Eco-Friendly Synthesis of Al2O3 Nanoparticles: Comprehensive Characterization Properties, Mechanics, and Photocatalytic Dye Adsorption Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extract
2.3. Synthesis of Al2O3 NPs
2.4. The Prepared Al2O3 NPs for Mechanical Properties
2.5. Characterization
2.6. Mechanical Properties of the Prepared Al2O3 NPs
2.7. Photocatalytic Degradation of RB and MB
3. Result and Discussion
3.1. UV–Vis Spectroscopy
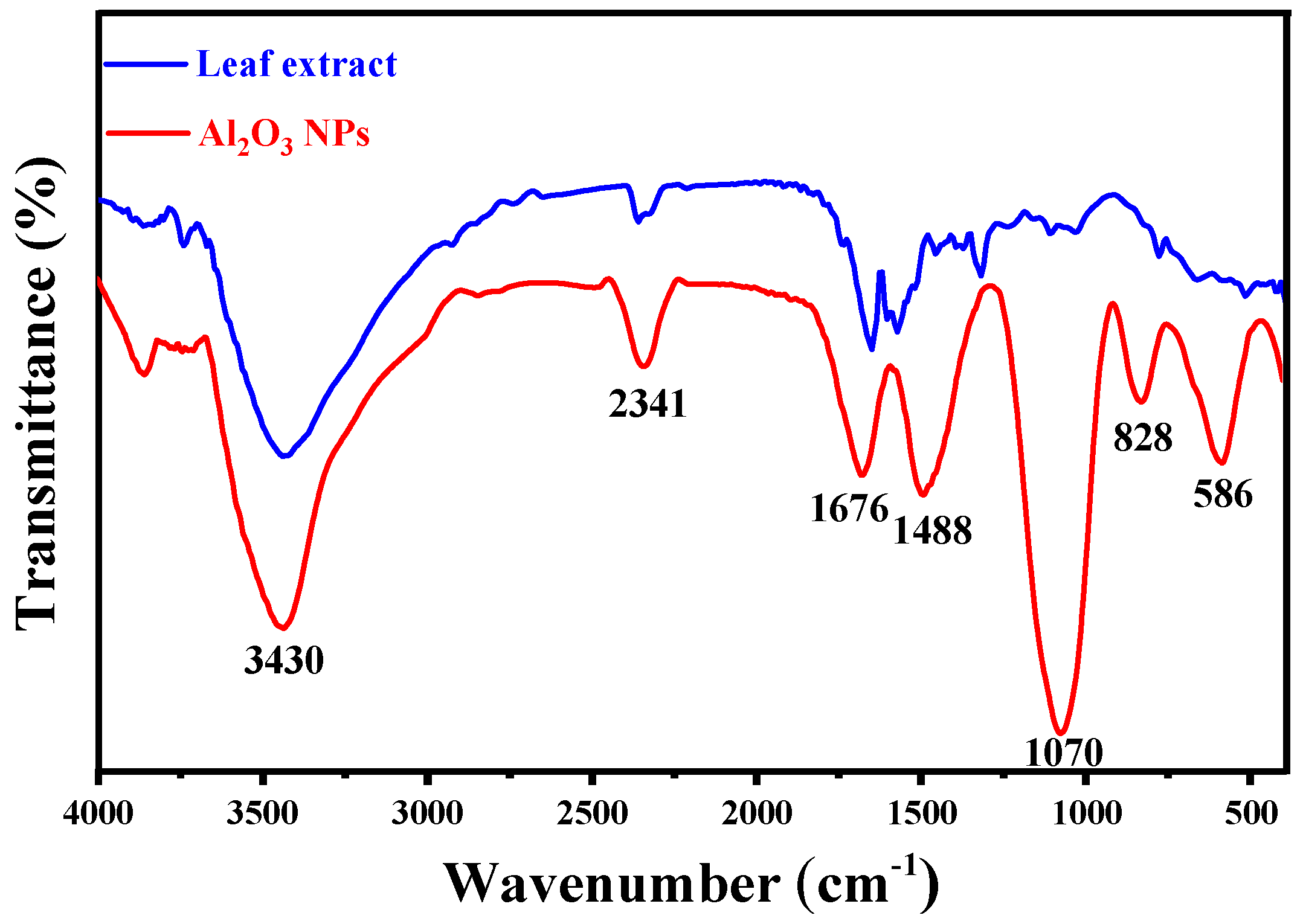
3.2. FTIR Spectroscopy Analysis
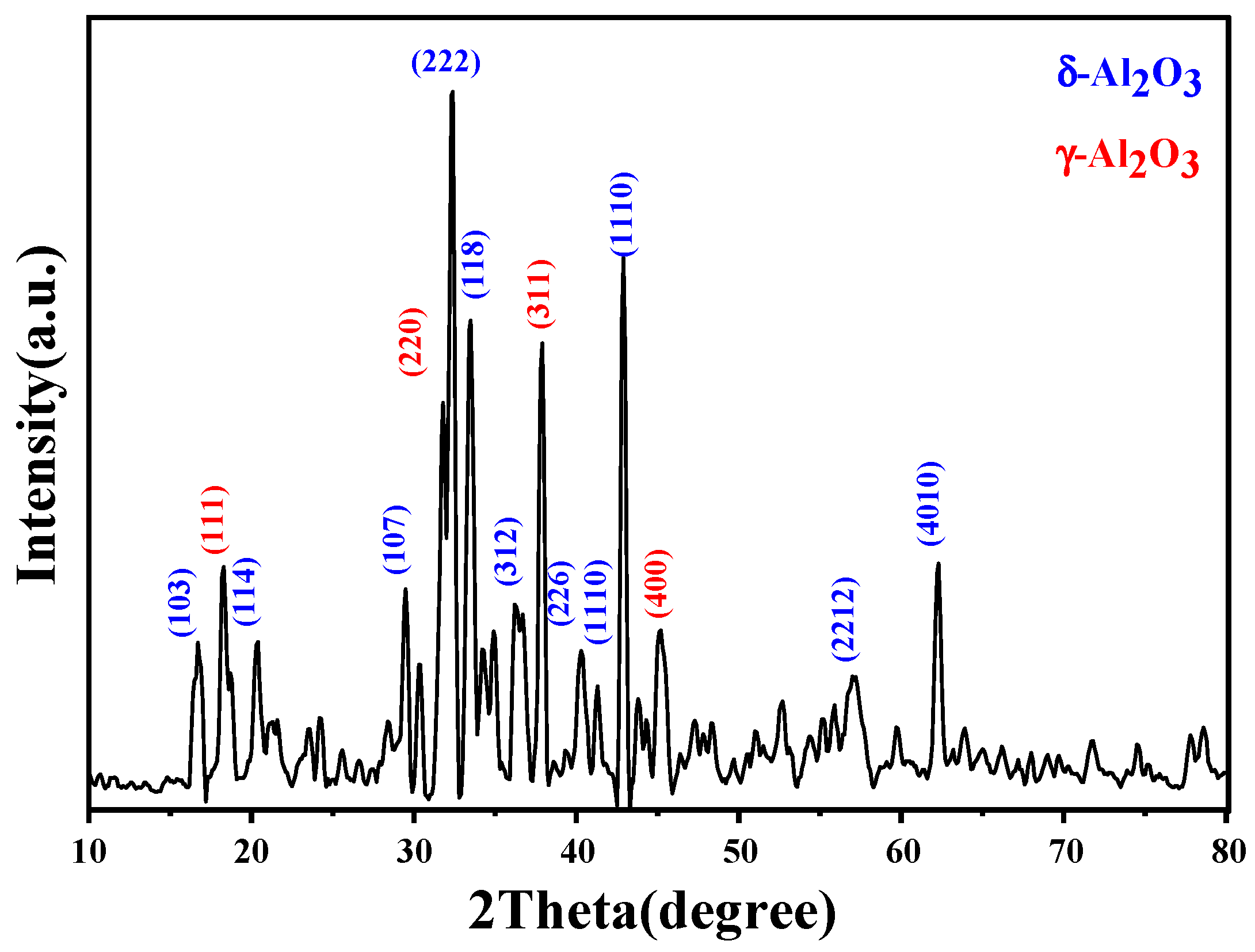
3.3. X-ray Diffraction
3.4. Morphological and Elemental Analysis
3.5. Mechanical Properties of the Prepared Al2O3 NPs
3.5.1. Flexion Test
3.5.2. Compression Test
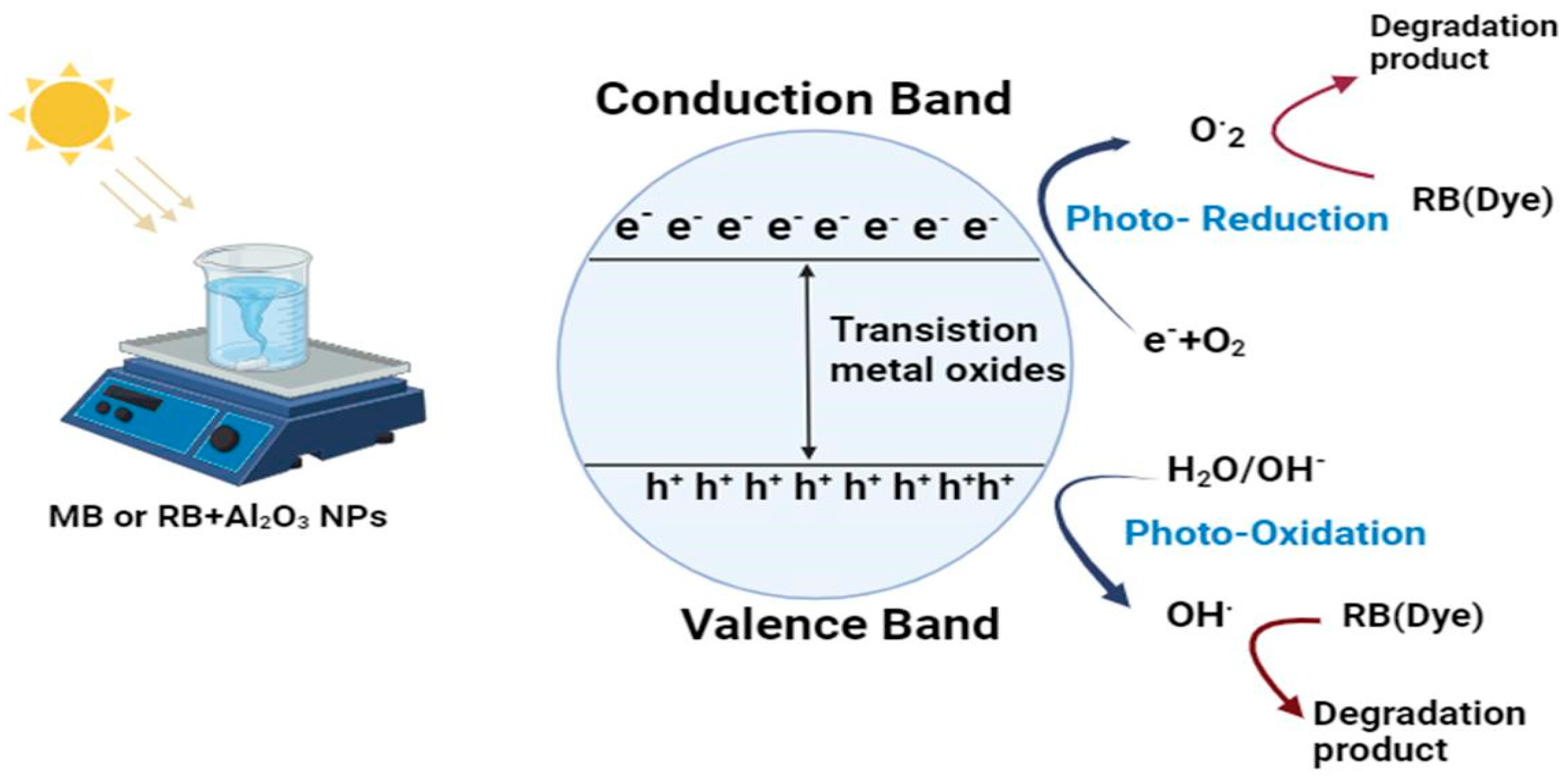
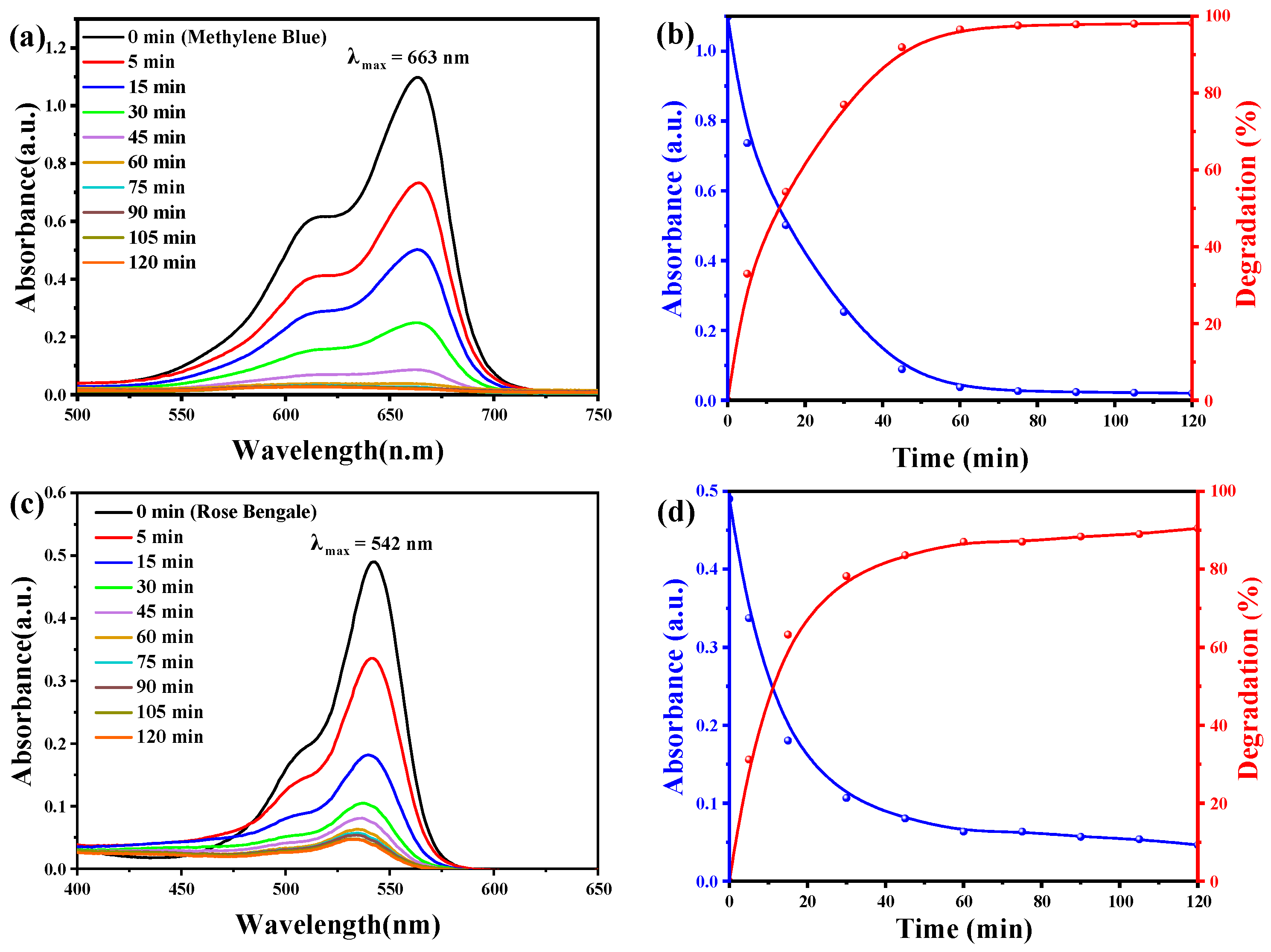
3.6. Photocatalytic Degradation of Methylene Blue and Rose Bengal
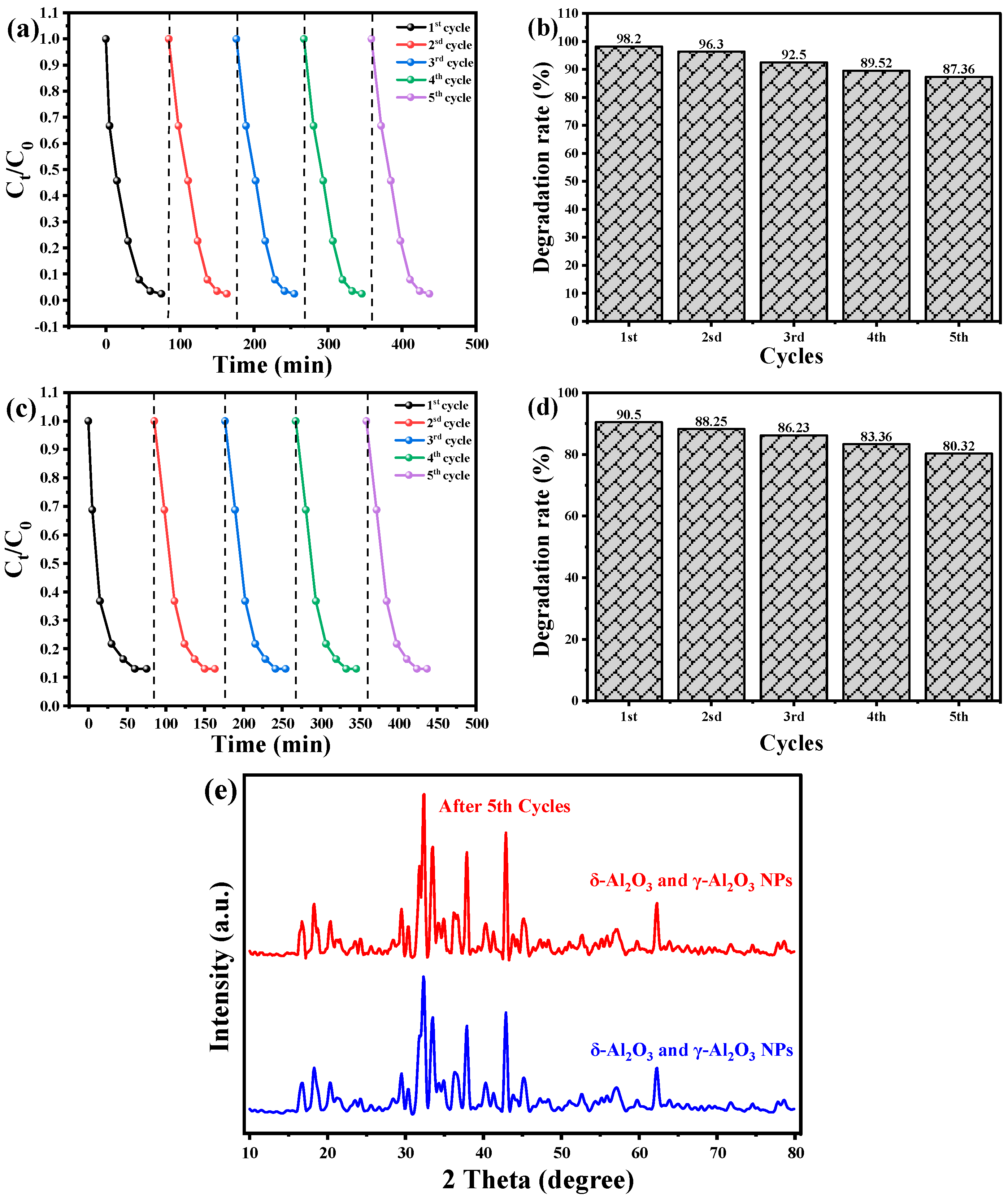
3.7. Recyclability and Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- del Carmen Gómez-Regalado, M.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E.; Zafra-Gómez, A. Bioaccumulation/bioconcentration of pharmaceutical active compounds in aquatic organisms: Assessment and factors database. Sci. Total Environ. 2023, 861, 160638. [Google Scholar] [CrossRef] [PubMed]
- Abba, E.; Shehu, Z.; Haruna, R.M. Green synthesis and characterization of CuO@SiO2 nanocomposite using gum Arabic (Acacia senegalensis) (L) against malaria vectors. Trends Sci. 2021, 18, 28. [Google Scholar] [CrossRef]
- Mishra, R.K.; Mentha, S.S.; Misra, Y.; Dwivedi, N. Emerging pollutants of severe environmental concern in water and wastewater: A comprehensive review on current developments and future research. Water-Energy Nexus 2023, 6, 74–95. [Google Scholar] [CrossRef]
- Bao, J.; Guo, S.; Fan, D.; Cheng, J.; Zhang, Y.; Pang, X. Sonoactivated Nanomaterials: A potent armament for wastewater treatment. Ultrason. Sonochemistry 2023, 99, 106569. [Google Scholar] [CrossRef] [PubMed]
- Zouari Ahmed, R.; Laouini, S.E.; Salmi, C.; Bouafia, A.; Meneceur, S.; Mohammed, H.A.; Chihi, S.; Alharthi, F.; Abdullah, J.A.A. Green synthesis of α-Fe2O3 and α-Fe2O3@Ag NC for degradation of rose Bengal and antimicrobial activity. Biomass Convers. Biorefinery 2023. [CrossRef]
- Koul, B.; Yadav, D.; Singh, S.; Kumar, M.; Song, M. Insights into the domestic wastewater treatment (DWWT) regimes: A review. Water 2022, 14, 3542. [Google Scholar] [CrossRef]
- Jiang, Z.; Cheng, B.; Zhang, L.; Zhang, Z.; Bie, C. A review on ZnO-based S-scheme heterojunction photocatalysts. Chin. J. Catal. 2023, 52, 32–49. [Google Scholar] [CrossRef]
- Sangor, F.I.; Al-Ghouti, M.A. Waste-to-value: Synthesis of nano-aluminum oxide (nano-γ- Al2O3) from waste aluminum foils for efficient adsorption of methylene blue dye. Case Stud. Chem. Environ. Eng. 2023, 8, 100394. [Google Scholar] [CrossRef]
- Marsili, V.; Mazzoni, F.; Marzola, I.; Alvisi, S.; Franchini, M. Intermittent water supply system rehabilitation through a multiphase methodology based on network analysis and hydraulic modeling. J. Water Resour. Plan. Manag. 2023, 149, 04023048. [Google Scholar] [CrossRef]
- Rehman, K.; Fatima, F.; Waheed, I.; Akash, M.S.H. Prevalence of exposure of heavy metals and their impact on health consequences. J. Cell. Biochem. 2018, 119, 157–184. [Google Scholar] [CrossRef]
- Pradhan, B.; Chand, S.; Chand, S.; Rout, P.R.; Naik, S.K. Emerging groundwater contaminants: A comprehensive review on their health hazards and remediation technologies. Groundw. Sustain. Dev. 2023, 20, 100868. [Google Scholar] [CrossRef]
- Miri, Z.; Haugen, H.J.; Loca, D.; Rossi, F.; Perale, G.; Moghanian, A.; Ma, Q. Review on the strategies to improve the mechanical strength of highly porous bone bioceramic scaffolds. J. Eur. Ceram. Soc. 2023, 44, 23–42. [Google Scholar] [CrossRef]
- Kam, O.R.; Bakouan, C.; Zongo, I.; Guel, B. Removal of Thallium from Aqueous Solutions by Adsorption onto Alumina Nanoparticles. Processes 2022, 10, 1826. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C. Enzyme-assisted extractions of polyphenols—A comprehensive review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-based green synthesis of metallic nanoparticles: Scientific curiosity or a realistic alternative to chemical synthesis? Nan-otechnol. Environ. Eng. 2016, 1, 4. [Google Scholar] [CrossRef]
- Rangasamy, P.; Hansiya, V.S.; Maheswari, P.U.; Suman, T.; Geetha, N. Phytochemical Analysis and Evaluation of In vitro Antioxidant and Anti-urolithiatic Potential of various fractions of Clitoria ternatea L. Blue Flowered Leaves. Asian J. Pharm. Anal. 2019, 9, 67–76. [Google Scholar] [CrossRef]
- Manikandan, V.; Jayanthi, P.; Priyadharsan, A.; Vijayaprathap, E.; Anbarasan, P.; Velmurugan, P. Green synthesis of pH-responsive Al2O3 nanoparticles: Application to rapid removal of nitrate ions with enhanced antibacterial activity. J. Photochem. Photobiol. A Chem. 2019, 371, 205–215. [Google Scholar] [CrossRef]
- Hassaan, M.A.; Meky, A.I.; Fetouh, H.A.; Ismail, A.M.; El Nemr, A. Central Composite Design and Mechanism of Antibiotic Ciprofloxacin Photodegradation under Visible Light by Green Hydrothermal Synthesized Cobalt-Doped Zinc Oxide Nanoparticles. Sci. Rep. 2024, 14, 9144. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.K.; Akhtar, M.S.; Ameen, S.; Srivastava, P.; Singh, G. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J. Alloys Compd. 2015, 632, 321–325. [Google Scholar] [CrossRef]
- Rao, V.N.; Kwon, H.; Lee, Y.; Ravi, P.; Ahn, C.W.; Kim, K.; Yang, J.-M. Synergistic integration of MXene nanosheets with CdS@ TiO2 core@ shell S-scheme photocatalyst for augmented hydrogen generation. Chem. Eng. J. 2023, 471, 144490. [Google Scholar]
- Amor, I.B.; Hemmami, H.; Laouini, S.E.; Ahmed, S.; Mohammed, H.A.; Abdullah, J.A.A.; Azooz, E.A.; Al-Mulla, E.A.J.; Alharthi, F. Enhancing oxidant and dye scavenging through MgO-based chitosan nanoparticles for potential antioxidant coatings and efficient photocatalysts. Biomass Convers. Biorefinery 2023, 1–15. [Google Scholar] [CrossRef]
- Zidane, Y.; Laouini, S.E.; Bouafia, A.; Meneceur, S.; Tedjani, M.L.; Alshareef, S.A.; Almukhlifi, H.A.; Al-Essa, K.; Al-Essa, E.M.; Rahman, M.M.; et al. Green synthesis of multifunctional MgO@AgO/Ag2O nanocomposite for photocatalytic degradation of methylene blue and toluidine blue. Front. Chem. 2022, 10, 1083596. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Weidner, S.; Wójtowicz, I.; Karmac, M.; Kosinska, A.; Rybarczyk, A. Influence of low-temperature stress on changes in the composition of grapevine leaf phenolic compounds and their antioxidant properties. Funct. Plant Sci. Biot 2010, 4, 90–96. [Google Scholar]
- Kaur, P.; Arora, S.; Singh, R. Isolation, characterization and biological activities of betulin from Acacia nilotica bark. Sci. Rep. 2022, 12, 9370. [Google Scholar] [CrossRef] [PubMed]
- Vilas-Boas, Â.; Valderrama, P.; Fontes, N.; Geraldo, D.; Bento, F. Evaluation of total polyphenol content of wines by means of voltammetric techniques: Cyclic voltammetry vs differential pulse voltammetry. Food Chem. 2019, 276, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Gherbi, B.; Laouini, S.E.; Meneceur, S.; Bouafia, A.; Hemmami, H.; Tedjani, M.L.; Thiripuranathar, G.; Barhoum, A.; Menaa, F. Effect of pH Value on the Bandgap Energy and Particles Size for Biosynthesis of ZnO Nanoparticles: Efficiency for Photocatalytic Adsorption of Methyl Orange. Sustainability 2022, 14, 11300. [Google Scholar] [CrossRef]
- Zlatić, G.; Martinović, I.; Pilić, Z.; Paut, A.; Mitar, I.; Prkić, A.; Čulum, D. Green inhibition of corrosion of aluminium alloy 5083 by Artemisia annua L. extract in artificial seawater. Molecules 2023, 28, 2898. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, P.; Subramaniyan, V.; Elavarasan, V.; Mohandoss, N.; Subramaniyan, P.; Vijayakumar, S. Biofabricated Aluminium Oxide Nanoparticles Derived from Citrus aurantium L.: Antimicrobial, Anti-Proliferation, and Photocatalytic Efficiencies. Sustainability 2023, 15, 1743. [Google Scholar]
- Natrayan, L.; Rao, Y.S.; Prasad, P.R.; Bhaskar, K.; Patil, P.P.; Abdeta, D.B. Biosynthesis-Based Al2O3 Nanofiller from Cymbopogon citratus Leaf/Jute/Hemp/Epoxy-Based Hybrid Composites with Superior Mechanical Properties. Bioinorg. Chem. Appl. 2023, 2023, 9299658. [Google Scholar] [CrossRef] [PubMed]
- Manogar, P.; Morvinyabesh, J.E.; Ramesh, P.; Jeyaleela, G.D.; Amalan, V.; Ajarem, J.S.; Allam, A.A.; Khim, J.S.; Vijayakumar, N. Biosynthesis and antimicrobial activity of aluminium oxide nanoparticles using Lyngbya majuscula extract. Mater. Lett. 2022, 311, 131569. [Google Scholar] [CrossRef]
- Songmene, V.; Khettabi, R.; Viens, M.; Kouam, J.; Hallé, S.; Morency, F.; Masounave, J.; Djebara, A. Mesure, Contrôle et Caractérisation des Nanoparticules: Procédure Appliquée à L’usinage et au Frottement Mécanique; Institut de Recherche Robert-Sauvé en Santé et en Sécurité du Travail (IRSST): Montréal, QC, Canada, 2014; 93p, ISBN 978-2-89631-716-5. [Google Scholar]
- Ghelani, D.; Faisal, S. Synthesis and characterization of Aluminium Oxide nanoparticles. Authorea 2022. [Google Scholar] [CrossRef]
- Roque-Ruiz, J.; Reyes-López, S. Synthesis of α- Al2O3 nanopowders at low temperature from aluminum formate by combustion process. J Mater. Sci Eng 2016, 6, 1–8. [Google Scholar]
- Djebaili, K.; Mekhalif, Z.; Boumaza, A.; Djelloul, A. XPS, FTIR, EDX, and XRD analysis of Al2O3 scales grown on PM2000 alloy. J. Spectrosc. 2015, 2015, 868109. [Google Scholar] [CrossRef]
- Prashanth, P.; Raveendra, R.; Krishna, R.H.; Ananda, S.; Bhagya, N.; Nagabhushana, B.; Lingaraju, K.; Naika, H.R. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion-derived α- Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef]
- Karunakaran, C.; Anilkumar, P.; Gomathisankar, P. Photoproduction of iodine with nanoparticulate semiconductors and insulators. Chem. Cent. J. 2011, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Anna, K.K.; Bogireddy, N.K.R.; Agarwal, V.; Bon, R.R. Synthesis of α and γ phase of aluminium oxide nanoparticles for the photocatalytic degradation of methylene blue under sunlight: A comparative study. Mater. Lett. 2022, 317, 132085. [Google Scholar] [CrossRef]
- Ezeibe Anderson, U.; Nleonu Emmanuel, C.; Onyemenonu Christopher, C.; Arrousse Nadia, N.C.C. Photocatalytic activity of aluminium oxide nanoparticles on degradation of ciprofloxacin. Saudi J. Eng. Technol. 2022, 7, 132–136. [Google Scholar]
- Makofane, A.; Motaung, D.E.; Hintsho-Mbita, N.C. Green Synthesis of Silver Deposited on Copper Ferrite Nanoparticles for the Photodegradation of Dye and Antibiotics. Appl. Surf. Sci. Adv. 2024, 21, 100601. [Google Scholar] [CrossRef]
- Taghavi Fardood, S.; Moradnia, F.; Ganjkhanlu, S.; Ouni, L.; Ramazani, A.; Sillanpää, M. Green Synthesis and Characterization of Spinel CoAl2O4 Nanoparticles: Efficient Photocatalytic Degradation of Organic Dyes. Inorg. Chem. Commun. 2024, 167, 112719. [Google Scholar] [CrossRef]
- Chihi, S.; Bouafia, A.; Meneceur, S.; Laouini, S.E.; Ahmed, R.Z. Effect of precursor concentration on the bandgap energy and particles size for green synthesis of hematite α-Fe2O3 nanoparticles by the aqueous extract of Moltkia ciliata and evaluation of the antibacterial activity. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Thakur, A.; Kaur, H.; Aguedal, H.; Singh, V.; Singh, V.; Goel, G. Biogenic Synthesis of Fe-Doped Al2O3 Nanoparticles Using Eichhornia Crassipes for the Remediation of Toxicant Malachite Green Dye: Kinetic and Thermodynamic Studies. Inorg. Chem. Commun. 2024, 163, 112340. [Google Scholar] [CrossRef]
- Guo, J.; Van Bui, H.; Valdesueiro, D.; Yuan, S.; Liang, B.; Van Ommen, J. Suppressing the Photocatalytic Activity of TiO2 Nanoparticles by Extremely Thin Al2O3 Films Grown by Gas-Phase Deposition at Ambient Conditions. Nanomaterials 2018, 8, 61. [Google Scholar] [CrossRef] [PubMed]
- Tai, L.T.; Dat, N.M.; Nam, N.T.H.; An, H.; Huong, L.M.; Cong, C.Q.; Hai, N.D.; Phong, M.T.; Hieu, N.H. Green Synthesis of Copper Oxide Nanoparticles for Photodegradation of Malachite Green and Antibacterial Properties under Visible Light. Opt. Mater. 2023, 136, 113489. [Google Scholar] [CrossRef]
- Jansanthea, P.; Inyai, N.; Chomkitichai, W.; Ketwaraporn, J.; Ubolsook, P.; Wansao, C.; Wanaek, A.; Wannawek, A.; Kuimalee, S.; Pookmanee, P. Green Synthesis of CuO/Fe2O3/ZnO Ternary Composite Photocatalyst Using Grape Extract for Enhanced Photodegradation of Environmental Organic Pollutant. Chemosphere 2024, 351, 141212. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.L.T.; Abdullah, C.A.C.; Chung, E.L.T.; Andou, Y. Recent progress in visible light-doped ZnO photocatalyst for pollution control. Int. J. Environ. Sci. Technol. 2023, 20, 5753–5772. [Google Scholar] [CrossRef]
- Lau, G.E.; Che Abdullah, C.A.; Wan Ahmad, W.A.; Assaw, S.; Zheng, A.L. Eco-Friendly Photocatalysts for Degradation of Dyes. Catalysts 2020, 10, 1129. [Google Scholar] [CrossRef]
- Goutam, S.P.; Saxena, G.; Roy, D.; Yadav, A.K.; Bharagava, R.N. Green Synthesis of Nanoparticles and Their Applications in Water and Wastewater Treatment. In Bioremediation of Industrial Waste for Environmental Safety; Springer Singapore: Singapore, 2020; pp. 349–379. [Google Scholar]
- Hussain, R.T.; Hossain, M.S.; Shariffuddin, J.H. Green Synthesis and Photocatalytic Insights: A Review of Zinc Oxide Nanoparticles in Wastewater Treatment. Mater. Today Sustain. 2024, 26, 100764. [Google Scholar] [CrossRef]
- Roy, S.; Tran, T.A.; Chowdhury, Z.Z.; Prathibha, B.S. Wastewater Treatment Using Green Synthesis; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781003342830. [Google Scholar]




| Samples | Plant |
|---|---|
| Young’s modulus (MPa) | 347.87 |
| Samples | Plant + Al2O3 NPs |
| Young’s modulus (MPa) | 530.32 |
| Sample | Plant |
|---|---|
| Young’s modulus (MPa) | 1139.23 |
| Sample | Plant + Al2O3 NPs |
| Young’s modulus (MPa) | 5242.88 |
| Metal NPs | Method of Synthesis | The Particle Size (nm) | Organic Pollutants | Time (min) | Dye Removal (%) | Ref. |
|---|---|---|---|---|---|---|
| Al2O3 | green method | 28 | MB | 150 | 89.1 | [28] |
| α-Al2O3 | sol–gel | 42 | MB | 4 h | 85 | [37] |
| γ-Al2O3 | precipitation | 36 | MB | 4 h | 91.6 | [37] |
| Al2O3 | sol–gel | / | MB | 30 | 95.9 | [31] |
| Al2O3 | green method | 50–100 | nitrate in aqueous solution | 105 | 94 | [17] |
| Al2O3 | chemical precipitation | / | ciprofloxacin | 1–5 h | 90 | [38] |
| Al2O3 | green method | 25.1 | MB | 120 | 98.2 | This work |
| Rose Bengal | 90.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gharbi, A.H.; Laouini, S.E.; Hemmami, H.; Bouafia, A.; Gherbi, M.T.; Ben Amor, I.; Hasan, G.G.; Abdullah, M.M.S.; Trzepieciński, T.; Abdullah, J.A.A. Eco-Friendly Synthesis of Al2O3 Nanoparticles: Comprehensive Characterization Properties, Mechanics, and Photocatalytic Dye Adsorption Study. Coatings 2024, 14, 848. https://doi.org/10.3390/coatings14070848
Gharbi AH, Laouini SE, Hemmami H, Bouafia A, Gherbi MT, Ben Amor I, Hasan GG, Abdullah MMS, Trzepieciński T, Abdullah JAA. Eco-Friendly Synthesis of Al2O3 Nanoparticles: Comprehensive Characterization Properties, Mechanics, and Photocatalytic Dye Adsorption Study. Coatings. 2024; 14(7):848. https://doi.org/10.3390/coatings14070848
Chicago/Turabian StyleGharbi, Ahlam Hacine, Salah Eddine Laouini, Hadia Hemmami, Abderrhmane Bouafia, Mohammed Taher Gherbi, Ilham Ben Amor, Gamil Gamal Hasan, Mahmood M. S. Abdullah, Tomasz Trzepieciński, and Johar Amin Ahmed Abdullah. 2024. "Eco-Friendly Synthesis of Al2O3 Nanoparticles: Comprehensive Characterization Properties, Mechanics, and Photocatalytic Dye Adsorption Study" Coatings 14, no. 7: 848. https://doi.org/10.3390/coatings14070848









