Influence of Deposition Temperature and WC Concentration on the Microstructure of Electroless Ni-P-WC Nanocomposite Coatings with Improved Hardness and Corrosion Resistance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Coating Preparation
- 1.
- Soaking in 1 M NaOH solution at 80 °C for 10 min, for degreasing and removal of liquids for long-term storage.
- 2.
- 3.
- Rinsing in acetone and ultrasounds using an Elmasonic S 30 (H) (Elma Schmidbauer GmbH, Singen, Germany) ultrasonic bath for 5 min, to remove any contamination from sandblasting.
- 4.
- Activation by pickling in a solution of HCl 37 wt.% further diluted by 50 vol%, to remove any residual non-catalytic superficial oxide.
2.2. Coating Characterization
2.3. Thermal Treatments
2.4. Vickers Microhardness Test and Instrumented Indentation Test
2.5. Potentiodynamic Polarization Test
3. Results and Discussion
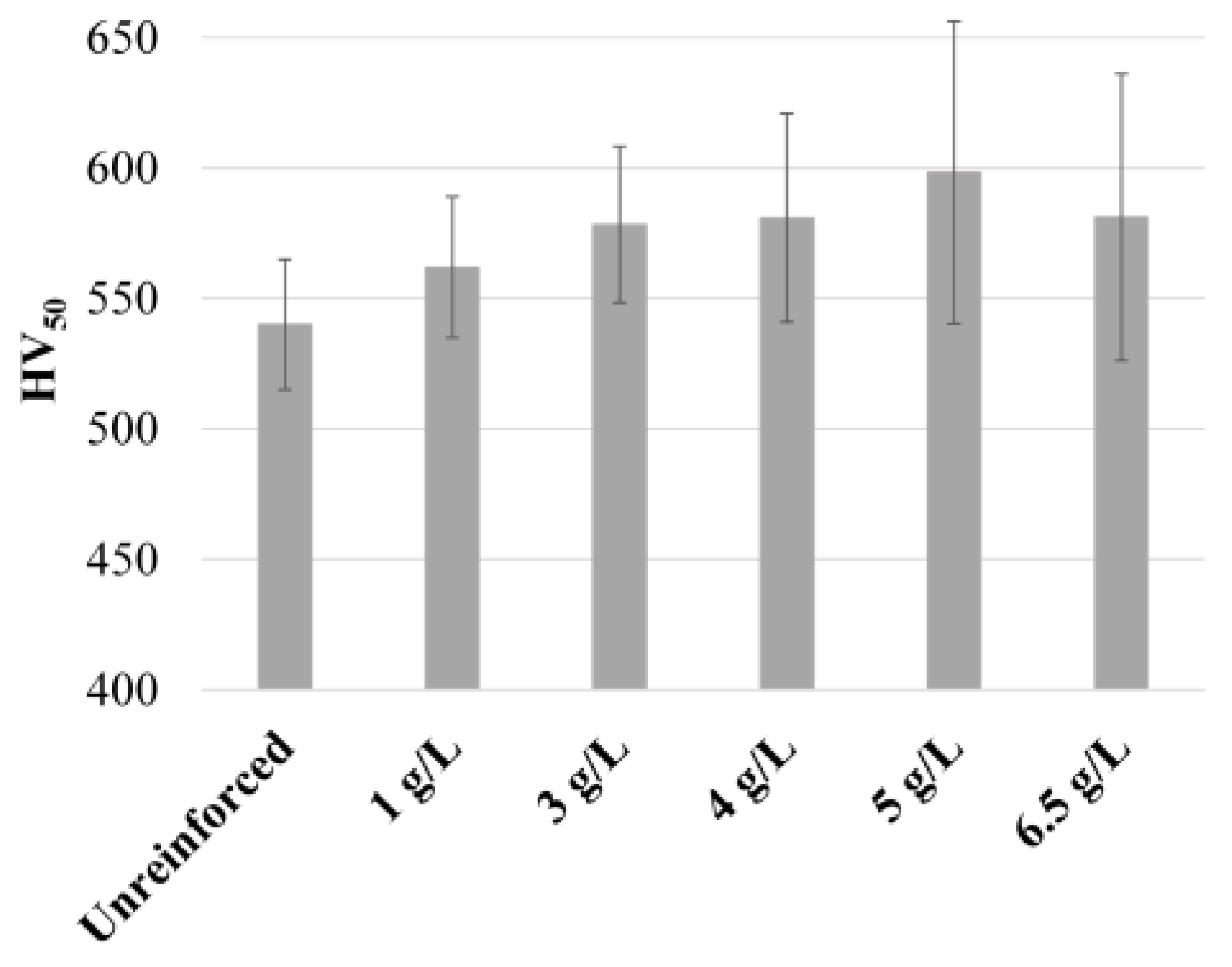
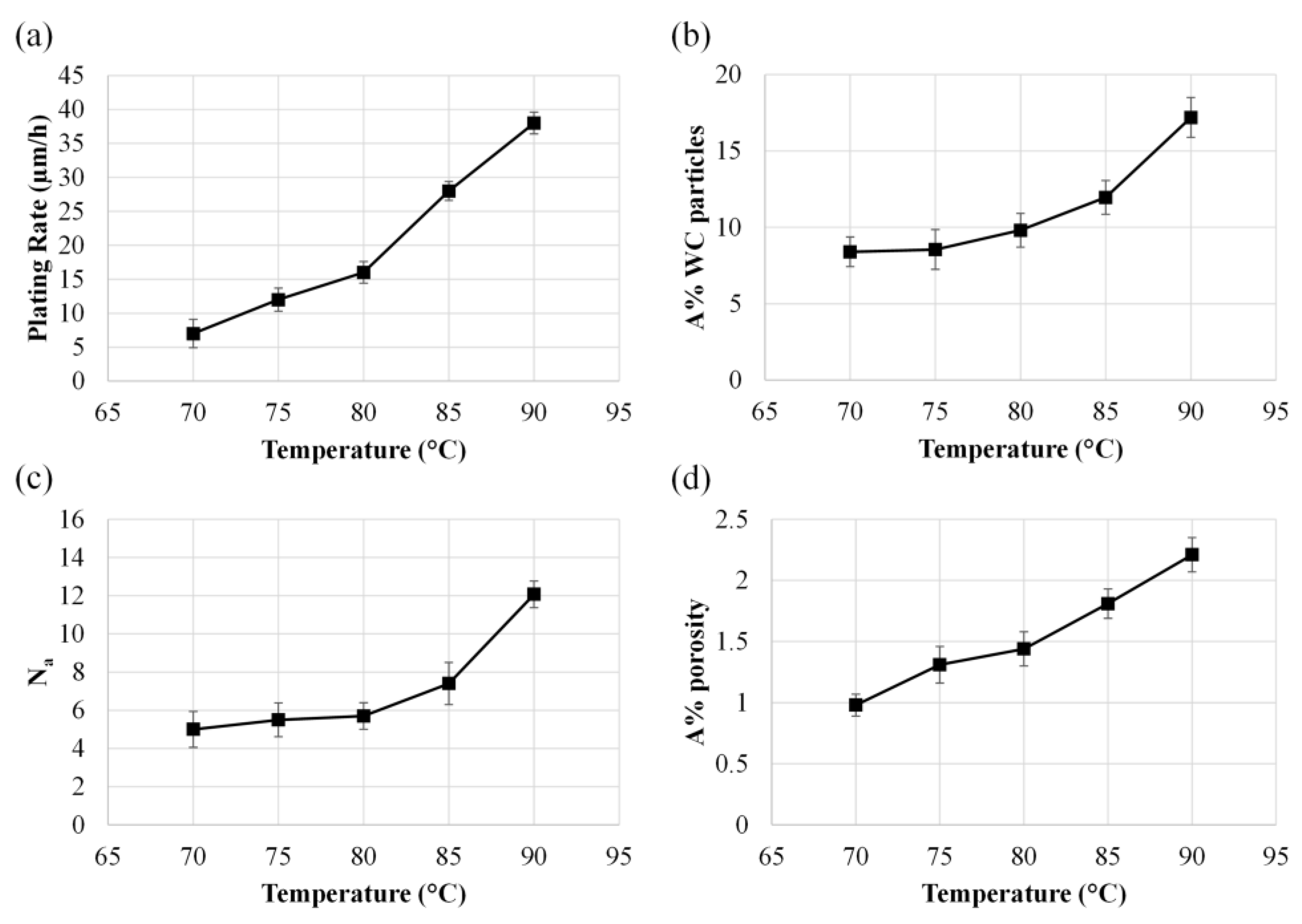
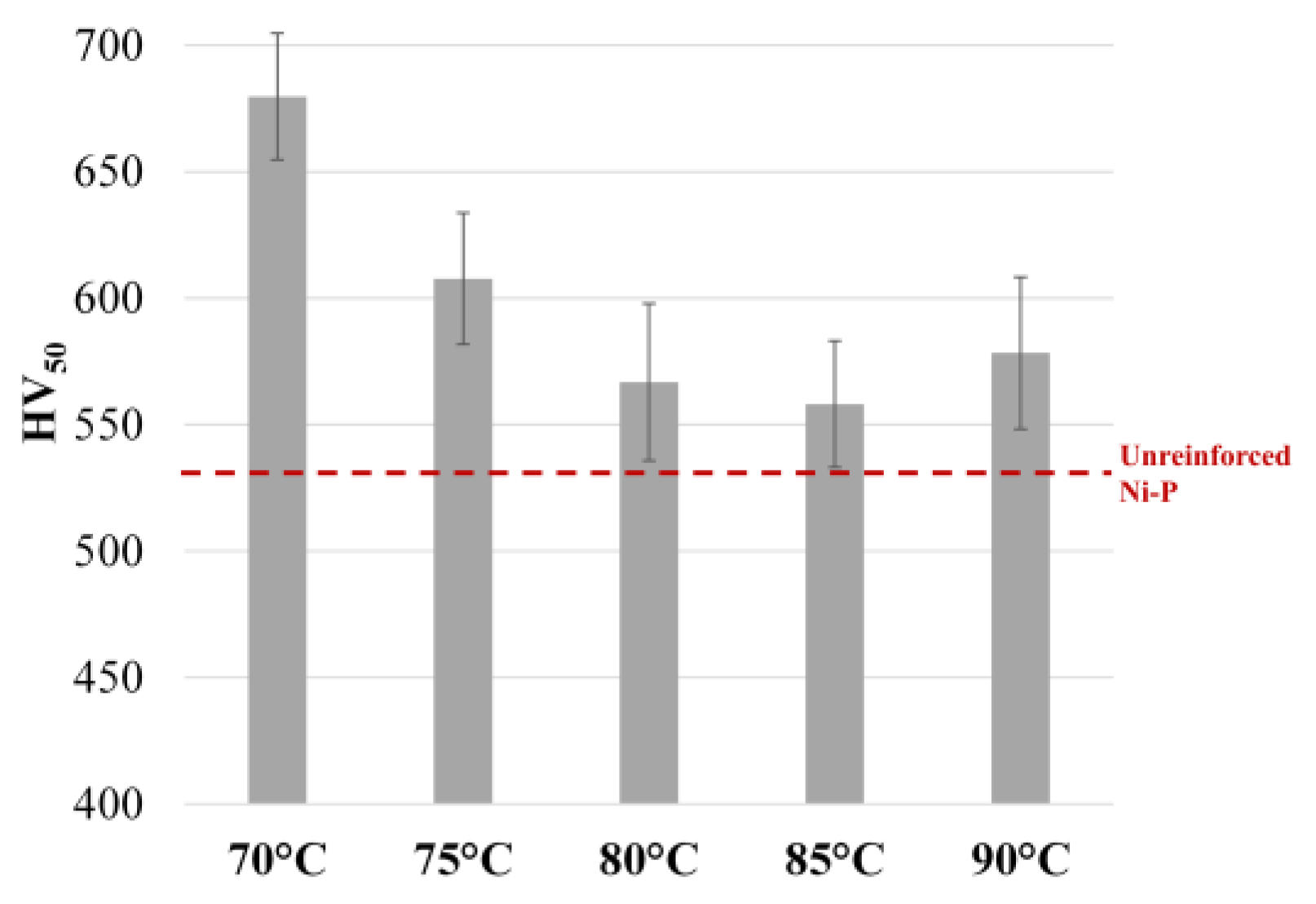
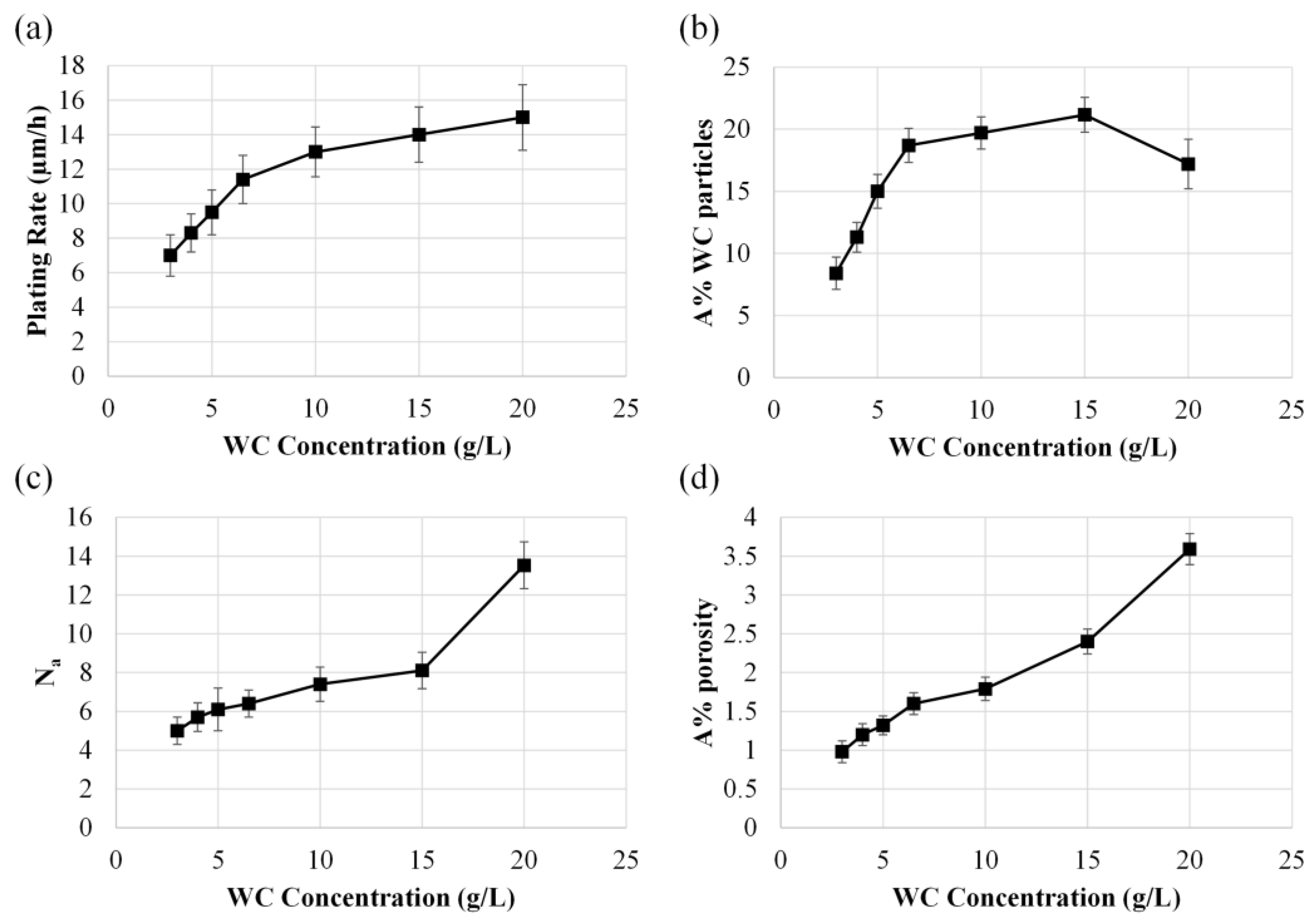
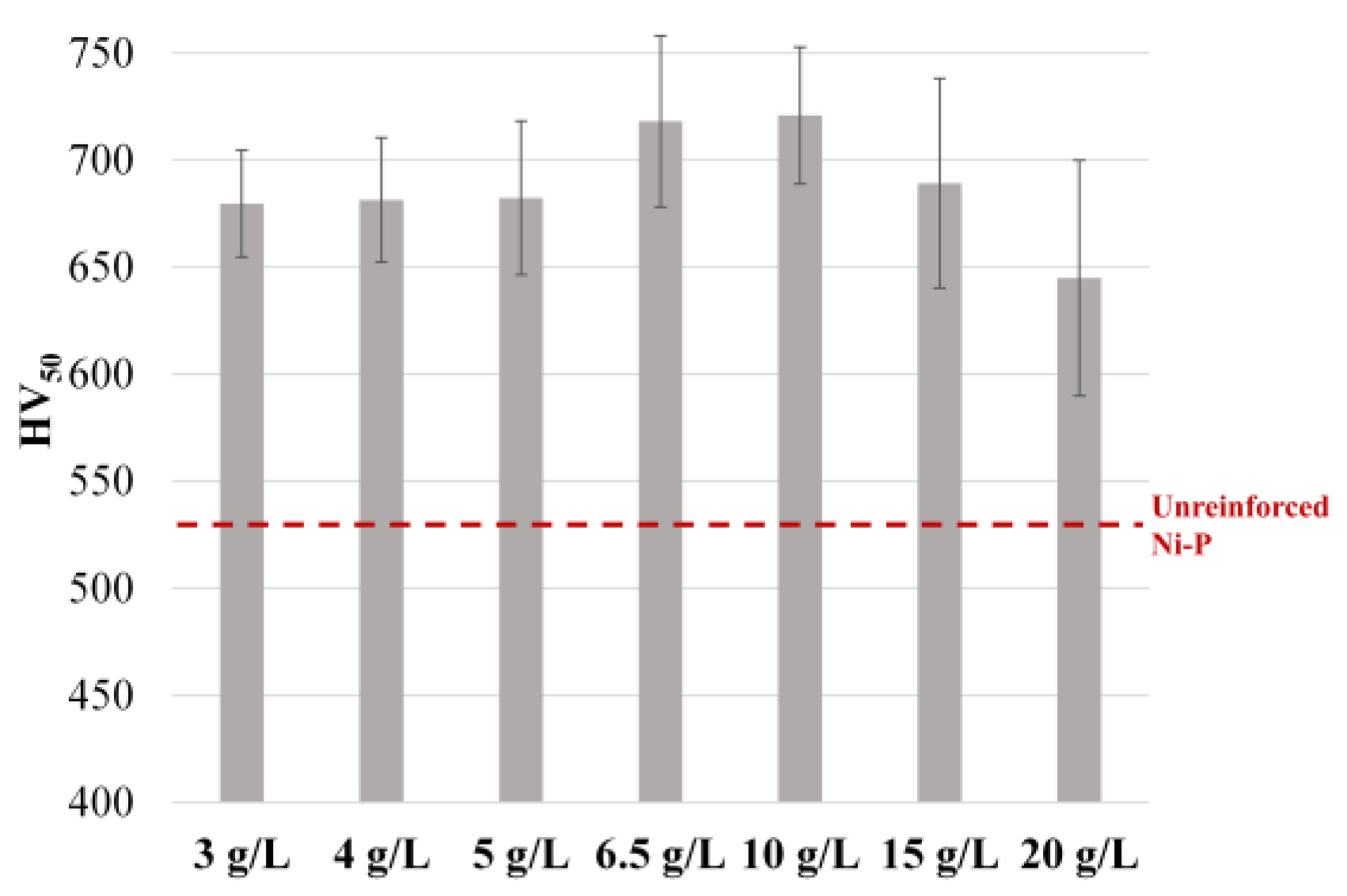
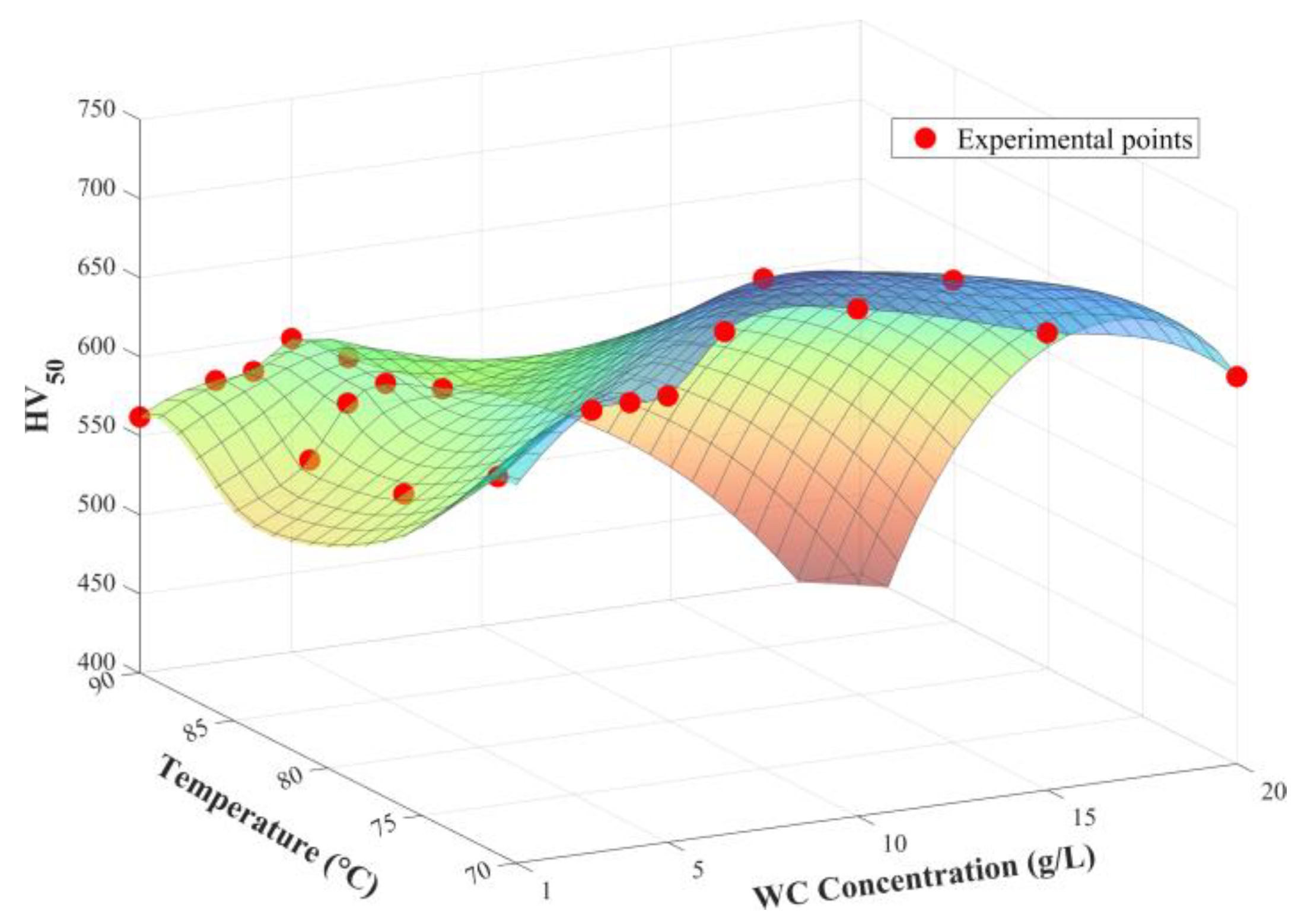
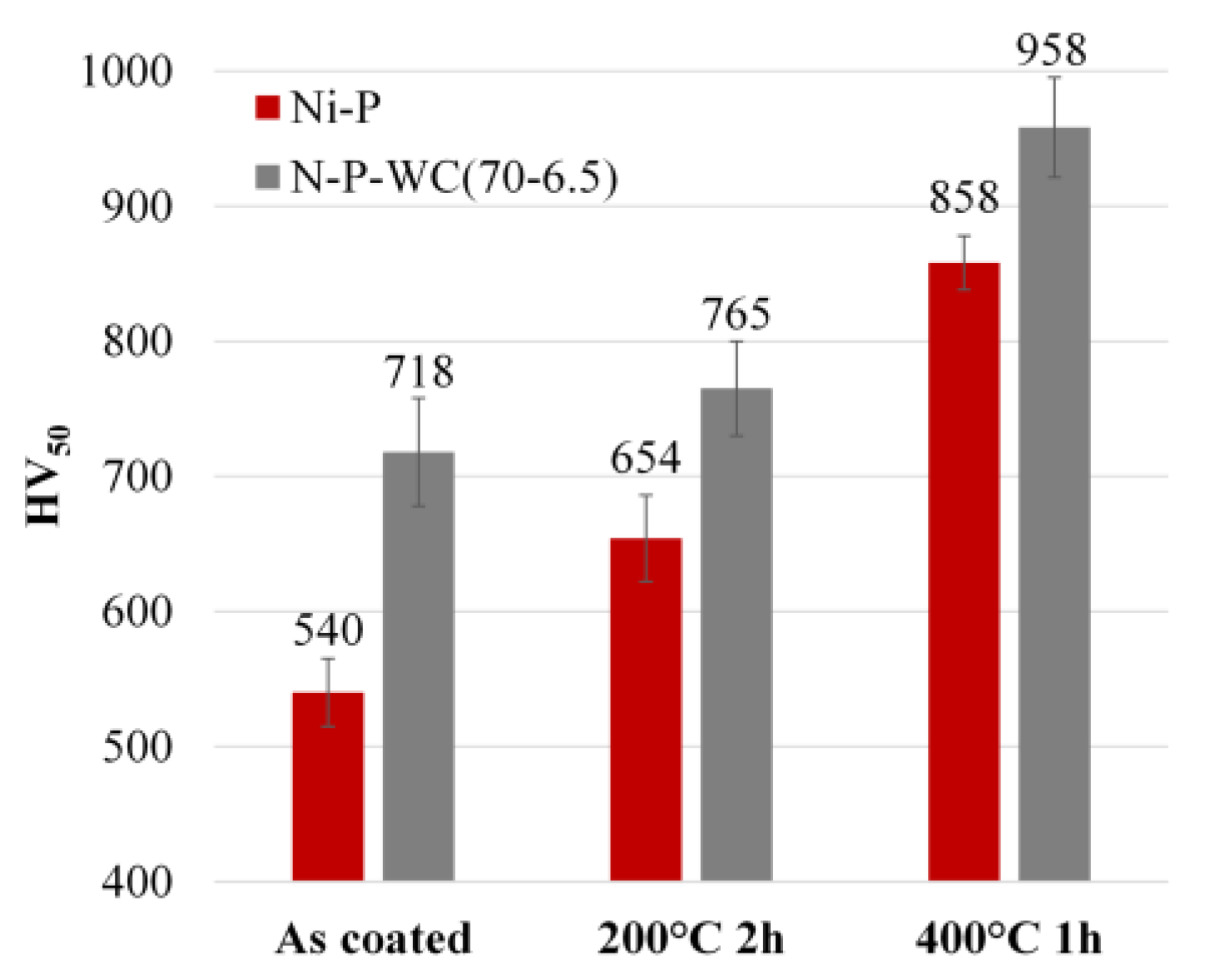
3.1. Effect of Temperature and WC Concentration on Microhardness
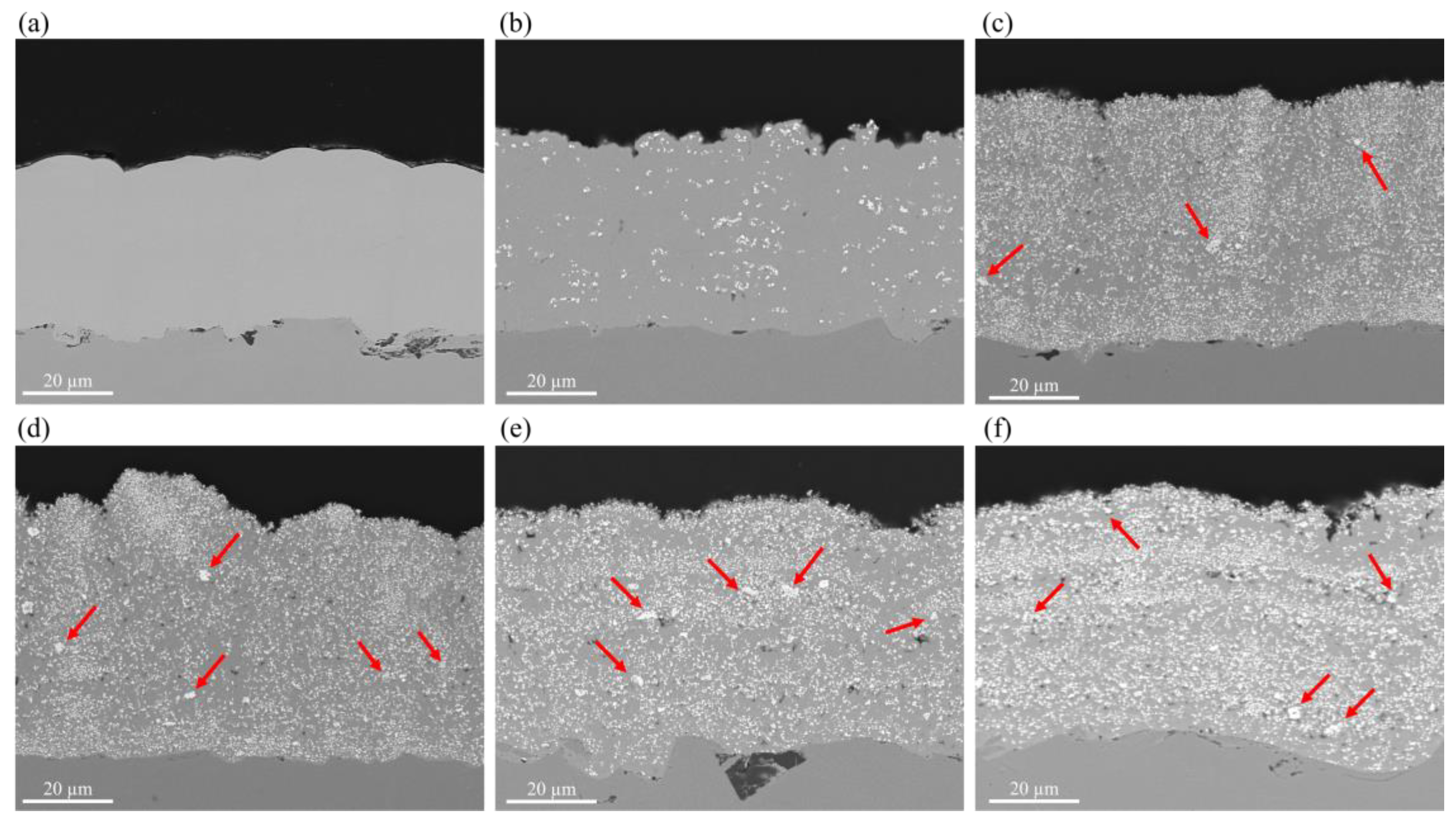
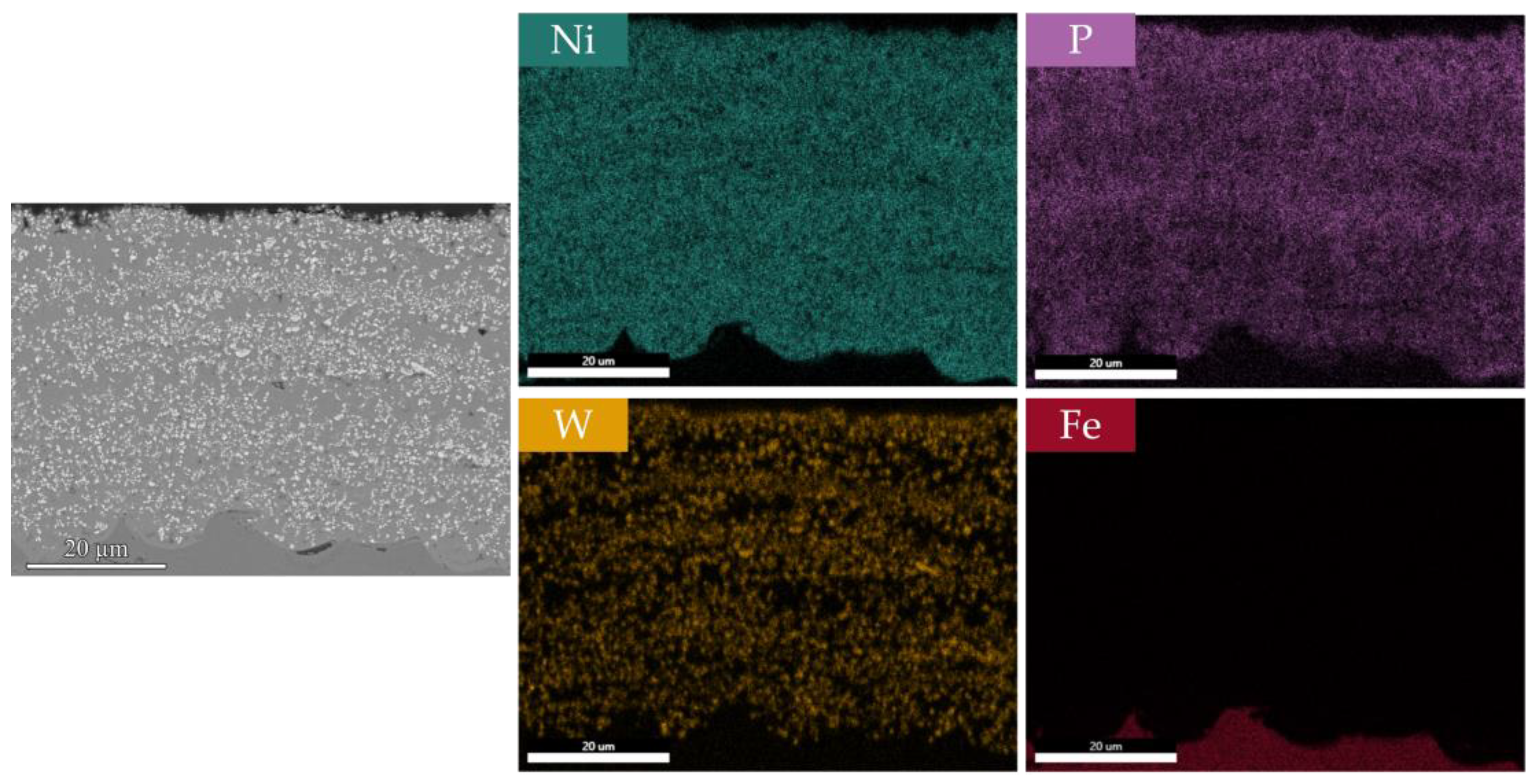
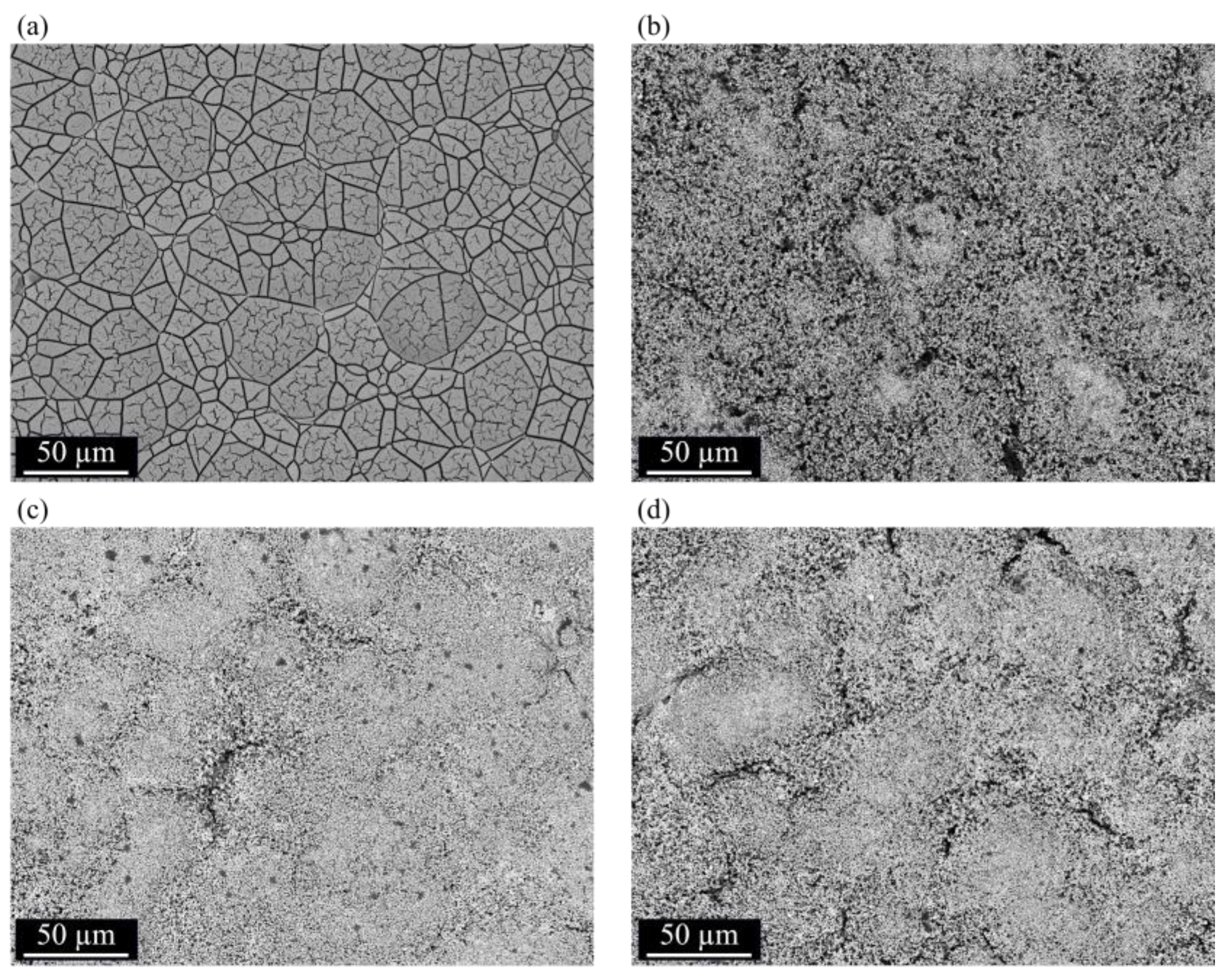
3.2. Surface Morphology, EDS Analysis, and Crystalline Structure
3.3. Thermal Treatments
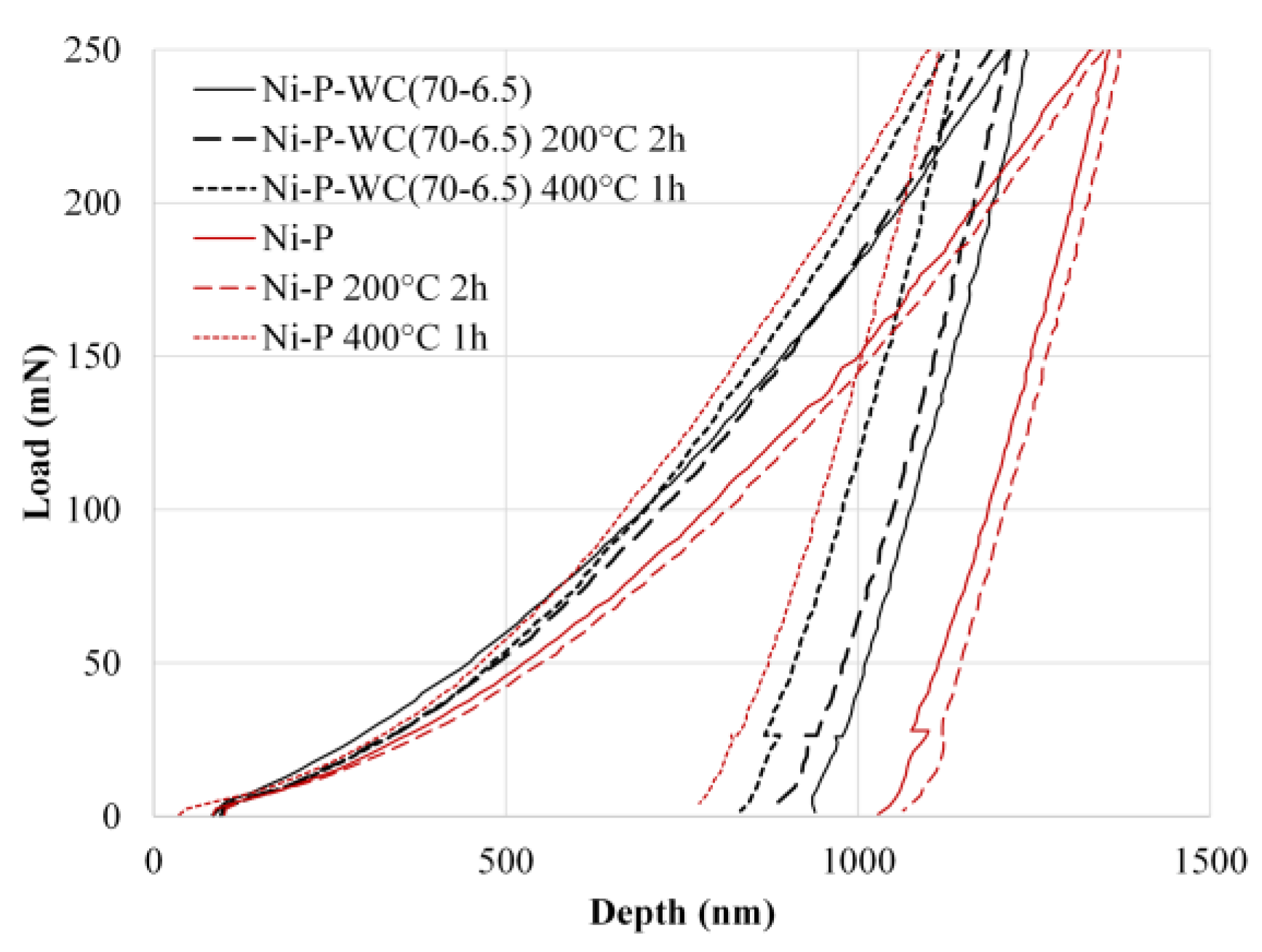
3.4. Instrumented Indentation
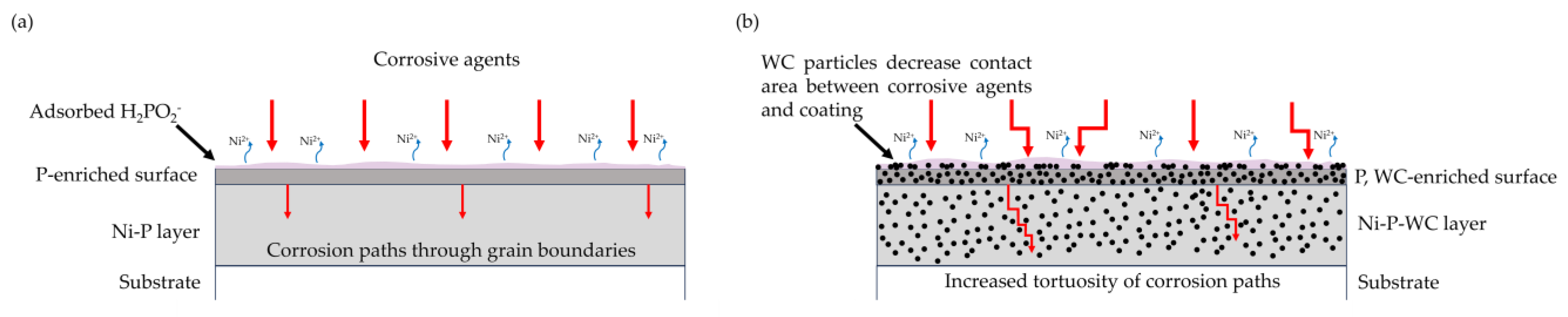
3.5. Potentiodynamic Polarization Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuentes, G.G. Surface Engineering and Micro-Manufacturing. In Micromanufacturing Engineering and Technology, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 459–486. ISBN 9780323312677. [Google Scholar]
- Johansen, R.; Footitt, A.; Le Vedrine, M.; Wakefield, L. Legislative Mapping Chromium (VI); European Environment Agency (EEA): Copenhagen, Denmark, 2020. [Google Scholar]
- European Chemical Agency ECHA to Prepare Restriction Proposal on Chromium (VI) Substances. Available online: https://echa.europa.eu/it/-/echa-to-prepare-restriction-proposal-on-chromium-vi-substances (accessed on 7 June 2024).
- Sharma, P.; Singh, S.P.; Parakh, S.K.; Tong, Y.W. Health Hazards of Hexavalent Chromium (Cr (VI)) and Its Microbial Reduction. Bioengineered 2022, 13, 4923–4938. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless Nickel Plating—A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Lelevic, A.; Walsh, F.C. Electrodeposition of Ni-P Alloy Coatings: A Review. Surf. Coat. Technol. 2019, 369, 198–220. [Google Scholar] [CrossRef]
- Sahoo, P.; Das, S.K. Tribology of Electroless Nickel Coatings—A Review. Mater. Des. 2011, 32, 1760–1775. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Electroless Ni-P and Ni-P-Al2O3 Nanocomposite Coatings and Their Corrosion and Wear Resistance. J. Mater. Eng. Perform. 2013, 22, 176–183. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Abdullah, A.M.; Hassan, M.K.; Mohamed, A.M.; Jarjoura, G.; Farhat, Z. Recent Advances in Electroless-Plated Ni-P and Its Composites for Erosion and Corrosion Applications: A Review. Emergent Mater. 2018, 1, 3–24. [Google Scholar] [CrossRef]
- Narayanan, T.S.N.S.; Seshadri, S.K.; Park, I.S.; Lee, M.H. Electroless Nanocomposite Coatings: Synthesis, Characteristics, and Applications. In Handbook of Nanoelectrochemistry; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 389–416. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990. [Google Scholar]
- Muraliraja, R.; Anthoni Sagaya Selvan, R.; Selvakumar, A.; Franco, M.; Tamilarasan, T.R.; Sanjith, U.; Sha, W.; Sudagar, J. A Review of Electroless Coatings on Non-Metals: Bath Conditions, Properties and Applications. J. Alloys Compd. 2023, 960, 170723. [Google Scholar] [CrossRef]
- Güler, O.; Kocaman, M.; Akçay, S.B.; Çuvalcı, H.; Varol, T. Structure and Tribological Properties of Composites Based on a Novolac Matrix with Ni–P Electrolytic Coating. Surf. Coat. Technol. 2022, 451, 129025. [Google Scholar] [CrossRef]
- Güler, O.; Çelebi, M.; Karabacak, A.H.; Çanakçı, A. The Effect of Advanced Electrodeposited Ni-P-Cu Coatings Obtained with Scrap Cu Plates on the Tribological Performance of Al Powder Metallurgy Products. Surf. Coat. Technol. 2023, 468, 129750. [Google Scholar] [CrossRef]
- Nickel Institute. Nickel Plating Handbook; Nickel Institute: Toronto, ON, Canada, 2022. [Google Scholar]
- Balaraju, J.N.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Structure and Phase Transformation Behaviour of Electroless Ni-P Composite Coatings. Mater. Res. Bull. 2006, 41, 847–860. [Google Scholar] [CrossRef]
- Gould, A.J.; Boden, P.J.; Harris, S.J. Phosphorus Distribution in Electroless Nickel Deposits. Surf. Technol. 1981, 12, 93–102. [Google Scholar] [CrossRef]
- Wang, X.C.; Cai, W.B.; Wang, W.J.; Liu, H.T.; Yu, Z.Z. Effects of Ligands on Electroless Ni-P Alloy Plating from Alkaline Citrate-Ammonia Solution. Surf. Coat. Technol. 2003, 168, 300–306. [Google Scholar] [CrossRef]
- Keong, K.G.; Sha, W.; Malinov, S. Crystallisation Kinetics and Phase Transformation Behaviour of Electroless Nickel-Phosphorus Deposits with High Phosphorus Content. J. Alloys Compd. 2002, 334, 192–199. [Google Scholar] [CrossRef]
- Graham, A.H.; Lindsay, R.W.; Read, H.J. Structure of Electroless Nickel. J. Electrochem. Soc. 1962, 109, 1200. [Google Scholar] [CrossRef]
- Johnson, C.E.; Ogburn, F. Hardness of Heat Treated Electroless Nickel. Surf. Technol. 1976, 4, 161. [Google Scholar] [CrossRef]
- Hadipour, A.; Bahrololoom, M.E.; Monirvaghefi, S.M.; Bahramkia, A.R. Evaluation of Wear Properties for Ni–P Electroless Coatings. Trans. Indian Inst. Met. 2016, 69, 1733–1743. [Google Scholar] [CrossRef]
- Staia, M.H.; Castillo, E.J.; Puchi, E.S.; Lewis, B.; Hintermann, H.E. Wear Performance and Mechanism of Electroless Ni-P Coating. Surf. Coat. Technol. 1996, 86, 598–602. [Google Scholar] [CrossRef]
- Duncan, R.N. The Effect of Solution Age on Corrosion Resistance of Electroless Nickel Deposits. Plat. Surf. Finish. 1996, 83, 64–68. [Google Scholar]
- Park, S.H.; Lee, D.N. A Study on the Microstructure and Phase Transformation of Electroless Nickel Deposits. J. Mater. Sci. 1988, 23, 1643–1654. [Google Scholar] [CrossRef]
- Apachitei, I.; Duszczyk, J. Autocatalytic Nickel Coatings on Aluminium with Improved Abrasive Wear Resistance. Surf. Coat. Technol. 2000, 132, 89–98. [Google Scholar] [CrossRef]
- Bozzini, B.; Martini, C.; Cavallotti, P.L.; Lanzoni, E. Relationships among Crystallographic Structure, Mechanical properties and Tribological Behaviour of Electroless Ni–P (9%)/B4C Films. Wear 1999, 225–229, 806–813. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Paglia, L.; Genova, V.; Conti, M.; Baiamonte, L.; Marra, F. The Effect of Composition and Heat Treatment on Microhardness of Ni-P and Ni-P-NanoZrO2 Coatings. Chem. Eng. Trans. 2023, 100, 433–438. [Google Scholar] [CrossRef]
- Shu, X.; Wang, Y.; Liu, C.; Aljaafari, A.; Gao, W. Double-Layered Ni-P/Ni-P-ZrO2 Electroless Coatings on AZ31 Magnesium Alloy with Improved Corrosion Resistance. Surf. Coat. Technol. 2015, 261, 161–166. [Google Scholar] [CrossRef]
- Sade, W.; Proença, R.T.; Moura, T.D.D.O.; Branco, J.R.T. Electroless Ni-P Coatings: Preparation and Evaluation of Fracture Toughness and Scratch Hardness. ISRN Mater. Sci. 2011, 2011, 1–6. [Google Scholar] [CrossRef]
- Shen, Y.F.; Xue, W.Y.; Liu, Z.Y.; Zuo, L. Nanoscratching Deformation and Fracture Toughness of Electroless Ni-P Coatings. Surf. Coat. Technol. 2010, 205, 632–640. [Google Scholar] [CrossRef]
- Khodaei, M.; Gholizadeh, A.M. SiC Nanoparticles Incorporation in Electroless NiP-Graphene Oxide Nanocomposite Coatings. Ceram. Int. 2021, 47, 25287–25295. [Google Scholar] [CrossRef]
- Chinchu, K.S.; Riyas, A.H.; Ameen Sha, M.; Geethanjali, C.V.; Saji, V.S.; Shibli, S.M.A. ZrO2–CeO2 Assimilated Electroless Ni–P Anti-Corrosion Coatings. Surfaces and Interfaces 2020, 21, 100704. [Google Scholar] [CrossRef]
- Amjad-Iranagh, S.; Zarif, M. TiO2 Nano-Particle Effect on the Chemical and Physical Properties of Ni-P-TiO2 Nanocomposite Electroless Coatings. J. Nanostructures 2020, 10, 415–423. [Google Scholar] [CrossRef]
- Chen, B.; Yan, F.; Guo, J.; Yan, M.F.; Zhang, Y. Attractive Effects of Re on Electroless Ni-P-TiN Nanocomposite Coating. Appl. Surf. Sci. 2021, 565, 150472. [Google Scholar] [CrossRef]
- Shahbazi, H.; Mahdavi, M.; Alirezaei, S.; Tabatabaei, F. Corrosion Behavior of Ni-P-Ag and Ni-P-Al2O3 Composite Coatings Ni-P-Ag and Ni-P-Al2O3 Composite. Mater. Res. Express 2019, 6, 085076. [Google Scholar] [CrossRef]
- Farhan, M.; Fayyaz, O.; Qamar, M.G.; Shakoor, R.A.; Bhadra, J.; Al-Thani, N.J. Mechanical and Corrosion Characteristics of TiC Reinforced Ni-P Based Nanocomposite Coatings. Mater. Today Commun. 2023, 36, 106901. [Google Scholar] [CrossRef]
- Zoikis-Karathanasis, A.; Pavlatou, E.A.; Spyrellis, N. The Effect of Heat Treatment on the Structure and Hardness of Pulse Electrodeposited NiP-WC Composite Coatings. Electrochim. Acta 2009, 54, 2563–2570. [Google Scholar] [CrossRef]
- Hamid, Z.A.; El Badry, S.A.; Aal, A.A. Electroless Deposition and Characterization of Ni-P-WC Composite Alloys. Surf. Coat. Technol. 2007, 201, 5948–5953. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Yu, J.; Huang, H.; Xu, B.H.; Liu, X.L.; Gao, Y.; Dong, X.L. Synthesis and Tribological Behavior of Electroless Ni-P-WC Nanocomposite Coatings. Surf. Coat. Technol. 2007, 201, 7246–7251. [Google Scholar] [CrossRef]
- Luo, H.; Leitch, M.; Behnamian, Y.; Ma, Y.; Zeng, H.; Luo, J.L. Development of Electroless Ni-P/Nano-WC Composite Coatings and Investigation on Its Properties. Surf. Coat. Technol. 2015, 277, 99–106. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wang, Q.Y.; Tang, Y.R.; Guo, H.C.; Xi, Y.C.; Dong, L.J.; Zheng, H. Preparation of NiP-WC Composite Coatings by Ultrasonically Assisted Electroless Plating and Their Characterization. Int. J. Electrochem. Sci. 2020, 15, 11821–11832. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Yao, Y. Preparation and Mechanical Properties of Electroless Nickel-Phosphorus-Tungsten Carbide Nanocomposite Coatings. J. Mater. Eng. Perform. 2014, 23, 193–197. [Google Scholar] [CrossRef]
- Testa, V.; Morelli, S.; Bolelli, G.; Benedetti, B.; Puddu, P.; Sassatelli, P.; Lusvarghi, L. Alternative Metallic Matrices for WC-Based HVOF Coatings. Surf. Coat. Technol. 2020, 402, 126308. [Google Scholar] [CrossRef]
- Conti, M.; Paglia, L.; Genova, V.; Pedrizzetti, G.; Baiamonte, L.; Marra, F. The Effects of Deposition Parameters on the Properties of NiCr Coatings Obtained by Electroless Plating. Chem. Eng. Trans. 2023, 100, 427–432. [Google Scholar] [CrossRef]
- A182/A182M–12a; Standard Specification for Forged or Rolled Alloy and Stainless Steel Pipe Flanges, Forged Fittings, and Valves and Parts for High-Temperature Service. American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2012.
- B183−79; Standard Practice for Preparation of Low-Carbon Steel for Electroplating (Reapproved 2014). American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2014.
- B322−99; Standard Guide for Cleaning Metals Prior to Electroplating (Reapproved 2014). American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2014.
- B 733–97; Specification for Autocatalytic (Electroless) Nickel-Phosphorus Coatings on Metal. American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2017.
- Genova, V.; Paglia, L.; Pulci, G.; Pedrizzetti, G.; Pranzetti, A.; Romanelli, M.; Marra, F. Medium and High Phosphorous Ni-P Coatings Obtained via an Electroless Approach: Optimization of Solution Formulation and Characterization of Coatings. Coatings 2023, 13, 1490. [Google Scholar] [CrossRef]
- Wang, J.; Bai, X.; Shen, X.; Liu, X.; Wang, B. Effect of Micro-Texture on Substrate Surface on Adhesion Performance of Electroless Ni–P Coating. J. Manuf. Process. 2022, 74, 296–307. [Google Scholar] [CrossRef]
- Baskaran, I.; Sankara Narayanan, T.S.N.; Stephen, A. Effect of Accelerators and Stabilizers on the Formation and Characteristics of Electroless Ni-P Deposits. Mater. Chem. Phys. 2006, 99, 117–126. [Google Scholar] [CrossRef]
- Wu, W.; Liu, J.; Miao, N.; Jiang, J.; Zhang, Y.; Zhang, L.; Yuan, N.; Wang, Q.; Tang, L. Influence of Thiourea on Electroless Ni–P Films Deposited on Silicon Substrates. J. Mater. Sci. Mater. Electron. 2019, 30, 7717–7724. [Google Scholar] [CrossRef]
- Park, I.C.; Kim, S.J. Effect of Lead Nitrate Concentration on Electroless Nickel Plating Characteristics of Gray Cast Iron. Surf. Coat. Technol. 2019, 376, 2–7. [Google Scholar] [CrossRef]
- ISO 21920-2; Geometrical Product Specifications (GPS)-Surface Texture: Profile-Part 2:Terms, Definitions and Surface Texture. ISO: Geneva, Switzerland, 2021.
- Apachitei, I.; Tichelaar, F.D.; Duszczyk, J.; Katgerman, L. The Effect of Heat Treatment on the Structure and Abrasive Wear Resistance of Autocatalytic NiP and NiP-SiC Coatings. Surf. Coat. Technol. 2002, 149, 263–278. [Google Scholar] [CrossRef]
- Pedrizzetti, G.; Paglia, L.; Genova, V.; Cinotti, S.; Bellacci, M.; Marra, F.; Pulci, G. Microstructural, Mechanical and Corrosion Characterization of Electroless Ni-P Composite Coatings Modified with ZrO2 Reinforcing Nanoparticles. Surf. Coat. Technol. 2023, 473, 129981. [Google Scholar] [CrossRef]
- Sahagian, D.L.; Proussevitch, A.A. 3D Particle Size Distributions from 2D Observations: Stereology for Natural Applications. J. Volcanol. Geotherm. Res. 1998, 84, 173–196. [Google Scholar] [CrossRef]
- B689−97; Standard Specification for Electroplated Engineering Nickel Coatings (Reapproved 2023). American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2023.
- Zhou, Q.J.; Qiao, L.J.; Qi, H.B.; Li, J.X.; He, J.Y.; Chu, W.Y. Hydrogen Blistering and Hydrogen-Induced Cracking in Amorphous Nickel Phosphorus Coating. J. Non-Cryst. Solids 2007, 353, 4011–4014. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, H.J.; Chen, Z.L. Study on Post-Treatments for Electroless Ni-P Coating. Surf. Eng. 2011, 27, 57–60. [Google Scholar] [CrossRef]
- Krishnan, K.H.; John, S.; Srinivasan, K.N.; Praveen, J.; Ganesan, M.; Kavimani, P.M. An Overall Aspect of Electroless Ni-P Depositions—A Review Article. Metall. Mater. Trans. A 2006, 37, 1917–1926. [Google Scholar] [CrossRef]
- E384–11; Test Method for Knoop and Vickers Hardness of Materials. American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2012.
- ISO STANDARD 14577-4:2016; Metallic Materials—Instrumented Test for Hardness and materials Parameters—Part 4: Test Method for Metallic and Nonmetallic Coatings. ISO: Geneva, Switzerland, 2016.
- Oliver, W.C.; Pharr, G.M. Measurement of Hardness and Elastic Modulus by Instrumented Indentation: Advances in Understanding and Refinements to Methodology. J. Mat. Res. 2004, 19, 3–20. [Google Scholar] [CrossRef]
- Genova, V.; Paglia, L.; Marra, F.; Bartuli, C.; Pulci, G. Pure Thick Nickel Coating Obtained by Electroless Plating: Surface Characterization and Wetting Properties. Surf. Coat. Technol. 2019, 357, 595–603. [Google Scholar] [CrossRef]
- Hamada, A.S.; Sahu, P.; Porter, D.A. Indentation Property and Corrosion Resistance of Electroless Nickel-Phosphorus Coatings Deposited on Austenitic High-Mn TWIP Steel. Appl. Surf. Sci. 2015, 356, 1–8. [Google Scholar] [CrossRef]
- ASTM G59–97; Standard Test Method for Conducting Potentiodynamic Polarization Resistance Measurements. American Society for Testing and Materials Designation: West Conshohocken, PA, USA, 2014.
- Alhamad, R.K.; Hussein, A.K.; Abbas, L.K. Improved Acrylonitrile-Butadiene-Styrene Surface Properties via Electroless (NiP-SiC) Nanocomposite Coating. Chem. Phys. Impact 2023, 7, 100375. [Google Scholar] [CrossRef]
- Ghavidel, N.; Allahkaram, S.R.; Naderi, R.; Barzegar, M.; Bakhshandeh, H. Corrosion and Wear Behavior of an Electroless Ni-P/Nano-SiC Coating on AZ31 Mg Alloy Obtained through Environmentally-Friendly Conversion Coating. Surf. Coat. Technol. 2020, 382, 125156. [Google Scholar] [CrossRef]
- Sheng, M.; Wang, Y.; Zhong, Q.; Wu, H.; Zhou, Q.; Lin, H. The Effects of Nano-SiO2 Additive on the Zinc Phosphating of Carbon Steel. Surf. Coat. Technol. 2011, 205, 3455–3460. [Google Scholar] [CrossRef]
- Czapczyk, K.; Zawadzki, P.; Wierzbicka, N.; Talar, R. Microstructure and Properties of Electroless Ni-p/Si3n4 Nanocomposite Coatings Deposited on the Aw-7075 Aluminum Alloy. Materials 2021, 14, 4487. [Google Scholar] [CrossRef]
- Sarret, M.; Müller, C.; Amell, A. Electroless NiP Micro- and Nano-Composite Coatings. Surf. Coat. Technol. 2006, 201, 389–395. [Google Scholar] [CrossRef]
- Abdel Gawad, S.; Baraka, A.; Morsi, M.; Ali Eltoum, M. Development of Electroless Ni-P-Al2O3 and Ni-P-TiO2 Composite Coatings from Alkaline Hypophosphite Gluconate Baths and Their Properties. Int. J. Electrochem. Sci. 2013, 8, 1722–1734. [Google Scholar] [CrossRef]
- He, Y.; Zhang, S.; He, Y.; Song, R.; Zhang, Z.; Liu, B.; Li, H.; Shangguan, J. Effects of Yttrium-Stabilized Zirconia (Different Yttrium Content) Doping on the Structure, Corrosion Resistance and Wear Resistance of Ni-P Electroless Coating. Colloids Surf. A Physicochem. Eng. Asp. 2022, 654, 130059. [Google Scholar] [CrossRef]
- Fayyad, E.M.; Hassan, M.K.; Rasool, K.; Mahmoud, K.A.; Mohamed, A.M.A.; Jarjoura, G.; Farhat, Z.; Abdullah, A.M. Novel Electroless Deposited Corrosion—Resistant and Anti-Bacterial NiP–TiNi Nanocomposite Coatings. Surf. Coat. Technol. 2019, 369, 323–333. [Google Scholar] [CrossRef]
- Grosjean, A.; Rezrazi, M.; Bercot, P.; Tachez, M. Adaptation of a Mathematical Model to the Incorporation of Silicon Carbide Particles in an Electroless Nickel Deposit. Met. Finish. 1998, 96, 14–17. [Google Scholar] [CrossRef]
- Ger, M.-D.; Hwang, B.J. Effect of Surfactants on Codeposition of PTFE Particles with Electroless Ni-P Coating. Mater. Chem. Phys. 2002, 76, 38–45. [Google Scholar] [CrossRef]
- Chen, W.-W.; Gao, W. Microstructures and Properties of Sol-Enhanced Nanostructured Metal-Oxide Composite Coatings. Progress Nat. Sci. Mater. Int. 2011, 21, 355–362. [Google Scholar] [CrossRef]
- Cheong, W.J.; Luan, B.L.; Shoesmith, D.W. The Effects of Stabilizers on the Bath Stability of Electroless Ni Deposition and the Deposit. Appl. Surf. Sci. 2004, 229, 282–300. [Google Scholar] [CrossRef]
- Medeliene, V. The Influence of B4C and SiC Additions on the Morphological, Physical, Chemical and Corrosion Properties of Ni Coatings. Surf. Coat. Technol. 2002, 154, 104–111. [Google Scholar] [CrossRef]
- Pinate, S.; Ghassemali, E.; Zanella, C. Strengthening Mechanisms and Wear Behavior of Electrodeposited Ni–SiC Nanocomposite Coatings. J. Mater. Sci. 2022, 57, 16632–16648. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Consideration of Orowan Strengthening Effect in Particulate-Reinforced Metal Matrix Nanocomposites: A Model for Predicting Their Yield Strength. Scr. Mater. 2006, 54, 1321–1326. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.L. Contribution of Orowan Strengthening Effect in Particulate-Reinforced Metal Matrix Nanocomposites. Mater. Sci. Eng. A 2008, 483–484, 148–152. [Google Scholar] [CrossRef]
- Kumaraguru, S.; Kumar, G.G.; Raghu, S.; Gnanamuthu, R.M. Fabrication of Ternary Ni-TiO2-TiC Composite Coatings and Their Enhanced Microhardness for Metal Finishing Application. Appl. Surf. Sci. 2018, 447, 463–470. [Google Scholar] [CrossRef]
- Gay, P.A.; Limat, J.M.; Steinmann, P.A.; Pagetti, J. Characterisation and Mechanical Properties of Electroless NiP-ZrO2 Coatings. Surf. Coat. Technol. 2007, 202, 1167–1171. [Google Scholar] [CrossRef]
- Malfatti, C.F.; Veit, H.M.; Menezes, T.L.; Zoppas Ferreira, J.; Rodriguês, J.S.; Bonino, J.P. The Surfactant Addition Effect in the Elaboration of Electrodepositated NiP-SiC Composite Coatings. Surf. Coat. Technol. 2007, 201, 6318–6324. [Google Scholar] [CrossRef]
- Nwoko, V.O.; Shreir, L.L. Electron Micrographic Examination of Electrodeposited Dispersion-Hardened Nickel. J. Appl. Electrochem. 1973, 3, 137–141. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Sankara Narayanan, T.S.N.; Seshadri, S.K. Electroless Ni-P Composite Coatings. J. Appl. Electrochem. 2003, 33, 807–816. [Google Scholar] [CrossRef]
- Bakhit, B.; Akbari, A. Effect of Particle Size and Co-Deposition Technique on Hardness and Corrosion Properties of Ni-Co/SiC Composite Coatings. Surf. Coat. Technol. 2012, 206, 4964–4975. [Google Scholar] [CrossRef]
- Niihara, K.; Nakihara, A. Strengthening and Toughening Mechanisms in Nanocomposite Ceramics. Ann. Chim. Fr. 1991, 16, 479–486. [Google Scholar]
- Elsener, B.; Crobu, M.; Scorciapino, M.A.; Rossi, A. Electroless Deposited Ni-P Alloys: Corrosion Resistance Mechanism. J. Appl. Electrochem. 2008, 38, 1053–1060. [Google Scholar] [CrossRef]
- Chintada, V.B.; Gurugubelli, T.R.; Koutavarapu, R. The Impact of Surfactants on the Properties of Electroless Ni–P–SiC Coatings. Mater. Chem. Phys. 2022, 291, 126682. [Google Scholar] [CrossRef]
- Sadreddini, S.; Salehi, Z.; Rassaie, H. Characterization of Ni-P-SiO2 Nano-Composite Coating on Magnesium. Appl. Surf. Sci. 2015, 324, 393–398. [Google Scholar] [CrossRef]
- Alirezaei, S.; Monirvaghefi, S.M.; Salehi, M.; Saatchi, A. Effect of Alumina Content on Surface Morphology and Hardness of Ni-P-Al2O3(α) Electroless Composite Coatings. Surf. Coat. Technol. 2004, 184, 170–175. [Google Scholar] [CrossRef]
- Ma, C.; Wu, F.; Ning, Y.; Xia, F.; Liu, Y. Effect of Heat Treatment on Structures and Corrosion Characteristics of Electroless Ni-P-SiC Nanocomposite Coatings. Ceram. Int. 2014, 40, 9279–9284. [Google Scholar] [CrossRef]
- Franco, M.; Sha, W.; Malinov, S.; Rajendran, R. Phase Composition, Microstructure and Microhardness of Electroless Nickel Composite Coating Co-Deposited with SiC on Cast Aluminium LM24 Alloy Substrate. Surf. Coat. Technol. 2013, 235, 755–763. [Google Scholar] [CrossRef]
- Sudagar, J.; Lian, J.; Sha, W. Electroless Nickel, Alloy, Composite and Nano Coatings—A Critical Review. J. Alloys Compd. 2013, 571, 183–204. [Google Scholar] [CrossRef]
- Islam, M.; Azhar, M.R.; Fredj, N.; Burleigh, T.D.; Oloyede, O.R.; Almajid, A.A.; Ismat Shah, S. Influence of SiO2 Nanoparticles on Hardness and Corrosion Resistance of Electroless Ni-P Coatings. Surf. Coat. Technol. 2015, 261, 141–148. [Google Scholar] [CrossRef]
- Shibli, S.M.A.; Chinchu, K.S.; Sha, M.A. Development of Nano-Tetragonal Zirconia-Incorporated Ni–P Coatings for High Corrosion Resistance. Acta Metall. Sin. Engl. Lett. 2019, 32, 481–494. [Google Scholar] [CrossRef]
- Djokić, S.S.; Djokić, N.S. Electroless Deposition of Metallic Powders. J. Electrochem. Soc. 2011, 158, D204. [Google Scholar] [CrossRef]
- Sliem, M.H.; Shahzad, K.; Sivaprasad, V.N.; Shakoor, R.A.; Abdullah, A.M.; Fayyaz, O.; Kahraman, R.; Umer, M.A. Enhanced Mechanical and Corrosion Protection Properties of Pulse Electrodeposited NiP-ZrO2 Nanocomposite Coatings. Surf. Coat. Technol. 2020, 403, 126340. [Google Scholar] [CrossRef]
- Liu, Z.W.; Sun, C.; Gauvin, R.; Wu, W.; Zeng, Y.; Demers, H. High Spatial Resolution EDS Mapping of Nanoparticles at Low Accelerating Voltage. J. Test. Eval. 2016, 44, 2285–2292. [Google Scholar] [CrossRef]
- Williams, D.B.; Michael, R.; Goldstem, I.; Romlg, A.D. Definition of the Spatial Resolution of X-Ray Microanalysis in Thin Foils. Ultramicroscopy 1992, 47, 121–132. [Google Scholar] [CrossRef]
- Agarwala, R.C.; Agarwala, V. Electroless Alloy/Composite Coatings: A Review. Sadhana 2003, 28, 475–493. [Google Scholar] [CrossRef]
- Samanta, S.; Vishwanath, K.; Mondal, K.; Dutta, M.; Singh, S.B. Electroless Amorphous NiP Coatings Over API X70 Steel: Resistance to Wear and Hydrogen Embrittlement. Met. Mater. Int. 2022, 28, 397–411. [Google Scholar] [CrossRef]
- He, Y.; Sun, W.T.; Wang, S.C.; Reed, P.A.S.; Walsh, F.C. An Electrodeposited Ni-P-WS2 Coating with Combined Super-Hydrophobicity and Self-Lubricating Properties. Electrochim. Acta 2017, 245, 872–882. [Google Scholar] [CrossRef]
- Dhakal, D.R.; Kshetri, Y.K.; Gyawali, G.; Kim, T.H.; Choi, J.H.; Lee, S.W. Understanding the Effect of Si3N4 Nanoparticles on Wear Resistance Behavior of Electroless Nickel-Phosphorus Coating through Structural Investigation. Appl. Surf. Sci. 2021, 541, 148403. [Google Scholar] [CrossRef]
- Van Swygenhoven, H.; Caro, A. Plastic Behavior of Nanophase Ni: A Molecular Dynamics Computer Simulation. Appl. Phys. Lett. 1997, 71, 1652–1654. [Google Scholar] [CrossRef]
- Schiotz, J.; Vegge, T.; Di Tolla, F.D.; Jacobsen, K.W. Atomic-Scale Simulations of the Mechanical Deformation of Nanocrystalline Metals. Phisical Rev. B 1999, 60, 11971–11983. [Google Scholar] [CrossRef]
- Nemane, V.; Chatterjee, S. Nanomechanical, Tribological, and Scratch Properties of Electroless Ni-B-W Alloy and Ni-B-W-SiC Composite Coatings. J. Tribol. 2022, 144, 051402. [Google Scholar] [CrossRef]
- Ahmadkhaniha, D.; Eriksson, F.; Zanella, C. Optimizing Heat Treatment for Electroplated Nip and NiP/SiC Coatings. Coatings 2020, 10, 1179. [Google Scholar] [CrossRef]
- Leyland, A.; Matthews, A. On the Significance of the H/E Ratio in Wear Control: A Nanocomposite Coating Approach to Optimised Tribological Behaviour. Wear 2000, 246, 1–11. [Google Scholar] [CrossRef]
- Bai, A.; Chuang, P.Y.; Hu, C.C. The Corrosion Behavior of Ni-P Deposits with High Phosphorous Contents in Brine Media. Mater. Chem. Phys. 2003, 82, 93–100. [Google Scholar] [CrossRef]
- Vitry, V.; Sens, A.; Kanta, A.F.; Delaunois, F. Wear and Corrosion Resistance of Heat Treated and As-Plated Duplex NiP/NiB Coatings on 2024 Aluminum Alloys. Surf. Coat. Technol. 2012, 206, 3421–3427. [Google Scholar] [CrossRef]
- Khodaei, M.; Gholizadeh, A.M. Surfactant-Free Commercial Electroless Bath with Low Concentration of SiC Nanoparticles to Prepare the NiP-SiC Nanocomposite Coatings. Mater. Res. Express 2021, 8, 055009. [Google Scholar] [CrossRef]
- Bigdeli, F.; Allahkaram, S.R. An Investigation on Corrosion Resistance of As-Applied and Heat Treated Ni-P/NanoSiC Coatings. Mater. Des. 2009, 30, 4450–4453. [Google Scholar] [CrossRef]
- Rabizadeh, T.; Allahkaram, S.R.; Zarebidaki, A. An Investigation on Effects of Heat Treatment on Corrosion Properties of Ni-P Electroless Nano-Coatings. Mater. Des. 2010, 31, 3174–3179. [Google Scholar] [CrossRef]
- Balaraju, J.N.; Selvi, V.E.; Grips, V.K.W.; Rajam, K.S. Electrochemical Studies on Electroless Ternary and Quaternary Ni-P Based Alloys. Electrochim. Acta 2006, 52, 1064–1074. [Google Scholar] [CrossRef]

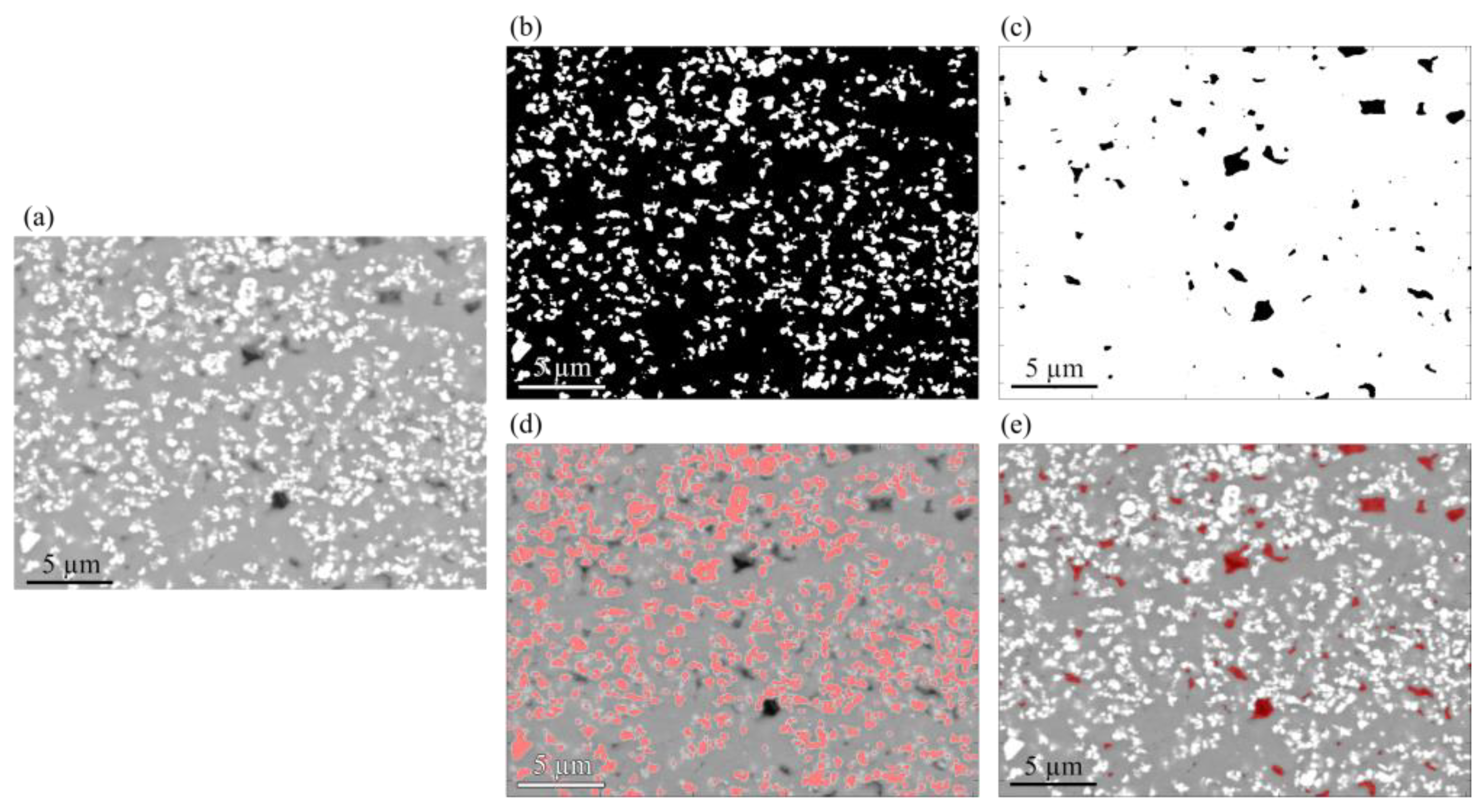
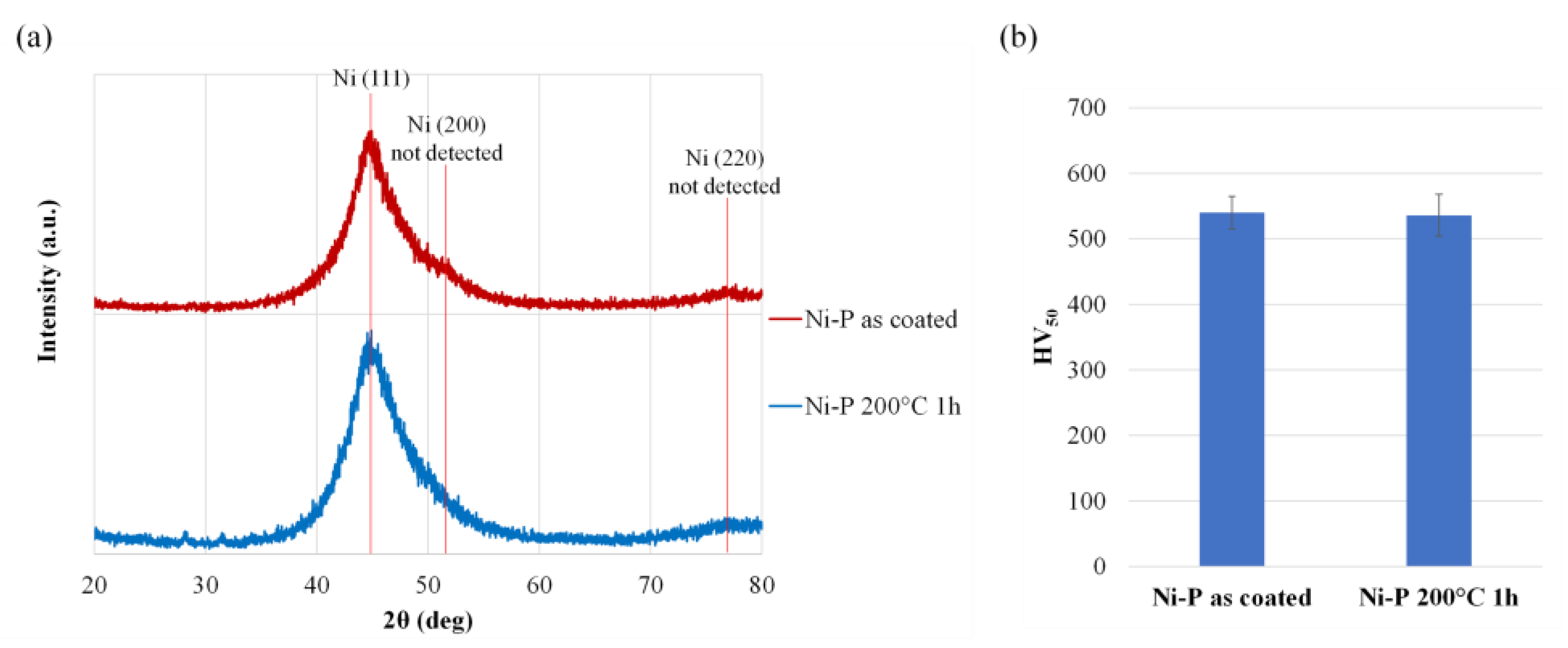
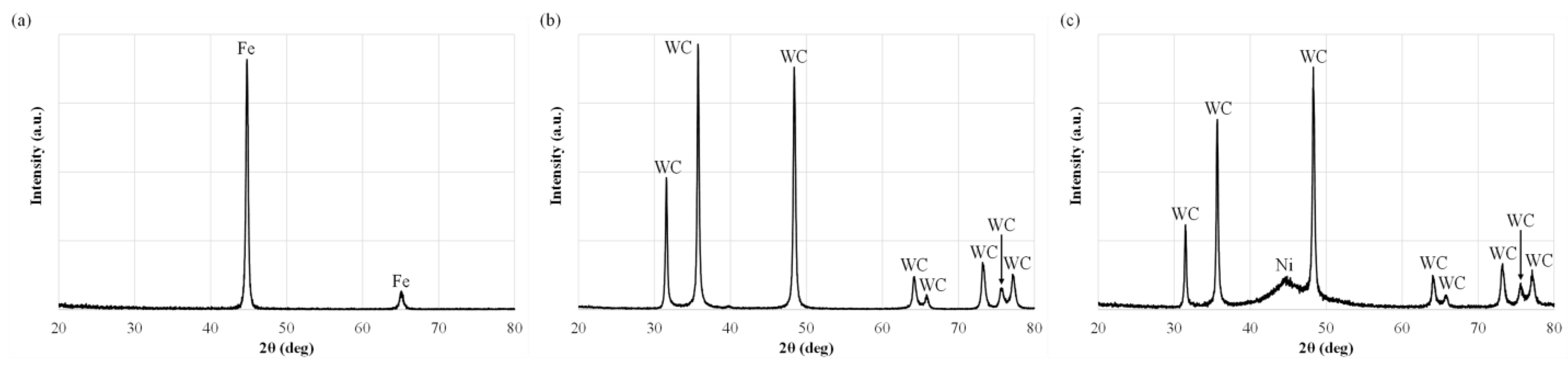
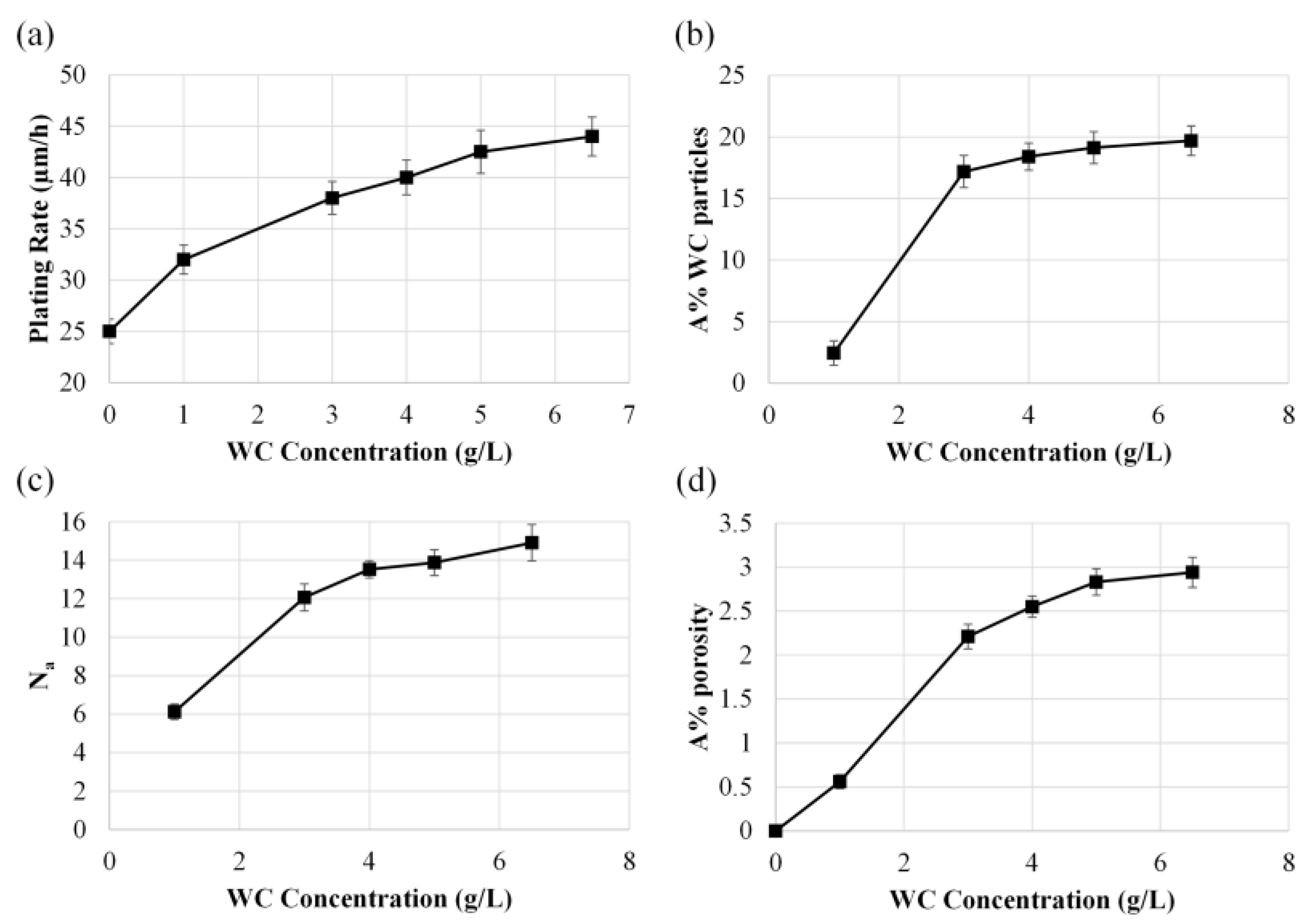








| Compound | Concentration (g/L) |
|---|---|
| Sodium hypophosphite monohydrate | 110 |
| Sodium acetate | 20 |
| Citric acid | 9 |
| Nickel sulphate hexahydrate | 25 |
| Thiourea | 8.5 ppm a |
| Temperature (°C) | WC Concentration (g/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 90 | 0 | 1 | 3 | 4 | 5 | 6.5 | |||
| 85 | 3 | 4 | 5 | 6.5 | |||||
| 80 | 3 | ||||||||
| 75 | 3 | 10 | 15 | ||||||
| 70 | 3 | 4 | 5 | 6.5 | 10 | 15 | 20 | ||
| Ni Crystallite Size (nm) | Ni3P Crystallite Size (nm) | |
|---|---|---|
| Ni-P 400 °C 1 h | 41.5 | 64.6 |
| Ni-P-WC(70-6.5) 400 °C 1 h | 41.4 | 56.5 |
| Hardness (GPa) | Young’s Modulus (GPa) | H/E Ratio | |
|---|---|---|---|
| Ni-P | 6.20 ± 0.27 | 136.9 ± 5.36 | 0.0453 ± 0.027 |
| Ni-P 200 °C 2 h | 6.93 ± 0.24 | 148.8 ± 7.2 | 0.0466 ± 0.0028 |
| Ni-P 400 °C 1 h | 9.35 ± 0.54 | 180.4 ± 10.3 | 0.0518 ± 0.0042 |
| Ni-P-WC(70-6.5) | 7.56 ± 0.44 | 153.7 ± 4.57 | 0.0492 ± 0.0032 |
| Ni-P-WC(70-6.5) 200 °C 2 h | 8.22 ± 0.35 | 162.2 ± 6.4 | 0.0507 ± 0.0029 |
| Ni-P-WC(70-6.5) 400 °C 1 h | 9.77 ± 0.35 | 181.2 ± 7.1 | 0.0539 ± 0.0029 |
| Ecorr (mV vs. SCE) | Icorr (µA/cm2) | βa (mV/Decade) | βc (mV/Decade) | Rp (kΩ ⋅ cm2) | |
|---|---|---|---|---|---|
| Ni-P | −436.45 | 7.061 | 200.05 | −200.08 | 6.151 |
| Ni-P+WC | −332.95 | 6.509 | 92.90 | −196.12 | 4.205 |
| Ni-P-WC 200 °C 2 h | −235.16 | 1.942 | 171.363 | −208.63 | 21.037 |
| Ni-P-WC 400 °C 1 h | −195.99 | 0.976 | 171.436 | −175.104 | 38.523 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrizzetti, G.; Genova, V.; Bellacci, M.; Scrinzi, E.; Brotzu, A.; Marra, F.; Pulci, G. Influence of Deposition Temperature and WC Concentration on the Microstructure of Electroless Ni-P-WC Nanocomposite Coatings with Improved Hardness and Corrosion Resistance. Coatings 2024, 14, 826. https://doi.org/10.3390/coatings14070826
Pedrizzetti G, Genova V, Bellacci M, Scrinzi E, Brotzu A, Marra F, Pulci G. Influence of Deposition Temperature and WC Concentration on the Microstructure of Electroless Ni-P-WC Nanocomposite Coatings with Improved Hardness and Corrosion Resistance. Coatings. 2024; 14(7):826. https://doi.org/10.3390/coatings14070826
Chicago/Turabian StylePedrizzetti, Giulia, Virgilio Genova, Michelangelo Bellacci, Erica Scrinzi, Andrea Brotzu, Francesco Marra, and Giovanni Pulci. 2024. "Influence of Deposition Temperature and WC Concentration on the Microstructure of Electroless Ni-P-WC Nanocomposite Coatings with Improved Hardness and Corrosion Resistance" Coatings 14, no. 7: 826. https://doi.org/10.3390/coatings14070826





