Fundamentals of Infrared Heating and Their Application in Thermosetting Polymer Curing: A Review
Abstract
1. Introduction
2. Heat Transfer Process
2.1. Classification and Selection of IR Heaters
2.1.1. Gas-Fired IR Heaters
2.1.2. Gas Catalytic IR Heaters
2.1.3. Electric IR Heaters
2.2. Monitoring Devices
2.2.1. Thermal Imaging
2.2.2. Resistance Temperature Detectors
2.2.3. Thermocouples
2.2.4. Heat Flux Sensors
2.3. Thermal Transfer Model
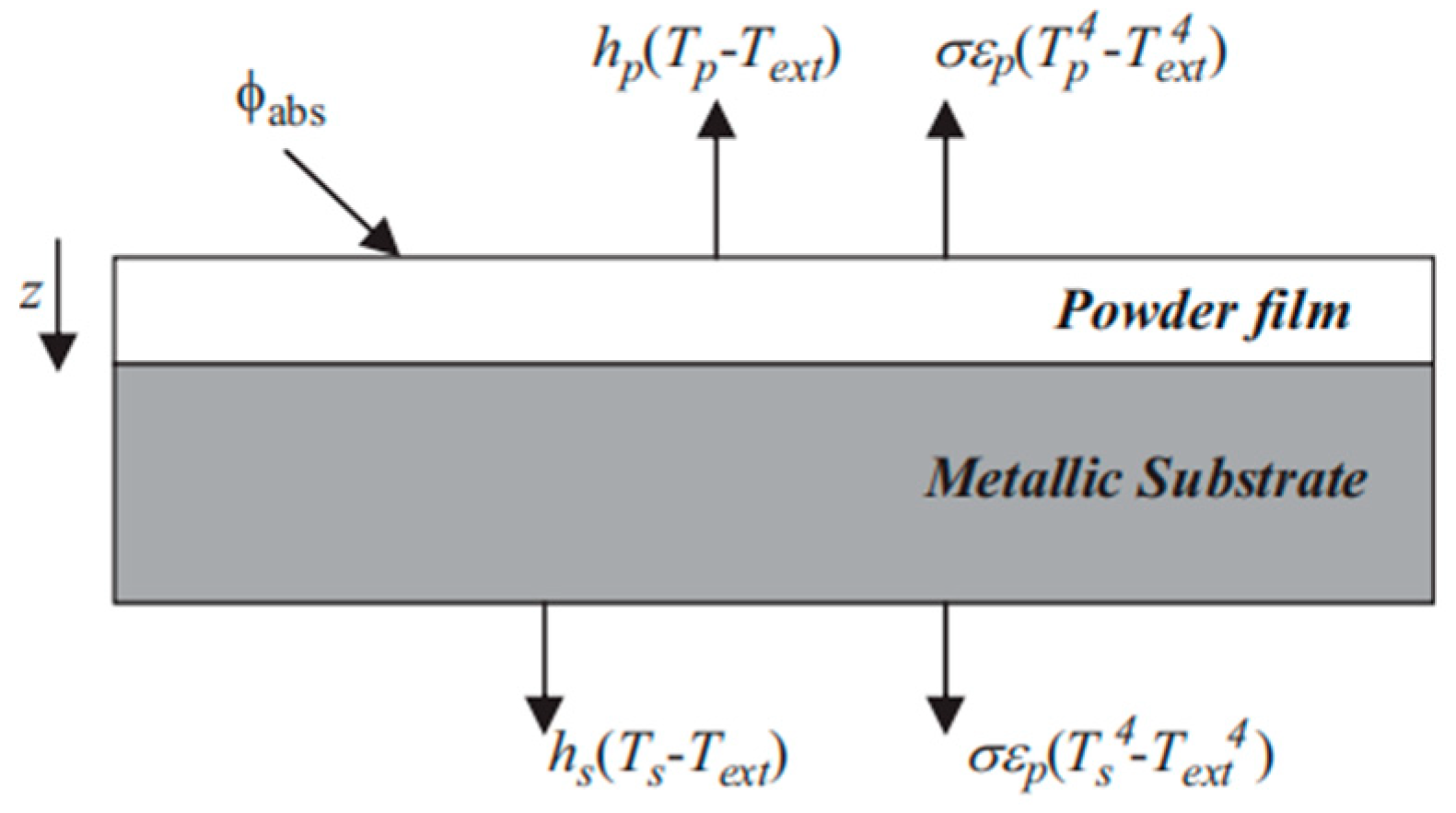
2.3.1. Surface Radiation Transport: Energy Balance and Boundary Conditions
- The model simulates the absorption of the IR energy by the upper surface of the composite via surface-to-surface radiation equations.
- The IR energy absorbed by the upper surface of the composite is integrated into the heat transfer equations as a heat flux boundary condition.
- Heat transfer within the part occurs through conduction to the bottom of the composite.
- The curing degree is calculated based on the heating rate.
- The exothermic heat of the resin is introduced to the heat equations as the heat source.
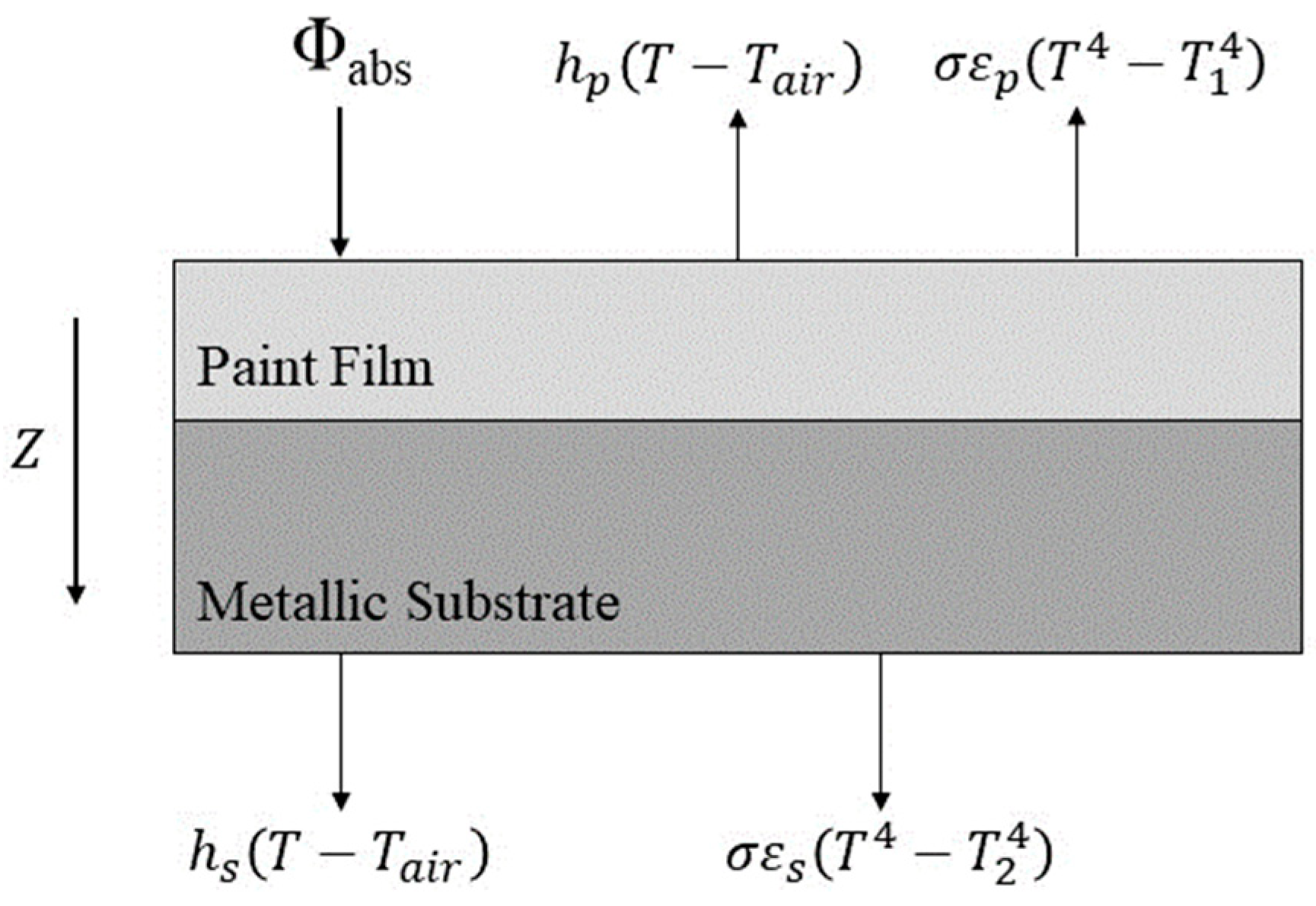
2.3.2. Volume Radiation Transport: Energy Balance and Boundary Conditions
- The film was assumed to be isotropic and homogeneous. Its temperature before curing was uniform and consistent. Its thickness did not change during curing.
- The density, thermal conductivity, specific heat capacity, and other physical properties of the coating and the substrate were considered constant and not affected by the temperature changes.
- The coating thickness was denoted as ‘ep’ and heat transfer occurred only in the Z direction.
- The heat transfer in the substrate was simplified as 1D heat transfer along the Z direction, assuming a uniform heat transfer process.
- The radiative effect of the air was not considered.
- IR rays have a specific penetration capability, allowing objects to be heated within this penetration distance. Considering the thickness of the coating (100 µm) within the IR penetration zone, the IR heating rate of the coating was assumed to be higher than that of hot-air heating. Therefore, it was assumed that the temperature increased simultaneously at every point in the coating. This simplified the heat transfer process by equating the IR radiation heating of the coating with that via an internal heat source.
- Owing to the small thickness of the coating, it was assumed that no IR radiation attenuation occurred along the thickness direction. Therefore, the intensity of the equivalent internal heat source within the coating was considered equal across the entire coating.
- The ambient temperature remains within 5% of its value during experimental testing, reflecting actual fluctuations.
- The area surrounding the coating and the substrate is treated as an insulated boundary, allowing heat exchange only with the surrounding air and not affecting the surface temperature.
2.4. Numerical Simulation Methods
2.4.1. Zone Method
2.4.2. Monte Carlo Method
2.4.3. Ray-Tracing Method
2.4.4. Discrete Ordinates Method
2.4.5. Discrete Transfer Method
2.4.6. Finite Volume Method
3. Curing Process
3.1. Monitoring Techniques and Models
3.1.1. Spectrum Analysis
3.1.2. Electrical Property Analysis
3.1.3. Thermal Property Analysis
3.1.4. Optical Fiber Measurement Analysis
3.1.5. Ultrasonic Analysis
3.1.6. Mechanical Property Analysis
3.2. Curing Theory
3.2.1. Flory–Stockmeyer Theory
3.2.2. Non-Equilibrium Thermodynamic Fluctuation Theory
3.2.3. Avrami Theory
3.2.4. DSC Kinetic Model
3.3. Non-Thermal Effect and Properties of Cured Products
3.3.1. Non-Thermal Effect
3.3.2. Properties of Cured Products
4. Discussion
5. Conclusions
- From the perspectives of energy consumption and wavelength matching, catalytic IR curing technology deserves further research and new application directions.
- For broadening the range of applications, particularly for curing materials (such as energetic materials) and substrates, the potential of IR curing technology for rapid curing at low temperatures needs to be further systematically explored.
- Undeniably, a lot more systematic explorations are further demanded to carry out research on curing monitoring techniques that match IR curing technology (volume absorption).
- Modeling of curing kinetics matching the characteristics of IR curing technology (volume absorption) should be pursued in the future.
- Quantification of the benefits of IR curing is still a complex task, and establishing a simple yet effective evaluation index would aid the research on IR curing technologies and engineering applications.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Cp | Heat capacity |
| e | Thickness |
| E | Spectral irradiance |
| h | Convective exchange coefficient |
| R | Universal gas constant |
| s | Metallic substrate |
| t | Time s |
| T | Temperature |
| x | Conversion degree |
| a | Absorption coefficient |
| αλ | Spectral absorption coefficient |
| e | Emissivity |
| λc | Thermal conductivity |
| λ | Wavelength |
| ρλ | Spectral reflectance |
| σ | Stefan–Boltzmann constant |
| Incident radiative flux | |
| Energy absorbed by the paint film | |
| Energy transmitted by the paint and reflected by the substrate | |
| Energy absorbed by the metallic substrate, | |
| Energy lost by radiative emission from the two faces of a system | |
| A(t) | Absorption peak areas of the group to be analysed |
| A(0) | Absorption peak areas of the group to be analysed at the beginning of the reaction |
| Complex dielectric coefficient | |
| Dielectric coefficient that indicates the storage capacity of the resin | |
| Dielectric loss or loss factor that indicates the energy dissipation part of the resin | |
| Reaction heat of the entire curing process | |
| Reaction heat of the curing reaction at time t | |
| Initial cured modulus of the resin system | |
| Fully cured modulus of the resin system | |
| Em(t) | Modulus at time t |
| Shear energy storage modulus at time t | |
| Shear energy storage modulus at the beginning of curing | |
| Shear energy storage modulus at end of curing | |
| Area below the loss peak at time t | |
| Area below the loss peak at the end of curing | |
| Curing degree at the gelation point | |
| Ratio of the A and B functional groups at the beginning of the reaction | |
| Functionalisation unit concentration | |
| Minimum torque on the experimental curing curve | |
| Maximum torque on the experimental curing curve | |
| Relaxation time | |
| Final elastic modulus | |
| G(t) | Elastic modulus at time t |
| Gelation time, which is indicated on the isothermal curing curve. | |
| Constant of the curing reaction rate after the gelation point | |
| Reaction rate intrinsic to the system | |
| Reaction rate catalysed by protons generated during the curing process. | |
| m, n | Order of reaction |
References
- Du, Z.; Wen, S.; Wang, J.; Yin, C.; Yu, D.; Luo, J. The Review of Powder Coatings. J. Mater. Sci. Chem. Eng. 2016, 04, 54–59. [Google Scholar] [CrossRef]
- Janardhanan, S.; Zvonkina, I.; Soucek, M.D. Powder Coating Technology. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk-Othmer, Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 1–16. ISBN 978-0-471-48494-3. [Google Scholar]
- Satit, P. Computational Fluid Dynamic (CFD) Modeling and Validation of Temperature Distribution in the Infrared Oven. Ph.D. Thesis, Heriot-Watt University, Edinburgh, UK, 2010. [Google Scholar]
- Yi, Y.; Salonitis, K.; Tsoutsanis, P.; Litos, L.; Patsavelas, J. Improving the Curing Cycle Time through the Numerical Modeling of Air Flow in Industrial Continuous Convection Ovens. Procedia CIRP 2017, 63, 499–504. [Google Scholar] [CrossRef]
- Maoult, Y.L.; Schmidt, F. Infrared Radiation Applied to Polymers Processes. In Heat Transfer in Polymer Composite Materials: Forming Processes; Wiley: Hoboken, NJ, USA, 2016; pp. 385–423. [Google Scholar]
- Abliz, D.; Duan, Y.; Steuernagel, L.; Xie, L.; Li, D.; Ziegmann, G. Curing Methods for Advanced Polymer Composites—A Review. Polym. Polym. Compos. 2013, 21, 341–348. [Google Scholar] [CrossRef]
- Jasso-Gastinel, C.F.; Vivero-Marin, J.M. Curing and Properties Comparison of an Unsaturated Polyester Resin, Varying Crosslinking Reaction Conditions. Polym. Compos. 2004, 25, 662–666. [Google Scholar] [CrossRef]
- Subasri, R.; Madhav, C.S.; Somaraju, K.R.C.; Padmanabham, G. Decorative, Hydrophobic Sol–Gel Coatings Densified Using near-Infrared Radiation. Surf. Coat. Technol. 2012, 206, 2417–2421. [Google Scholar] [CrossRef]
- Barletta, M. Flash IR Pre-Curing of the Decorative Layer in Metal-Flake Powder Coatings. Prog. Org. Coat. 2011, 72, 498–510. [Google Scholar] [CrossRef]
- Kumar, P.K.; Raghavendra, N.V.; Sridhara, B.K. Optimization of Infrared Radiation Cure Process Parameters for Glass Fiber Reinforced Polymer Composites. Mater. Des. 2011, 32, 1129–1137. [Google Scholar] [CrossRef]
- Blanc, D.; Laurent, P.; Andrieu, J.; Gerard, J.F. Convective and Radiant (IR) Curing of Bulk and Waterborne Epoxy Coatings as Thin Layers. Part II: Infrared Curing. Polym. Eng. Sci. 1999, 39, 2487–2497. [Google Scholar] [CrossRef]
- Deans, J.; Kögl, M. The Curing of Powder Coatings Using Gaseous Infrared Heaters: An Analytical Model to Assess the Process Thermal Efficiency. Int. J. Therm. Sci. 2000, 39, 762–769. [Google Scholar] [CrossRef]
- Chern, B.-C.; Moon, T.J.; Howell, J.R. On-Line Processing of Unidirectional Fiber Composites Using Radiative Heating: I. Model. J. Compos. Mater. 2002, 36, 1905–1934. [Google Scholar] [CrossRef]
- Kim, J.; Moon, T.J.; Howell, J.R. Transient Thermal Modeling of In-Situ Curing During Tape Winding of Composite Cylinders. J. Heat Transf. 2003, 125, 137–146. [Google Scholar] [CrossRef]
- Véchot, L.; Bombard, I.; Laurent, P.; Lieto, J. Experimental and Modelling Study of the Radiative Curing of a Polyester-Based Coating. Int. J. Therm. Sci. 2006, 45, 86–93. [Google Scholar] [CrossRef]
- Bombard, I.; Laurent, P.; Lieto, J.; Jeandel, G. A Model of the Infrared Cure of Powder Coatings Based on Surface Absorptivities In-Situ Measurements. J. Coat. Technol. Res. 2008, 5, 353–363. [Google Scholar] [CrossRef]
- Basu, S.K.; Shah, B.; Kia, H.G. The Effect of Oven-Heat Flux on Powder-Coating Issues of Sheet Molding Compound Panels. Ind. Eng. Chem. Res. 2009, 48, 1638–1649. [Google Scholar] [CrossRef]
- Bombard, I.; Da Silva, B.; Dufour, P.; Laurent, P. Experimental Predictive Control of the Infrared Cure of a Powder Coating: A Non-Linear Distributed Parameter Model Based Approach. Chem. Eng. Sci. 2010, 65, 962–975. [Google Scholar] [CrossRef]
- Nakouzi, S.; Pancrace, J.; Schmidt, F.M.; Le Maoult, Y.; Berthet, F. Curing Simulation of Composites Coupled with Infrared Heating. Int. J. Mater. Form. 2010, 3, 587–590. [Google Scholar] [CrossRef]
- Kumar, P.K.; Raghavendra, N.V.; Sridhara, B.K. Development of Infrared Radiation Curing System for Fiber Reinforced Polymer Composites: An Experimental Investigation. Indian J. Eng. Mater. Sci. 2011, 18, 24–30. [Google Scholar]
- Mabbett, I.; Elvins, J.; Gowenlock, C.; Glover, C.; Jones, P.; Williams, G.; Worsley, D. Addition of Carbon Black NIR Absorber to Galvanised Steel Primer Systems: Influence on NIR Cure of Polyester Melamine Topcoats and Corrosion Protection Characteristics. Prog. Org. Coat. 2014, 77, 494–501. [Google Scholar] [CrossRef]
- Nakouzi, S.; Berthet, F.; Le Maoult, Y.; Schmidt, F. Simulations of an Infrared Composite Curing Process. Key Eng. Mater. 2013, 554–557, 1517–1522. [Google Scholar] [CrossRef]
- Schmitz, C.; Gökce, B.; Jakobi, J.; Barcikowski, S.; Strehmel, B. Integration of Gold Nanoparticles into NIR-Radiation Curable Powder Resin. ChemistrySelect 2016, 1, 5574–5578. [Google Scholar] [CrossRef]
- Choi, J.W.; Chun, W.P.; Oh, S.H.; Lee, K.J.; Kim, S.I. Experimental Studies on a Combined near Infrared (NIR) Curing System with a Convective Oven. Prog. Org. Coat. 2016, 91, 39–49. [Google Scholar] [CrossRef]
- Krasnovskii, A.N.; Kazakov, I.A. The Study of the Heating and Curing of a Composite Material during the Production of a Composite Reinforcement: Analysis of the Efficiency of an Infrared Heating System. Polym. Sci. Ser. D 2017, 10, 372–375. [Google Scholar] [CrossRef]
- Orth, T.; Krahl, M.; Parlevliet, P.; Modler, N. Optical Thermal Model for LED Heating in Thermoset-Automated Fiber Placement. Adv. Manuf. Polym. Compos. Sci. 2018, 4, 73–82. [Google Scholar] [CrossRef]
- Genty, S.; Tingaut, P.; Aufray, M. Fast Polymerization at Low Temperature of an Infrared Radiation Cured Epoxy-Amine Adhesive. Thermochim. Acta 2018, 666, 27–35. [Google Scholar] [CrossRef]
- Schmitz, C.; Strehmel, B. NIR LEDs and NIR Lasers as Feasible Alternatives to Replace Oven Processes for Treatment of Thermal-Responsive Coatings. J. Coat. Technol. Res. 2019, 16, 1527–1541. [Google Scholar] [CrossRef]
- Zhilyaev, I.; Brauner, C.; Queloz, S.; Jordi, H.; Lüscher, R.; Conti, S.; Conway, R. Controlled Curing of Thermoset Composite Components Using Infrared Radiation and Mathematical Modelling. Compos. Struct. 2021, 259, 113224. [Google Scholar] [CrossRef]
- Uday, M.; KiranKumar, P. Heat Transfer Studies for Infrared Radiation Assisted Curing in Polymer Composites. J. Phys. Conf. Ser. 2020, 1473, 012027. [Google Scholar] [CrossRef]
- Genty, S.; Tingaut, P.; Tendero, C.; Aufray, M. Effects of Infrared Radiation on the Mechanical Properties of an Epoxy-Amine Adhesive Using a Central Composite Design Method. Int. J. Adhes. Adhes. 2022, 112, 102990. [Google Scholar] [CrossRef]
- Stojanović, I.; Logar, M.; Turkalj, L.; Cindrić, I.; Kurtela, M.; Franjić, H. Influence of Catalytic Infrared Radiation on the Protective Properties of Industrial Epoxy Primers. Materials 2023, 16, 6551. [Google Scholar] [CrossRef]
- Yuan, Y.; Pan, S.; Wang, T.; Xia, L.; Liu, Y.; Wang, X.; Li, L.; Wang, T. Experimental and Numerical Investigations on Curing a Polyester-Based Powder Coating by Catalytic Infrared Radiation. Appl. Sci. 2023, 13, 2187. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P. (Eds.) Fundamentals of Heat and Mass Transfer, 7th ed.; Wiley: Hoboken, NJ, USA, 2011; ISBN 978-0-470-50197-9. [Google Scholar]
- Pan, Z.; Atungulu, G.G. (Eds.) Infrared Heating for Food and Agricultural Processing; Contemporary Food Engineering; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4200-9097-0. [Google Scholar]
- Wilson, C.; McGranaghan, G. Infrared Heating Comes of Age. Reinf. Plast. 2014, 58, 43–47. [Google Scholar] [CrossRef]
- Al-Dabbas, M.A. Heating by Catalytic Gas Infrared Rays. Energy Eng. 2011, 108, 26–45. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Tzou, D.-Y.; Huang, Z.-S.; Lee, C.-Y.; Chang, J.-K. High Performance Infrared Heaters Using Carbon Fiber Filaments Decorated with Alumina Layer by Microwave-Assisted Method. J. Taiwan Inst. Chem. Eng. 2016, 59, 521–525. [Google Scholar] [CrossRef]
- Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Food science and technology international series; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA, 2005; ISBN 978-0-12-676757-5. [Google Scholar]
- Kamel, A.; Pascal, D.; Isabelle, B.; Pierre, L. Model Predictive Control of a Powder Coating Curing Process: An Application of the MPC@CB Software. In Proceedings of the 2007 Chinese Control Conference, Zhangjiajie, China, 26–31 July 2007; pp. 630–634. [Google Scholar]
- Nakouzi, S.; Pancrace, J.; Schmidt, F.M.; Le Maoult, Y.; Berthet, F. Infrared Curing Simulations of Liquid Composites Molding; American Institute of Physics: Belfast, UK, 2011; pp. 1125–1130. [Google Scholar]
- Nakouzi, S.; Berthet, F.; Delaunay, D.; Le Maoult, Y.; Schmidt, F.; Sobotka, V. Optimization of the Incident IR Heat Flux upon a 3D Geometry Composite Part (Carbon/Epoxy). Key Eng. Mater. 2012, 504–506, 1085–1090. [Google Scholar] [CrossRef]
- Salagnac, P.; Dutournié, P.; Glouannec, P. Curing of Composites by Radiation and Natural Convection in an Autoclave. AIChE J. 2004, 50, 3149–3159. [Google Scholar] [CrossRef]
- Haglund, J.; Jeppsson, F.; Melander, E.; Pendrill, A.-M.; Xie, C.; Schönborn, K.J. Infrared Cameras in Science Education. Infrared Phys. Technol. 2016, 75, 150–152. [Google Scholar] [CrossRef]
- Lodeiro, M.J.; Mulligan, D.R. Cure Monitoring Techniques for Polymer Composites, Adhesives and Coatings; NPL Measurement Good Practice Guide No 75; NPL: Middlesex, UK, 2005. [Google Scholar]
- Kumari, N.; Sathiya, S. Performance Enhanced Nonlinearity Compensation of Thermocouple Using Convolutional Neural Network. J. Inst. Eng. India Ser. B 2023. [Google Scholar] [CrossRef]
- Carlomagno, G.; De Luca, L.; Cardone, G.; Astarita, T. Heat Flux Sensors for Infrared Thermography in Convective Heat Transfer. Sensors 2014, 14, 21065–21116. [Google Scholar] [CrossRef]
- Hall, M.; Zeng, X.; Shelley, T.; Schubel, P. In Situ Thermoset Cure Sensing: A Review of Correlation Methods. Polymers 2022, 14, 2978. [Google Scholar] [CrossRef]
- Xu, G.; Huang, Y.; Dong, B.; Quan, Y.; Yin, Q.; Chai, J. Design and Performance Evaluation of a Novel Thin-Film Heat Flux Sensor. Case Stud. Therm. Eng. 2023, 47, 103121. [Google Scholar] [CrossRef]
- Khan, M.I.H.; Batuwatta-Gamage, C.P.; Karim, M.A.; Gu, Y. Fundamental Understanding of Heat and Mass Transfer Processes for Physics-Informed Machine Learning-Based Drying Modelling. Energies 2022, 15, 9347. [Google Scholar] [CrossRef]
- Dai, G.; Huangfu, J.; Wang, X.; Du, S.; Zhao, T. A Review of Radiative Heat Transfer in Fixed-Bed Particle Solar Receivers. Sustainability 2023, 15, 9918. [Google Scholar] [CrossRef]
- Incropera, F.P.; DeWitt, D.P.; Bergman, T.L.; Lavine, A.S. (Eds.) Principles of Heat and Mass Transfer, 7th ed.; International Student Version; Wiley: Hoboken, NJ, USA, 2013; ISBN 978-0-470-50197-9. [Google Scholar]
- Hu, G.; Zhang, Y.; Du, W.; Long, J.; Qian, F. Zone Method Based Coupled Simulation of Industrial Steam Cracking Furnaces. Energy 2019, 172, 1098–1116. [Google Scholar] [CrossRef]
- Zhang, J. Modern Monte Carlo Methods for Efficient Uncertainty Quantification and Propagation: A Survey. WIREs Comput. Stat. 2021, 13, e1539. [Google Scholar] [CrossRef]
- Tan, H.; Liu, L.; Yi, H.; Zhao, J.; Qi, H.; Tan, J. Recent Progress in Computational Thermal Radiative Transfer. Chin. Sci. Bull. 2009, 54, 4135–4147. [Google Scholar] [CrossRef]
- Cosson, B.; Schmidt, F.; Le Maoult, Y.; Bordival, M. Infrared Heating Stage Simulation of Semi-Transparent Media (PET) Using Ray Tracing Method. Int. J. Mater. Form. 2011, 4, 1–10. [Google Scholar] [CrossRef]
- Zhao, J.M.; Liu, L.H. Radiative Transfer Equation and Solutions. In Handbook of Thermal Science and Engineering; Kulacki, F.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–46. ISBN 978-3-319-32003-8. [Google Scholar]
- Krishna, N.A.; Mishra, S.C. Discrete Transfer Method Applied to Radiative Transfer in a Variable Refractive Index Semitransparent Medium. J. Quant. Spectrosc. Radiat. Transf. 2006, 102, 432–440. [Google Scholar] [CrossRef]
- Gong, G.; Li, K.; Xie, G. The application of discrete transfer method in the simulation of radiation heating or cooling. Int. J. Archit. Sci. 2003, 4, 1–5. [Google Scholar]
- Coelho, P.J. Numerical Simulation of Radiative Heat Transfer from Non-Gray Gases in Three-Dimensional Enclosures. J. Quant. Spectrosc. Radiat. Transf. 2002, 74, 307–328. [Google Scholar] [CrossRef]
- Nirgudkar, H.; Kumar, S.; Srivastava, A. Solution of Radiative Transfer Equation Using Discrete Transfer Method for Two-Dimensional Participating Medium. Int. Commun. Heat Mass Transf. 2015, 61, 88–95. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. ‘Cure Index’ for Thermoset Composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Kiamanesh, A. The Effect of Micro and Nano-Sized Particles on Mechanical and Adhesion Properties of a Clear Polyester Powder Coating. Prog. Org. Coat. 2013, 76, 1625–1632. [Google Scholar] [CrossRef]
- Hardis, R.; Jessop, J.L.P.; Peters, F.E.; Kessler, M.R. Cure Kinetics Characterization and Monitoring of an Epoxy Resin Using DSC, Raman Spectroscopy, and DEA. Compos. Part Appl. Sci. Manuf. 2013, 49, 100–108. [Google Scholar] [CrossRef]
- Castell, P.; Wouters, M.; Fischer, H.; De With, G. Kinetic Studies of a UV-Curable Powder Coating Using Photo-DSC, Real-Time FTIR and Rheology. J. Coat. Technol. Res. 2007, 4, 411–423. [Google Scholar] [CrossRef]
- Tang, Z.S.; Lim, Y.Y.; Smith, S.T.; Izadgoshasb, I. Development of Analytical and Numerical Models for Predicting the Mechanical Properties of Structural Adhesives under Curing Using the PZT-Based Wave Propagation Technique. Mech. Syst. Signal Process. 2019, 128, 172–190. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karabela, M.M.; Varkopoulou, E.A.; Sideridou, I.D. Cure Kinetics Study of Two Epoxy Systems with Fourier Tranform Infrared Spectroscopy (FTIR) and Differential Scanning Calorimetry (DSC). J. Macromol. Sci. Part A 2012, 49, 630–638. [Google Scholar] [CrossRef]
- Garden, L.H.; Hayward, D.; Pethrick, R.A. Dielectric Non-Destructive Testing Approach to Cure Monitoring of Adhesives and Composites. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2007, 221, 521–533. [Google Scholar] [CrossRef]
- Kwon, J.W.; Chin, W.S.; Lee, D.G. In Situ Cure Monitoring of Adhesively Bonded Joints by Dielectrometry. J. Adhes. Sci. Technol. 2003, 17, 2111–2130. [Google Scholar] [CrossRef]
- Maistros, G.M.; Bucknall, C.B. Modeling the Dielectric Behavior of Epoxy Resin Blends during Curing. Polym. Eng. Sci. 1994, 34, 1517–1528. [Google Scholar] [CrossRef]
- Bilyeu, B.; Brostow, W.; Menard, K.P. Epoxy thermosets and their applications II. Thermal analysis. J. Mater. Educ. 2000, 22, 107–130. [Google Scholar]
- Ramis, X.; Cadenato, A.; Morancho, J.M.; Salla, J.M. Curing of a Thermosetting Powder Coating by Means of DMTA, TMA and DSC. Polymer 2003, 44, 2067–2079. [Google Scholar] [CrossRef]
- Mafi, R.; Mirabedini, S.M.; Attar, M.M.; Moradian, S. Cure Characterization of Epoxy and Polyester Clear Powder Coatings Using Differential Scanning Calorimetry (DSC) and Dynamic Mechanical Thermal Analysis (DMTA). Prog. Org. Coat. 2005, 54, 164–169. [Google Scholar] [CrossRef]
- Gedan-Smolka, M.; Lehmann, D.; Çetin, S. Basic Investigations for Development of New Curing Mechanisms for Powder Coatings. Prog. Org. Coat. 1998, 33, 177–185. [Google Scholar] [CrossRef]
- Olivier, P.; Mulle, M.; Paris, C.; Collombet, F. Carbon/Polymeric Composites Autoclave Cure Monitoring with Optical Fiber Bragg Grating (FBG) Sensors. In Wiley Encyclopedia of Composites; Wiley: Hoboken, NJ, USA, 2012; pp. 1–11. ISBN 978-0-470-12828-2. [Google Scholar]
- Cusano, A.; Breglio, G.; Giordano, M.; Calabrò, A.; Cutolo, A.; Nicolais, L. An Optoelectronic Sensor for Cure Monitoring in Thermoset-Based Composites. Sens. Actuators Phys. 2000, 84, 270–275. [Google Scholar] [CrossRef]
- Raišutis, R.; Kažys, R.; Mažeika, L. Application of the Ultrasonic Pulse-Echo Technique for Quality Control of the Multi-Layered Plastic Materials. NDT E Int. 2008, 41, 300–311. [Google Scholar] [CrossRef]
- Lionetto, F.; Maffezzoli, A. Polymer Characterization by Ultrasonic Wave Propagation. Adv. Polym. Technol. 2008, 27, 63–73. [Google Scholar] [CrossRef]
- Pavlopoulou, S.; Soutis, C.; Staszewski, W.J. Cure Monitoring through Time–Frequency Analysis of Guided Ultrasonic Waves. Plast. Rubber Compos. 2012, 41, 180–186. [Google Scholar] [CrossRef]
- Jang, B.Z.; Shih, W.K. TECHNIQUES FOR CURE MONITORING OF THERMOSET RESINS AND COMPOSITES—A REVIEW. Mater. Manuf. Process. 1990, 5, 301–331. [Google Scholar] [CrossRef]
- Zhou, B.; Shen, M.; Wang, Q.; Chen, Q. Kinetic Study of Polysulfide-acrylate Click Reaction by DEA and DMA. Polym. Adv. Technol. 2011, 22, 2374–2381. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zou, S.; Liu, H.; Wang, G.; Cheng, Z.; Yang, Z.; Xue, C.; Liu, J.; Yang, S.; et al. Dynamic Test of the Continuously Variable Weak Force by a Torsion Pendulum with Pre-Applied Stress. Measurement 2024, 228, 114341. [Google Scholar] [CrossRef]
- Liao, X.; Jiao, Y.; Xie, J.; Chen, L. Fiber-Level Modeling of 3D Braided Preforms Using Virtual Braiding Method. Compos. Struct. 2024, 334, 117988. [Google Scholar] [CrossRef]
- Zheng, S.X.; Chen, H.S. Correlations of Rheological Methods to Coatings’ Performance. Prog. Org. Coat. 2023, 177, 107403. [Google Scholar] [CrossRef]
- Omrani, A.; Rostami, A.; Ravari, F.; Ehsani, M. The Relationship between Composition and Electrical Properties of Brass Covered by a Nanocomposite Coating of Glycerol Diglycidyl Ether: An EIS and DMTA Study. Prog. Org. Coat. 2013, 76, 360–366. [Google Scholar] [CrossRef]
- Dang, T.D.; Do, T.K.M.; Vu, M.D.; Le, N.L.; Vu, T.H.; Vu, H.N. Nonlinear Torsional Buckling of Corrugated Core Sandwich Toroidal Shell Segments with Graphene-Reinforced Coatings in Temperature Change Using the Ritz Energy Method. Appl. Math. Model. 2024, 126, 739–752. [Google Scholar] [CrossRef]
- Osterhold, M.; Niggemann, F. Viscosity–Temperature Behaviour of Powder Coatings. Prog. Org. Coat. 1998, 33, 55–60. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Khan, S.A. Real-Time FTIR and in Situ Rheological Studies on the UV Curing Kinetics of Thiol-Ene Polymers. Macromolecules 1997, 30, 7322–7328. [Google Scholar] [CrossRef]
- Ivankovic, M.; Incarnato, L.; Kenny, J.M.; Nicolais, L. Curing Kinetics and Chemorheology of Epoxy/Anhydride System. J. Appl. Polym. Sci. 2003, 90, 3012–3019. [Google Scholar] [CrossRef]
- Kim, S.-W.; Lu, M.-G.; Shim, M.-J. The Isothermal Cure Kinetic of Epoxy/Amine System Analyzed by Phase Change Theory. Polym. J. 1998, 30, 90–94. [Google Scholar] [CrossRef]
- Ton-That, M.-T.; Ngo, T.-D.; Ding, P.; Fang, G.; Cole, K.C.; Hoa, S.V. Epoxy Nanocomposites: Analysis and Kinetics of Cure. Polym. Eng. Sci. 2004, 44, 1132–1141. [Google Scholar] [CrossRef]
- Chen, D.Z.; He, P.S.; Pan, L.J. Cure Kinetics of Epoxy-Based Nanocomposites Analyzed by Avrami Theory of Phase Change. Polym. Test. 2003, 22, 689–697. [Google Scholar] [CrossRef]
- Konishi, T.; Miyamoto, Y. Polymer Crystallization Process at High Supercooling: Crystallization with Nodular Aggregation Region near the Glass Transition. Polymer 2024, 304, 127131. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, X.; Zhu, X.; Luo, C.; Li, D.; Liu, Q.; Wang, K. Curing Kinetics Study of Thermosetting Resin Material with Ultra-Low Dielectric Loss for Advanced Electronic Packaging. Polym. Test. 2024, 130, 108312. [Google Scholar] [CrossRef]
- Narishige, K.; Katsunuma, T.; Honda, M.; Yatsuda, K. EUV Resist Curing Technique for LWR Reduction and Etch Selectivity Enhancement; Zhang, Y., Ed.; Semantic: San Jose, CA, USA, 2012; p. 83280N. [Google Scholar]
- Safarpour, M.A.; Omrani, A.; Afsar, S.; Zare-Hossein-Abadi, D. Study of Cure Kinetics of Epoxy/DDS/Nanosized (SiO2/TiO2) System by Dynamic Differential Scanning Calorimetry. Polym. Adv. Technol. 2011, 22, 718–723. [Google Scholar] [CrossRef]
- Knischka, R.; Lehmann, U.; Stadler, U.; Mamak, M.; Benkhoff, J. Novel Approaches in NIR Curing Technology. Prog. Org. Coat. 2009, 64, 171–174. [Google Scholar] [CrossRef]
- Hay, J.N.; O’Gara, P. Recent Developments in Thermoset Curing Methods. Proc. Inst. Mech. Eng. Part G J. Aerosp. Eng. 2006, 220, 187–195. [Google Scholar] [CrossRef]
- Sawyer, M. Infrared Curing Systems Offer Alternative to Tried-and-True Convection Heat Sources. Met. Finish. 2006, 104, 9–11. [Google Scholar] [CrossRef]
- Alpay, Y.; Uygur, I.; Kilincel, M. On the Optimum Process Parameters of Infrared Curing of Carbon Fiber-Reinforced Plastics. Polym. Polym. Compos. 2020, 28, 433–439. [Google Scholar] [CrossRef]
- Bordival, M.; Schmidt, F.M.; Maoult, Y.L.; Velay, V. Optimization of Preform Temperature Distribution for the Stretch-blow Molding of PET Bottles: Infrared Heating and Blowing Modeling. Polym. Eng. Sci. 2009, 49, 783–793. [Google Scholar] [CrossRef]
- Monteix, S.; Schmidt, F.; Le Maoult, Y.; Ben Yedder, R.; Diraddo, R.W.; Laroche, D. Experimental Study and Numerical Simulation of Preform or Sheet Exposed to Infrared Radiative Heating. J. Mater. Process. Technol. 2001, 119, 90–97. [Google Scholar] [CrossRef]
- Monteix, S.; Le Maoult, Y.; Schmidt, F.; Arcens, J.P. Quantitative Infrared Thermography Applied to Blow Moulding Process: Measurement of a Heat Transfer Coefficient. Quant. InfraRed Thermogr. J. 2004, 1, 133–150. [Google Scholar] [CrossRef]
- Endruweit, A.; Johnson, M.S.; Long, A.C. Curing of Composite Components by Ultraviolet Radiation: A Review. Polym. Compos. 2006, 27, 119–128. [Google Scholar] [CrossRef]
- Alcón, N.; Tolosa, A.; Rodríguez, M.T.; Moreno, C. Development of Photoluminescent Powder Coatings by UV Curing Process. Prog. Org. Coat. 2010, 68, 88–90. [Google Scholar] [CrossRef]
- Czachor-Jadacka, D.; Pilch-Pitera, B. Progress in Development of UV Curable Powder Coatings. Prog. Org. Coat. 2021, 158, 106355. [Google Scholar] [CrossRef]
- Patil, R.S.; Thomas, J.; Patil, M.; John, J. To Shed Light on the UV Curable Coating Technology: Current State of the Art and Perspectives. J. Compos. Sci. 2023, 7, 513. [Google Scholar] [CrossRef]
- Yuan, Q.; Yang, M.-B.; Mai, Y.-W. Ultraviolet Curing of Glass Fibre Reinforced Polyester Composites. Adv. Compos. Lett. 2000. [Google Scholar] [CrossRef]
- Li, M.; Tucker, C.L., III. Optimal Curing for Thermoset Matrix Composites: Thermochemical and Consolidation Considerations. Polym. Compos. 2002, 23, 739–757. [Google Scholar] [CrossRef]
- Broyer, E.; Macosko, C.W. Heat Transfer and Curing in Polymer Reaction Molding. AIChE J. 1976, 22, 268–276. [Google Scholar] [CrossRef]
- Matsuzaki, R.; Kobara, T.; Yokoyama, R. Efficient Estimation of Thermal Conductivity Distribution during Curing of Thermoset Composites. Adv. Compos. Mater. 2021, 30, 34–49. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Y.C.; Huang, P. Effect of Cure Cycle on Curing Process and Hardness for Epoxy Resin. 2009. Express Polym. Lett. 2009, 3, 534–541. [Google Scholar] [CrossRef]
- Reboredo, M.M.; Vazquez, A. Curing of Thermosetting Polymers by an External Fluid. Polym. Eng. Sci. 1995, 35, 1521–1526. [Google Scholar] [CrossRef]
- Capehart, T.W.; Kia, H.G.; Abujoudeh, T. Cure Simulation of Thermoset Composite Panels. J. Compos. Mater. 2006, 41, 1339–1360. [Google Scholar] [CrossRef]
- Bird, D.; Caravaca, E.; Laquidara, J.; Luhmann, K.; Ravindra, N.M. Formulation of Curable Resins Utilized in Stereolithography. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; The Minerals, Metals & Materials Series; Springer International Publishing: Cham, Switzerland, 2019; pp. 1575–1587. ISBN 978-3-030-05860-9. [Google Scholar]
- Guo, H.; Xu, S.; Gao, H.; Geng, X.; An, C.; Xu, C.; Li, Q.; Wang, S.; Ye, B.; Wang, J. CL-20 Based Ultraviolet Curing Explosive Composite with High Performance. Propellants Explos. Pyrotech. 2019, 44, 935–940. [Google Scholar] [CrossRef]



| Material | Substrate | Condition | IR Source | Reference |
|---|---|---|---|---|
| DGEBA-type epoxy system | Zinc steel plates | Near-IR (NIR) panel heaters | [11] | |
| Powder coating | Gaseous IR heaters | [12] | ||
| Unidirectional fiber composites | IR heaters | [13] | ||
| AS4/3501-6 epoxy resin prepreg | AISI 304 stainless steel | IR heaters | [14] | |
| Glass fiber-reinforced unsaturated polyester-styrene system | 85 °C/3 h | Long-wavelength IR radiation, medium-wavelength IR radiation | [7] | |
| Polyester + TGIC powder coating/polyester-based powder coating | Steel standard test panels | 255 °C/105 s | IR radiation (SIR, MIR, NIR) | [15] |
| Polyester-based system powder/epoxy–polyester-based system | steel panels | 255 °C/105 s | IR radiation (SIR, MIR, NIR) | [16] |
| Polyester epoxy hybrid powder primer | SMC (sheet molding compound) panels and sheet metal panels | IR heaters | [17] | |
| Thermosetting powders/polyester-based system | Steel standard test panels | 255 °C/105 s | IR lamps (MWIR, SWIR, NIR) | [18] |
| Glass fiber-reinforced DGEBA-type epoxy system | IR heaters | [10] | ||
| Epoxy resin (RTM-6) | IR heaters | [19] | ||
| BADGE type epoxy resin/4-4′-aminophenylmethylaniline | 28–85 °C/150 min and 28–150 °C/146 min | IR heater (3–8 μm wavelength, 2 kW capacity) | [20] | |
| Epoxy polyester/polyurethane powders | Metal sheets | 1.5 kW/30 s or 2 kW/20–30 s | IR radiation setting the power of IR lamps within the range of 1.0–2.0 kW | [9] |
| Decorative hydrophobic coatings | 250 °C/30 min | NIR radiation | [8] | |
| Polyestermelamine paints/highly absorbent pigments | Steel galvanized with zinc | Convective ovens, NIR heaters | [21] | |
| Carbon fiber-reinforced epoxy matrix | IR heaters | [22] | ||
| Gold nanoparticles integrated into a maleate polyester | NIR | [23] | ||
| Solvent-blended acrylic resin (polymer matrix)/melamine as hardener | Steel sheet | NIR heating module with 12 tungsten-halogen lamps/2990 K and 4.3 kW | [24] | |
| Composites made of epoxide thermoset resin | IR heaters | [5] | ||
| Polymer–composite rods reinforced with fibers | Ceramic IR heaters | [25] | ||
| Thermoset-automated fiber placement | LED-based heating unit | [26] | ||
| Poly-epoxide adhesive/epoxy pre-polymer | 50 °C/40 min | IR heaters | [27] | |
| One-component thermoset coatings | 175 °C/15 min | NIR heaters | [28] | |
| Catalyzed cyanate ester resin | Aluminum sheet | 260 °C | Medium wavelength heaters (3–7 µm) | [29] |
| Fiber-reinforced polymer composites | 28–85 °C/10–60 min | IR heaters | [30] | |
| Polyepoxy adhesive/epoxy prepolymer | IR heaters | [31] | ||
| Solvent-borne epoxy primers | Gas catalytic IR heaters | [32] | ||
| Polyester-based powder coatings/triglycidyl isocyanurate (TGIC) polyester system | 220 °C/3 min and 230 °C/2 min | Catalytic IR heaters | [33] |
| Gas-Fired IR Heaters | Gas Catalytic IR Heaters | Electric IR Heaters | |
|---|---|---|---|
| Wavelength range (μm) | 2–4.5 | 3–7 | 0.7–1.5 |
| Temperature rating (°C) | 800–1200 | 350–500 | 500–1000 |
| Thermal efficiency (%) | 80%–85% | 85%–90% | 70%–80% |
| Response time | Medium | Slow | Fast |
| Working lifespan (Years) | 10–15 | 15–20 | 5–10 |
| Advantages | High heat output, efficient large-area heating | Uniform heat distribution, energy-efficient | Fast response time, precise temperature control |
| Limitations | Low precision, high operating temperature | Slow response time, low temperature range | High operating cost, short lifespan |
| Literature | Deans et al., 1999 [2] | Yi et al., 2023 [6] | [16] [40] [18] [15] [29] [13] [19] [41] [42] [10] [27] [43] |
| Material Properties | Typical Instrument | Limitations | Reference |
|---|---|---|---|
| Thermal property | Thermocouple Pyrometer temperature | Low accuracy and restricted temperature range | [18] [16] [40] [15] [29] [22] [27] [43] |
| IR camera | Accuracy affected by the ambient temperature, distance, and geometry | [22] | |
| Electric property | RTD | Fragile and somewhat destructive | |
| Heat flux | Thermopile calorimetric | High calibration complexity and high sensitivity to thermal regulation | [18] [16] |
| Model | Heat Transfer Method | Geometric Model | Controller | Software | Reference | ||
|---|---|---|---|---|---|---|---|
| Radiation | Radiation and Convection | 3D/2D Model | 1D Model | ||||
| Surface absorption | √ | √ | PID | Matlab | [16] | ||
| √ | √ | MPC | - | [18] | |||
| √ | √ | PID & MPC | - | [40] | |||
| √ | √ | PID | COMSOL | [29] | |||
| √ | √ | - | - | [13] | |||
| √ | √ | - | COMSOL | [19] | |||
| √ | √ | - | COMSOL | [41] | |||
| √ | √ | SQP | Matlab & COMSOL | [42] | |||
| √ | √ | - | COMSOL | [22] | |||
| √ | √ | - | - | [12] | |||
| √ | √ | - | FLUENT | [43] | |||
| Volume absorption | √ | √ | Matlab | [15] | |||
| √ | √ | ANSYS | [33] | ||||
| √ | √ | - | - | [13] | |||
| √ | √ | - | - | [27] | |||
| Calculation Method | Complex Shapes | Anisotropic Scattering | Inhomogeneous Media and Variable Parameters | Semi-Transparent Interfaces | Calculation Accuracy | Reference |
|---|---|---|---|---|---|---|
| Zone Method | − | − | +/− | − | Good | |
| MCM | + | + | +/− | ++ | Good | [12] |
| RTM | −− | ++ | +/− | ++ | Very good | [41] |
| DTM | − | −− | +/− | +/− | Good | |
| DOM | + | + | + | +/− | Moderate | |
| FVM | + | + | + | + | Good |
| Method Classification | Representative Measurement Methods | Measured Physical Quantity | Reference |
|---|---|---|---|
| Chemical reactions | Chemical titration | Chemical group concentration | |
| IR, Raman, and other spectroscopic methods | Spectral signal intensity of chemical bonds | ||
| Thermal properties | Differential thermal analysis (DSC, DTA) | Heat fusion changes during curing | [19] [41] [42] [22] [18] [16] [40] [15] [42] [27] [43] |
| Electrical properties | Dielectric analysis (DEA) | Dielectric loss and ionic conductivity, and resistance | [29] |
| Mechanical properties | Dynamic spring method, dynamic thermo-mechanical analysis, dynamic torsion vibration method | Correlation of mechanical modulus and mechanical loss | |
| Fiber optic-based measurements | Fiber optic monitoring | Change in refractive index or absorption of signal waves | |
| Ultrasound measurements | Ultrasonic monitoring | Longitudinal ultrasonic velocity and attenuation | |
| Other methods | Viscosity method, hardness method, swelling method | Corresponding physical properties |
| Theory | Main Feature | Reference |
|---|---|---|
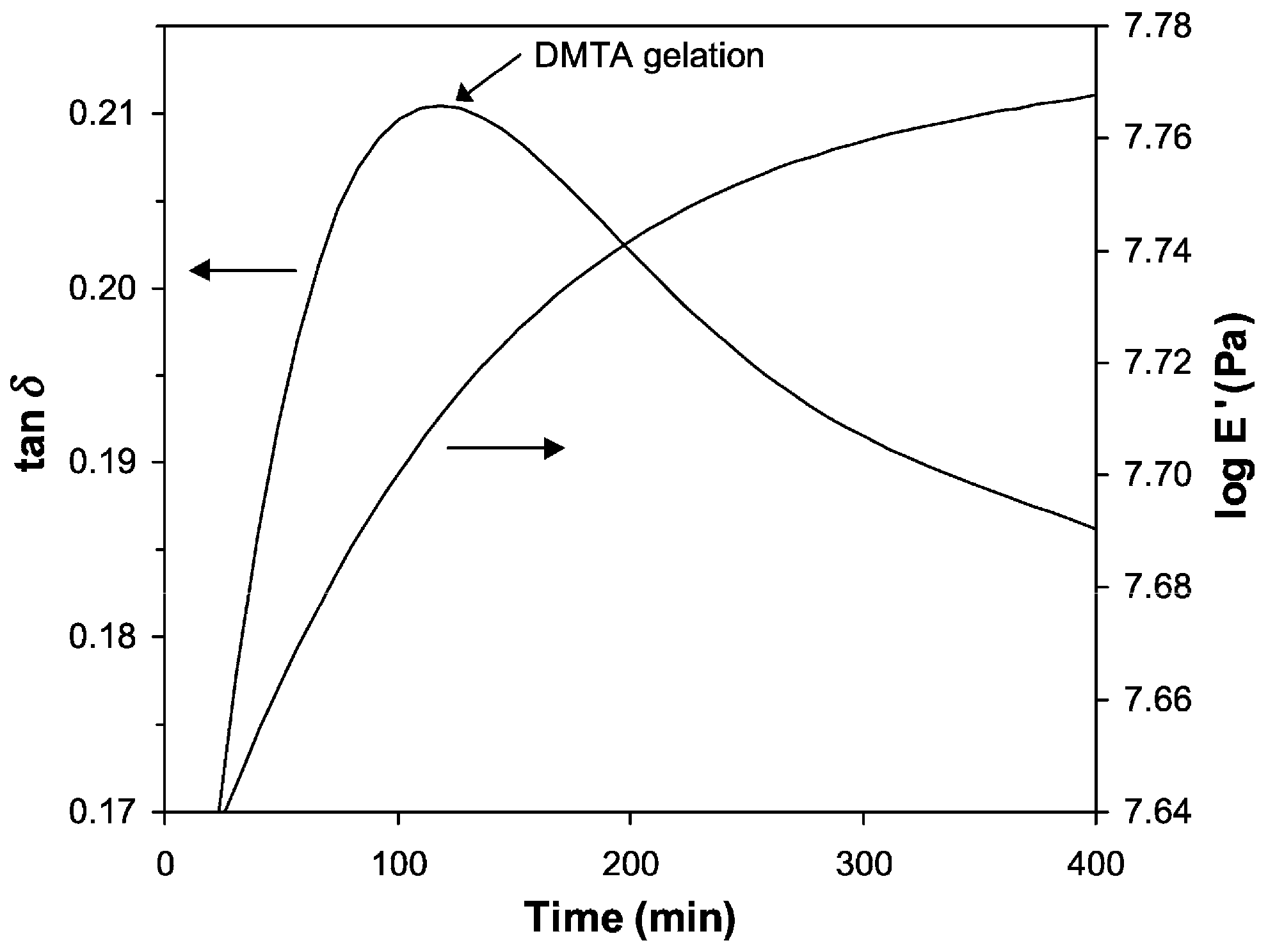
| Gelation theory | The critical conditions of gelation are derived theoretically; the chemical conversion at the gelation point depends only on the curing components in the system and is not related to the reaction temperature and experimental conditions. | [27] |
| DSC kinetic model | The reaction details in the curing process are ignored, and the entire curing process is considered a virtual macroscopic reaction. The kinetic characteristics of the virtual reaction (e.g., reaction order, activation energy, etc.) are investigated from a macroscopic perspective. | [18] [16] [40] [15] [29] [19] [41] [42] [22] [43] |
| Avrami theory | The solidification process is compared with the crystallization process of polymers. The micro-mechanism of the solidification process is analyzed using the crystallization kinetics equation. | |
| Non-equilibrium thermodynamic fluctuation theory | This theory connects the curing degree with the physical and mechanical properties of the resin and can predict their change during curing. |
| Absorption Model | Monitoring Device | Curing Theory | Reference |
|---|---|---|---|
| Surface absorption | DSC | DSC kinetic model | [19] [41] [42] [22] [18] [16] [40] [15] [42] [43] |
| DEA | DSC kinetic model | [29] | |
| Volume absorption | DSC | Gelation theory | [27] |
| Curing Method | Material | Penetration | Applicability | Cost | Reference |
|---|---|---|---|---|---|
| Infrared | No special requirements for polymers, good applicability, universality, and potential | Highly affected by physical properties, low penetration into carbon fiber and glass/natural fiber polymer | Potential for fast curing, good at handling flexible workpieces, high controllability | Lower investment, running, and maintenance costs | [99] [100] [101] [102] [103] |
| Ultraviolet | Need to add photoinitiators to polymers, can be used for curing energetic materials | Can be hindered by fillers, very limited penetration in semi- and non-transparent polymers | Fast-curing, good at handling flexible workpieces, high controllability | High cost of photoinitiators and acrylic toxicity | [104] [105] [106] [107] [108] |
| Convection | No special requirements for polymers, good applicability, universality, and potential | Faces the issue of ‘surface heating’ mechanism | High controllability but limited by curing time | Lower investment, running, and maintenance costs | [109] [110] [111] [112] [113] [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Xia, L.; Ni, M.; Pan, S.; Luo, C. Fundamentals of Infrared Heating and Their Application in Thermosetting Polymer Curing: A Review. Coatings 2024, 14, 875. https://doi.org/10.3390/coatings14070875
Wang T, Xia L, Ni M, Pan S, Luo C. Fundamentals of Infrared Heating and Their Application in Thermosetting Polymer Curing: A Review. Coatings. 2024; 14(7):875. https://doi.org/10.3390/coatings14070875
Chicago/Turabian StyleWang, Tongzhao, Liang Xia, Minrui Ni, Song Pan, and Chuyi Luo. 2024. "Fundamentals of Infrared Heating and Their Application in Thermosetting Polymer Curing: A Review" Coatings 14, no. 7: 875. https://doi.org/10.3390/coatings14070875
APA StyleWang, T., Xia, L., Ni, M., Pan, S., & Luo, C. (2024). Fundamentals of Infrared Heating and Their Application in Thermosetting Polymer Curing: A Review. Coatings, 14(7), 875. https://doi.org/10.3390/coatings14070875






