Enhanced Coagulation for Algae Removal Using Composite Al-Based Coagulants: Collaborative Optimization Mechanism of Aluminum Morphology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Water Samples
2.2. Preparation of the Used Coagulants
2.3. Jar Test Experiments and the Analysis of Characteristics of Flocs
2.4. Scanning Electron Microscope (SEM) Analysis for Flocs
2.5. EOM Analysis
3. Results and Discussion
3.1. Characteristics of Raw Water
3.2. Coagulation Performance
3.2.1. Effect of Coagulant Dosage
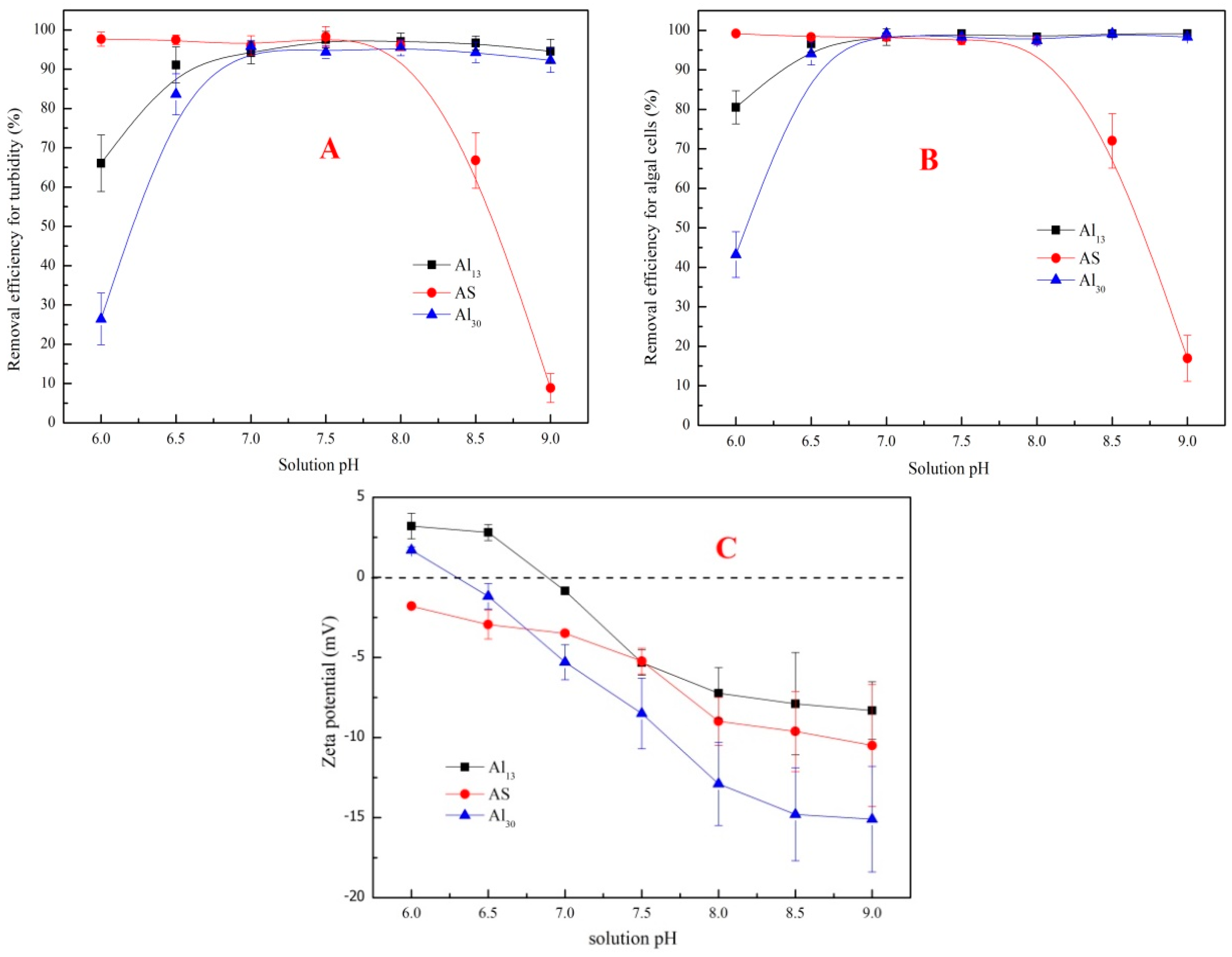
3.2.2. Effect of Solution pH
3.3. Characterization of Flocs
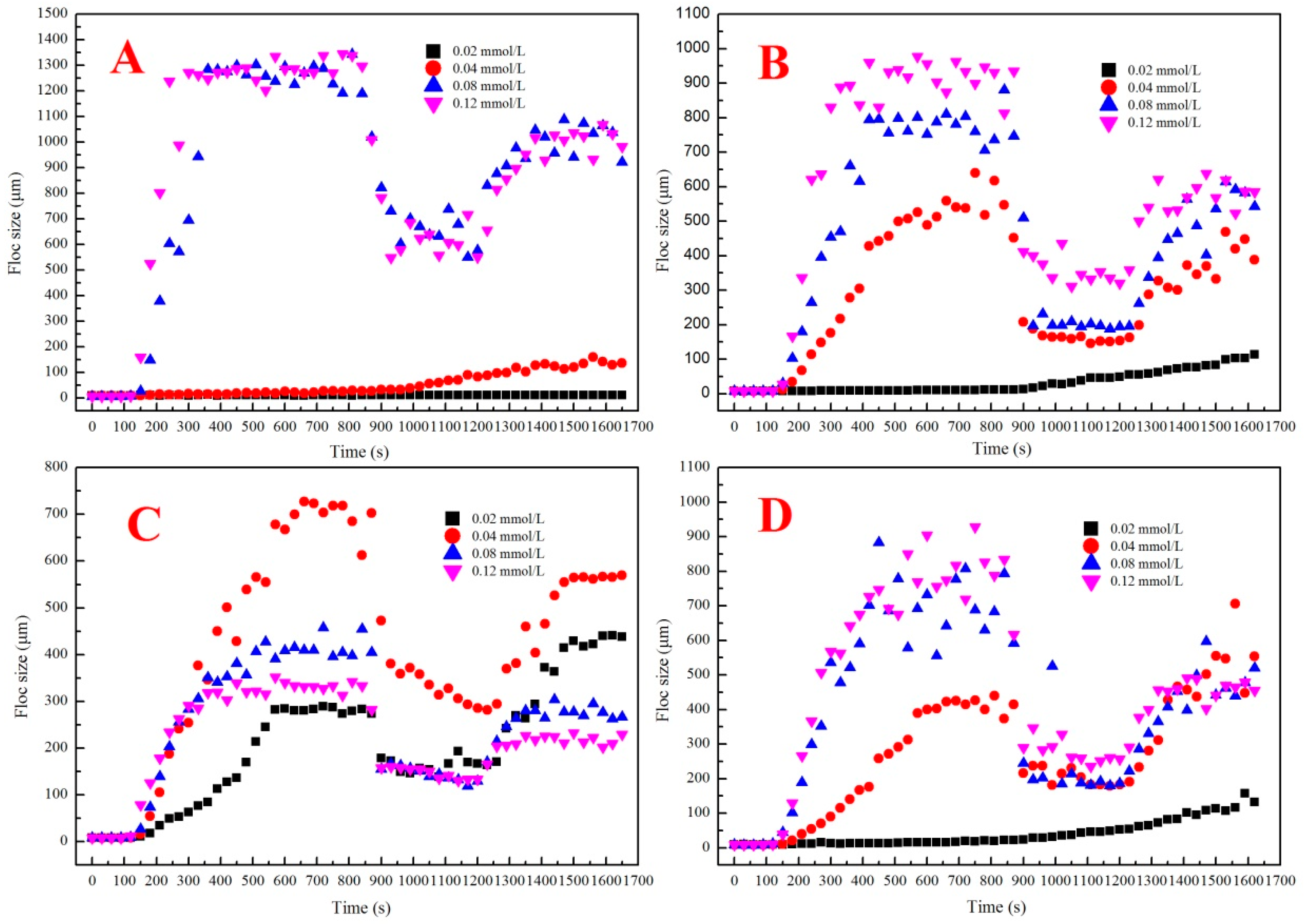
3.3.1. Properties of Flocs Formed by Al13, AS, Al30, and PAC
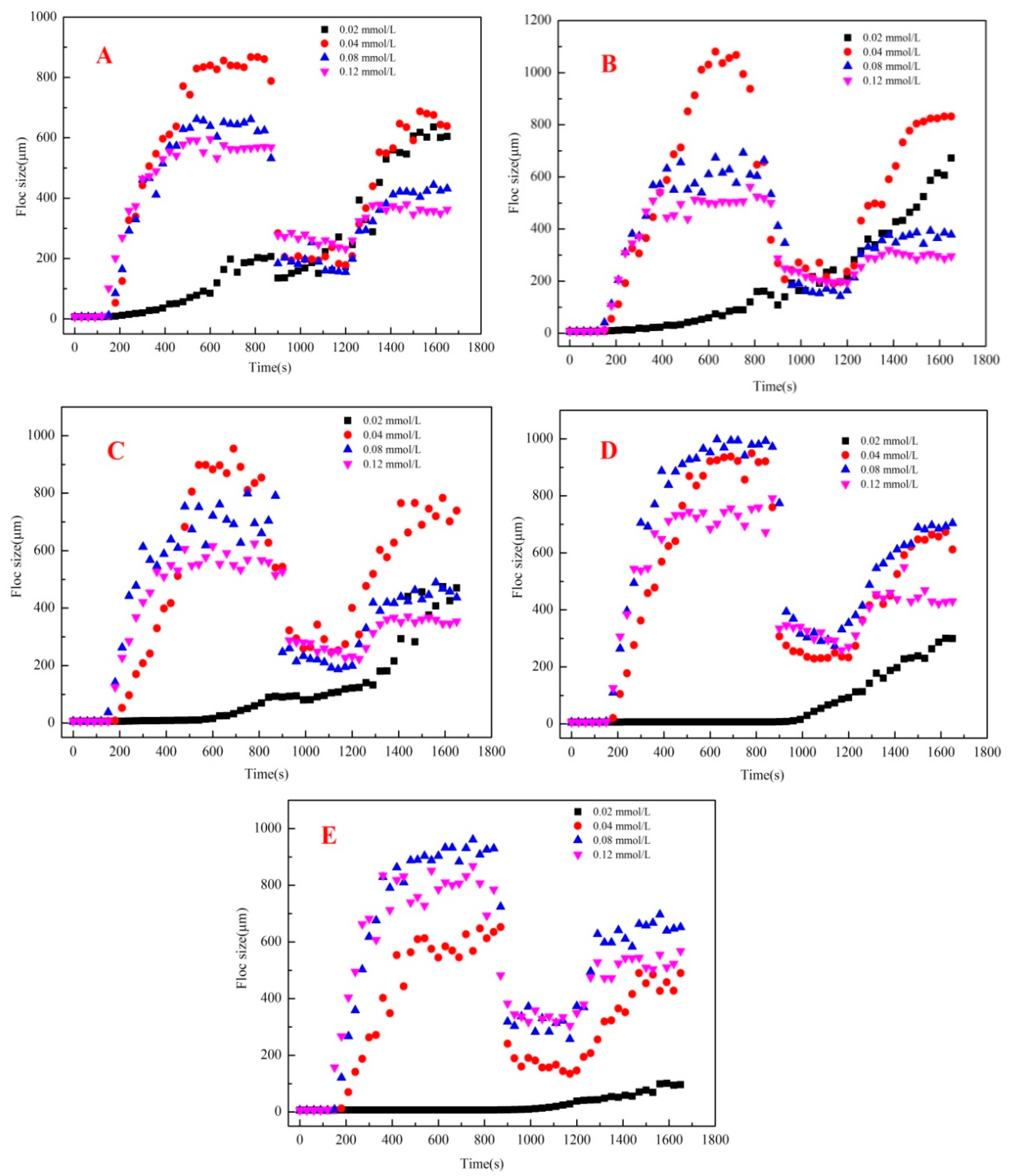
3.3.2. Properties of Flocs Formed by Al13/Al30 Composite Coagulants
3.3.3. Properties of Flocs Formed by PDADMAC/Al13 Composite Coagulants
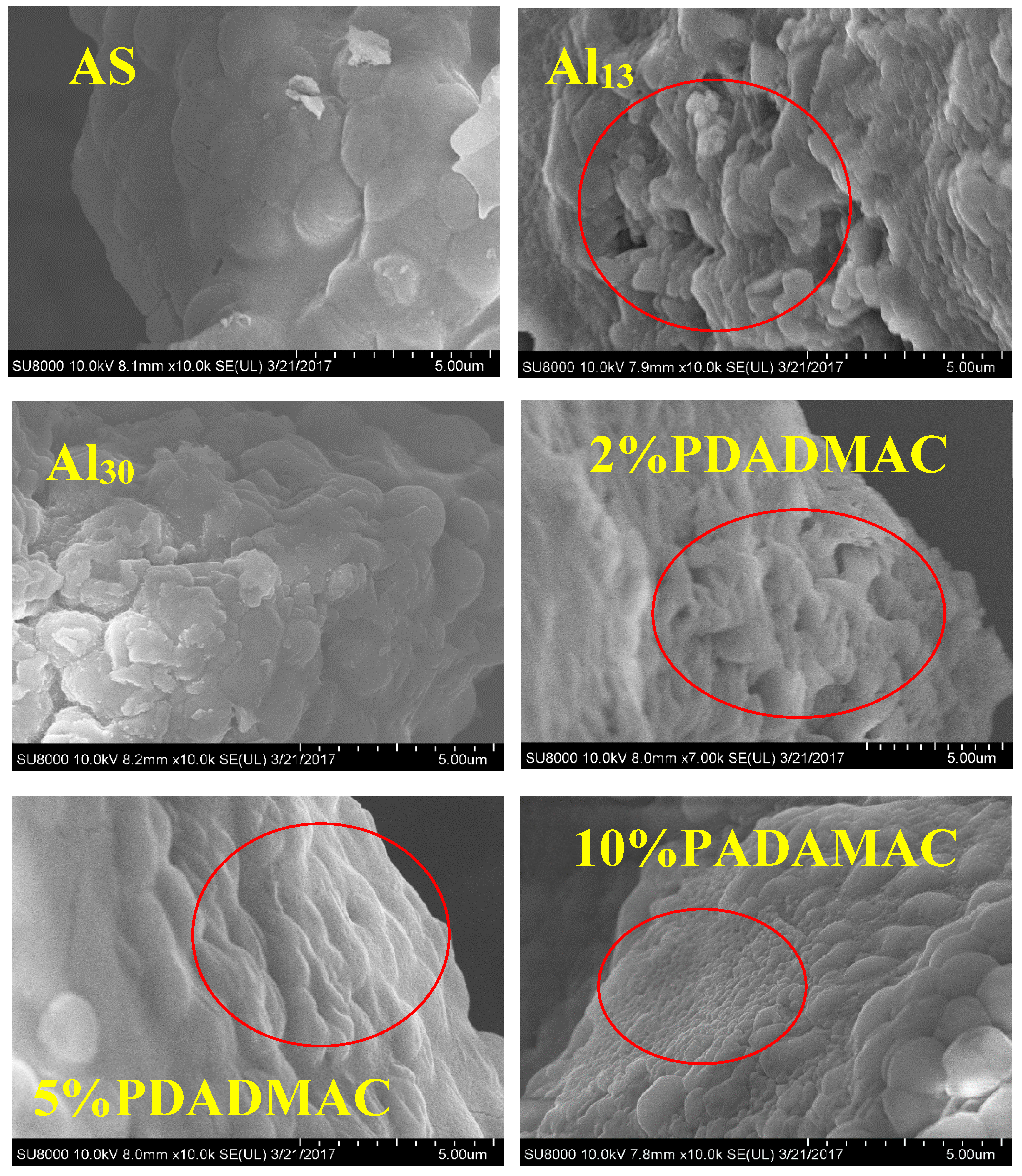
3.3.4. SEM Image for the Flocs under the Optimum Dosage
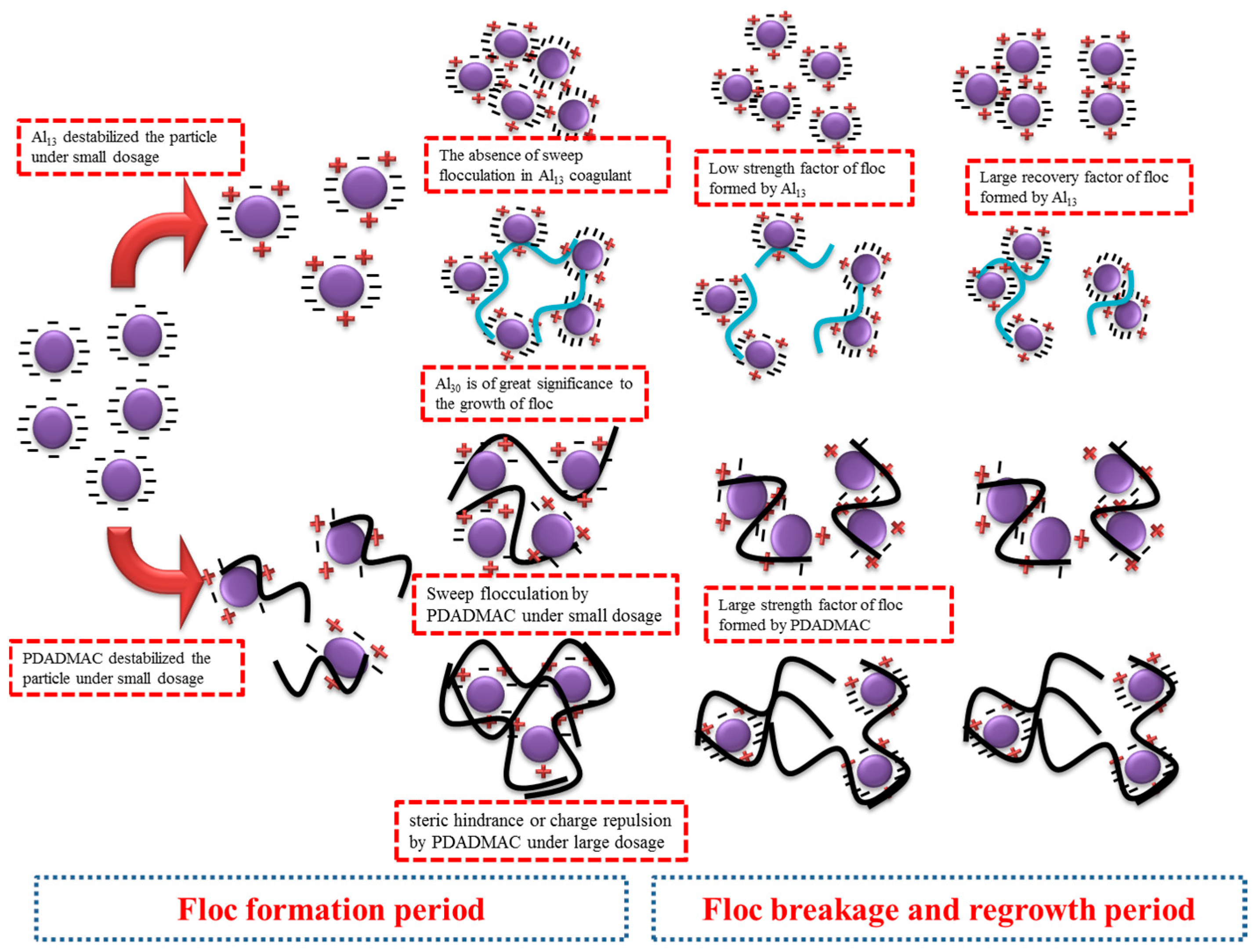
3.4. Mechanism of Collaborative Optimization Process
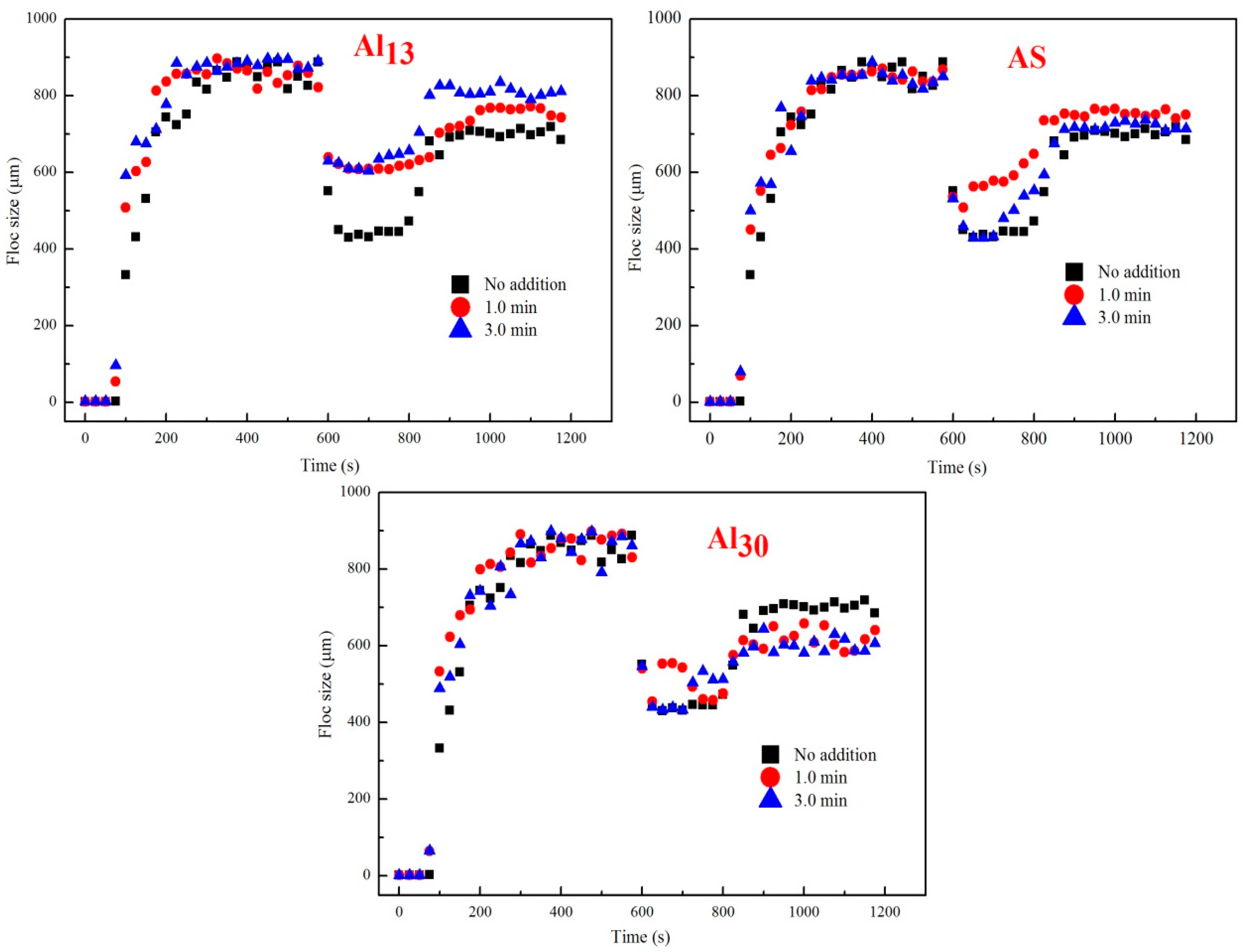
3.5. Coagulation Performance in the Second Addition
4. Conclusions
- Coagulation mainly removed the SMP component in the EOM of M. aeruginosa, and the SMP was mainly removed by the action of electrical neutralization.
- Al13 played an important role in the destabilization and regeneration of algal flocs. A high content of Al13 could make the flocs destabilize rapidly, and the generated flocs have a relatively high recovery factor. Al30 played an important role in the growth and strength of flocs, and the formed flocs had a larger particle size and strength factor. The two coagulants achieved the optimal effect when the ratio of Al13:Al30 = 7:3, and the speed and strength of the formed flocs were both the largest.
- The strength factor of flocs was the result of the combined action of organic matter and coagulant. When the dosage of coagulant increased, on the one hand, the coagulation mechanism transitioned from the action of electrical neutralization to the action of entrapment and sweeping, and on the other hand, it led to a decrease in the content of EOM in algae. Thus, the strength factor of flocs increased.
- The role of PDADMAC coagulant aid in the coagulation process was affected by the dosage. Under the condition of low dosage, its high positive charge and large molecular weight could effectively enhance the electrical neutralization ability and entrapment and sweeping ability of the coagulant. When the dosage was high, the charge reversal phenomenon caused by the excessive positive charge in the system and the steric hindrance phenomenon of large molecules also caused the coagulation efficiency to decrease.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Henderson, R.; Parsons, S.A.; Jefferson, B. The impact of algal properties and pre-oxidation on solid–liquid separation of algae. Water Res. 2008, 42, 1827–1845. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Yang, X.; Ma, J.; Shang, C.; Zhao, Q. Characterization of algal organic matter and formation of DBPs from chlor(am)ination. Water Res. 2010, 44, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Sayinli, B.; Dong, Y.; Park, Y.; Bhatnagar, A.; Mika, S. Recent progress and challenges facing ballast water treatment—A review. Chemosphere 2022, 291, 132776. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xu, H.; Wang, X.; Wang, D.; Duan, J.; Men, B. Influence of coagulation process on the ultrafiltration performance—The roles of Al species and characteristics of algae-laden water. Sep. Purif. Technol. 2017, 183, 32–42. [Google Scholar] [CrossRef]
- Li, L.; Gao, N.; Deng, Y.; Yao, J.; Zhang, K. Characterization of intracellular & extracellular algae organic matters (AOM) of Microcystic aeruginosa and formation of AOM-associated disinfection byproducts and odor & taste compounds. Water Res. 2012, 46, 1233–1240. [Google Scholar] [PubMed]
- Peng, Y.; Yang, X.; Ren, B.; Zhang, Z.; Deng, X.; Yin, W.; Zhou, S.; Yang, S. Algae removal characteristics of the ultrasonic radiation enhanced drinking water treatment process. J. Water Process Eng. 2023, 55, 104154. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Yao, H.; Liu, B.; Ding, M.; Lin, T.; Mo, T.; Gao, L.; Zhang, L. Insights into the role of prechlorination in algae-laden raw water distribution process: Algal organic matter and microcystin-LR release, extracellular polymeric substances (EPS) aggregation, and pipeline biofilm communities. J. Hazard. Mater. 2023, 443, 130306. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lim, J.; Zhan, M.; Hong, S. UV-LED/PMS preoxidation to control fouling caused by harmful marine algae in the UF pretreatment of seawater desalination. Desalination 2019, 467, 219–228. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, D.; Song, X.; Ma, L.; Qi, W.; Li, W. Biofouling metallic membranes in algae-laden water treatment: Performance, mechanism, and the role of the pre-coated layer. J. Membr. Sci. 2024, 691, 122254. [Google Scholar] [CrossRef]
- Liao, X.; Liu, J.; Yang, M.; Ma, H.; Yuan, B.; Huang, C. Evaluation of disinfection by-product formation potential (DBPFP) during chlorination of two algae species-Blue-green Microcystis aeruginosa and diatom Cyclotella meneghiniana. Sci. Total Environ. 2015, 532, 540–547. [Google Scholar] [CrossRef]
- Liu, B.; Wang, Q.; Al-Dhabi, N.A.; Zhang, C.; Tang, W.; Deng, L.; Mao, X.; Chang, H. Mechanistic insight into peracetic acid-enhanced coagulation for algae-laden water treatment. J. Environ. Chem. Eng. 2024, 12, 112041. [Google Scholar] [CrossRef]
- Lin, D.; Zhuang, Z.; Yu, N.; Wang, Z.; Song, W.; Du, X. Comprehensive effects of microplastics on algae-laden surface water treatment by coagulation-ultrafiltration combined process: Algae cultivation, coagulation performance and membrane fouling development. Sci. Total Environ. 2024, 924, 171553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Cai, Z.; Xie, L.; Zhang, Y.; Tang, L.; Zhou, Q.; Qiang, Z.; Zhang, H.; Zhang, D.; Pan, X. Comparison of coagulative colloidal microbubbles with monomeric and polymeric inorganic coagulants for tertiary treatment of distillery wastewater. Sci. Total Environ. 2019, 694, 133649. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Ma, J.; Guan, X.; Song, A.; Cui, Y. Effect of basicity on coagulation performance of polyferric chloride applied in eutrophicated raw water. Desalination 2009, 247, 518–529. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, J.; Li, Z. Enhancing pollutants removal in hospital wastewater: Comparative analysis of PAC coagulation vs. bio-contact oxidation, highlighting the impact of outdated treatment plants. J. Hazard. Mater. 2024, 471, 134340. [Google Scholar] [CrossRef] [PubMed]
- Balbinoti, J.R.; Jorge, R.M.M.; Junior, R.E.D.S.; Balbinoti, T.C.V.; Coral, L.A.D.A.; de Jesus Bassetti, F. Treatment of low-turbidity water by coagulation combining Moringa oleifera Lam and polyaluminium chloride (PAC). J. Environ. Chem. Eng. 2024, 12, 111624. [Google Scholar] [CrossRef]
- Xu, H.; Jiao, R.; Xiao, F.; Wang, D. Relative importance of hydrolyzed Al species (Ala, Alb, Alc) on residual Al and effects of nano-particles (Fe-surface modified TiO2 and Al2O3) on coagulation process. Colloids Surf. A Physicochem. Eng. Asp. 2014, 446, 139–150. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Zhao, X.; Gao, N.; Fu, T. The characteristics of sludge from enhanced coagulation processes using PAC/PDMDAAC composite coagulants in treatment of micro-polluted raw water. Sep. Purif. Technol. 2015, 147, 125–131. [Google Scholar] [CrossRef]
- Wang, D.S.; Zhao, Y.M.; Yan, M.Q.; Chow, C.W.K. Removal of DBP precursors in micro-polluted source waters: A comparative study on the enhanced coagulation behavior. Sep. Purif. Technol. 2013, 118, 271–278. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, J.; Xin, F.; Wang, Y.; Liu, Z.; Wei, J.; Sun, X.; Li, P.; Cao, X.; Zheng, X. Compensatory growth of Microcystis aeruginosa after copper stress and the characteristics of algal extracellular organic matter (EOM). Chemosphere 2024, 352, 141422. [Google Scholar] [CrossRef]
- Wang, X.; Xu, H.; Jiao, R.; Ma, G.; Wang, D. Coagulation removal of phosphorus from a southern China reservoir in different stages of algal blooms: Performance evaluation and AlP matching principle analysis. Sci. Total Environ. 2021, 782, 146849. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.; Lan, H.; Liu, R.; Liu, H.; Qu, J. Efficient Microcystis aeruginosa removal by moderate photocatalysis-enhanced coagulation with magnetic Zn-doped Fe3O4 particles. Water Res. 2020, 171, 115448. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liu, B.; Qu, F.; Ding, A.; Liang, H.; Zhao, Y.; Li, G. Control of ultrafiltration membrane fouling caused by algal extracellular organic matter (EOM) using enhanced Al coagulation with permanganate. Sep. Purif. Technol. 2017, 172, 51–58. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Y.; Hu, M.; Lei, T.; Tian, Z.; Yang, W.; Yang, Z.; Graham, J.D. Influence of DOM characteristics on the flocculation removal of trace pharmaceuticals in surface water by the successive dosing of alum and moderately hydrophobic chitosan. Water Res. 2022, 213, 118163. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Gao, B.Y.; Zhang, G.Z.; Phuntsho, S.; Wang, Y.; Yue, Q.Y.; Li, Q.; Shon, H.K. Comparative study of floc characteristics with titanium tetrachloride against conventional coagulants: Effect of coagulant dose, solution pH, shear force and break-up period. Chem. Eng. J. 2013, 233, 70–79. [Google Scholar] [CrossRef]
- Xia, W.; Wang, Y.; Shen, S.; Yang, H. Efficient and rapid settling removal of algae by using a novel actinia-shaped composite coagulant. Chem. Eng. J. 2023, 470, 144116. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, D.; Xu, Z.; Liu, Y.; Jiao, R.; Wang, D. Study on the effects of organic matter characteristics on the residual aluminum and flocs in coagulation processes. J. Environ. Sci. 2018, 63, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tang, M.; Xiong, X.; Li, Y.; Wang, L.; Feng, X. Removal of algae from ballast water by cationic flocculant-composite titanium xerogel coagulant: Differences in freshwater and seawater. Sep. Purif. Technol. 2024, 337, 126317. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Yan, K.; Wang, Z.; Torres, O.L.; Guo, R.; Chen, J. A new disposal method for systematically processing of ceftazidime: The intimate coupling UV/algae-algae treatment. Chem. Eng. J. 2017, 314, 152–159. [Google Scholar] [CrossRef]
- Li, C.-P.; Du, M. Role of solvents in coordination supramolecular systems. Chem. Commun. 2011, 47, 5958–5972. [Google Scholar] [CrossRef]
- Chen, Z.; Luan, Z.; Fan, J.; Zhang, Z.; Peng, X.; Fan, B. Effect of thermal treatment on the formation and transformation of Keggin Al13 and Al30 species in hydrolytic polymeric aluminum solutions. Colloids Surf. A Physicochem. Eng. Asp. 2007, 292, 110–118. [Google Scholar] [CrossRef]
- Wang, D.; Sun, W.; Xu, Y.; Tang, H.; Gregory, J. Speciation stability of inorganic polymer flocculant–PACl. Colloids Surf. A Physicochem. Eng. Asp. 2004, 243, 1–10. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B.; Wang, Y.; Zhang, Q.; Yue, Q. Influences of polysilicic acid in Al13 species on floc properties and membrane fouling in coagulation/ultrafiltration hybrid process. Chem. Eng. J. 2012, 181–182, 407–415. [Google Scholar] [CrossRef]
- Xu, W.; Gao, B.; Yue, Q.; Wang, Q. Effect of preformed and non-preformed Al13 species on evolution of floc size, strength and fractal nature of humic acid flocs in coagulation process. Sep. Purif. Technol. 2011, 78, 83–90. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation-Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 375, 701–5710. [Google Scholar] [CrossRef] [PubMed]
- Jiao, R.; Xu, H.; Xu, W.; Yang, X.; Wang, D. Influence of coagulation mechanisms on the residual aluminum—The roles of coagulant species and MW of organic matter. J. Hazard. Mater. 2015, 290, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Tabatabai, S.A.A.; Schippers, J.C.; Kennedy, M.D. Effect of coagulation on fouling potential and removal of algal organic matter in ultrafiltration pretreatment to seawater reverse osmosis. Water Res. 2014, 59, 283–294. [Google Scholar] [CrossRef]


| Indicator | Turbidity (NTU) | UV680 | DOC (mg/L) | Zeta Potential (mV) | Protein (%) | HA (%) | SMP (%) |
|---|---|---|---|---|---|---|---|
| value | 6.77 | 0.118 | 0.571 | −27.1 | 17.08 | 36.92 | 46.00 |
| Coagulants | Coagulant Dosage (mmol/L) | Before Breakage (μm) | After Breakage (μm) | After Regrowth (μm) | Sf (%) | Rf (%) |
|---|---|---|---|---|---|---|
| AS | 0.08 | 1194 | 578 | 1014 | 52.61 | 60.58 |
| 0.12 | 1317 | 540 | 1003 | 48.59 | 53.62 | |
| PAC | 0.04 | 580 | 152 | 447 | 26.21 | 68.92 |
| 0.08 | 770 | 192 | 572 | 24.93 | 65.74 | |
| 0.12 | 897 | 336 | 564 | 37.46 | 40.64 | |
| Al13 | 0.02 | 282 | 131 | 435 | 58.86 | 201.324 |
| 0.04 | 687 | 310 | 566 | 45.12 | 67.90 | |
| 0.08 | 422 | 132 | 275 | 31.27 | 49.31 | |
| 0.12 | 329 | 134 | 216 | 40.73 | 42.05 | |
| Al30 | 0.04 | 411 | 187 | 563 | 45.49 | 171.69 |
| 0.08 | 667 | 184 | 460 | 28.58 | 51.49 | |
| 0.12 | 819 | 251 | 463 | 30.65 | 37.32 |
| Coagulants | Coagulant Dosage (mmol/L) | Before Breakage (μm) | After Breakage (μm) | After Regrowth (μm) | Sf (%) | Rf (%) |
|---|---|---|---|---|---|---|
| Al13:Al30 = 9:1 | 0.04 | 853 | 177 | 659 | 20.75 | 71.31 |
| 0.08 | 640 | 154 | 431 | 24.06 | 56.99 | |
| 0.12 | 569 | 232 | 356 | 40.74 | 36.79 | |
| Al13:Al30 = 7:3 | 0.04 | 1038 | 196 | 829 | 18.88 | 75.17 |
| 0.08 | 628 | 142 | 376 | 22.61 | 51.54 | |
| 0.12 | 500 | 195 | 293 | 32.13 | 30.28 | |
| Al13:Al30 = 5:5 | 0.04 | 803 | 400 | 737 | 29.11 | 83.62 |
| 0.08 | 697 | 199 | 465 | 28.55 | 53.41 | |
| 0.12 | 570 | 231 | 354 | 40.52 | 36.28 | |
| Al13:Al30 = 3:7 | 0.04 | 911 | 232 | 651 | 25.46 | 61.70 |
| 0.08 | 973 | 354 | 694 | 36.33 | 54.92 | |
| 0.12 | 720 | 270 | 427 | 37.5 | 34.88 | |
| Al13:Al30 = 1:9 | 0.04 | 632 | 134 | 449 | 21.20 | 63.25 |
| 0.08 | 921 | 257 | 662 | 27.90 | 60.99 | |
| 0.12 | 761 | 304 | 523 | 39.94 | 47.92 |
| Coagulants | Coagulant Dosage (mmol/L) | Before Breakage (μm) | After Breakage (μm) | After Regrowth (μm) | Sf (%) | Rf (%) |
|---|---|---|---|---|---|---|
| PDADMAC:Al13 = 2.0% | 0.04 | 865 | 190 | 604 | 21.96 | 61.33 |
| 0.08 | 631 | 201 | 448 | 31.85 | 57.44 | |
| 0.12 | 398 | 174 | 290 | 43.72 | 51.78 | |
| PDADMAC:Al13 = 5.0% | 0.04 | 913 | 242 | 668 | 26.51 | 63.48 |
| 0.08 | 448 | 143 | 304 | 31.92 | 52.78 | |
| 0.12 | 352 | 143 | 250 | 40.63 | 51.19 | |
| PDADMAC:Al13 = 10.0% | 0.04 | 755 | 158 | 585 | 20.93 | 71.52 |
| 0.08 | 455 | 158 | 313 | 34.72 | 52.18 | |
| 0.12 | 185 | 130 | 138 | 70.27 | 14.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, D.; Zhang, G.; Li, W.; Zhu, N.; Bo, J.; Meng, X.; Chen, Y.; Qin, Y.; Liu, H. Enhanced Coagulation for Algae Removal Using Composite Al-Based Coagulants: Collaborative Optimization Mechanism of Aluminum Morphology. Coatings 2024, 14, 857. https://doi.org/10.3390/coatings14070857
Zhou Y, Zhang D, Zhang G, Li W, Zhu N, Bo J, Meng X, Chen Y, Qin Y, Liu H. Enhanced Coagulation for Algae Removal Using Composite Al-Based Coagulants: Collaborative Optimization Mechanism of Aluminum Morphology. Coatings. 2024; 14(7):857. https://doi.org/10.3390/coatings14070857
Chicago/Turabian StyleZhou, Yangyuan, Dawei Zhang, Guosheng Zhang, Weiying Li, Ningzheng Zhu, Jinpei Bo, Xiangzhou Meng, Yao Chen, Yu Qin, and Huajie Liu. 2024. "Enhanced Coagulation for Algae Removal Using Composite Al-Based Coagulants: Collaborative Optimization Mechanism of Aluminum Morphology" Coatings 14, no. 7: 857. https://doi.org/10.3390/coatings14070857





