Improving Surface Antimicrobial Performance by Coating Homogeneous PDA-Ag Micro–Nano Particles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of PDA-Ag Composite Particles
2.3. Deposition of PDA-Ag onto TC4 Substrates
2.4. Antimicrobial Properties of TC4@PDA-Ag
2.5. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.F.; Zhang, J.Y.; Wang, Y.B.; Li, C.M.; Lu, Z.S.; Hu, X.F.; Xu, L.Q. Deposition of catechol-functionalized chitosan and silver nanoparticles on biomedical titanium surfaces for antibacterial application. Mater. Sci. Eng. C 2019, 98, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; He, X.; Xiao, J.; Yuan, C.; Bai, X. Covalent bonding of AgNPs to 304 stainless steel by reduction in situ for antifouling applications. Appl. Surf. Sci. 2018, 452, 201–209. [Google Scholar] [CrossRef]
- Lv, L.; Deegan, A.; Musa, F.; Xu, T.; Yang, Y. The effects of biomimetic ally conjugated VEGF on osteogenesis and angiogenesis of MSCs (human and rat) and HUVECs co-cultu remodels. Colloids Surf. B Biointerfaces 2018, 167, 550–559. [Google Scholar]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Yavari, S.A.; Castenmiller, S.M.; van Strijp, J.A.G.; Croes, M. Combating implant infections: Shifting focus from bacteria to host. Adv. Mater. 2020, 32, 2002962. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Yang, F.; Tang, H. Recent advance in polymer coatings combating bacterial adhesion and biofilm formation. Chin. J. Chem. 2022, 40, 2988–3000. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Y.; Han, R.; Yu, Q.; Yang, R.; Zhang, X. Improving antifouling functions of titanium alloys by robust slippery liquid-infused porous surfaces with tailored multiscale structures. Chem. Eng. J. 2023, 478, 147342. [Google Scholar] [CrossRef]
- Wei, Z.; Li, K.; Wang, S.; Wen, L.; Xu, L.; Wang, Y.; Chen, Z.; Li, W.; Qiu, H.; Li, X.; et al. Controllable AgNPs encapsulation to construct biocompatible and antibacterial titanium implant. Front. Bioeng. Biotechnol. 2022, 10, 1056419. [Google Scholar] [CrossRef]
- Yue, D.; Jiang, X.; Yu, H.; Sun, D. In-situ fabricated hierarchical nanostructure on titanium alloy as highly stable and durable super-lubricated surface for anti-biofouling in marine engineering. Chem. Eng. J. 2023, 463, 142389. [Google Scholar] [CrossRef]
- Higaki, Y.; Kobayashi, M.; Murakami, D.; Takahara, A. Anti-fouling behavior of polymer brush immobilized surfaces. Polym. J. 2016, 48, 325–331. [Google Scholar] [CrossRef]
- Song, J.; Zhu, Y.; Zhang, J.; Yang, J.; Du, Y.; Zheng, W.; Wen, C.; Zhang, Y.; Zhang, L. Encapsulation of AgNPs within zwitterionic hydrogels for highly efficient and antifouling catalysis in biological environments. Langmuir 2019, 35, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Morinobu, M.; Tsuchiya, A.; Kishida, R.; Kawashita, M.; Ishikawa, K. Antibacterial and osteogenic thin films on Ti-6Al-4V surface formed bypassivation process in copper hydroxide solution. Sci. Technol. Adv. Mater. 2024, 25, 2303327. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jiang, M.; Zhao, J.; Cai, Y.; Li, X.; Yang, X.; Jiang, H.; Sun, Y.; Wei, N.; Liu, Y.; et al. Polyelectrolyte-based antifouling and pH-responsive multilayer coatings for reverse osmosis membrane. Colloids Surf. A Physicochem. Eng. Asp. 2023, 679, 132642. [Google Scholar] [CrossRef]
- Christoff-Tempesta, T.; Deiss-Yehiely, E.; Dromel, P.C.; Uliassi, L.D.; Chazot, C.A.C.; Postelnicu, E.; Hart, A.J.; Spector, M.; Hammond, P.T.; Ortony, J.H. Antifouling surface coatings from self-assembled zwitterionic aramid amphiphile nanoribbons. Adv. Mater. Interfaces 2022, 9, 2200311. [Google Scholar] [CrossRef]
- Chen, L.; Duan, Y.; Cui, M.; Huang, R.; Su, R.; Qi, W.; He, Z. Biomimetic surface coatings for marine antifouling: Natural antifoulants, synthetic polymers and surface microtopography. Sci. Total Environ. 2021, 766, 144469. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Liu, J.; Shen, G.; Zheng, X.; Pei, Y.; Tang, K. In-situ synthesis and immobilization of silver nanoparticles on microfibrillated cellulose for long-term antibacterial applications. Cellulose 2021, 28, 6287–6303. [Google Scholar] [CrossRef]
- He, M.; Li, W.; Chen, J.; Zhang, Z.; Wang, X.; Yang, G. Immobilization of silver nanoparticles on cellulose nanofibrils incorporated into nanofiltration membrane for enhanced desalination performance. mpj Clean Water 2022, 5, 64. [Google Scholar] [CrossRef]
- Kumari, R.; Barsainya, M.; Singh, D.P. Biogenic synthesis of silver nanoparticle by using secondary metabolites from Pseudomonas aeruginosa DM1 and its anti-algal effect on Chlorella vulgaris and Chlorella pyrenoidosa. Environ. Sci. Pollut. Res. 2017, 24, 4645–4654. [Google Scholar] [CrossRef]
- Debiemme-Chouvy, C.; Cachet, H. Electrochemical (pre)treatments to prevent biofouling. Curr. Opin. Electrochem. 2018, 11, 48–54. [Google Scholar] [CrossRef]
- Hirsch, U.M.; Teuscher, N.; Rühl, M.; Heilmann, A. Plasma-enhanced magnetron sputtering of silver nanoparticles on reverse osmosis membranes for improved antifouling properties. Surf. Interfaces 2019, 16, 1–7. [Google Scholar] [CrossRef]
- Ferraris, S.; Spriano, S. Antibacterial titanium surfaces for medical implants. Mater. Sci. Eng. C 2016, 61, 965–978. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Goh, P.S.; Wong, T.W.; Zhang, L.; Ismail, A.F. In-situ complexation of silver nanoparticle on thin film composite reverse osmosis membrane for improving desalination and anti-biofouling performance. Desalination 2024, 569, 117040. [Google Scholar] [CrossRef]
- Qi, L.; Liu, Z.; Wang, N.; Hu, Y. Facile and efficient in situ synthesis of silver nanoparticles on diverse filtration membrane surfaces for antimicrobial performance. Appl. Surf. Sci. 2018, 456, 95–103. [Google Scholar] [CrossRef]
- Suriati1, G.; Mariatti, M.; Azizan, A. Synthesis of silver nanoparticles by chemical reduction method: Effect of reducing agent and surfactant concentration. Int. J. Automot. Mech. Eng. 2014, 10, 1920–1927. [Google Scholar] [CrossRef]
- Chumachenko, V.; Kutsevol, N.; Rawiso, M.; Schmutz, M.; Blanck, C. In situ formation of silver nanoparticles in linear and branched polyelectrolyte matrices using various reducing agents. Nanoscale Res. Lett. 2014, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Swensson, B.; Ek, M.; Gray, D.G. In situ preparation of silver nanoparticles in paper by reduction with alkaline glucose solutions. ACS Omega 2018, 3, 9449–9452. [Google Scholar] [CrossRef] [PubMed]
- Pauzi, N.; Mohamad, S.; Ghazali, S.; Jamari, S.S. Evaluation of glucose reduction for silver nanoparticles synthesis with nanocrystalline cellulose matrix. BioNanoScience 2023, 13, 1695–1702. [Google Scholar] [CrossRef]
- Chen, K.; Wang, F.; Liu, S.; Wu, X.; Xu, L.; Zhang, D. In situ reduction of silver nanoparticles by sodium alginate to obtain silver-loaded composite wound dressing with enhanced mechanical and antimicrobial property. Int. J. Biol. Macromol. 2020, 148, 501–509. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426. [Google Scholar] [CrossRef]
- Wang, J.; Cui, Y.; Zhang, B.; Sun, S.; Xu, H.; Yao, M.; Wu, D.; Wang, Y. Polydopamine-Modified functional materials promote bone regeneration. Mater. Des. 2024, 238, 112655. [Google Scholar] [CrossRef]
- Qiu, W.; Wu, G.; Xu, Z. Robust coatings via catechol-amine codeposition: Mechanism, kinetics, and application. ACS Appl. Mater. Interfaces 2018, 10, 5902–5908. [Google Scholar] [CrossRef] [PubMed]
- Bekalé, L.; Barazzouk, S.; Hotchandani, S. Nanosilver could usher in nextgeneration photoprotective agents for magnesium porphyrins. Part. Part. Syst. Charact. 2014, 31, 843–850. [Google Scholar] [CrossRef]
- Lingamgunta, S.; Xiao, Y.; Choi, H.; Christiea, G.; Fruk, L. Microwave-enhanced antibacterial activity of polydopamine–silver hybrid nanoparticles. RSC Adv. 2024, 14, 8331–8340. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Guo, D.; Han, S.; Fu, Z.; Lyu, S.; Li, J.; Lu, Y. Polydopamine-assisted immobilization of metallic nanoparticles confined regionally in bamboo microchannels as continuous-flow microreactors for enhanced catalysis. Chem. Eng. J. 2024, 492, 152327. [Google Scholar] [CrossRef]
- Luo, H.; Gu, C.; Zheng, W.; Dai, F.; Wang, X.; Zheng, Z. Facile synthesis of novel size-controlled antibacterial hybrid spheres using silver nanoparticles loaded with poly-dopamine spheres. RSC Adv. 2015, 5, 13470–13477. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, G.; Xia, T.; Li, Z.; Zhao, K.; Deng, Z.; Guo, D.; Peng, B. Bioinspired synthesis of polydopamine/Ag nanocomposite particles with antibacterial activities. Mater. Sci. Eng. C 2015, 55, 155–165. [Google Scholar] [CrossRef]
- Nieto, C.; Marcelo, G.; Vega, M.; del Valle, E.M.M. Antineoplastic behavior of polydopamine nanoparticles prepared in different water/alcohol media. Colloids Surf. B Biointerfaces 2021, 199, 111506. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, D.; Bhattacharya, A.; Dutta, T.; Nag, S.; Wulferding, D.; Lemmens, P.; Pal, S.K. Nano MOF entrapping hydrophobic photosensitizer for dual-stimuli-responsive unprecedented therapeutic action against drug-resistant bacteria. ACS Appl. Bio Mater. 2019, 2, 1772–1780. [Google Scholar] [CrossRef]
- Niyonshuti, I.I.; Krishnamurthi, V.R.; Okyere, D.; Song, L.; Benamara, M.; Tong, X.; Wang, Y.; Chen, J. Polydopamine surface coating synergizes the antimicrobial activity of silver nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 39937–40986. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Li, J.; Li, D.; Wang, Q.; Teng, W. Dopamine-assisted co-deposition of hydroxyapatite-functionalised nanoparticles of polydopamine on implant surfaces to promote osteogenesis in environments with high ROS levels. Mater. Sci. Eng. C 2021, 131, 112473. [Google Scholar] [CrossRef]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.-J.; Freeman, B.D.; Park, H.B. Oxygen concentration control of dopamine-induced high uniformity surface coating chemistry. ACS Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Du, C.; He, X.; Zhang, C.; Yuan, C. Modification of a derived antimicrobial peptide on steel surface for marine bacterial resistance. Appl. Surf. Sci. 2020, 510, 145512. [Google Scholar] [CrossRef]
- Qi, X.; Huang, Y.; You, S.; Xiang, Y.; Cai, E.; Mao, R.; Pan, W.; Tong, X.; Dong, W.; Ye, F.; et al. Engineering robust Ag-decorated polydopamine nano-photothermal platforms to combat bacterial infection and prompt wound healing. Adv. Sci. 2022, 9, 2106015. [Google Scholar] [CrossRef] [PubMed]
- Cant, N.E.; Critchley, K.; Zhang, H.-L.; Evans, S.D. Surface functionalisation for the self-assembly of nanoparticle/polymer multilayer films. Thin Solid Film. 2003, 426, 31–39. [Google Scholar] [CrossRef]
- Cao, P.; Yuan, C.; Xiao, J.; He, X.; Bai, X. A biofilm resistance surface yielded by grafting of antimicrobial peptides on stainless steel surface. Surf. Interface Anal. 2018, 50, 516–521. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Das, S.S.; Khatoon, A.; Ansari, M.T.; Afzal, M.; Hasnain, M.S.; Nayak, A.K. Bactericidal activity of silver nanoparticles: A mechanistic review. Mater. Sci. Energy Technol. 2020, 3, 756–769. [Google Scholar] [CrossRef]
- Pasquina-Lemonche, L.; Burns, J.; Turner, R.D.; Kumar, S.; Tank, R.; Mullin, N.; Wilson, J.S.; Chakrabarti, B.; Bullough, P.A.; Foster, S.J.; et al. The architecture of the Gram-positive bacterial cell wall. Nature 2020, 582, 294–297. [Google Scholar] [CrossRef]
- Panáček, A.; Kvítek, L.; Smékalová, M.; Večeřová, R.; Kolář, M.; Röderová, M.; Dyčka, F.; Šebela, M.; Prucek, R.; Tomanec, O.; et al. Bacterial resistance to silver nanoparticles and how to overcome it. Nat. Nanotechnol. 2018, 13, 65–71. [Google Scholar] [CrossRef]
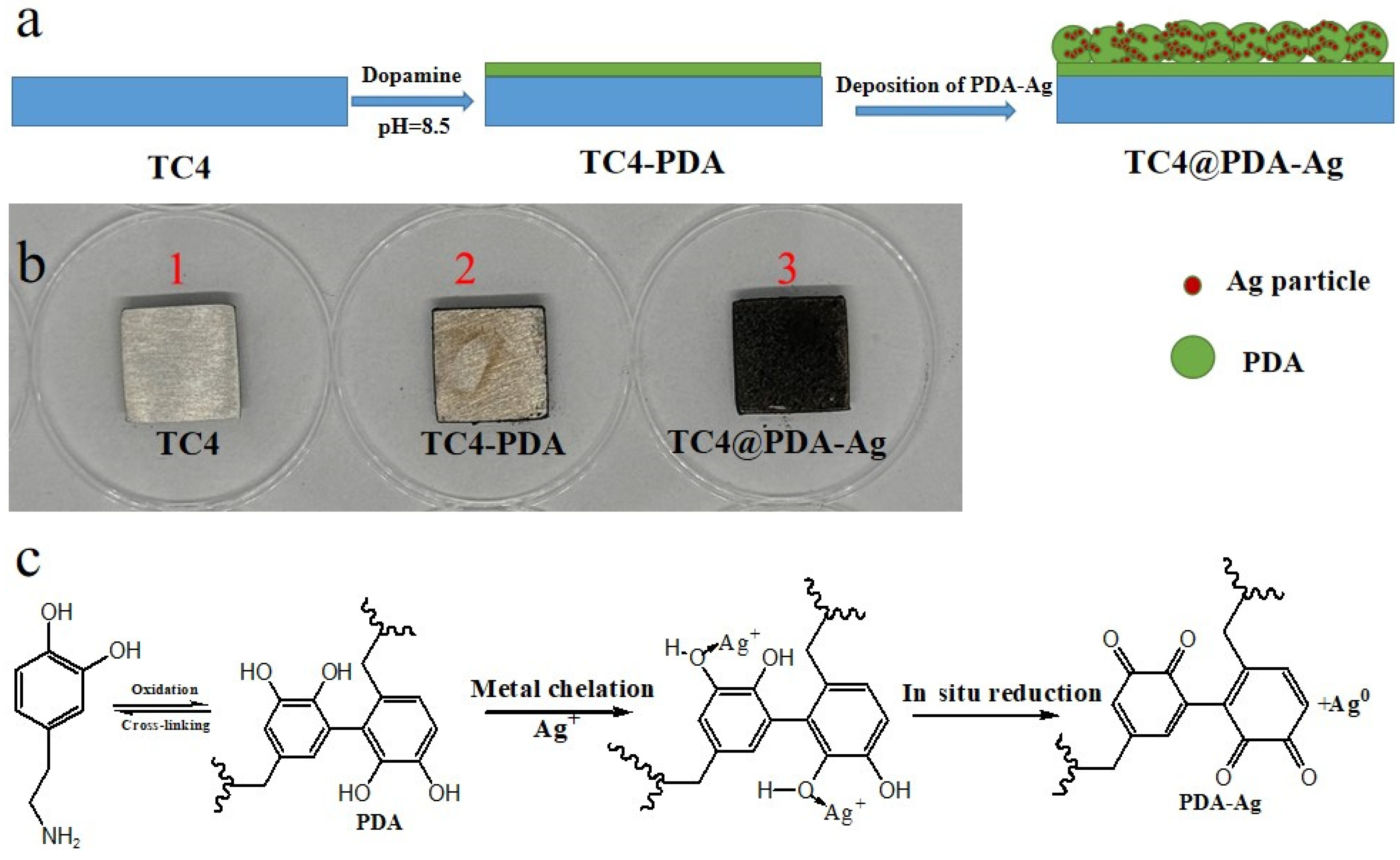
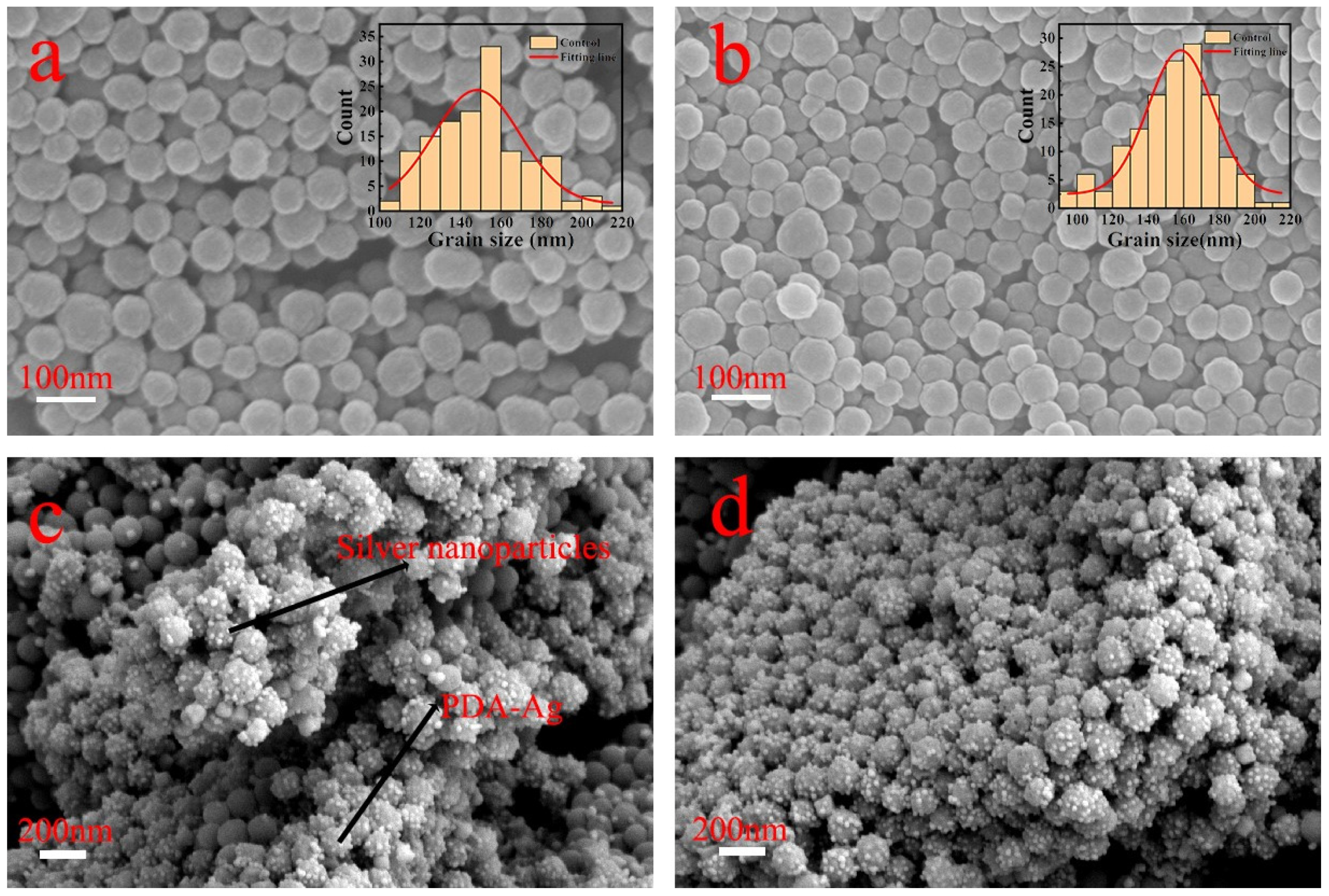

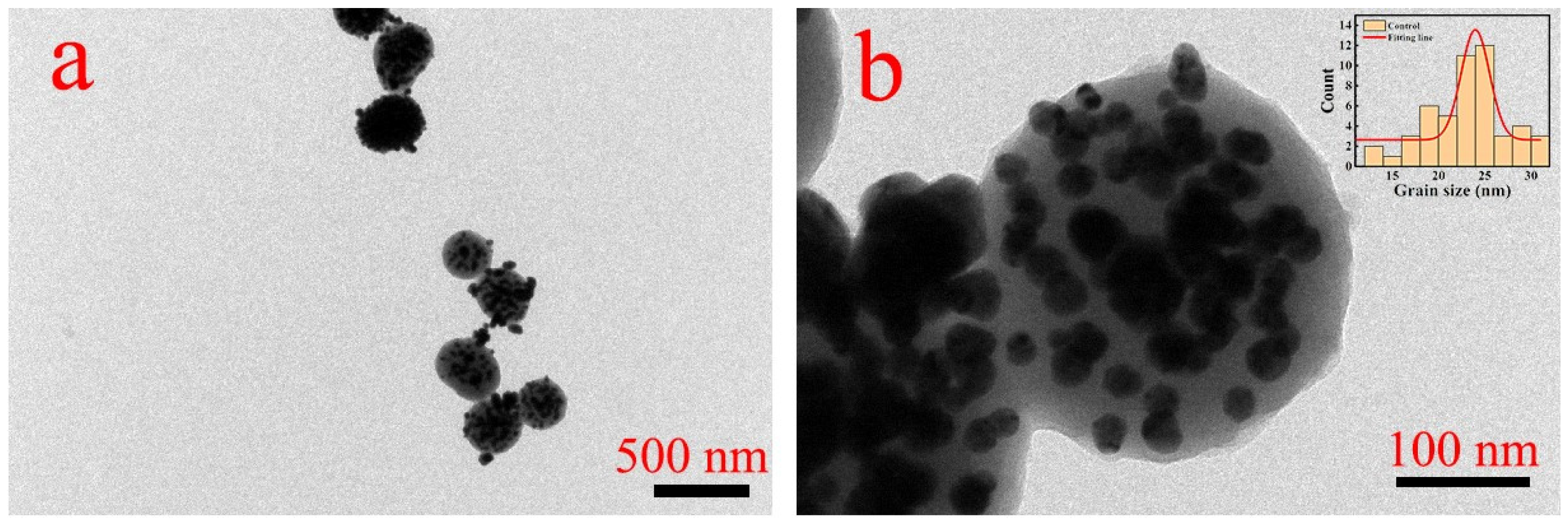


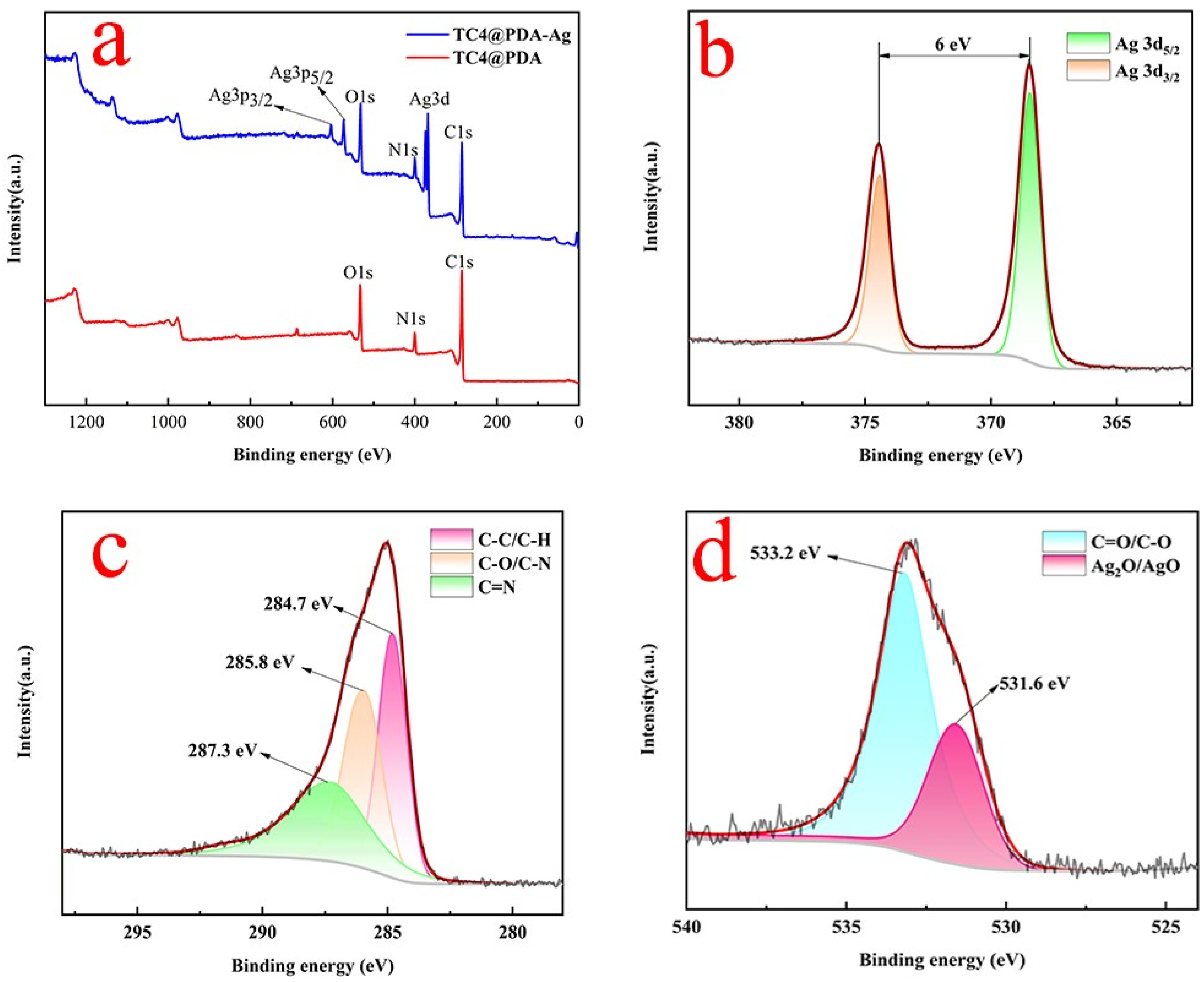
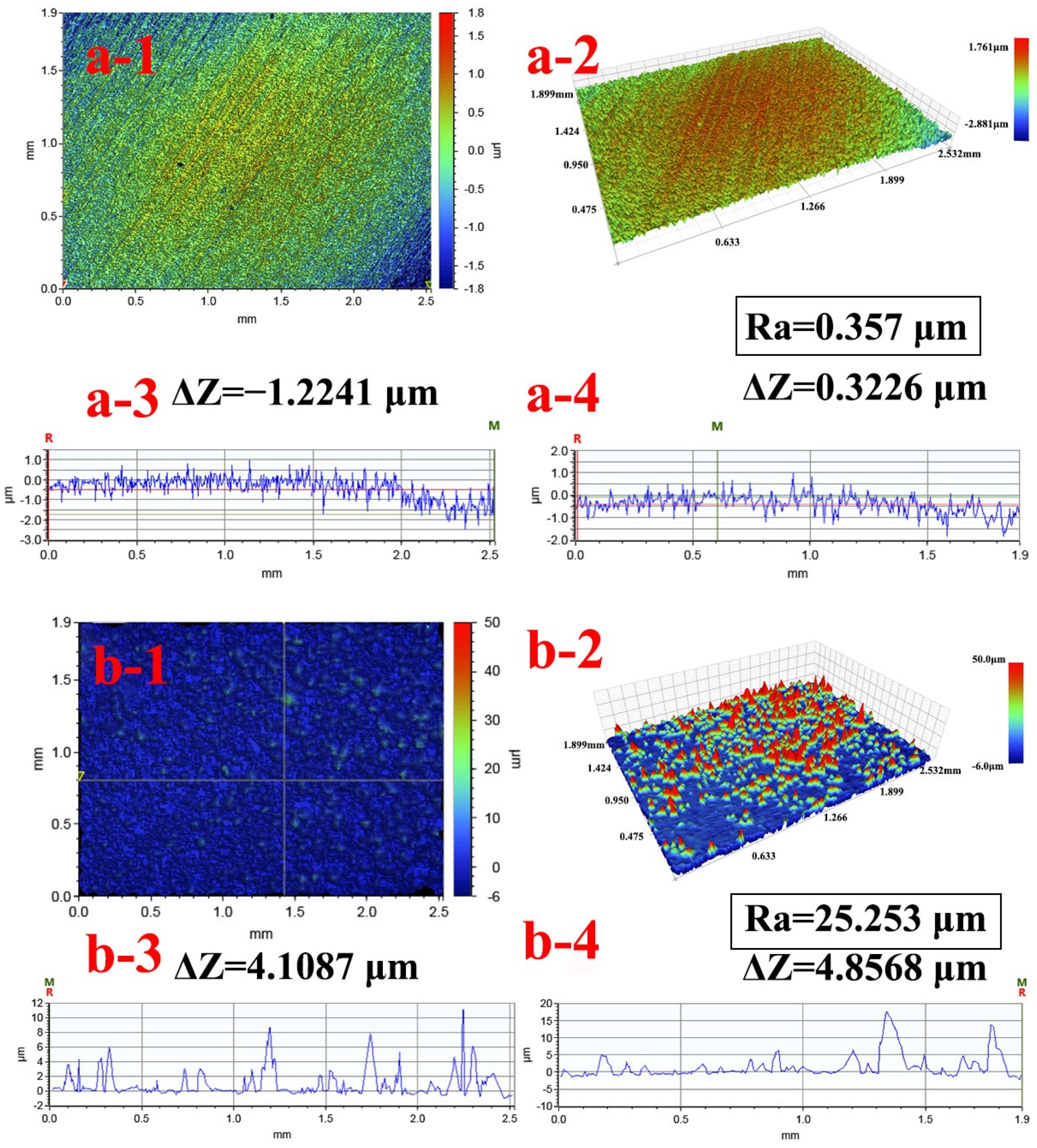


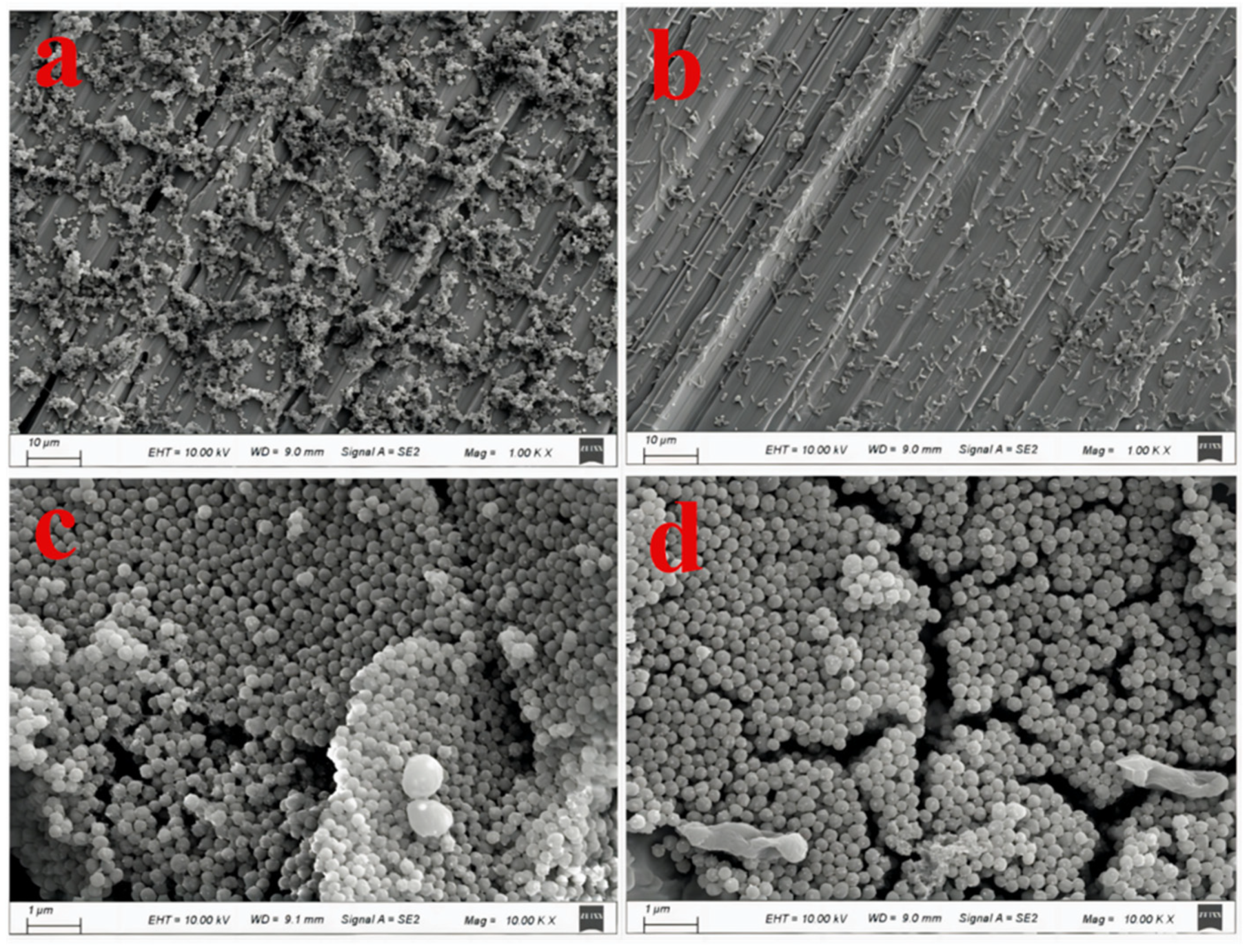
| Elements | C | N | O | Ag | Ti | Al + V |
|---|---|---|---|---|---|---|
| TC4 | 4.96 | 0 | 42.96 | 0 | 44.91 | 7.17 |
| TC4@PDA-Ag | 9.09 | 1.75 | 8.96 | 80.20 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Meng, F.; Cao, Z. Improving Surface Antimicrobial Performance by Coating Homogeneous PDA-Ag Micro–Nano Particles. Coatings 2024, 14, 887. https://doi.org/10.3390/coatings14070887
Wang S, Meng F, Cao Z. Improving Surface Antimicrobial Performance by Coating Homogeneous PDA-Ag Micro–Nano Particles. Coatings. 2024; 14(7):887. https://doi.org/10.3390/coatings14070887
Chicago/Turabian StyleWang, Shuilin, Fanping Meng, and Zhimin Cao. 2024. "Improving Surface Antimicrobial Performance by Coating Homogeneous PDA-Ag Micro–Nano Particles" Coatings 14, no. 7: 887. https://doi.org/10.3390/coatings14070887




