramic dental materials are explored. Fiberglass 2022, 49, 1–6. [Google Scholar]
- Chong, E.; Pelletier, M.H.; Mobbs, R.J.; Walsh, W.R. The design evolution of interbody cages in anterior cervical discectomy and fusion: A systematic review. BMC Musculoskelet. Disord. 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.; Lee, J.; Li, L.; Seol, Y.J.; Lee, Y.M.; Koo, K.T. A preclinical study comparing single-and double-root 3d-printed Ti-6Al-4V implant. Sci. Rep. 2023, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Bruno, V.; Berti, C.; Barausse, C.; Badino, M.; Gasparro, R.; Ippolito, D.R.; Felice, P. Clinical Relevance of Bone Density Values from CT Related to Dental Implant Stability: A Retrospective Study. BioMed Res. Int. 2018, 2018, 6758245. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Franco, S.; Levrini, L.; Castellani, D.; Pagliani, L.; Rea, M.; Modena, C.; Sandri, G.; Longhi, C. Tapered, Double-Lead Threads Single Implants Placed in Fresh Extraction Sockets and Healed Sites of the Posterior Jaws: A Multicenter Randomized Controlled Trial with 1 to 3 Years of Follow-Up. Biomed. Res. Int. 2017, 2017, 8017175. [Google Scholar] [CrossRef] [PubMed]
- Maglione, M.; Bevilacqua, L.; Dotto, F.; Costantinides, F.; Lorusso, F.; Scarano, A. Observational Study on the Preparation of the Implant Site with Piezosurgery vs. Drill: Comparison between the Two Methods in terms of Postoperative Pain, Surgical Times, and Operational Advantages. Biomed. Res. Int. 2019, 2019, 8483658. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kato, S.; Bengazi, F.; Urbizo Velez, J.; Tumedei, M.; Kotsu, M.; Botticelli, D. Healing at implant installed in osteotomies prepared either with a piezoelectric device or drills: An experimental study in dogs. Oral Maxillofac. Surg. 2021, 25, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Clauser, T.; Lin, G.H.; Lee, E.; Del Fabbro, M.; Wang, H.L.; Testori, T. Risk of early implant failure in grafted and non-grafted sites: A systematic review and meta-analysis. Int. J. Oral Implantol. 2022, 15, 31–41. [Google Scholar]
- Munakata, M.; Kataoka, Y.; Yamaguchi, K.; Sanda, M. Risk Factors for Early Implant Failure and Selection of Bone Grafting Materials for Various Bone Augmentation Procedures: A Narrative Review. Bioengineering 2024, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- French, D.; Grandin, H.M.; Ofec, R. Retrospective cohort study of 4591 dental implant: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Laleman, I.; Lambert, F. Implant connection and abutment selection as a predisposing and/or precipitating factor for peri-implant diseases: A review. Clin. Implant. Dent. Relat. Res. 2023, 25, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.S.; Liu, K.C.; Hung, M.C.; Lin, H.Y.; Chuang, S.L.; Lin, P.J.; Chang, J.Z. A cross-sectional study for prevalence and risk factors of peri-implant marginal bone loss. J. Prosthet. Dent. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Tang, H.; Gao, X.L.; McGrath, C.; Mattheos, N. Patients’ expectations to dental implant: A systematic review of the literature. Health Qual. Life Outcomes 2014, 12, 153. [Google Scholar] [CrossRef] [PubMed]
- McCrea, S.J.J. An Analysis of Patient Perceptions and Expectations to Dental Implant: Is There a Significant Effect on Long-Term Satisfaction Levels. Int. J. Dent. 2017, 2017, 8230618. [Google Scholar] [CrossRef] [PubMed]
- Kashbour, W.A.; Rousseau, N.S.; Thomason, J.M.; Ellis, J.S. Provision of information on dental implant treatment: Patients’ thoughts and experiences. Clin. Oral Implant. Res. 2018, 29, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Chrcanovic, B.; Jacobsson, M.; Wennerberg, A. Osseointegration of implant—A biological and clinical overview. JSM Dent. Surg. 2017, 2, 1022–1028. [Google Scholar]
- Amengual-Peñafiel, L.; Córdova, L.A.; Constanza Jara-Sepúlveda, M.; Brañes-Aroca, M.; Marchesani-Carrasco, F.; Cartes-Velásquez, R. Osteoimmunology drives dental implant osseointegration: A new paradigm for implant dentistry. Jpn. Dent. Sci. Rev. 2021, 57, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Feng, Y.; Cheng, H.; Li, D. The role of macrophages in osseointegration of dental implant: An experimental study in vivo. J. Biomed. Mater. Res. Part A 2020, 108, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, D.M.; Santos, S.G.; Lamghari, M.; Barbosa, M.A. The two faces of metal ions: From implant rejection to tissue repair/regeneration. Biomaterials 2016, 84, 262–275. [Google Scholar] [CrossRef]
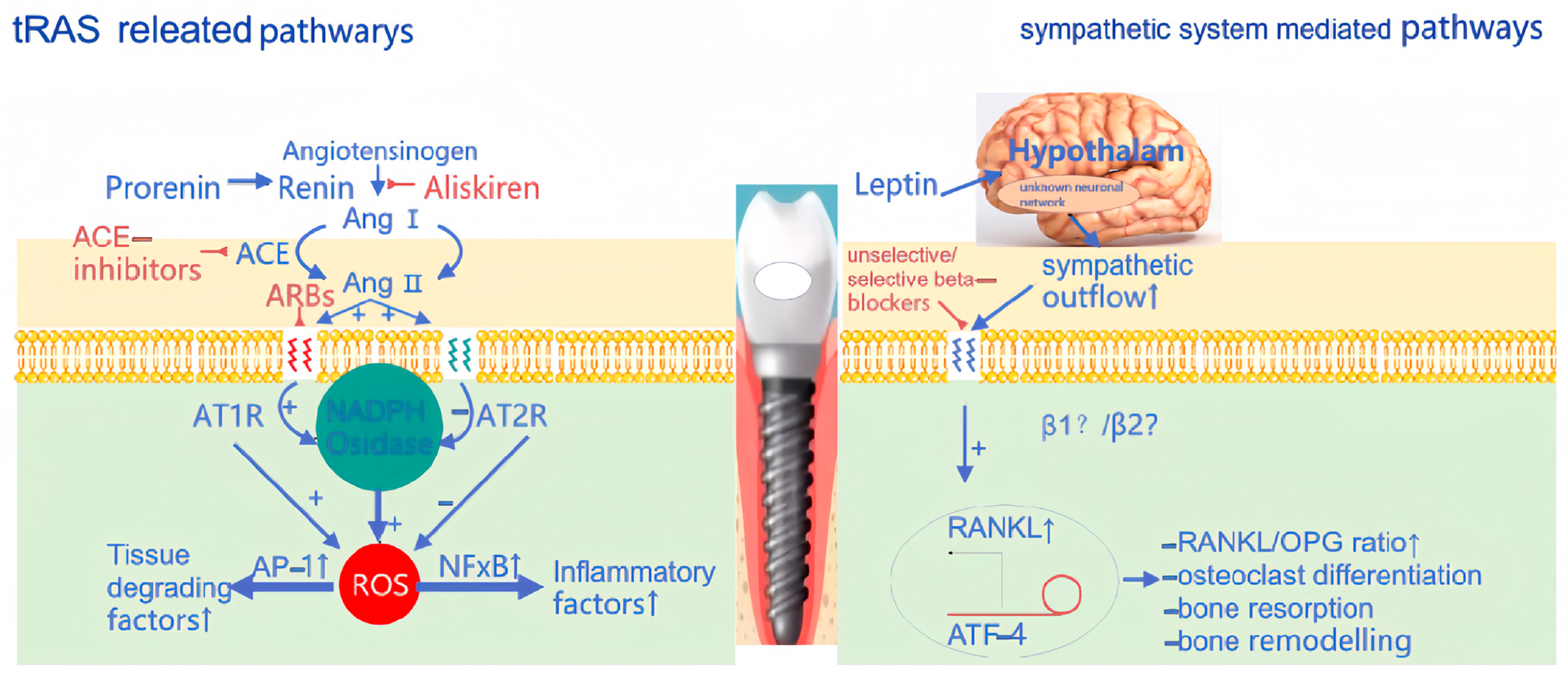
- Saravi, B.; Vollmer, A.; Lang, G.; Adolphs, N.; Li, Z.; Giers, V.; Stoll, P. Impact of renin-angiotensin system inhibitors and beta-blockers on dental implant stability. Int. J. Implant. Dent. 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Monasterio, G.; Cavalla, F.; Córdova, L.A.; Hernández, M.; Heymann, D.; Garlet, G.P.; Sorsa, T.; Pärnänen, P.; Lee, H.M.; et al. Osteoimmunology of Oral and Maxillofacial Diseases: Translational Applications Based on Biological Mechanisms. Front. Immunol. 2019, 10, 1664. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
| Study | 3D Printing Method | Results | References |
|---|---|---|---|
| Osteogenesis of 3D-Printed porous Ti-6Al-4V implant with different pore sizes. | SLM | SLM was able to fabricate porous Ti6Al4V implants with proper mechanical properties analogous to human bone. | [26] |
| Micro/nano topography of selective laser melting titanium inhibits osteoclastogenesis via mediation of macrophage polarization. | SLM | Both in vivo and in vitro studies revealed that the SLA- and SAH-treated SLM-Ti implants significantly inhibited peri-implant osteoclast activity. | [27] |
| Micro-/nano-topography of selective laser melting titanium enhances adhesion and proliferation. | SLM | Creating appropriate micro-/nano-topographies affected cell behavior and increased the stability of the SLM-Ti. | [28] |
| Osteoinduction of porous Ti implants with a channel structure fabricated by selective laser melting. | SLM | The RP-based SLM technique was very effective for investigating the influence of pore size on osteoinduction. | [29] |
| A biomimetic hierarchical structure on selective laser melting titanium with enhanced hydrophobic surface. | SLM | The SLM-Ti had a superhydrophilic surface, and the contact angles quickly reduced to zero upon complete wetting. | [30] |
| Improved osseointegration of 3D-printed Ti-6Al-4V implants with a micro/nano surface topography. | EBM | After acid etching and anodic oxidation, the hydrophilicity and bioactivity of EBM-Ti were improved. | [31] |
| Osteoconductivity of bioactive Ti-6Al-4V implants with lattice-shaped interconnected large pores fabricated by electron beam melting. | EBM | EBM-Ti with NaOH, CaCl2, heating, and water treatment, with lattice-like pores, with superior mechanical properties and biological activity. | [32] |
| Electron beam-melted, free-form-fabricated titanium alloy implants: Material surface characterization and early bone response in rabbits. | EBM | Compared with machined Ti, EBM-Ti implants had higher surface roughness, thicker surface oxides, and better osteogenic activity. | [33] |
| Mechanical properties of selective laser sintering pure titanium and Ti-6Al-4V, and its anisotropy. | SLS | The SLS process with Ti-6Al-4V powder had great performance for the fabrication of dental prostheses. | [34] |
| In vitro and vivo comparisons of the porous Ti-6Al-4V alloys fabricated by the selective laser melting technique and sintering technique. | SLS | Microstructure and mechanical properties of the SLS porous Ti-6Al-4V were more similar to the cancellous bone, without obvious stress shielding. | [35] |
| 3D laser-printed porous Ti-6Al-4V dental implants for compromised bone support. | SLS | In micro-CT analysis, new bone formation and osseointegration within the SLS-ITRI implants were observed. | [36] |
| By Structure Classification | One-Stage Implant | Two-Stage Non-Embedded Implant | Two-Stage Embedded Implant |
|---|---|---|---|
| Planting patterns | The appropriate position is selected in the alveolar bone, the implant is placed in the hole, and the screw is closed; 7–10 days later the stitches are removed. | Non-invasive surgery (gum surgery): The implant is directly inserted into the alveolar bone and mounted on a healing abutment. The implant is directly or indirectly exposed in the mouth. | First surgery: To ensure that the bone tissue has a long enough time to heal, we place the implant in the alveolar bone and bury it completely under the soft tissue, without exposing it to the mouth. Second procedure: After the bone has healed, the abutment is attached to the implant, the gums are cut, and the abutment is connected to the implant using the implant’s center screw. |
| Merits | Strong function: Can restore tooth function, chewing function is better than traditional dentures. No grinding: Artificial root restoration, without grinding healthy teeth next to it. High comfort level: No foreign body sensation, and conducive to maintaining oral hygiene. | The implant soft tissue and bone tissue have the same healing time, which is more favorable for healing soft tissue. The implant and periodontal tissue heal well, with improved initial stability. The need for secondary surgery shortens the dental implant time and reduces secondary trauma to the oral cavity. The non-submersible implant has a higher joint plane and only one joint, thus providing a more favorable biological width and gingival margin height. | High stability: Higher implant stability due to adequate healing time between implant and bone tissue. Difficult to infection: The risk of infection is reduced because the implant is completely embedded under the soft tissue. Good bone integration: The closer bonding between the implant and the bone tissue is conducive to long-term stability. Good long-term results: The long-term results are usually better because the connection between the implant and the abutment is better. High success rate: Due to the above advantages, the success rate of two-stage embedded implants is usually higher. |
| Shortcomings | Long operation cycle: It usually takes 3 to 6 months between primary and secondary operations to facilitate the full integration of the implant and the alveolar bone. Postoperative discomfort: After the first phase of the surgery, the mouth may be swollen and painful. | As implants are directly exposed to the oral environment, they may be more susceptible to infection by the oral bacteria. During healing, the implant may be affected by the bite force, thus affecting its stability. | Two operations are required: Compared to a one-stage implant, the two-stage embedded implant requires two operations, increasing the risk and complexity of the procedure. Long healing time: Due to the need to wait for bone tissue and implant healing, the entire treatment process is longer, usually taking several months. Higher cost: The cost of two-stage embedded implants is usually higher due to the need for two surgeries and longer treatment times. |
| Term | Ultra-Short | Short | Standard | Long |
|---|---|---|---|---|
| Measurements | ≤6 mm | >6 mm to <10 mm | 10 mm to <13 mm | ≥13 mm |
| Comments | 6 mm or less | From more than 6 mm to less than 10 mm | From 10 mm to less than 13 mm | 13 mm or more |
| Term | Ultra-Narrow | Narrow | Standard | Wide |
|---|---|---|---|---|
| Measurements | <3.0 mm | ≥3.0 mm to ≤3.75 mm | ≥3.75 mm to ≤5 mm | ≥5 mm |
| Comments | Less than 3.0 mm | From 3.0 mm to less than 3.75 mm | From 3.75 mm to less than 5 mm | 5.0 mm or more |
| Dental Implant Materials | Advantages | Disadvantages |
|---|---|---|
| PMMA-ZrO biomimetic nanocomposites | Strong weather resistance Wide variety Non-toxic Environmental protection Good overall mechanical properties Tensile properties | The fracture toughness and curvature strength are much higher than those of traditional dental ceramics Poor wear resistance High cost Poor heat resistance |
| Zirconia–glass ceramic composite material | High density High hardness Low thermal expansion coefficient High melting point Good corrosion resistance Good insulation Biocompatibility | Easy to damage Affects the service life The preparation process requirements are high High price |
| Titanium (chemical) | Light weight High strength High-temperature resistance (to be used at higher temperatures for a long time) Low-temperature resistance (to maintain good ductility and toughness) Non-toxic The new dental restorative material titanium has good biocompatibility and strong strength The tribological properties of the natural teeth are very good | May cause allergies High raw material cost High weight Low hardness Difficult to process Easy to oxidize Expensive Difficult to refine |
| Glass ceramics | Excellent lithium silicate glass ceramics High bending strength Transparency High biocompatibility Repair versatility Comfort to wear | Easy to damage Affects the service life The preparation process requirements are high High price |
| Resin matrix composite | A series of resin composites with uniform size dispersion Low water absorption Good biocompatibility was prepared for modified alumina nanoparticles | Poor durability Unstable color Easy color |
| Silicon nitride | Good antibacterial properties No biotoxicity Good biocompatibility Mechanical properties Good osseoconjugation High mechanical strength High fracture rate | Low elasticity Heat shock Low wear resistance Can cause a foreign body reaction |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Pan, Y.; Gao, Y.; Pang, H.; Sun, H.; Cheng, L.; Liu, J. Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications. Coatings 2024, 14, 781. https://doi.org/10.3390/coatings14070781
Gao J, Pan Y, Gao Y, Pang H, Sun H, Cheng L, Liu J. Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications. Coatings. 2024; 14(7):781. https://doi.org/10.3390/coatings14070781
Chicago/Turabian StyleGao, Jingjing, Yang Pan, Yuting Gao, Hanyu Pang, Haichuan Sun, Lijia Cheng, and Juan Liu. 2024. "Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications" Coatings 14, no. 7: 781. https://doi.org/10.3390/coatings14070781
APA StyleGao, J., Pan, Y., Gao, Y., Pang, H., Sun, H., Cheng, L., & Liu, J. (2024). Research Progress on the Preparation Process and Material Structure of 3D-Printed Dental Implants and Their Clinical Applications. Coatings, 14(7), 781. https://doi.org/10.3390/coatings14070781







