Smoke Suppression Properties of Fe2O3 on Intumescent Fire-Retardant Coatings of Styrene–Acrylic Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Intumescent Fire-Retardant Coatings
2.3. Cone Calorimeter (CCT)
2.4. Smoke Density Analyzer (SDA)
2.5. Thermal Gravimetric Analysis (TGA)
2.6. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Cone Calorimeter Test
3.1.1. Heat Release Rate (HRR)
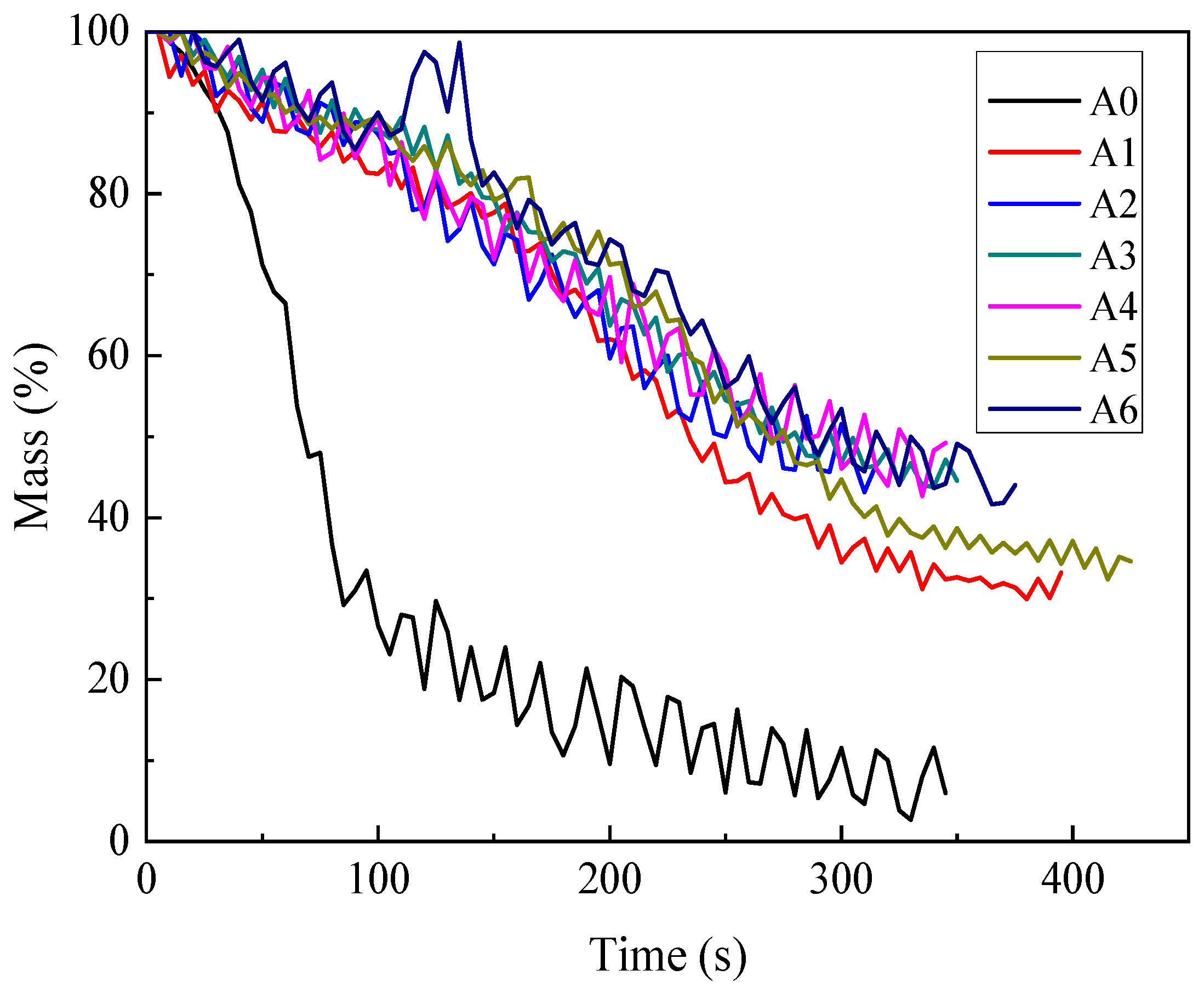
3.1.2. Mass
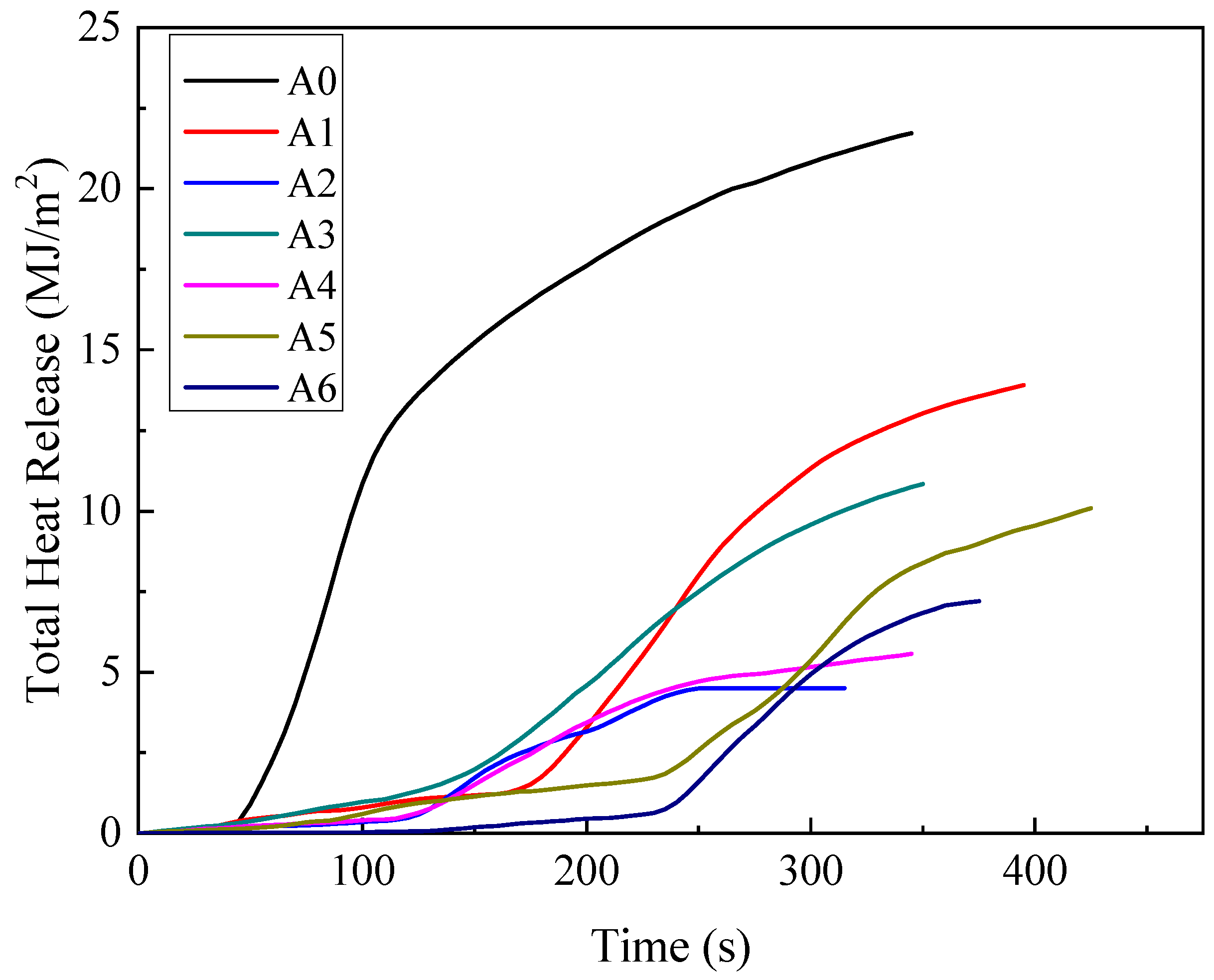
3.1.3. Total Heat Release (THR)
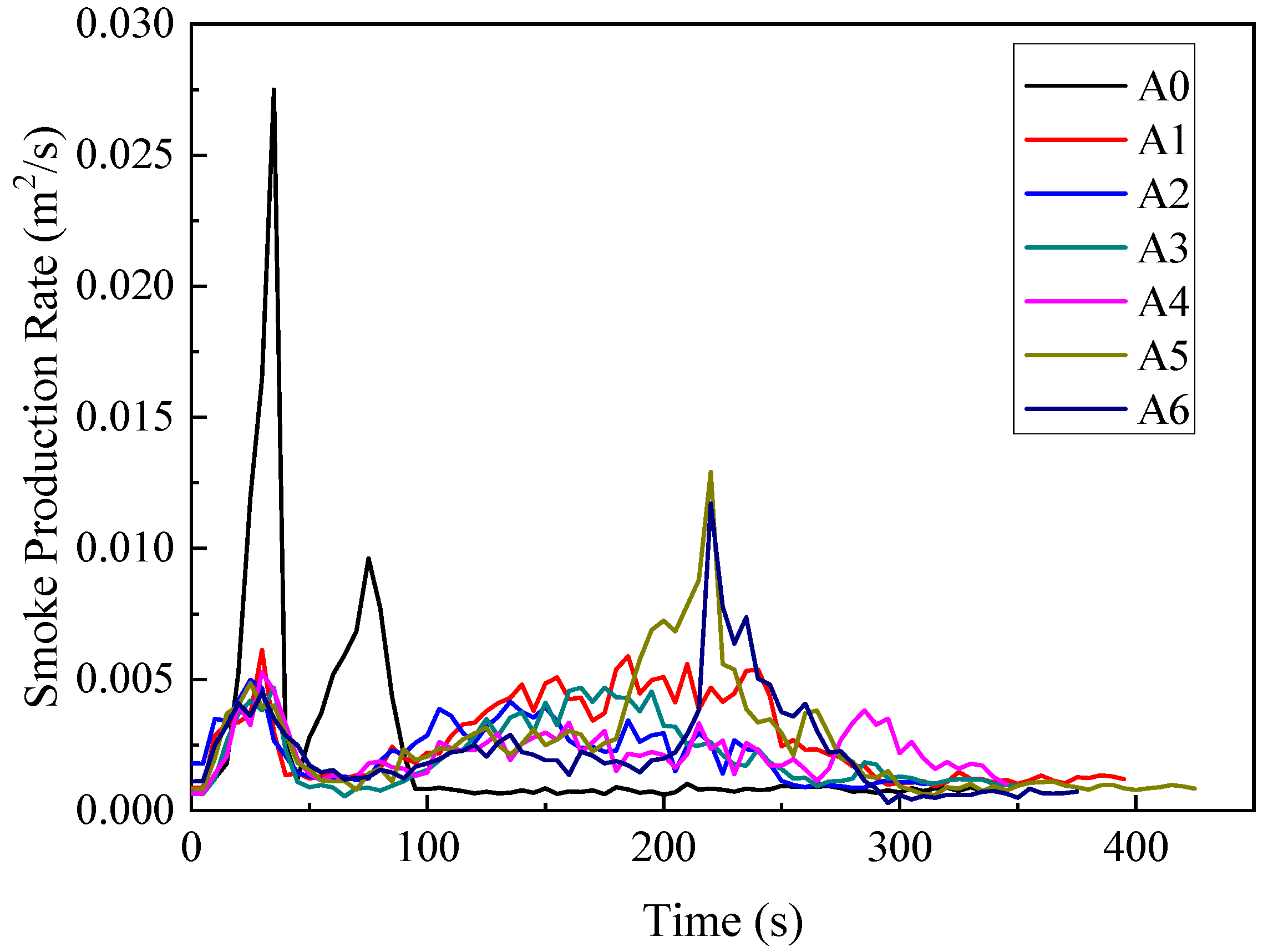
3.1.4. Smoke Production Rate (SPR)
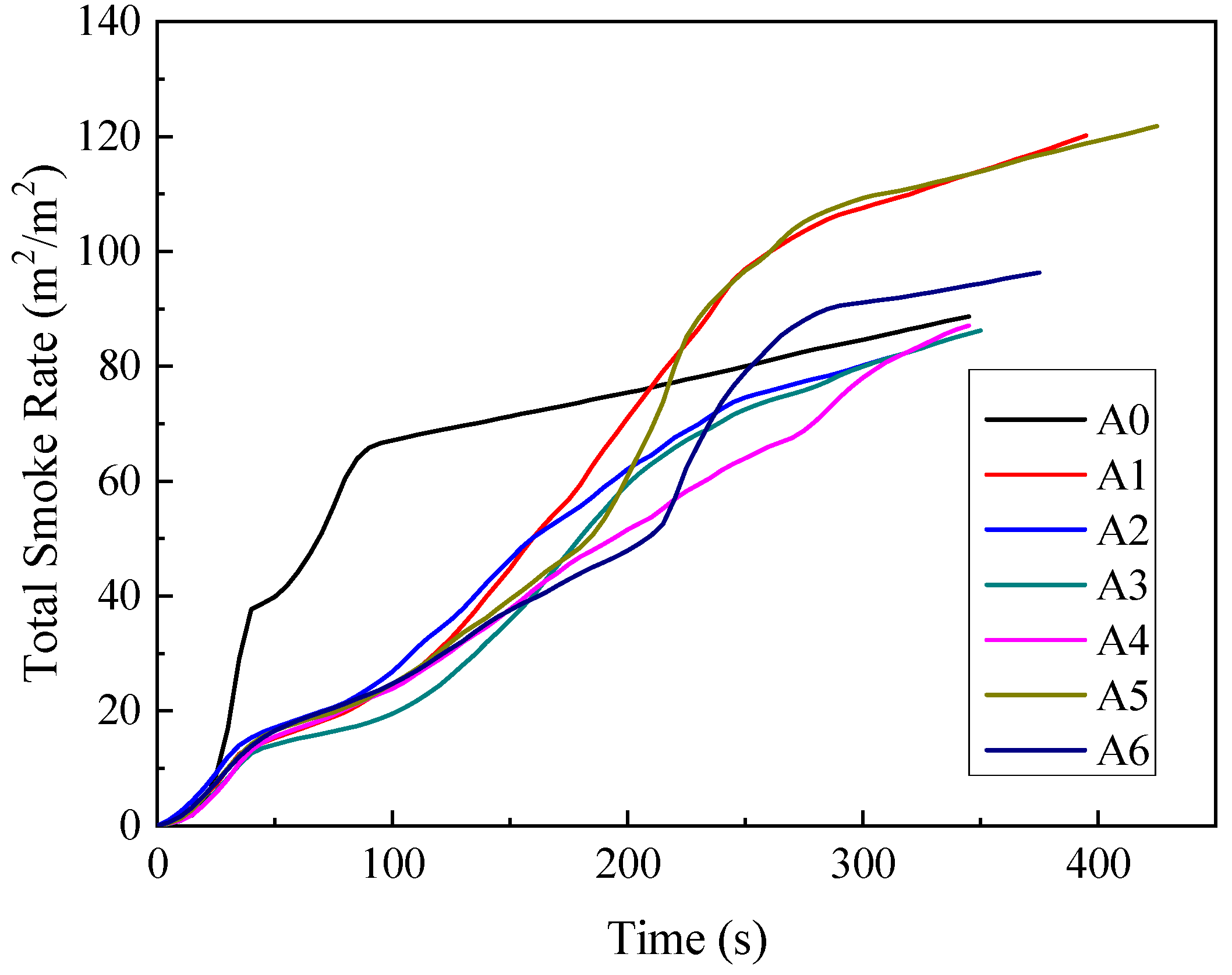
3.1.5. Total Smoke Release (TSR)
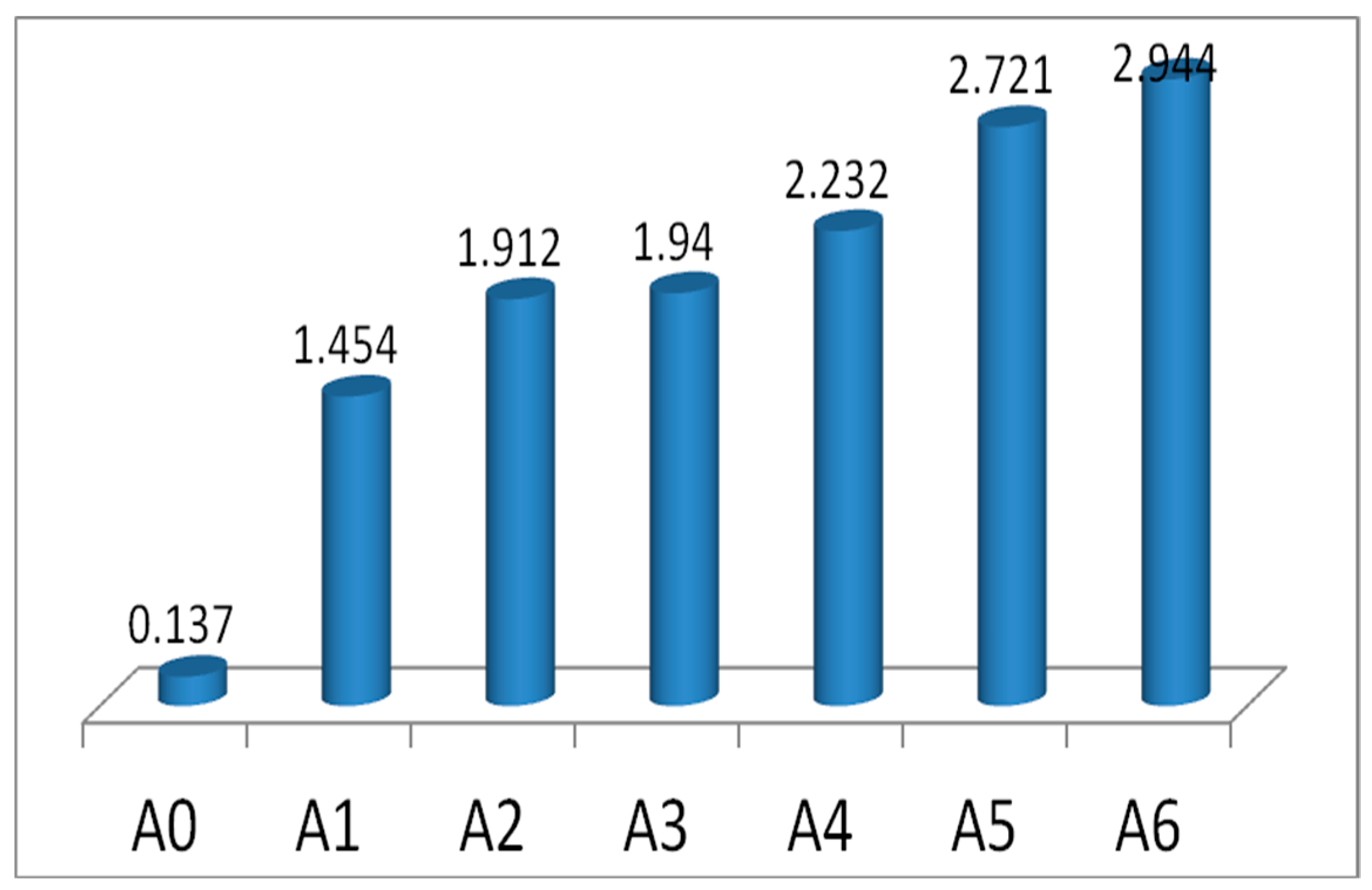
3.1.6. Fire Performance Index (FPI) and Fire Growth Index (FGI)
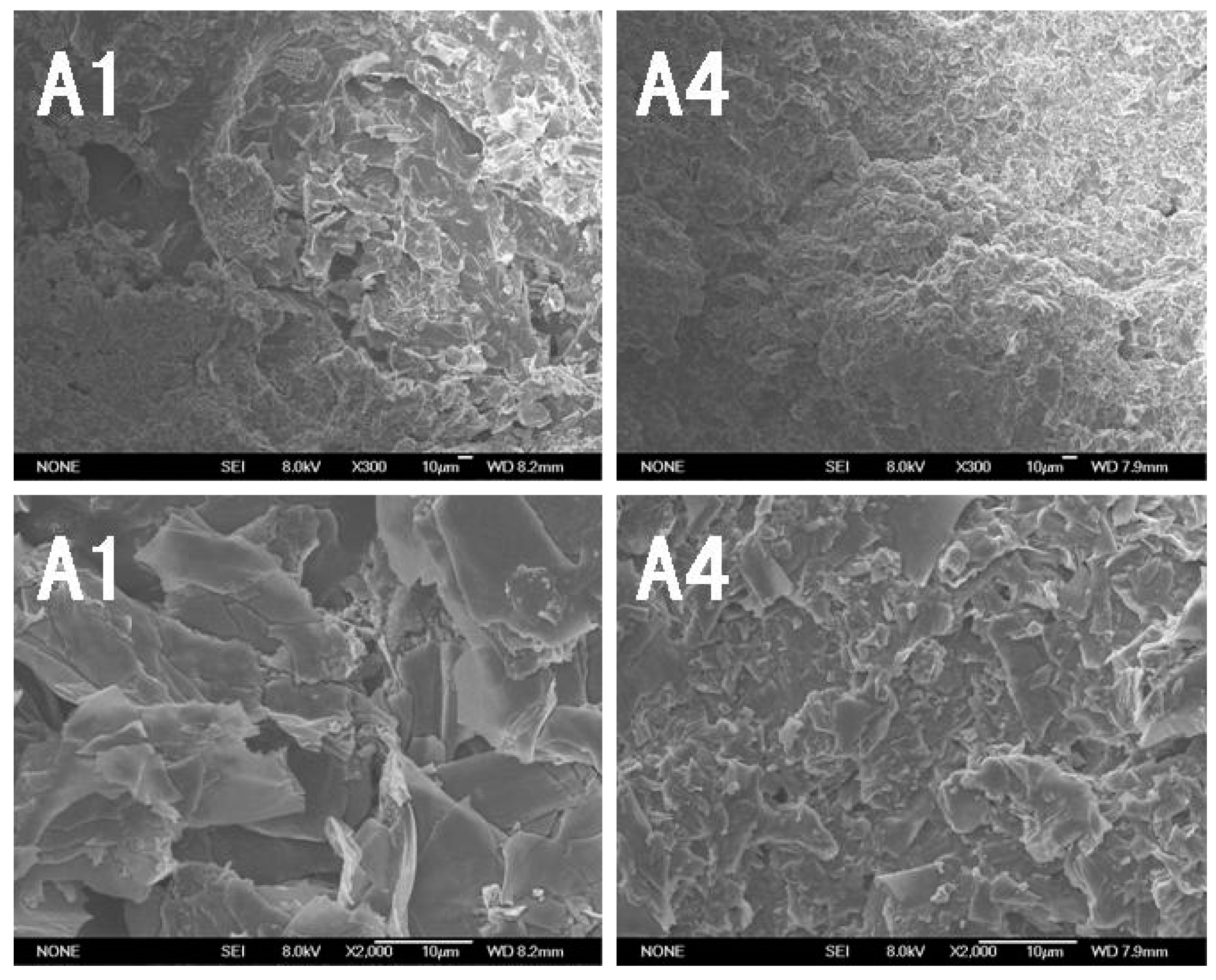
3.1.7. Morphology of Residues
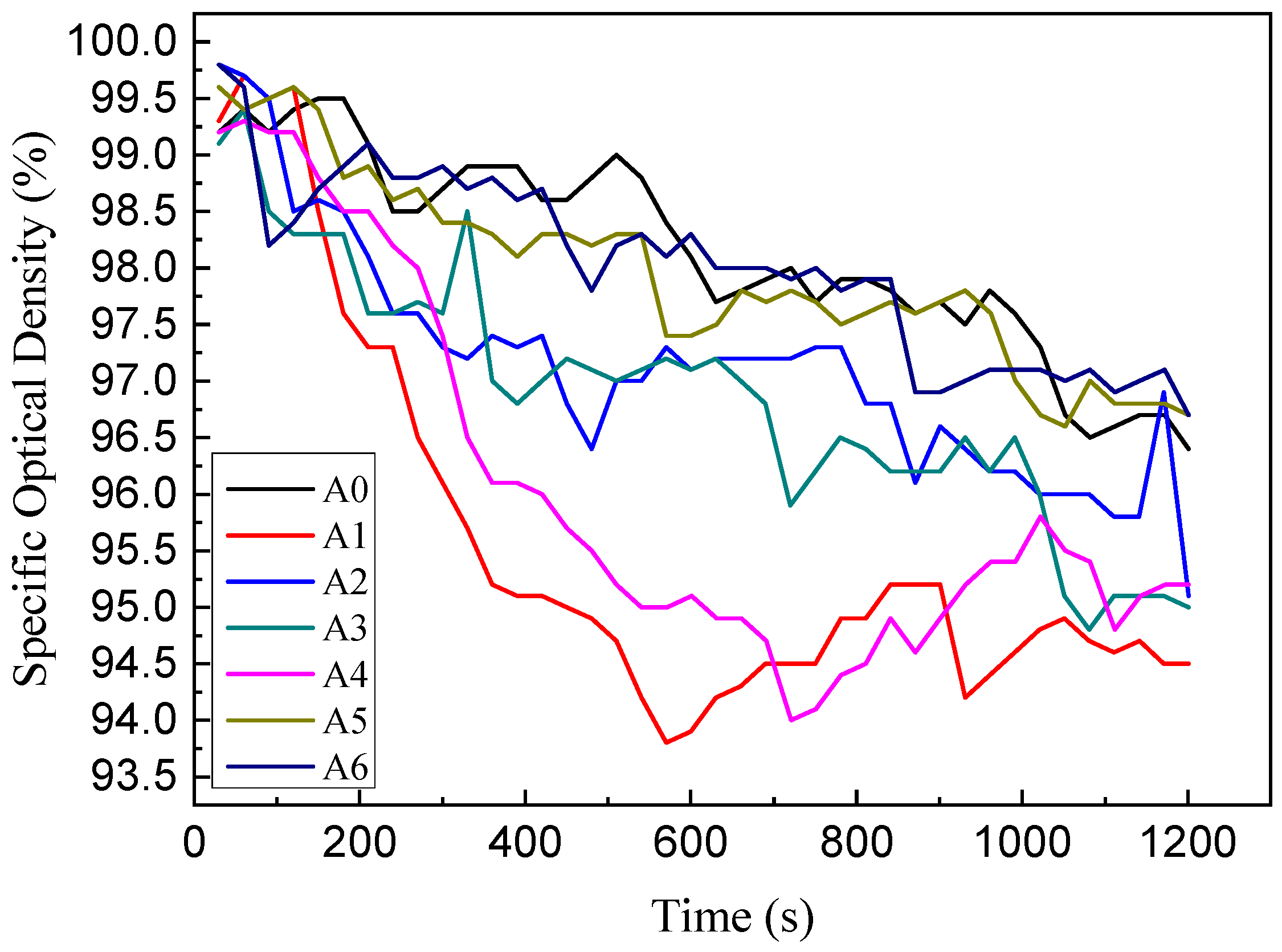
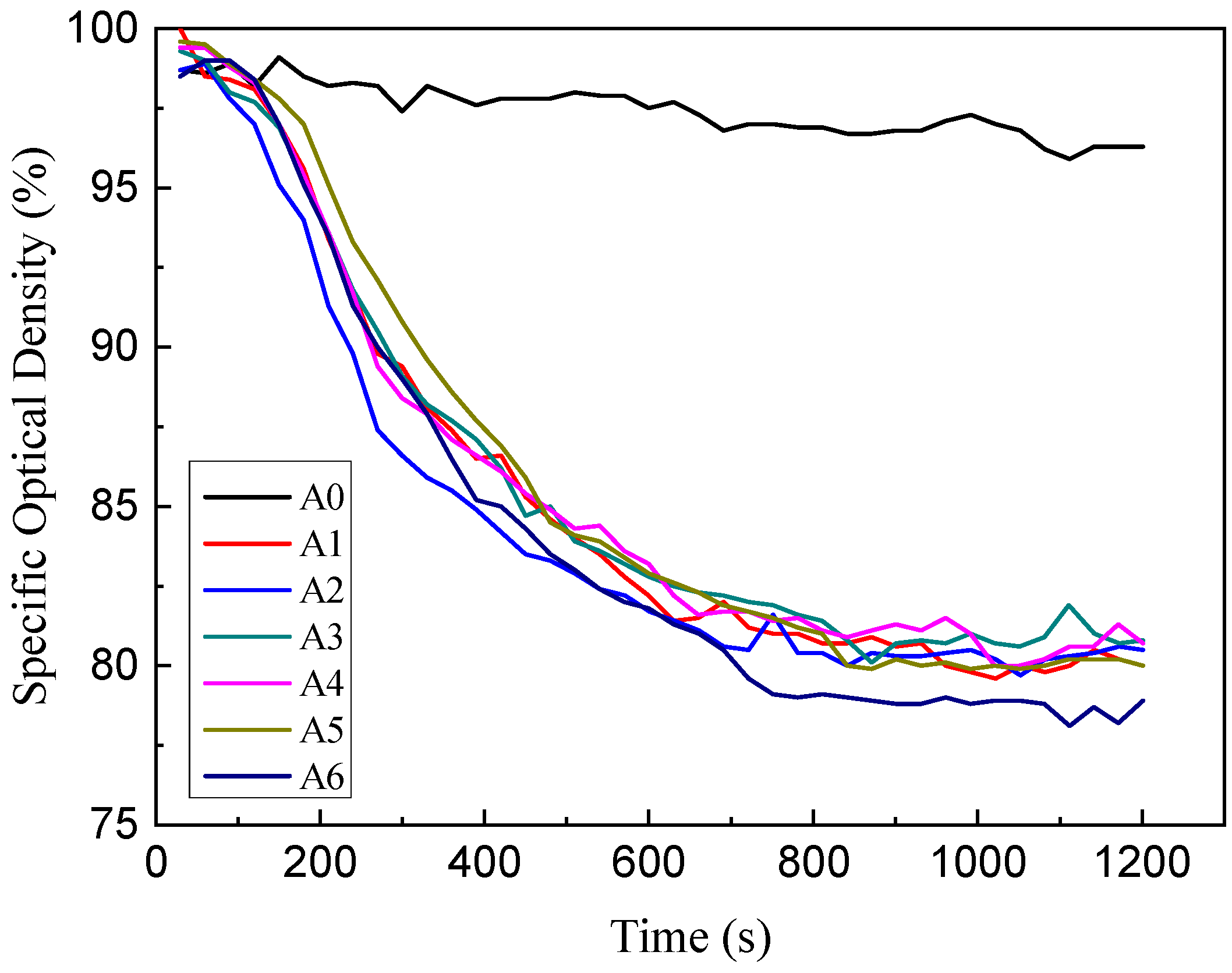
3.2. Smoke Density Analyzer Test
Specific Optical Density (SOD)
3.3. Thermal–Gravimetric Analysis Test
3.4. Scanning Electron Microscopy (SEM)
4. Conclusions
- (1)
- FeOOH can significantly reduce the HRR, THR, SPR, and TSR of paint samples, improve the quality of the carbon residue, reduce the smoke production of the samples, and change the structure of the carbonization residue;
- (2)
- The weight of char residue increases with the increase in the addition of FeOOH;
- (3)
- Samples containing 2% FeOOH/IFR have a greater effect on the fire- and smoke suppression properties of fire-retardant paint samples.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, H.D.; Song, L.; Hu, Y. A review on flame retardant technology in China. Part II: Flame retardant polymeric nanocomposites and coatings. Polym. Adv. Technol. 2011, 22, 379–394. [Google Scholar] [CrossRef]
- Carty, P.; White, S. The effect of temperature on char formation in polymer blends: An explanation of the role of the smoke suppressant FeOOH acting in ABS/CPVC polymer blends. Polym. Degrad. Stab. 2002, 75, 173–184. [Google Scholar] [CrossRef]
- Sharma, S.K.; Singh, M.P.; Saxena, N.K.; Srivastava, S.K. Assessment of the effectiveness of smoke suppressants using a He-Ne laser in a flow system. Fire Mater. 1993, 17, 271–277. [Google Scholar] [CrossRef]
- Powell, D.A.; Martin, K.G. Smoke and fire assessment with the fire propagation test. Fire Mater. 1976, 1, 97–102. [Google Scholar] [CrossRef]
- Formicola, C.; Fenzo, A.D.; Zarrelli, M.; Giordano, M.; Antonucci, V. Zinc-based compounds as smoke suppressant agents for an aerospace epoxy matrix. Polym. Int. 2011, 60, 304–311. [Google Scholar] [CrossRef]
- Kim, S. Flame retardancy and smoke suppression of magnesium hydroxide filled polyethylene. J. Polym. Science. Part B Polym. Phys. 2003, 41, 936–944. [Google Scholar] [CrossRef]
- Chen, X.L.; Hu, Y.; Song, L. Thermal behaviors of a novel UV cured flame retardant coatings containing phosphorus, nitrogen and silicon. Polym. Eng. Sci. 2008, 48, 116–123. [Google Scholar] [CrossRef]
- Rhys, J.A. Intumescent coatings and their uses. Fire Mater. 1980, 4, 154–156. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Wang, M.Y.; Hall, M.E.; Sunmonu, F.; Pearson, J.S. Flame retardant textile back-coatings. Part 2. Effectiveness of phosphorus-containing flame retardants in textile back-coating formulations. Polym. Int. 2000, 49, 1079–1091. [Google Scholar] [CrossRef]
- Kandola, B.K.; Horrocks, A.R. Complex char formation in flame-retarded fibre-intumescent combinations II: Thermal analytical studies. Polym. Degrad. Stab. 1996, 54, 289–303. [Google Scholar] [CrossRef]
- Chiu, S.H.; Wang, W.K. The dynamic flammability and toxicity of magnesium hydroxide filled intumescent fire polypropylene. J. Appl. Polym. Sci. 1998, 67, 989–995. [Google Scholar] [CrossRef]
- Hu, X.; Li, W.; Wang, Y. Synthesis and characterization of a novel nitrogen-containing flame retardant. J. Appl. Polym. Sci. 2004, 94, 1556–1561. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.; Wang, J.; Song, Y.P.; Wang, Y.Z. A novel intumescent flame-retardant LDPE system and its thermo-oxidative degradation and flame-retardant mechanisms. Polym. Adv. Technol. 2008, 19, 1566–1575. [Google Scholar] [CrossRef]
- Skinner, G.A.; Haines, P.J. Molybdenum compounds as flame-retardants and smoke-suppressants in halogenated polymers. Fire Mater. 1986, 10, 63–69. [Google Scholar] [CrossRef]
- Kong, Q.H.; Zhang, J.H.; Ma, J.J.; Yi, C.W.; Li, F.C.; Liu, H.; Lu, W.L. Flame retardant and smoke suppressant of Fe-organophilic montmorillonite in polyvinyl chloride nanocomposites. Chin. J. Chem. 2008, 26, 2278–2284. [Google Scholar] [CrossRef]
- Ning, Y.; Guo, S. Flame-retardant and smoke-suppressant properties of zinc borate and aluminum trihydrate-filled rigid PVC. J. Appl. Polym. Sci. 2000, 77, 3119–3127. [Google Scholar] [CrossRef]
- Cusack, P.A.; Hornsby, P.R. Zinc stannate-coated fillers: Novel flame retardants and smoke suppressants for polymeric materials. J. Vinyl Addit. Technol. 1999, 5, 21–30. [Google Scholar] [CrossRef]
- Carty, P.A. Docherty, Iron-containing compounds as flame retarding/smoke-suppressing additives for PVC. Fire Mater. 1988, 12, 109–113. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, J. Study on the flame-retarded poly (methyl methacrylate) by triphenylphosphate and nano-poly (phenyl silsesquioxane) spheres. Adv. Polym. Technol. 2011, 30, 33–40. [Google Scholar] [CrossRef]
- Morgan, A.B.; Bundy, M. Cone calorimeter analysis of UL-94 V-rated plastics. Fire Mater. 2007, 31, 257–283. [Google Scholar] [CrossRef]
- ISO 13943:2010; Fire Safety—Vocabulary. German Committee on Electrical Engineering, Electronics and Information Technology (DIN) and Information technology (DIN): Norm, Germany, 2010.
- ISO 5659-2(2006); Plastics-Smokegeneration Part 2: Determination of Optical Density by a Single-Chamber Test. Standards Policy and Strategy Committee of UK: London, UK, 2007.
- Schartel, B.; Hull, T.R. Development of fire-retarded materials-Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiao, C.M. Flammability and thermal degradation of epoxy acrylate modified with phosphorus-containing compounds. Polym. Adv. Technol. 2010, 21, 490–495. [Google Scholar] [CrossRef]
- Babrauskas, V.; Peacock, R.D. Heat release rate: The single most crucial variable in fire hazard. Fire Saf. J. 1992, 18, 255–272. [Google Scholar] [CrossRef]
- Jiao, C.M.; Zhang, J.; Zhang, F. Combustion behavior of intumescent flame retardant polypropylene composites. J. Fire Sci. 2008, 26, 455–469. [Google Scholar] [CrossRef]
- Gao, M.; Wu, W.; Yan, Y. Thermal degradation and flame retardancy of epoxy resins containing intumescent flame retardant. J. Therm. Anal. Calorim. 2009, 95, 605–608. [Google Scholar] [CrossRef]
- Almeras, X.; Bras, M.L.; Hornsby, P.; Bourbigot, S.; Marosi, G.; Keszei, S.; Poutch, F. Effect of fillers on the fire retardancy of intumescent polypropylene compounds. Polym. Degrad. Stab. 2003, 82, 325–331. [Google Scholar] [CrossRef]
- Jiao, C.M.; Chen, X.L. Flammability and thermal degradation of intumescent flame-retardant polypropylene composites. Polym. Eng. Sci. 2010, 10, 767–772. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiao, C.M.; Zhang, J. Thermal and combustion behavior of ethylene-vinyl acetate/aluminum trihydroxide/Fe-montmorillonite composites. Polym. Eng. Sci. 2012, 52, 414–419. [Google Scholar] [CrossRef]
- Mercado, L.A.; Reina, J.A.; Galia, M. Flame retardant epoxy resins based on diglycidyloxymethylphenylsilane. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 5580–5587. [Google Scholar] [CrossRef]
- Chen, X.L.; Jiao, C.M. Thermal degradation characteristics of a novel flame retardant coating using TG-IR technique. Polym. Degrad. Stab. 2008, 93, 2222–2225. [Google Scholar] [CrossRef]
- Usta, N. Investigation of fire behavior of rigid polyurethane foams containing fly ash and intumescent flame retardant using a cone calorimeter. J. Appl. Polym. Sci. 2012, 124, 3372–3382. [Google Scholar] [CrossRef]
- Carty, P.; White, S.; Creghton, J.R. TG and flammability studies on polymer blends containing acrylonitrile-butadiene-styrene and chlorinated poly(vinyl chloride). J. Therm. Anal. Calorim. 2001, 63, 679–687. [Google Scholar] [CrossRef]
- Fang, S.L.; Hu, Y.; Song, L.; Zhan, J.; He, Q.L. Mechanical properties, fire performance and thermal stability of magnesium hydroxide sulfate hydrate whiskers flame retardant silicone rubber. J. Mater. Sci. 2008, 43, 1057–1062. [Google Scholar] [CrossRef]
- Jiao, C.M.; Chen, X.L.; Zhang, J. Synergistic effects of Fe2O3 with layered double hydroxides in EVA/LDH composites. J. Fire Sci. 2009, 27, 465–479. [Google Scholar] [CrossRef]
- Ullah, S.; Ahmad, F.; Yusoff, P.S.M.M. Effect of boric acid and melamine on the intumescent fire-retardant coating composition for the fire protection of structural steel substrates. J. Appl. Polym. Sci. 2013, 128, 2983–2993. [Google Scholar] [CrossRef]




| Sample Code | A0 (Blank Board) | A1 | A2 | A3 | A4 | A5 | A6 |
|---|---|---|---|---|---|---|---|
| Styrene–acrylic emulsion/wt% | 0 | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 | 21.0 |
| IFR/wt% | 0 | 49.0 | 48.5 | 48.0 | 47.0 | 45.0 | 41.0 |
| FeOOH/wt% | 0 | 0 | 0.5 | 1.0 | 2.0 | 4.0 | 8.0 |
| Distilled water/wt% | 0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Hydroxyethyl cellulose/wt% | 0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Ethanediol/wt% | 0 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| Antifoaming agent/wt% | 0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Film-forming agent/wt% | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, F.; Song, Q.; Ma, L. Smoke Suppression Properties of Fe2O3 on Intumescent Fire-Retardant Coatings of Styrene–Acrylic Emulsion. Coatings 2024, 14, 850. https://doi.org/10.3390/coatings14070850
Dong F, Song Q, Ma L. Smoke Suppression Properties of Fe2O3 on Intumescent Fire-Retardant Coatings of Styrene–Acrylic Emulsion. Coatings. 2024; 14(7):850. https://doi.org/10.3390/coatings14070850
Chicago/Turabian StyleDong, Fang, Qingfeng Song, and Liyong Ma. 2024. "Smoke Suppression Properties of Fe2O3 on Intumescent Fire-Retardant Coatings of Styrene–Acrylic Emulsion" Coatings 14, no. 7: 850. https://doi.org/10.3390/coatings14070850






