Anti-Corrosion SiOx-Doped DLC Coating for Raster Steel Linear Scales
Abstract
:1. Introduction
2. Materials and Methods
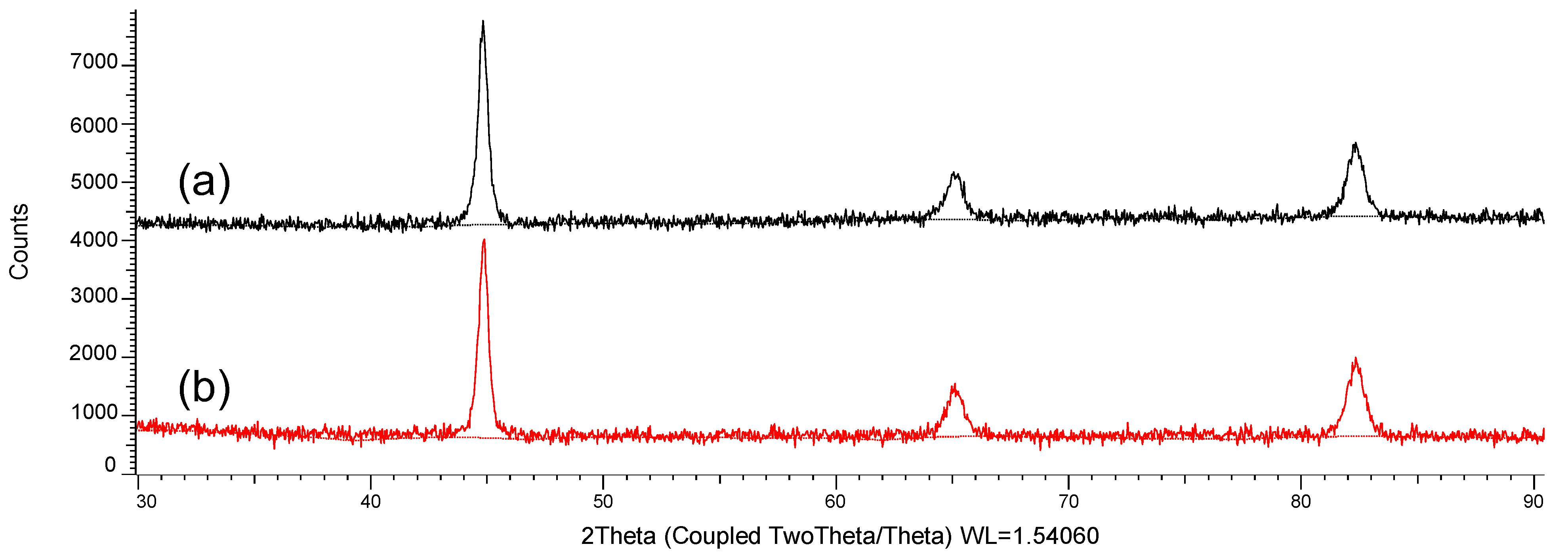
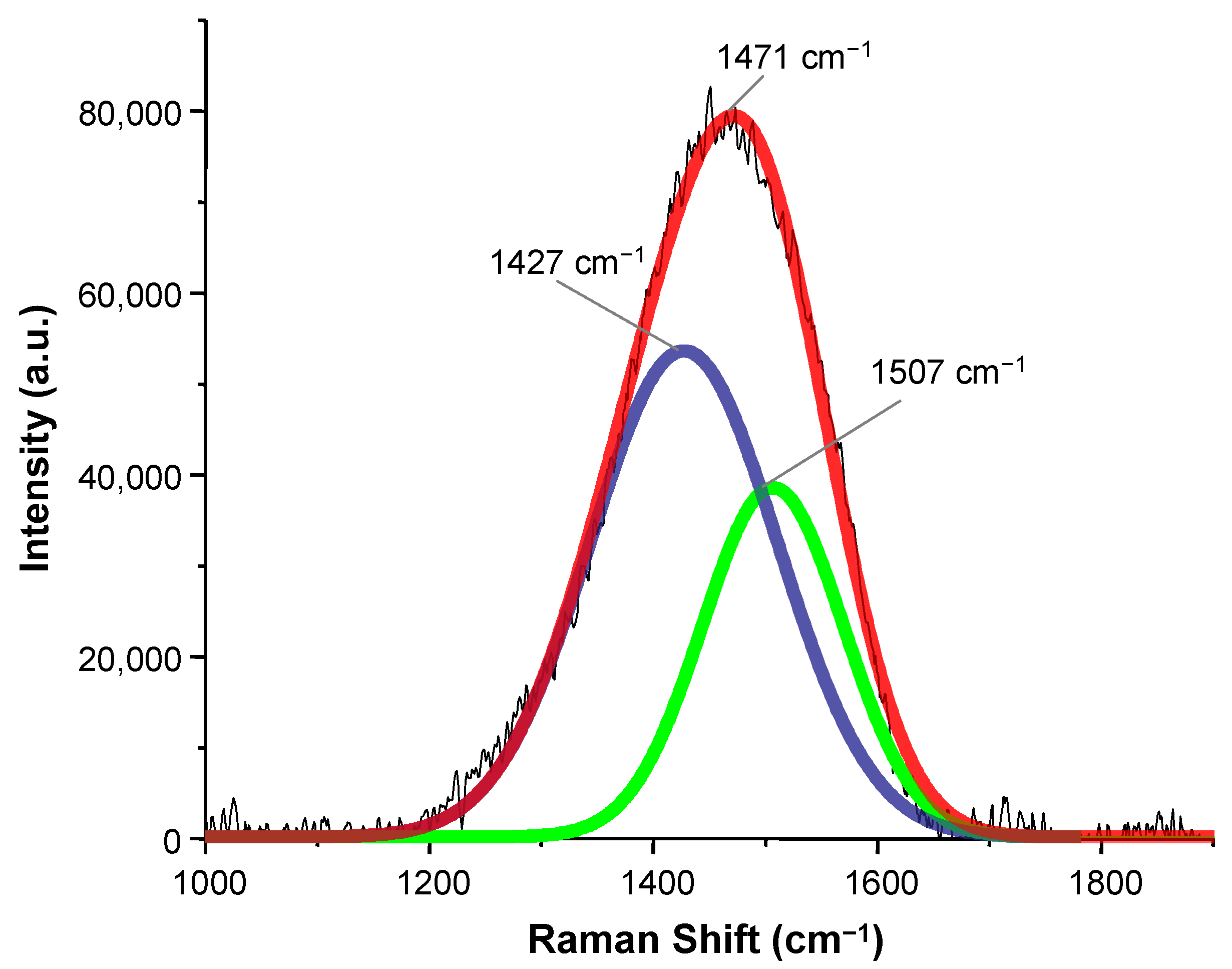
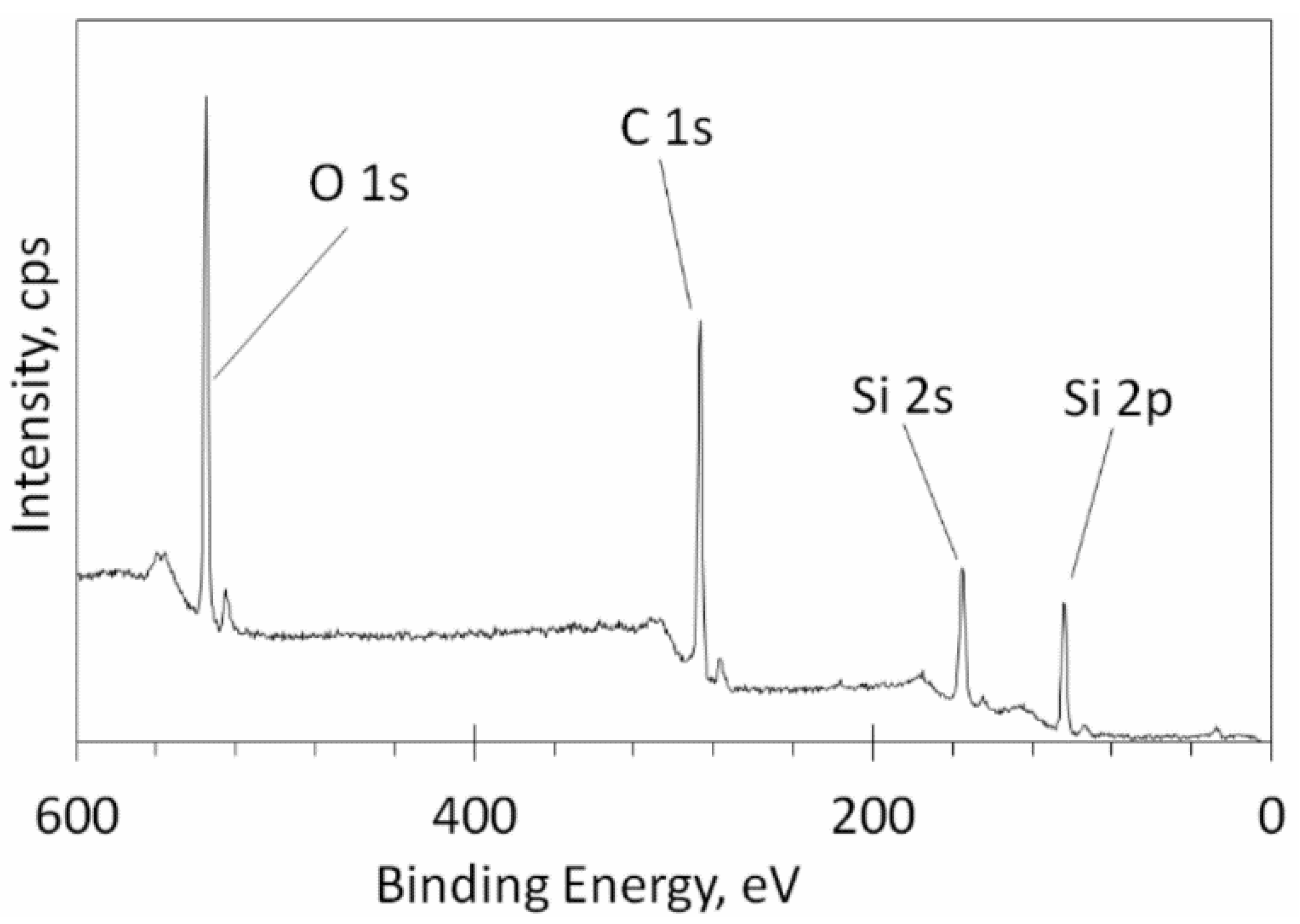
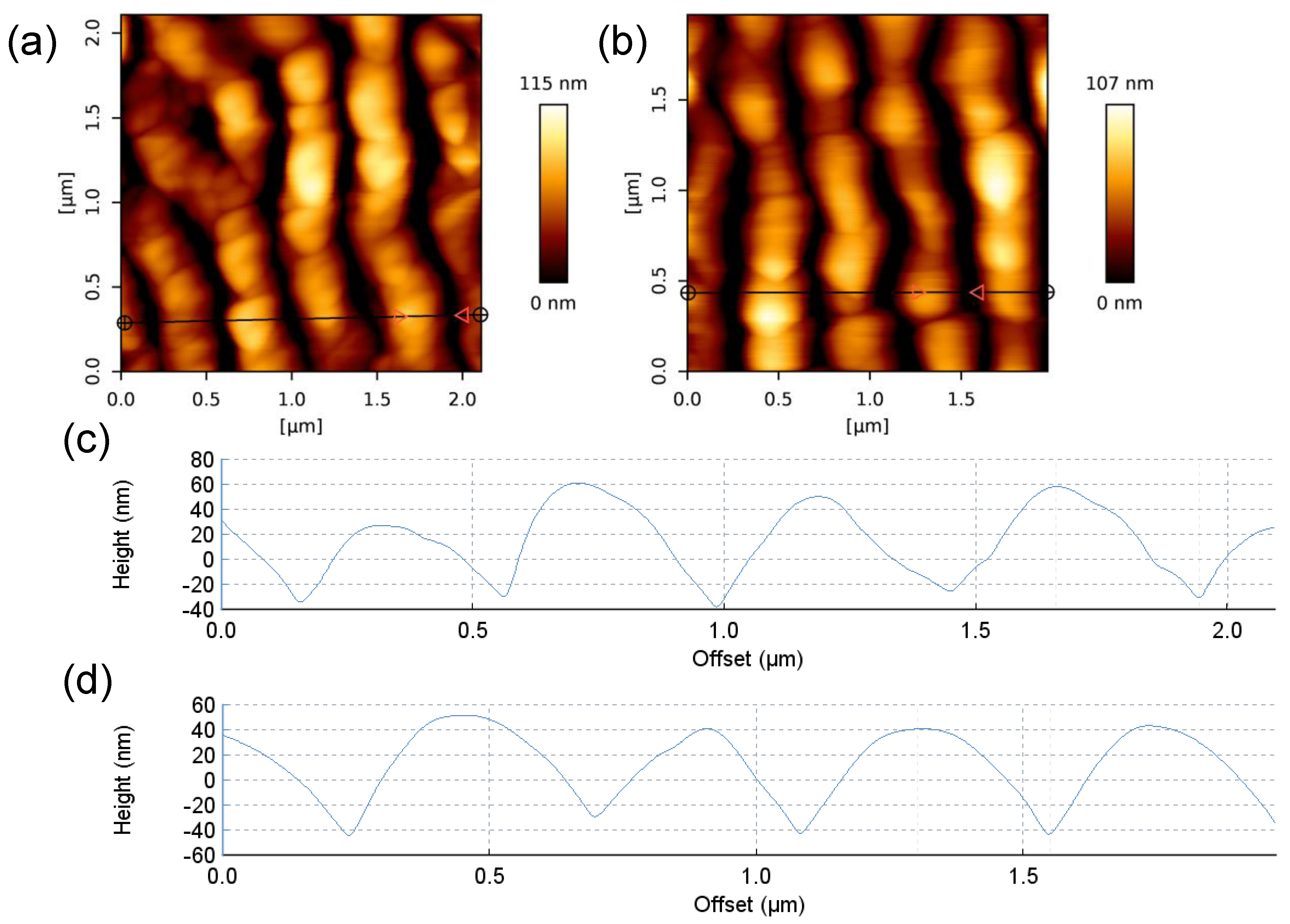
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ou, Z.; Huang, M.; Zhao, F. The fluence threshold of femtosecond laser blackening of metals: The effect of laser-induced ripples. Opt. Laser Technol. 2016, 79, 79–87. [Google Scholar] [CrossRef]
- Santamaria, M.; Tranchida, G.; Di Franco, F. Corrosion resistance of passive films on different stainless steel grades in food and beverage industry. Corros. Sci. 2020, 173, 108778. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, X.; Shi, Y.; Zhang, W.; Gu, Y.; Feng, C.; Volodymyr, K. Correlation between the microstructure and corrosion behaviour of copper/316 L stainless-steel dissimilar-metal welded joints. Corros. Sci. 2021, 191, 109729. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, B.; Wei, S.; Wang, Y.; Zhang, S. Effect of microstructure on the corrosion-friction behavior of low-carbon martensitic stainless steel. Tribol. Int. 2024, 194, 109560. [Google Scholar] [CrossRef]
- Sun, M.; Xu, X.; Li, J.; Yang, L.; Liu, X.; Du, C.; Zhao, T.; Li, X. The influence of microstructures on the corrosion resistance of Cr-Mo-Sn low alloy steel in a tropical marine atmospheric. Corros. Sci. 2024, 233, 112058. [Google Scholar] [CrossRef]
- Dearnaley, G.; Arps, J.H. Biomedical applications of diamond-like carbon (DLC) coatings: A review. Surf. Coat. Technol. 2005, 200, 2518–2524. [Google Scholar] [CrossRef]
- Zhao, Q.; Mou, Z.; Zhang, B.; Zhang, X.; Wang, Z.; Wang, K.; Gao, K.; Jia, Q. Revealing the corrosion resistance of amorphous carbon films under heat shock via annealing. Diam. Relat. Mater. 2020, 102, 107692. [Google Scholar] [CrossRef]
- Dalibón, E.; Escalada, L.; Simison, S.; Forsich, C.; Heim, D.; Brühl, S. Mechanical and corrosion behavior of thick and soft DLC coatings. Surf. Coat. Technol. 2017, 312, 101–109. [Google Scholar] [CrossRef]
- Csorbai, H.; Kovach, G.; Pető, G.; Csikvari, P.; Karacs, A.; Solyom, A.; Hárs, G.; Kalman, E. Diamond–DLC double layer used in corrosive protective coating. Appl. Surf. Sci. 2007, 253, 5070–5075. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Nakamura, M.; Zanocco, M.; Zhu, W.; Pezzotti, G.; Andreatta, F. Corrosion and scratch resistance of DLC coatings applied on chromium molybdenum steel. Surf. Coat. Technol. 2019, 378, 124944. [Google Scholar] [CrossRef]
- Radi, P.A.; Vieira, A.; Manfroi, L.; de Farias Nass, K.C.; Ramos, M.A.R.; Leite, P.; Martins, G.V.; Jofre, J.B.F.; Vieira, L. Tribocorrosion and corrosion behavior of stainless steel coated with DLC films in ethanol with different concentrations of water. Ceram. Int. 2019, 45, 9686–9693. [Google Scholar] [CrossRef]
- Hatada, R.; Flege, S.; Bobrich, A.; Ensinger, W.; Baba, K. Surface modification and corrosion properties of implanted and DLC coated stainless steel by plasma based ion implantation and deposition. Surf. Coat. Technol. 2014, 256, 23–29. [Google Scholar] [CrossRef]
- Feng, X.; Hu, H.; Gui, B.; Guo, F.; Wang, K.; Zheng, Y.; Zhou, H. Corrosion behavior of W-DLC and DLC films deposited on plasma nitrided CF170 steel in H2SO4 solution. Vacuum 2022, 204, 111385. [Google Scholar] [CrossRef]
- Ramos, B.; Vicente, F.; Hammes, G.; Bendo, T.; Binder, C. Enhancing corrosion and tribology performance of stainless steel with DLC coatings: Effects of doping and multilayered structures. Surf. Coat. Technol. 2024, 477, 130334. [Google Scholar] [CrossRef]
- Zhang, T.; Deng, Q.; Liu, B.; Wu, B.; Jing, F.; Leng, Y.; Huang, N. Wear and corrosion properties of diamond like carbon (DLC) coating on stainless steel, CoCrMo and Ti6Al4V substrates. Surf. Coat. Technol. 2015, 273, 12–19. [Google Scholar] [CrossRef]
- Nagai, T.; Hiratsuka, M.; Alanazi, A.; Nakamori, H.; Hirakuri, K. Anticorrosion of DLC coating in acid solutions. Appl. Surf. Sci. 2021, 552, 149373. [Google Scholar] [CrossRef]
- Wei, X.; Feng, H.; Liu, Z.; Chen, Z.; Yin, P.; Lu, S.; Ding, J.; Du, N.; Li, X.; Zhang, G. Insight into the corrosion behaviors and mechanism of the self-healing Si/N-DLC films in oilfield environment. Corros. Sci. 2024, 231, 111989. [Google Scholar] [CrossRef]
- Meškinis, Š.; Šlapikas, K.; Gudaitis, R.; Tamulevičius, S.; Kopustinskas, V.; Guobienė, A.; Niaura, G. SiOx-doped DLC films: Charge transport, dielectric properties and structure. Vacuum 2008, 82, 617–622. [Google Scholar] [CrossRef]
- Meškinis, Š.; Tamulevičienė, A. Structure, properties and applications of diamond like nanocomposite (SiOx containing DLC) films: A review. Mater. Sci. 2011, 17, 358–370. [Google Scholar] [CrossRef]
- Wu, X.; Suzuki, M.; Ohana, T.; Tanaka, A. Characteristics and tribological properties in water of Si-DLC coatings. Diam. Relat. Mater. 2008, 17, 7–12. [Google Scholar] [CrossRef]
- Barve, S.; Chopade, S.; Kar, R.; Chand, N.; Deo, M.; Biswas, A.; Patel, N.; Rao, G.; Patil, D.; Sinha, S. SiOx containing diamond like carbon coatings: Effect of substrate bias during deposition. Diam. Relat. Mater. 2017, 71, 63–72. [Google Scholar] [CrossRef]
- Kumar, N.; Barve, S.; Chopade, S.; Kar, R.; Chand, N.; Dash, S.; Tyagi, A.; Patil, D. Scratch resistance and tribological properties of SiOx incorporated diamond-like carbon films deposited by rf plasma assisted chemical vapor deposition. Tribol. Int. 2015, 84, 124–131. [Google Scholar] [CrossRef]
- Grenadyorov, A.; Zhulkov, M.; Solovyev, A.; Oskomov, K.; Semenov, V.; Chernyavskiy, A.; Sirota, D.; Karmadonova, N.; Malashchenko, V.; Litvinova, L. Surface characterization and biological assessment of corrosion-resistant aC: H: SiOx PACVD coating for Ti-6Al-4V alloy. Mater. Sci. Eng. C 2021, 123, 112002. [Google Scholar] [CrossRef] [PubMed]
- Yin, P.; Wei, X.; Shang, L.; Lu, Z.; Zhang, G. Design of low-friction and anti-corrosion aC: H: SiOx films. Diam. Relat. Mater. 2021, 118, 108512. [Google Scholar] [CrossRef]
- Rajak, D.K.; Kumar, A.; Behera, A.; Menezes, P.L. Diamond-like carbon (DLC) coatings: Classification, properties, and applications. Appl. Sci. 2021, 11, 4445. [Google Scholar] [CrossRef]
- Kadowaki, M.; Saengdeejing, A.; Muto, I.; Chen, Y.; Masuda, H.; Katayama, H.; Doi, T.; Kawano, K.; Miura, H.; Sugawara, Y. First-principles analysis of the inhibitive effect of interstitial carbon on an active dissolution of martensitic steel. Corros. Sci. 2020, 163, 108251. [Google Scholar] [CrossRef]
- Mallik, A.K.; Dandapat, N.; Ghosh, P.; Ganguly, U.; Jana, S.; Das, S.; Guha, K.; Rebello, G.; Lahiri, S.K.; Datta, S. Deposition and characterization of diamond-like nanocomposite coatings grown by plasma enhanced chemical vapour deposition over different substrate materials. Bull. Mater. Sci. 2013, 36, 193–202. [Google Scholar] [CrossRef]
- Wu, J.; Chen, L.; Ning, C.; Wu, G.; Lu, Z.; Shang, L.; Wu, Z.; Zhang, G. Tribo-mechanism of amorphous carbon films under corrosion solution and various mechanical loads. Diam. Relat. Mater. 2021, 114, 108318. [Google Scholar] [CrossRef]
- Lazauskas, A.; Grigaliūnas, V.; Meškinis, Š.; Ecarla, F.; Baltrusaitis, J. Surface morphology, cohesive and adhesive properties of amorphous hydrogenated carbon nanocomposite films. Appl. Surf. Sci. 2013, 276, 543–549. [Google Scholar] [CrossRef]
- Irmer, G.; Dorner-Reisel, A. Micro-Raman studies on DLC coatings. Adv. Eng. Mater. 2005, 7, 694–705. [Google Scholar] [CrossRef]
- Xu, N.; Tsang, S.H.; Tan, C.W.; Teo, H.T.; Wang, X.; Tay, B.K. The effect of bonding structure of diamond like carbon (DLC) film with surface nickel coating upon laser annealing. Prog. Nanotechnol. Nanomater. 2013, 12, 35–39. [Google Scholar]
- Kalish, R.; Lifshitz, Y.; Nugent, K.; Prawer, S. Thermal stability and relaxation in diamond-like-carbon. A Raman study of films with different sp 3 fractions (ta-C to a-C). Appl. Phys. Lett. 1999, 74, 2936–2938. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Quintián, F.P.; Calarco, N.; Lutenberg, A.; Lipovetzky, J. Performance of an optical encoder based on a nondiffractive beam implemented with a specific photodetection integrated circuit and a diffractive optical element. Appl. Opt. 2015, 54, 7640–7647. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Chen, B.; Xiao, P.; Chen, X.; Cai, N.; Ling, B.W.-K. Absolute optical imaging position encoder. Measurement 2015, 67, 42–50. [Google Scholar] [CrossRef]
- Lee, C.-H.; Huang, H.-J.; Chang, J.-P.; Chen, Y.-C. Incremental optical encoder based on a sinusoidal transmissive pattern. IEEE Photonics J. 2021, 14, 1–6. [Google Scholar] [CrossRef]





| Substrate | Stainless Steel |
|---|---|
| Reagents | Hexamethyldisiloxane (C6H18Si2O) + H2 |
| Base pressure | 2 × 10−4 Pa |
| Work pressure | 2 × 10−2 Pa |
| Ion beam energy | 1000 eV |
| Ion beam current density | 50 μA/cm2 |
| Deposition time | 10 min |
| O 1s | C 1s | Si 2p |
|---|---|---|
| 27.27% | 52.29% | 20.44% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazauskas, A.; Grigaliūnas, V.; Jucius, D.; Meškinis, Š.; Andrulevičius, M.; Guobienė, A.; Vasiliauskas, A.; Kasparaitis, A. Anti-Corrosion SiOx-Doped DLC Coating for Raster Steel Linear Scales. Coatings 2024, 14, 818. https://doi.org/10.3390/coatings14070818
Lazauskas A, Grigaliūnas V, Jucius D, Meškinis Š, Andrulevičius M, Guobienė A, Vasiliauskas A, Kasparaitis A. Anti-Corrosion SiOx-Doped DLC Coating for Raster Steel Linear Scales. Coatings. 2024; 14(7):818. https://doi.org/10.3390/coatings14070818
Chicago/Turabian StyleLazauskas, Algirdas, Viktoras Grigaliūnas, Dalius Jucius, Šarūnas Meškinis, Mindaugas Andrulevičius, Asta Guobienė, Andrius Vasiliauskas, and Albinas Kasparaitis. 2024. "Anti-Corrosion SiOx-Doped DLC Coating for Raster Steel Linear Scales" Coatings 14, no. 7: 818. https://doi.org/10.3390/coatings14070818
APA StyleLazauskas, A., Grigaliūnas, V., Jucius, D., Meškinis, Š., Andrulevičius, M., Guobienė, A., Vasiliauskas, A., & Kasparaitis, A. (2024). Anti-Corrosion SiOx-Doped DLC Coating for Raster Steel Linear Scales. Coatings, 14(7), 818. https://doi.org/10.3390/coatings14070818








