Abstract
This study investigated the biochemical and microbiological properties of Cotton–Copper composite materials obtained using magnetron sputtering technology. Copper particles were precisely distributed on the fabric surface, ensuring free airflow without the need to create additional layers. The Cotton–Copper composite materials were subjected to physiochemical and biological investigations. The physiochemical analysis included the elemental analysis of composites (C, N, O, S, Cu) and analyses of their microscopic and surface properties (specific surface area and total pore volume). The biological investigations consisted of microbiological and biochemical–hematological tests, including evaluation of the activated partial thromboplastin time and prothrombin time. Experiments showed significant effectiveness of the antibacterial material against representative strains of fungi and bacterial species. We also demonstrated the ability of the cotton–copper material to interact directly with the plasmid DNA.
Keywords:
antimicrobial activity; cellulose; coagulation; coating; composite; copper; cotton; plasmid DNA; sputtering 1. Introduction
Plasma technology is revolutionizing the surface modification of materials, especially textiles [1]. As a result, fabrics acquire new properties such as hydrophilicity or hydrophobicity, enhanced dye absorption capacity, and the ability to clean surfaces without the use of chemicals. Additionally, plasma treatment enhances the adhesion of other materials to fabrics, facilitating the creation of specialized finishes such as flame retardancy, UV protection, increased hardness, and antibacterial properties [2,3,4,5,6,7].
Recent advances in antimicrobial textile technology have significantly impacted the medical field, as textiles provide an excellent environment for the colonization, transmission, and spread of microorganisms [8,9,10,11,12,13]. Under favorable humidity and temperature conditions, textiles become an ideal environment for the development of pathogens. This negatively affects their quality and durability, leading to potential discoloration, unpleasant odors, and reduced mechanical strength of the fabric [11,14]. The latest research on fabrics focuses on developing innovative features that enhance their market appeal, improve quality and functionality, and respond to the changing needs and expectations of consumers [15]. By using different molecules with different structures, textile materials can be modified, giving them new properties. These modified fabrics are primarily used in the medical industry, wound healing, and hospital environments, providing protection against microorganisms [16].
Since March 2008, the United States Environmental Protection Agency has recognized copper as a natural source of protection against microorganisms [17,18,19,20]. A distinctive characteristic of this metal is its enduring antibacterial effect, capable of significantly reducing the number of harmful bacteria after brief contact. Copper possesses the ability to both transfer and accept electrons, initiating oxidation and reduction reactions within microbial cells. The effectiveness of copper is contingent upon its concentration, determining whether microorganisms will be inhibited or allowed to proliferate further [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39].
Cotton–copper composites have attracted significant global interest as promising antibacterial materials due to their unique combination of properties. Scientists have extensively explored their potential applications across various fields, driven by the urgent need for effective antimicrobial solutions amidst increasing concerns about antibiotic resistance [40,41]. Several notable advancements highlight the growing interest in this composite material. Pérez-Alvarez et al. synthesized copper nanoparticles via green methods using cotton fibers and water as a solvent, eliminating the need for toxic reducing agents [42]. Zhao et al. described an environmentally friendly and cost-effective process for producing copper–cotton composites through electroless plating [43]. Qian et al. addressed the challenge of enhancing the durability and wash resistance of copper-containing materials. They devised a straightforward, cost-effective method for integrating copper ions into cotton fabrics at the molecular level, facilitating the efficient production of textiles with antiviral and antibacterial properties. This molecular integration enables these textiles to endure repeated washing and wearing, essential for practical applications [10]. Similarly, Sedighi et al. developed an economical in situ synthesis of copper nanoparticles on cotton fabric using a chemical reduction approach. They investigated the hydrophobicity, mechanical properties, and antibacterial efficacy of the samples. The sustained antibacterial effectiveness of the fabrics containing copper nanoparticles even after 30 washing cycles underscores their potential for use in textile and medical applications, demonstrating the robust stability of the nanoprocessed fabric [44]. Despite these advancements, there remain several challenges in the development and application of cotton–copper composites. Our research aims to contribute to the field of cotton–copper composites by examining their impact on blood clotting mechanisms. Specifically, we focus on evaluating their effects on activated partial thromboplastin time (aPTT) and prothrombin time (PT). Additionally, we investigate their interactions with plasmid DNA using a plasmid relaxation assay, which may elucidate their antimicrobial properties. Our study seeks to expand the understanding of the biomedical applications of cotton–copper composites and assess their potential utility in textiles and wound dressing materials.
The article presents a method for producing antibacterial cotton textiles using magnetron sputtering technology, which generates a stable metallic copper coating on fabric (COT–Cu). The COT–Cu material, comprising cotton as the primary component and magnetron-sputtered copper particles, has the potential to exhibit several important properties. The degradability and stability of this material are crucial factors influencing its environmental impact [45,46]. Cotton, being a natural plant fiber, is biodegradable, which facilitates the biological decomposition of the material [47,48,49,50,51]. In contrast, sputtered copper, as a metal, does not undergo traditional biodegradation but can corrode under specific atmospheric conditions, particularly when exposed to moisture and chemical agents [52,53,54]. The intricate composition of cotton and copper results in varying durability of the individual components, influencing their degradation processes based on usage conditions and their relative proportions. The stability of COT–Cu materials is another key consideration. Cotton is relatively chemically stable [55], meaning it is resistant to most common cleaning agents and does not undergo destructive reactions with them [56]. However, microorganisms can degrade cotton fibers, potentially compromising the overall structural stability of the material [57,58]. Additionally, the antimicrobial properties of copper can protect the cotton material from microbial degradation, further enhancing its stability [10,59,60]. Conversely, copper is known for its corrosion resistance, although its durability can be affected by atmospheric conditions and the presence of certain chemicals [61]. These materials can demonstrate good mechanical stability due to the strength of cotton fibers combined with the additional properties of copper, such as resistance to wear and friction. The complex composition of COT–Cu materials defines their degradability and stability, which is critical for their various applications.
This study focuses on qualitatively assessing the effectiveness of copper as an antimicrobial agent on cotton fabrics. Evidence supporting the validity of the antimicrobial concept includes studies examining its antifungal and antibacterial effects. Through magnetron sputtering, various samples were analyzed to determine the deposited copper amount. The aim of the research was to investigate the impact of the obtained materials on the blood plasma coagulation process in the initial stage of wound treatment while maintaining their antibacterial properties. Diagnostic tests including aPTT (to determine fibrin clot formation time) and PT (to determine prothrombin time) were used for this purpose. We also performed a plasmid relaxation assay, which is a simple method for determining the potential of the tested material to directly interaction with DNA. As a result of these interactions, it is possible to induce DNA breaks, resulting in a change in electrophoretic mobility of the plasmid DNA. Herein, we analyzed the potential of cotton and cotton–copper composites to interact with DNA after 24 h incubation. Moreover, the process of obtaining copper-coated materials (COT–Cu) was found to be simple, cost-effective, and applicable to various types of cotton fabrics. This methodology has the potential to expand the applications of cellulosic materials, introducing new possibilities. These findings hold significance, particularly in utilizing plasma technology to confer antibacterial properties to textiles, with implications extending to the production of medical materials.
2. Materials and Methods
2.1. Materials
- Cotton material was purchased from Andropol S.A. (Andrychów, Poland). Unbleached 100% plain woven cotton fabric with a weight of 200 g/m2 was used. The amount of weft yarn in the longitudinal direction per unit length was 20 yarns/cm, and the amount of warp yarns in the transverse direction per unit length was 16 yarns/cm. Before testing, cotton fabric samples were subjected to pre-treatment with an anionic wetting–washing agent—Periwet WLV (Dr. Petry GmbH, Reutlingen, Germany)—2 g/L, at 98 °C for 30 min, using laboratory dyeing machine RED KROME (Ugolini S.R.L., Schio, Italy).
- The target copper was purchased from Testbourne Ltd. (Basingstoke, UK) and was 99.99% pure. The copper shield had dimensions of 798 × 122 × 6 mm.
- Dia-PTT and Dia-PT reagents and calcium chloride (CaCl2) solution with a concentration of 0.025 M were purchased from Diagon Kft based in Budapest (Hungary).
- Bacterial and fungal cultures were purchased from Microbiologics (St. Cloud, MN, USA). Bacterial strains included Escherichia coli (ATCC 25922) and Staphylococcus aureus (ATCC 6538), while fungal strains included Chaetomium globosum (ATCC 6205) and Aspergillus niger van Tieghem (ATCC 6275).
2.2. Methods
2.2.1. Magnetron Sputtering
The cotton was modified using a DC magnetron sputtering system provided by P.P.H. Jolex sc. (Częstochowa, Poland). A target copper disc purchased from Testbourne Ltd. from Basingstoke (UK) was used for the coating modification (Table 1). The distance of the target from the substrate was 15 cm, and the coating deposition process took place in an argon atmosphere. To optimize the process, the disc discharge power was varied from 0.5 to 1 kW. In order to obtain differences in copper content, two different time variants of copper deposition were used: 20 min (sample designation: COT–Cu(0.5kW/20), COT–Cu(1kW/20)) and 40 min (COT–Cu(0.5kW/40), COT–Cu(1kW/40)). The size of the sprayed sample was 60 cm × 20 cm.

Table 1.
Parameters of magnetron sputtering of copper on cotton fabric.
2.2.2. Analysis of the Morphology and Chemical Composition
Microscopic Analysis
For microscopic analysis of the fiber materials, a Tescan Vega 3 scanning electron microscope (SEM) was utilized. The SEM was manufactured by Tescan Analytics (Brno, Czech Republic), and equipped with an EDS X-ray microanalyzer from Oxford Instruments, based in Abingdon (UK). Surface topography studies using SEM were conducted under high vacuum conditions with a probe beam energy of 15 keV. SEM observations were made at magnifications ranging from 5000× to 10,000×. The performance of the EDS system was evaluated by measuring resolution using core standards provided by Oxford Instruments, defined in accordance with ISO 15632:2012 [62].
Atomic Absorption Spectrometry with Flame Excitation (FAAS)
To determine the copper content in COT–Cu samples, mineralization was performed using a single-module Magnum II microwave mineralizer from Ertec, based in Wrocław, Poland. Copper(II) ions were then quantified using flame atomic absorption spectrometry (FAAS) with a Thermo Scientific Thermo Solar M6 spectrometer (Midland, ON, Canada). The spectrometer was equipped with a 100 mm diameter titanium burner and coded lamps featuring single-element hollow cathodes. Background correction was carried out using a D2 deuterium lamp.
2.2.3. Antibacterial and Antifungal Tests
The antibacterial effect of the obtained materials was tested in accordance with the PN-EN ISO 20645:2006 standard [63] on Gram-negative bacteria (Escherichia coli, ATCC 25922) and Gram-positive bacteria (Staphylococcus aureus, ATCC 6538) using the diffusion method on Muller Hinton agar. The procedure began by pouring the agar onto sterilized Petri dishes and allowing it to solidify. Agar media surfaces were inoculated with bacterial broth cultures (ATCC 25922: 1.2 × 108 CFU/mL, ATCC 6538: 1.7 × 108 CFU/mL) and stored at 4 °C before analysis. Material samples were placed on inoculated agar and incubated at 37 °C for 24 h. The diameter of the zone around the materials indicates the inhibition of microbial growth. At the same time, the same tests were performed for control samples—unmodified cotton samples. The research procedure closely followed the methodology described above. To evaluate the antifungal effect of the obtained materials, tests were conducted according to the PN-EN 14119:2005 standard [64] using the fungus Chaetomium globosum (ATCC 6205) and Aspergillus niger van Tieghem (ATCC 6275). Samples were placed on agar plates inoculated with C. globosum/A. niger and then incubated at 29 °C for 14 days. The antifungal effect was assessed by analyzing the extent of fungal growth in the zone of contact between the agar and the sample, as well as on the surface of the samples, and by measuring the potential inhibition zone around the sample. All tests were performed in duplicate. Concurrently, similar tests were conducted for control samples (unmodified cotton).
2.2.4. Plasmid Relaxation Assay
The plasmid relaxation assay was performed following the procedure by Juszczak et al. [65]. The pUC19 plasmid was isolated from the DH5α E. coli cells with AxyPrep Plasmid Miniprep Kit (Axygen, Corning, NY, USA) according to the manufacturer’s instructions. The isolated plasmid quantity and quality were determined using the A260/A280 ratio and gel electrophoresis, respectively. The native form of pUC19 exists mainly in the supercoiled form (CCC), which is characterized by a relatively high electrophoretic mobility. The plasmid was digested with the restrictase PstI (New English Biolabs, Ipswich, MA, USA) to induce the linear (L) form. Topological differences between CCC and L forms of the plasmid account for their different electrophoretic mobilities. The plasmid (50 ng μL−1) was incubated for 24 h with cotton–copper composite materials (COT–Cu). Then, the samples were subjected to 1% agarose gel electrophoresis with ethidium bromide staining, visualization under UV light (302 nm), scanning by a CCD camera, and analysis with the GeneTools software, version number 4.3.9.0. from Syngene (Cambridge, UK). During electrophoresis, we also separated 4 μL of 1 kb DNA ladder (GeneRuler 1 kb DNA Ladder, Thermo Scientific, Waltham, MA, USA).
2.2.5. Assessment of Blood Coagulation Factors (aPTT and PT)
Human plasma, which had been frozen and freeze-dried, was dissolved in deionized water. Samples weighing 1 mg were added to 200 µL of plasma and, after centrifugation, incubated for 15 min at a controlled temperature of 37 °C. For the assessment of aPTT, the Dia-PTT reagent containing kaolin, cephalin, and 0.025 M calcium chloride (CaCl2) solution was prepared. aPTT measurements were performed using a K-3002 OPTIC coagulometer. For each sample, 50 μL of plasma and 50 μL of Dia-PTT suspension were obtained and then placed in the coagulometer thermostat to maintain the temperature at 37 °C. After 3 min of incubation, 50 µL of 0.025 M CaCl2 solution was added to start the measurement.
For prothrombin time (PT) assessment, 100 μL of plasma sample was incubated for 2 min at a controlled temperature of 37 °C. Subsequently, 100 µL of Dia-PTT suspension was added, and the measurement was initiated. The Dia-PTT suspension contained rabbit brain tissue thromboplastin, calcium ions, and a preservative. Before each use, the Dia-PTT suspension was thoroughly mixed to ensure the accuracy of the results.
2.2.6. Air Permeability
Air permeability analysis was conducted on a monolayer of samples in accordance with the EN ISO 9237:1998 standard [66]. The measurements were performed using an FX 3300 TEXTEST AG permeability tester from Schwerzenbach, Switzerland. The fabric, with a diameter of 20 cm2, was subjected to air pressure levels of 100 and 200 Pa during testing. To ensure accuracy, the final reported value for each sample was determined as the average of ten individual measurements.
2.2.7. Surface Properties and Pore Volume
The specific surface area was assessed using the Brunauer, Emmett, and Teller (BET) method. Surface properties and pore volume were evaluated with an Autosorb-1 instrument from Quantachrome Instruments, Boynton Beach, FL, USA. Nitrogen was used as the adsorbate, and measurements were conducted at 77 K. For each test, 1–2 g of the sample was accurately weighed. Prior to analysis, the samples were dried at 105 °C for 24 h and subsequently degassed at ambient temperature. Each measurement was performed in duplicate, and the results were reported as the average of the two readings.
3. Results and Discussion
3.1. Deposition of Copper Nanoparticles on Cotton Fabric
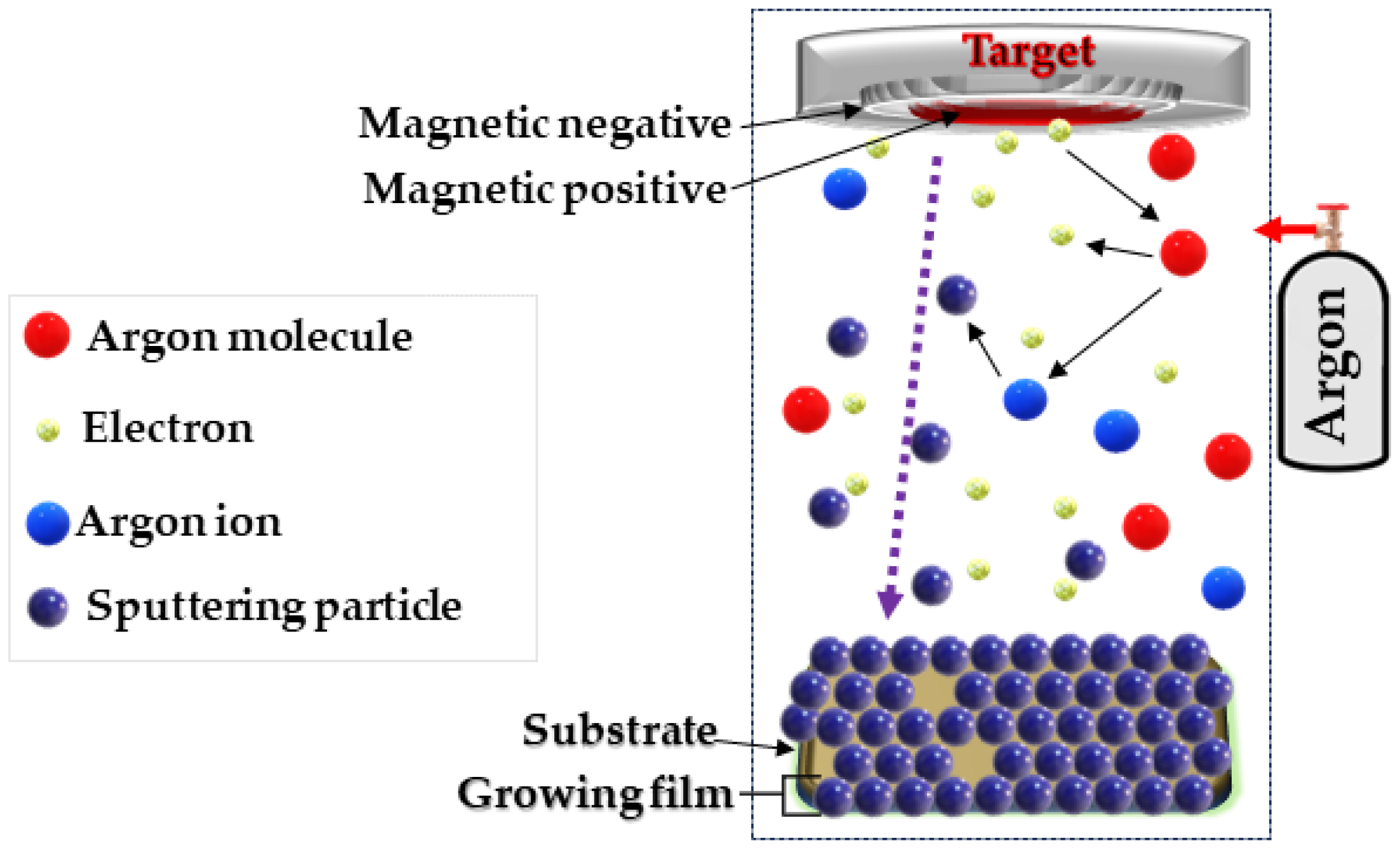
Magnetron sputtering (MS) is a method that allows precise deposition of layers on various substrates. Using this technology, we proposed creating a thin-layer cotton–copper material (Figure 1).
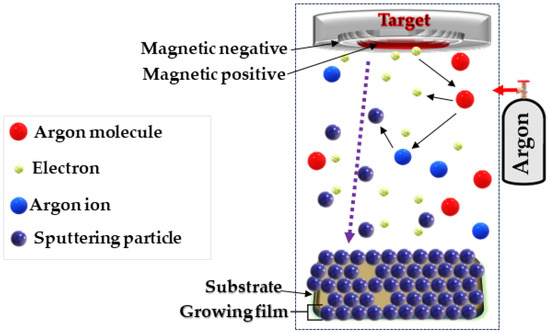
Figure 1.
Schematic diagram of the DC magnetron sputtering system used.
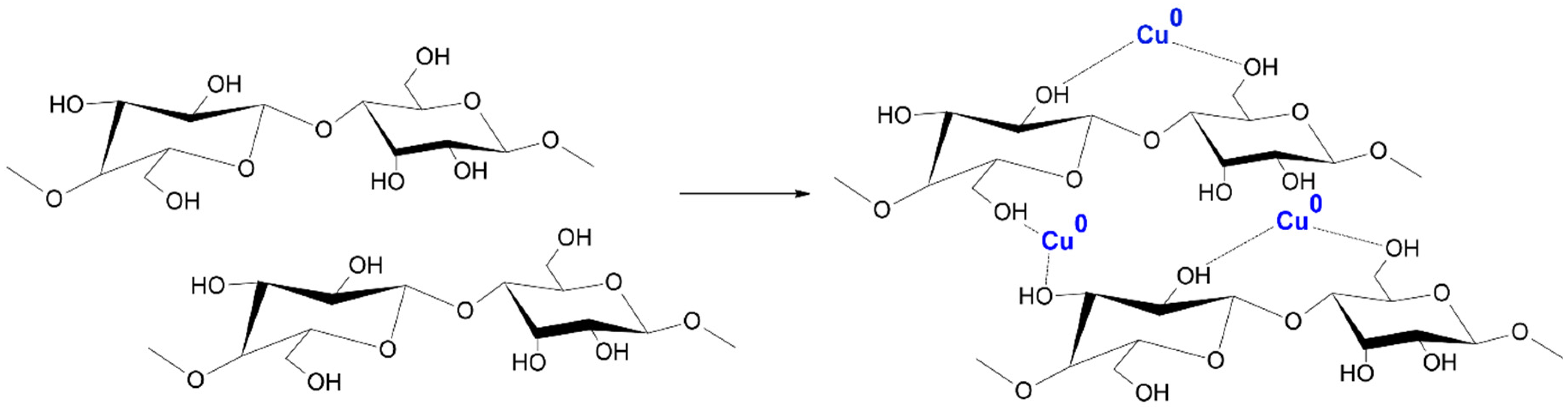
The magnetron sputtering system consisted of several key components [67,68,69,70], including a copper sputter target deposited on a cotton substrate and a non-reactive gas (Ar+). The process began with the introduction of process gas, i.e., argon, into the process chamber. Then, when an electrical voltage was applied, the gas was ionized, which led to the formation of a plasma containing ions and electrons. Argon ions collided with the surface of the copper sputtering disk, causing the separation of copper atoms, which were carried away as atoms, ions, and cations. The atomic stream of copper floating in the process chamber was deposited on a prepared cotton substrate. This process enabled the construction of a thin layer atom by atom, which provided control over the thickness and structure of the coating. When copper particles reach the fiber surface, adsorption occurs, whereby the particles adhere to functional groups present on the cotton surface, such as hydroxyl groups (-OH) and others. These functional groups act as chemically active sites on the cotton surface, facilitating the attraction and binding of copper particles through chemical bonds and electrostatic interactions (Figure 2) [71]. During the process, key parameters such as gas pressure, magnetron power (0.5–1 kW), and process duration (10–40 min) were carefully monitored. This made it possible to obtain layers of uniform thickness and controlled properties that meet the assumed requirements.
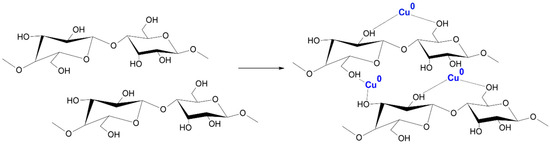
Figure 2.
Probable mechanism of COT–Cu formation.
3.2. Physical Properties of the Obtained COT–Cu Materials
3.2.1. Flame Atomic Absorption Spectrometry (FAAS)
The analysis of the copper content in cotton–copper materials (COT–Cu) obtained by magnetron sputtering was carried out using Flame Induced Atomic Absorption Spectrometry (FAAS), and the results are presented in Table 2.

Table 2.
Results of determining the copper content in the tested samples.
The magnetron time variants of copper deposition used to coat cotton fabrics with copper particles play a key role in the concentration of copper in the samples and extend sputtering time, significantly increasing copper deposition efficiency. Greater sputtering power enables maximum deposition of copper ions, which are crucial for the durability and effectiveness of the coating (COT–Cu(1kW/40)). Thanks to meticulous design and supervision of coating processes, uniform and durable copper layers are ensured on the surface of the material, which is crucial for their antibacterial properties.
3.2.2. Microscopic Analysis
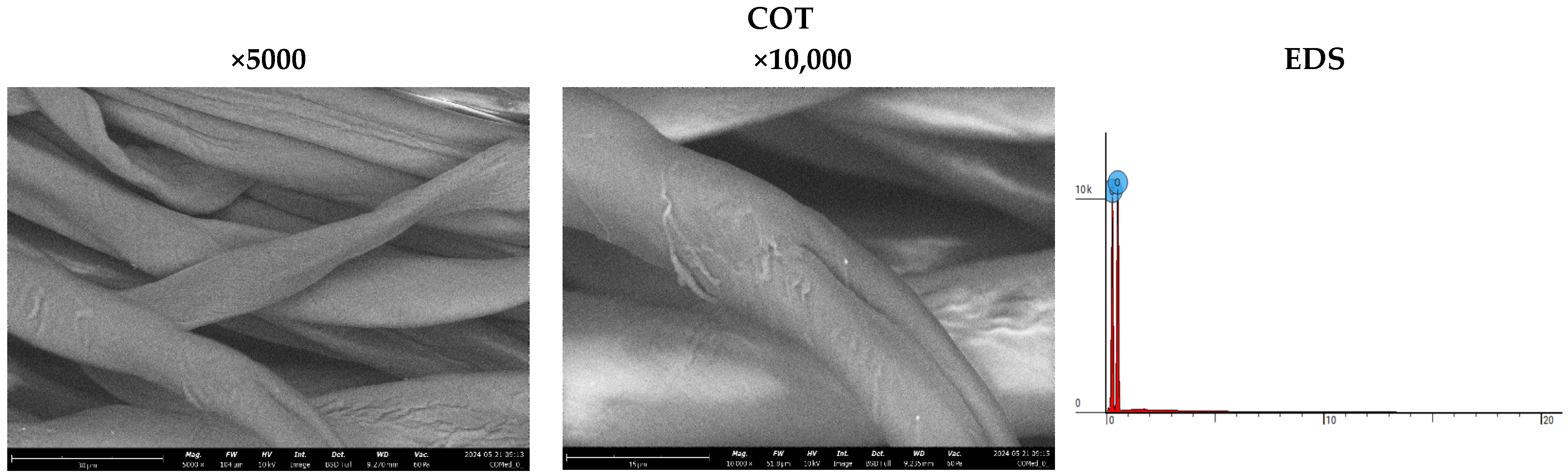
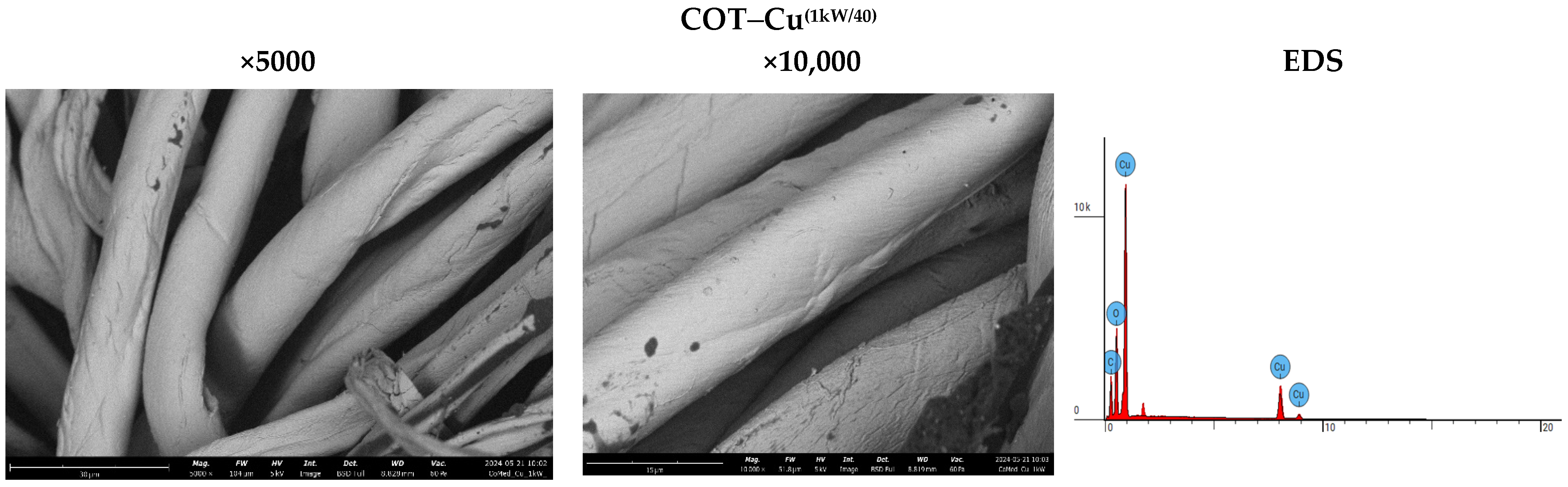
The analysis of sample surfaces before and after the modification process is a critical step in evaluating the effectiveness of applying a copper coating to cotton fabric. Microscopic observations reveal significant changes in surface structure that may impact the material’s antimicrobial properties (Figure 3). Morphological analysis further aids in identifying potential alterations in fabric fiber structure resulting from the modification process. Detailed microscopic examinations also facilitate assessing the uniformity of the coating and the possible formation of copper particle agglomerates. The results affirm the achievement of a uniform and durable copper coating, pivotal for the material’s effectiveness in antimicrobial applications. SEM (scanning electron microscopy) images of unmodified cotton fabrics (COT) reveal regular and parallel fibers with relatively smooth surfaces (Figure 3). SEM images of COT–Cu materials obtained through the magnetron sputtering process illustrate changes in the surface structure of the samples. The newly applied coating layer is visibly distributed evenly over the entire surface of the fibers. Notably, no fiber damage or cracks are observed in the case of COT–Cu samples (Figure 4 and Figure 5).
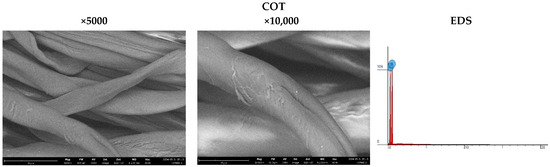
Figure 3.
Images taken using a scanning electron microscope (SEM) (magnification: 5000×; 10,000×) and EDS spot analysis diagrams of cotton samples (COT).

Figure 4.
Images taken using a scanning electron microscope (SEM) (magnification: 5000×; 10,000×) and EDS spot analysis diagrams of cotton–copper samples (COT–Cu(0.5kW/40)).
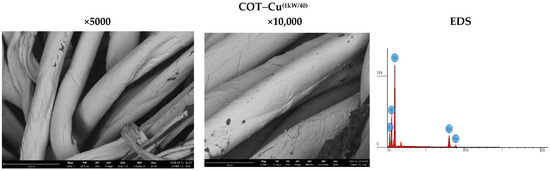
Figure 5.
Images taken using a scanning electron microscope (SEM) (magnification: 5000×; 10,000×) and EDS spot analysis diagrams of cotton–copper samples (COT–Cu(1kW/40)).
The COT–Cu samples were exposed to an electron beam using a scanning electron microscope, which enabled detailed energy-dispersive X-ray spectroscopy (EDS) to be performed. This technique allowed for not only the chemical characterization of the obtained materials but also a detailed analysis of their elemental surface composition. The EDS results facilitated the identification of elements present in the sample by analyzing the position of peaks in the X-ray spectrum, where the signal intensity was directly proportional to the concentration of a given element in the sample. The analyses performed are summarized in Table 3. and illustrated in Figure 3, Figure 4 and Figure 5, providing detailed information on the distribution and amounts of individual elements in the tested samples. The EDS analysis showed that both the duration and power of magnetron sputtering influence the copper concentrations in the tested materials. Copper concentration was higher in samples sputtered for 40 min compared to those sputtered for 20 min. In contrast, sputtering power had a minor effect on the copper concentration, although samples sputtered at 1 kW exhibited slightly higher copper concentrations than those sputtered at 0.5 kW. These results indicate that a longer sputtering time has a greater impact on the copper content in the material than variations in magnetron power.

Table 3.
Energy-dispersive X-ray spectroscopy (EDS) experimental data.
3.2.3. Analysis of Surface Properties and Pore Volume
Table 4 shows the results of the analysis of the specific surface area and total pore volume of uncoated cotton fabric (COT) and cotton–copper (COT–Cu) composites. Significant changes in these parameters were observed as a result of the copper deposition process using the magnetron sputtering method. The specific surface area of the cotton fabric decreased from 0.7345 m2/g to 0.5061–0.7176 m2/g in the copper-coated samples; this may be due to the filling of the pores on the fiber surface with deposited copper. Similarly, the total pore volume decreased from 7.408 × 10⁻3 cm3/g for uncoated cotton to 2.519–3.222 × 10⁻3 cm3/g for copper-coated samples. This phenomenon may be attributed to the reduction in porosity of the cotton fibers caused by the presence of deposited copper. Higher magnetron power leads to a thicker copper layer and greater changes in specific surface area and total pore volume.

Table 4.
Specific surface area and total pore volume of a sample of unmodified cotton and COT–Cu materials.
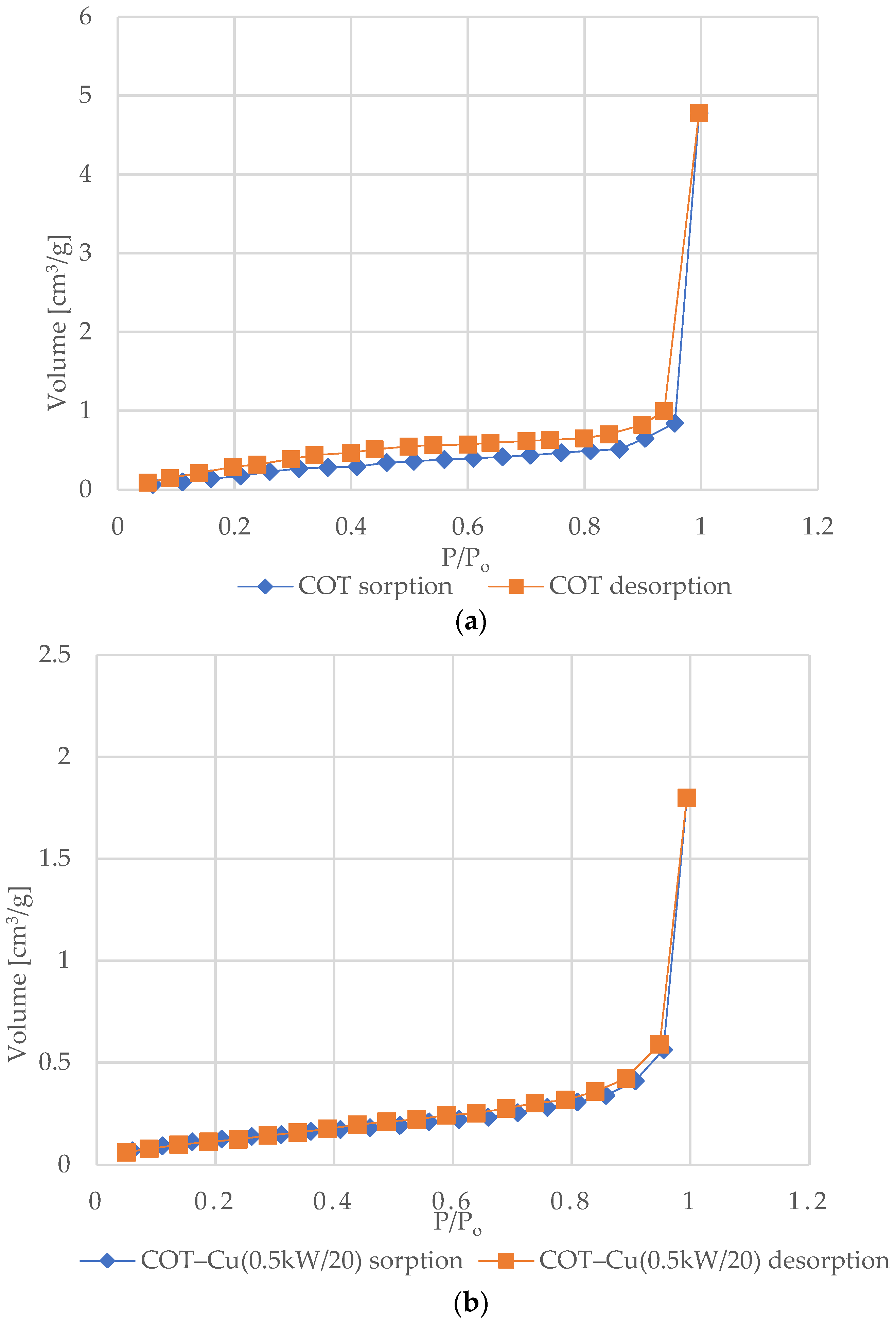
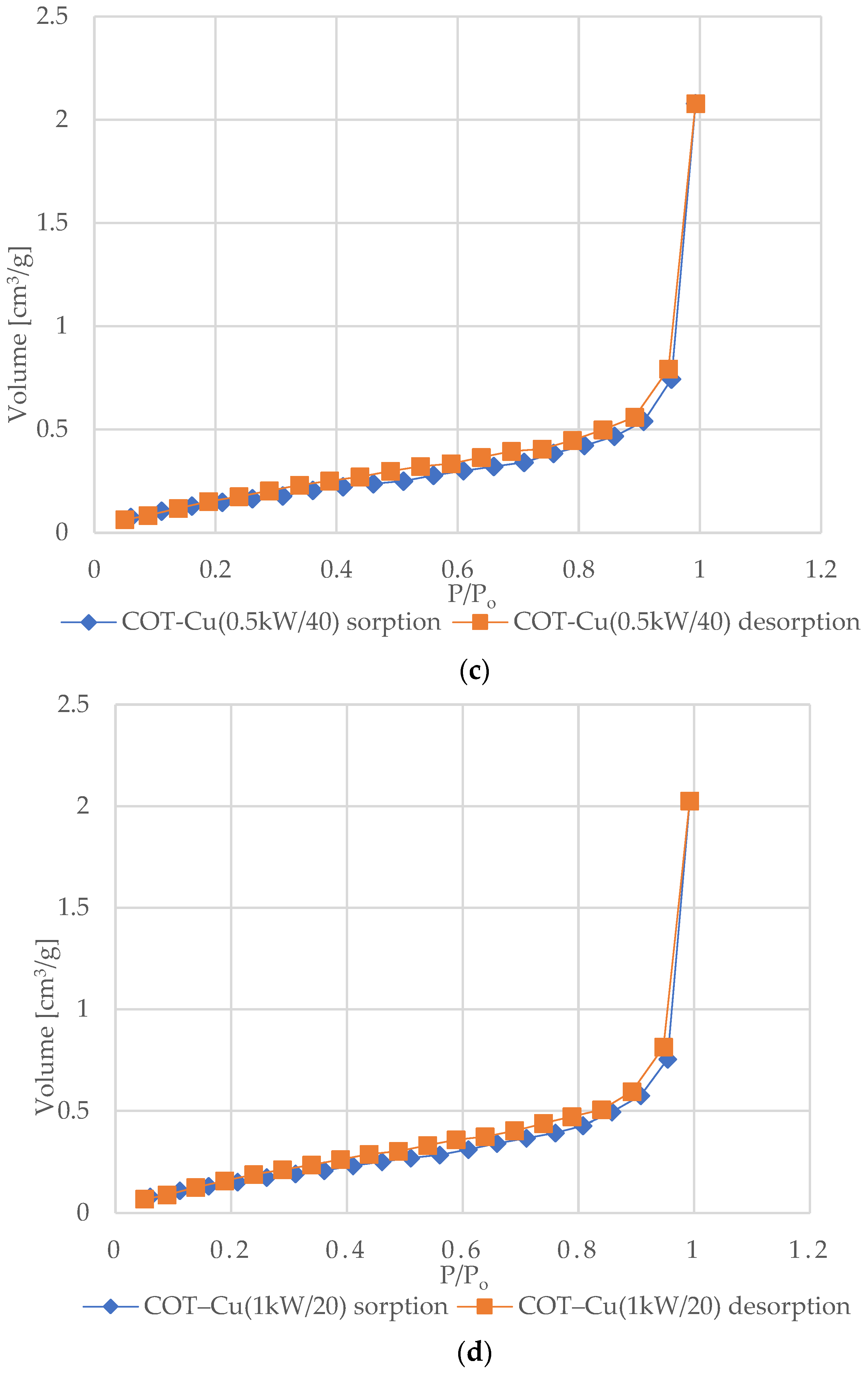
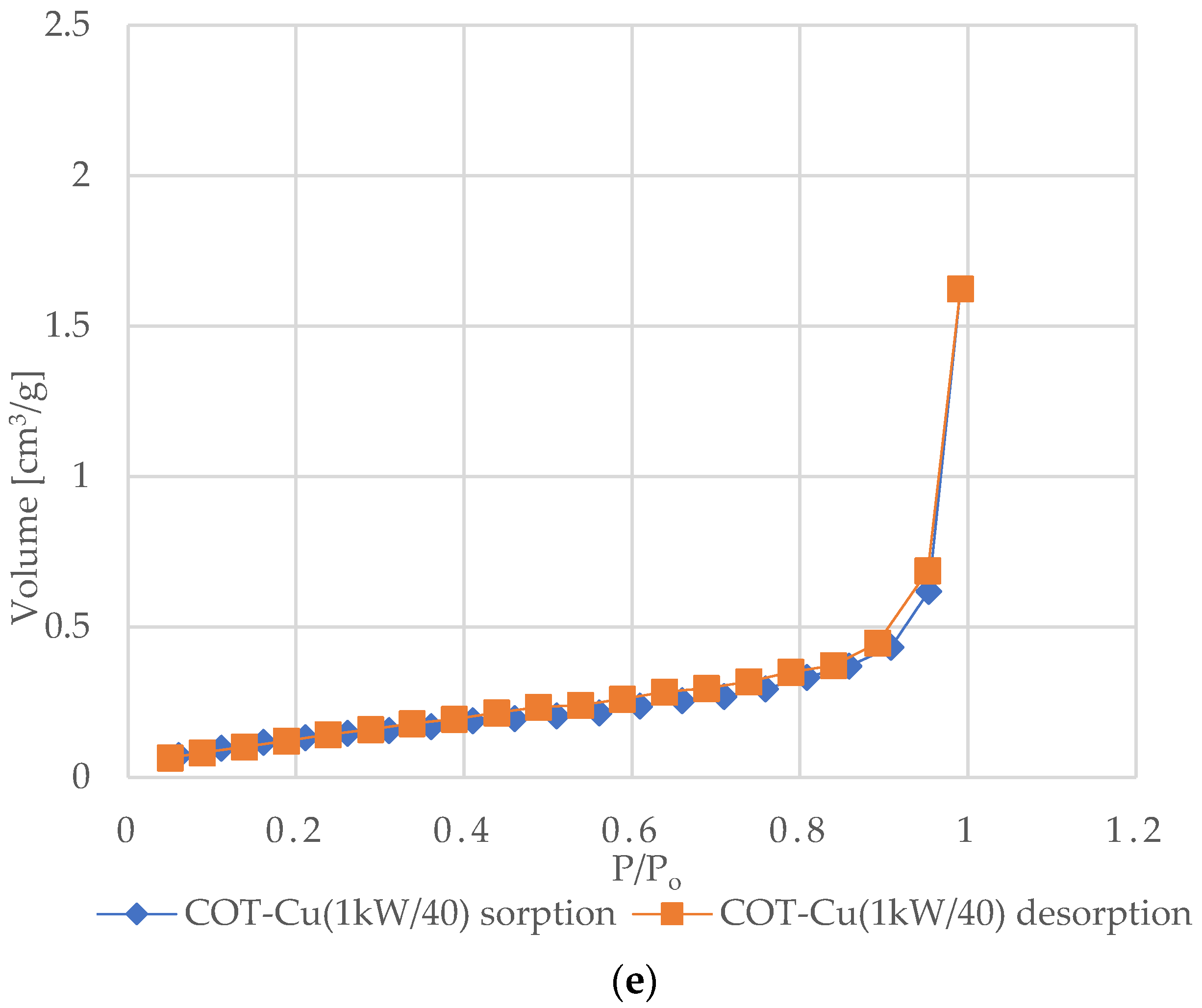
Figure 6 shows the nitrogen (N2) adsorption and desorption isotherms for samples of unmodified COT and COT–Cu materials. The characteristic shape of the type III isotherm, consistent with the IUPAC classification, indicates weak interactions between the adsorbent and the adsorbate, where the adsorbed molecules cluster mainly around optimal places on the surface of non-porous or macroporous solid materials [72,73,74,75]. The amount of adsorbed adsorbate after reaching the saturation pressure is finite (p/p0 = 1), which suggests a multilayer adsorption mechanism over the entire pressure range. The appearance of a type III isotherm is often associated with the presence of non-porous or macroporous structures [76,77]. Analysis of the graphs indicates an exponential increase in the amount of adsorbate with increasing pressure, and the rate of this increase may initially be limited at low relative pressure values, but at values close to p/p0 = 1, there is a significant increase in the amount of adsorbed adsorbate [78]. The appearance of H3-type hysteresis loops suggests the presence of materials characterized by slotted pores that tend to retain part of the adsorbate even at lower pressures. However, as the pressure increases, the amount of adsorbate increases rapidly, which is characteristic of slotted pores [79,80,81].
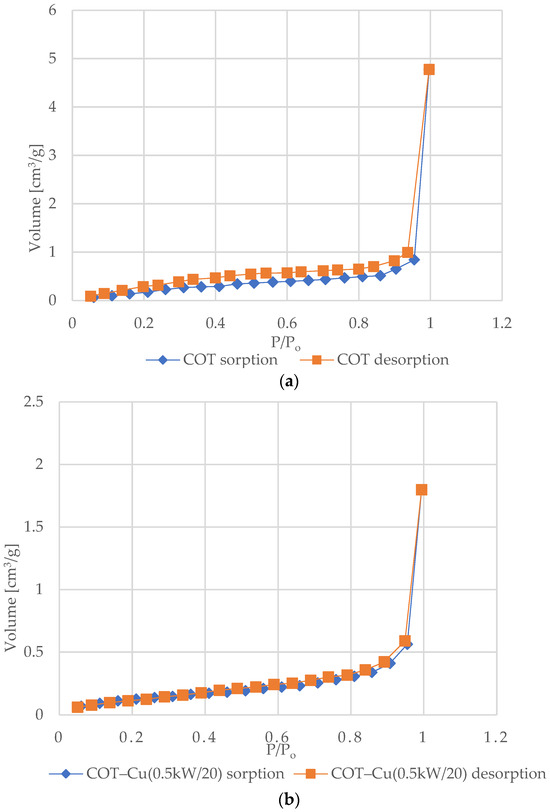
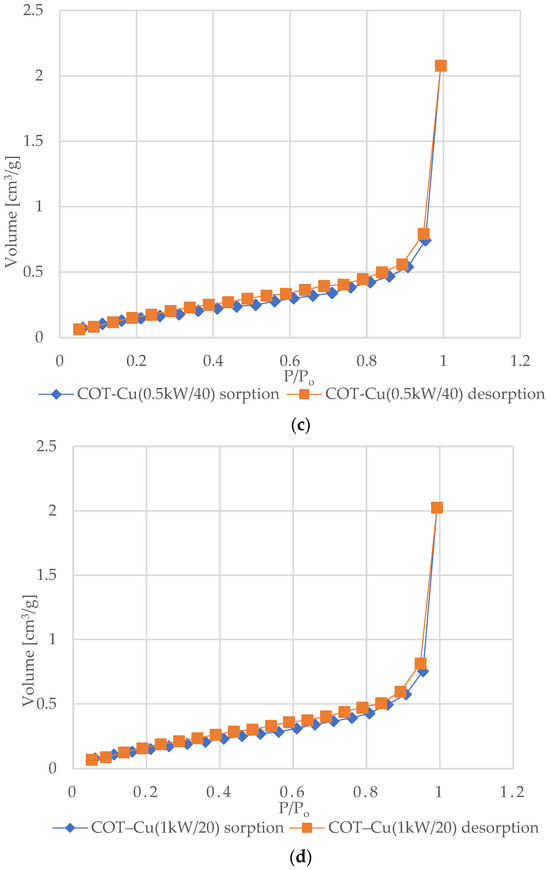
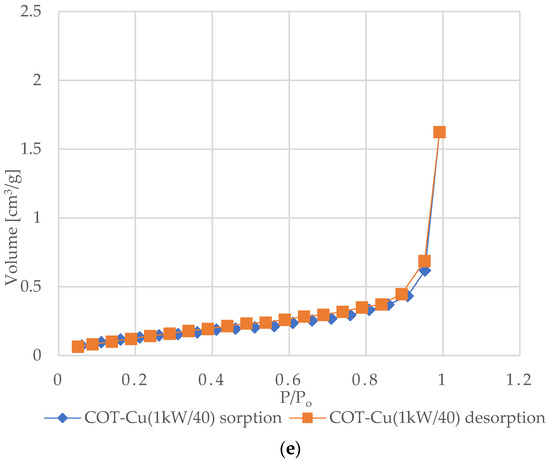
Figure 6.
N2 adsorption–desorption isotherms for COT and COT–Cu samples: (a) COT; (b) COT–Cu(0.5kW/20); (c) COT–Cu(0.5kW/40); (d) COT–Cu(1kW/20); (e) COT–Cu(1kW/40).
3.2.4. Air Permeability
Based on the data presented in Table 5, it can be inferred that the air permeability at various parameters of copper sputtering on cotton fibers, using different power and time settings, shows minimal variation. Specifically, the average air permeability values at a pressure drop of 100 Pa for COT, COT–Cu(0.5kW/20), COT–Cu(0.5kW/40), COT–Cu(1kW/20), and COT–Cu(1kW/40) samples were measured as 810, 805, 800, 806, and 801 mm/s, respectively. These results indicate that despite slight adjustments in sputtering conditions (power and duration), there is no significant alteration in air permeability.

Table 5.
Airflow resistance of the samples was evaluated according to the PN-EN ISO 9237:1998 standard [66]. The results were measured in duplicate and are presented as the mean value ± approximately 2% deviation.
This observation can be attributed to the structural integrity of the cotton substrate during the copper sputtering process under the specified parameters (power and time). It is hypothesized that the magnetron sputtering of copper does not induce substantial changes in the pore geometry of the material, thus resulting in only marginal differences in air permeability across samples with varying sputtering parameters.
3.3. Biological Properties
3.3.1. Antibacterial Effect
The antimicrobial efficacy of copper is attributable to its oxidative processes, resulting in the sequential formation of copper oxides and the subsequent release of copper ions into the surrounding environment. The rate at which copper ions and oxides are released from metallic copper is directly linked to their antimicrobial effectiveness, with metallic copper being the most potent, followed by cuprous oxide and cupric oxide [82,83]. CuO demonstrates bactericidal activity attributed to its capability to catalyze the production of reactive oxygen species (ROS), which play a crucial role in oxidative stress mechanisms against microbial cells. Similarly, transition metal oxides such as CuO [84,85], ZnO [86,87,88], and TiO2 [89,90] manifest antibacterial properties due to their photoactivation potential. This photoinduced process facilitates the generation of bactericidal ROS upon exposure to light, which oxidatively damages bacterial lipids, disrupts cellular integrity, interferes with intracellular constituents, and consequently leads to cellular demise [91].
To evaluate the antibacterial and antifungal properties of the COT–Cu materials obtained, tests were conducted with Gram-negative bacteria (E. coli), Gram-positive bacteria (S. aureus), and representative fungi species (C. globosum, A. niger), following the EN-ISO 20645:2006 [63] and EN-ISO 14119:2005 [64] standards. Detailed results of the microbiological analyses are presented in Table 6. This method allowed for assessing the antibacterial and antifungal activity of the materials obtained and evaluating their potential antibacterial and antifungal properties. Analyzing this data enables the assessment of the effectiveness of COT–Cu and their potential impact on microorganisms, Gram-negative and Gram-positive bacteria, which is crucial for ensuring the safety and hygiene of using these materials.

Table 6.
Results of antibacterial activity tests on COT–Cu materials based on PN-EN ISO 20645:2006 [63] and EN-ISO 14119:2005 [64] standards.
In the unmodified control material (COT), a significant increase in bacterial and fungal colonies was observed spreading over the entire surface of the samples placed in Petri dishes (Table 6). Conversely, cotton–copper materials exhibited inhibitory properties against E. coli, S. aureus, and the fungi C. Globosum and A. Niger. Distinct inhibition zones ranging from 1 to 3 mm in diameter were observed, with no visible growth (Table 6). The results of tests conducted in accordance with EN ISO 20645:2006 [63] and EN 14119:2005 [64] standards confirmed the effective antibacterial protection of copper-infused cotton (COT–Cu) against various microorganisms. To illustrate the microbiological properties, we have included Figure 7 as an example demonstrating tests for antimicrobial activity against Candida globosum. These findings are consistent with previous studies on the antimicrobial properties of copper complexes [78,92], highlighting their potential in enhancing biosecurity and mitigating against the development of pathogens.

Figure 7.
Representative sample images depicting the results of antimicrobial activity tests against Candida globosum using (a) COT and (b) COT–Cu(1kW/40). Zones of inhibition of fungal growth are observed in Petri dishes.
3.3.2. Plasmid Relaxation Assay
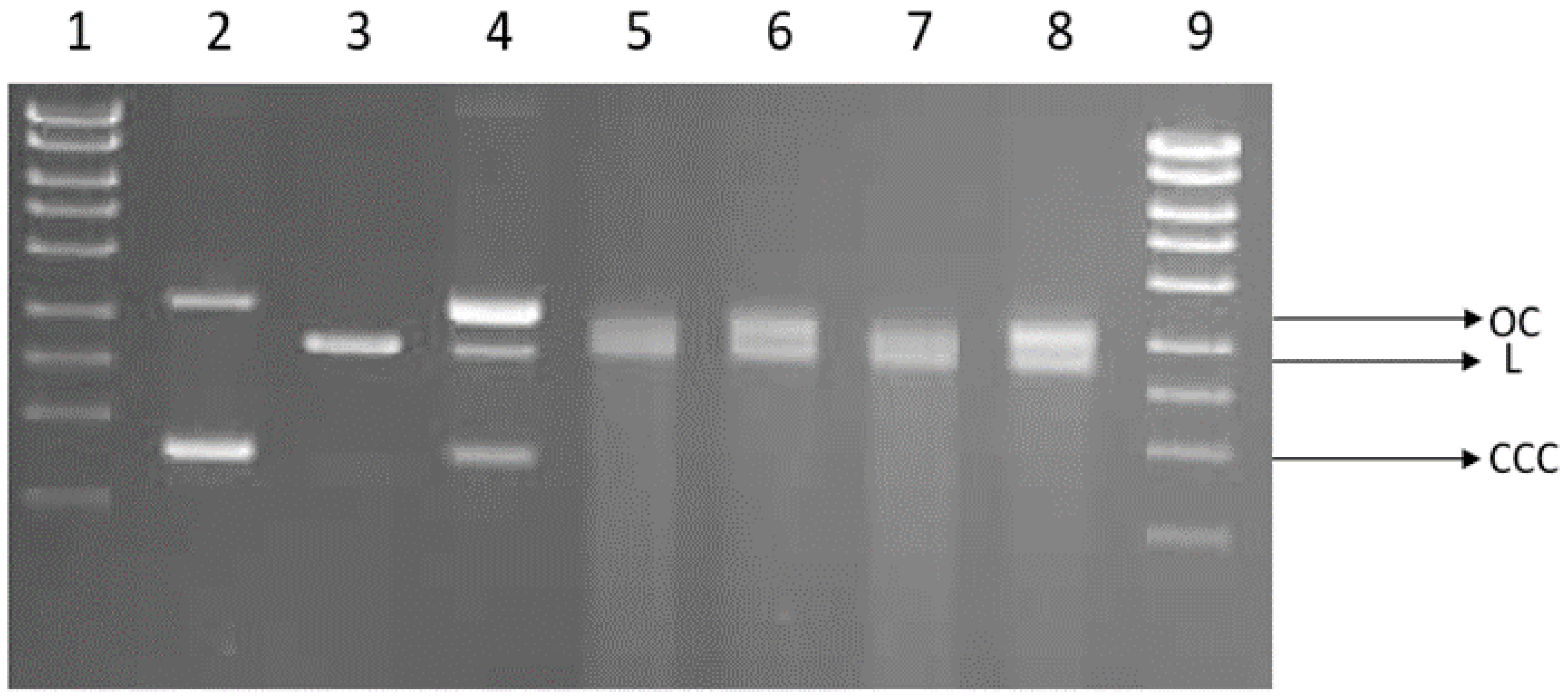
We investigated the possibility of direct interaction of cotton and cotton–copper composites with DNA. For this purpose, we used the plasmid relaxation assay. Results obtained from electrophoretic mobility shift analysis (EMSA) showed that the pUC19 plasmid, which we isolated from the DH5α E. coli cells, is presented mainly in supercoiled form (CCC). Overnight treatment at 37 °C with restrictase PstI led to a linear form (L) of the plasmid. Incubation of the CCC form with cotton–copper composites showed a possibility of DNA adducts or breaks, which affected topological changes in the plasmid.
We demonstrated the induction of single-strand DNA breaks by cotton in vitro. After incubation, the OC and L forms of the plasmid appear in cotton (COT) (Figure 8). Linear and open circular forms are present in COT–Cu samples; for these probes, we can also observe smears indicating significant DNA degradation. DNA degradation occurs at a similar level regardless of the power and duration of magnetron sputtering.
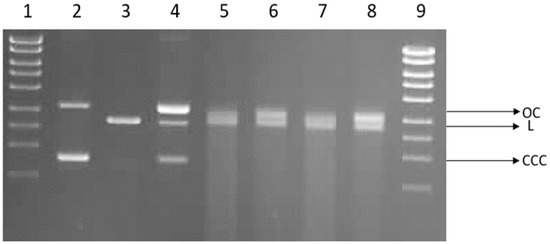
Figure 8.
Plasmid relaxation assay. pUC19 plasmid was incubated for 24 h (37 °C) with cotton and cotton–copper composites (COT–Cu(0.5kW/20), COT–Cu(0.5kW/40), COT–Cu(1kW/20), COT–Cu(1kW/40)) and then separated on a 1% agarose gel, stained with ethidium bromide, and visualized under UV light. Line 1—DNA ladder; line 2—pUC19 plasmid (the supercoiled form, CCC); line 3—pUC19 plasmid incubated with restrictase PstI (the linear form, L); lines 4–8—pUC19 plasmid incubated with COT, COT–Cu(0.5kW/20), COT–Cu(0.5kW/40), COT–Cu(1kW/20), COT–Cu(1kW/40), respectively; line 9—DNA ladder. OC—the open circular form of plasmid DNA.
Copper’s antimicrobial power has been recognized for millennia. Ancient civilizations used copper solutions to treat wounds and purify water [93]. Copper, especially in its Cu2+ form, exhibits potential to interact with DNA [94,95]. Moreover, copper can trigger reactions similar to the Fenton reaction, generating reactive oxygen species (ROS), including extremely reactive hydroxyl radicals (•HO) [96]. Our findings suggest that cotton–copper materials might directly interact with bacterial DNA. This interaction could explain the observed antimicrobial activity against the tested bacteria. However, it is important to note that DNA interaction does not necessarily translate to genotoxicity (DNA damage) in humans. Even if copper from the composites interacts with human DNA, our organisms have robust DNA repair mechanisms, like base excision repair (BER), that eliminate damage and maintain genome stability [97]. Additionally, cellular antioxidant systems protect our cells from the harmful effects of ROS [98].
3.3.3. Effect on Blood Plasma Coagulation: aPTT and PT Measurements
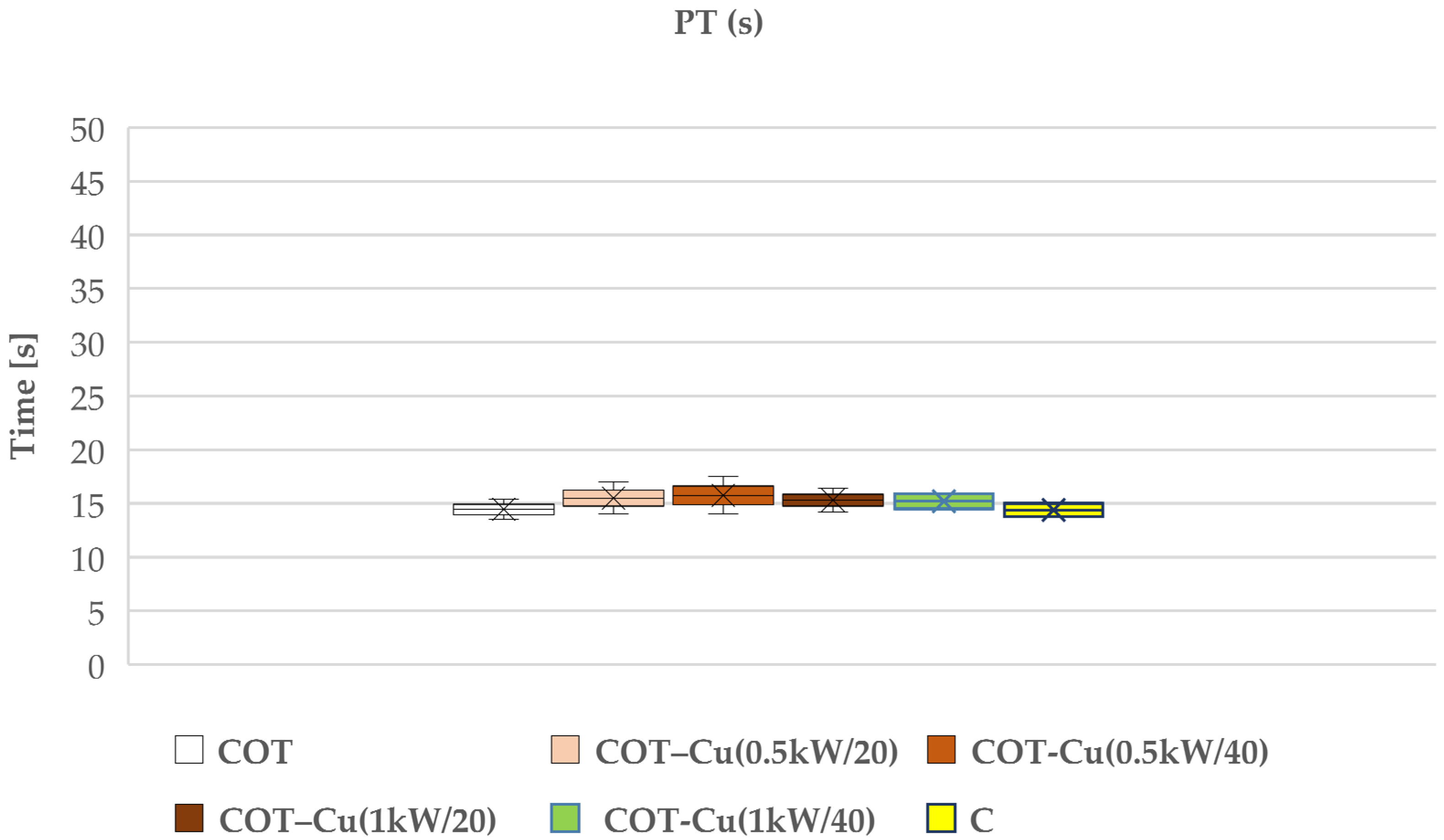
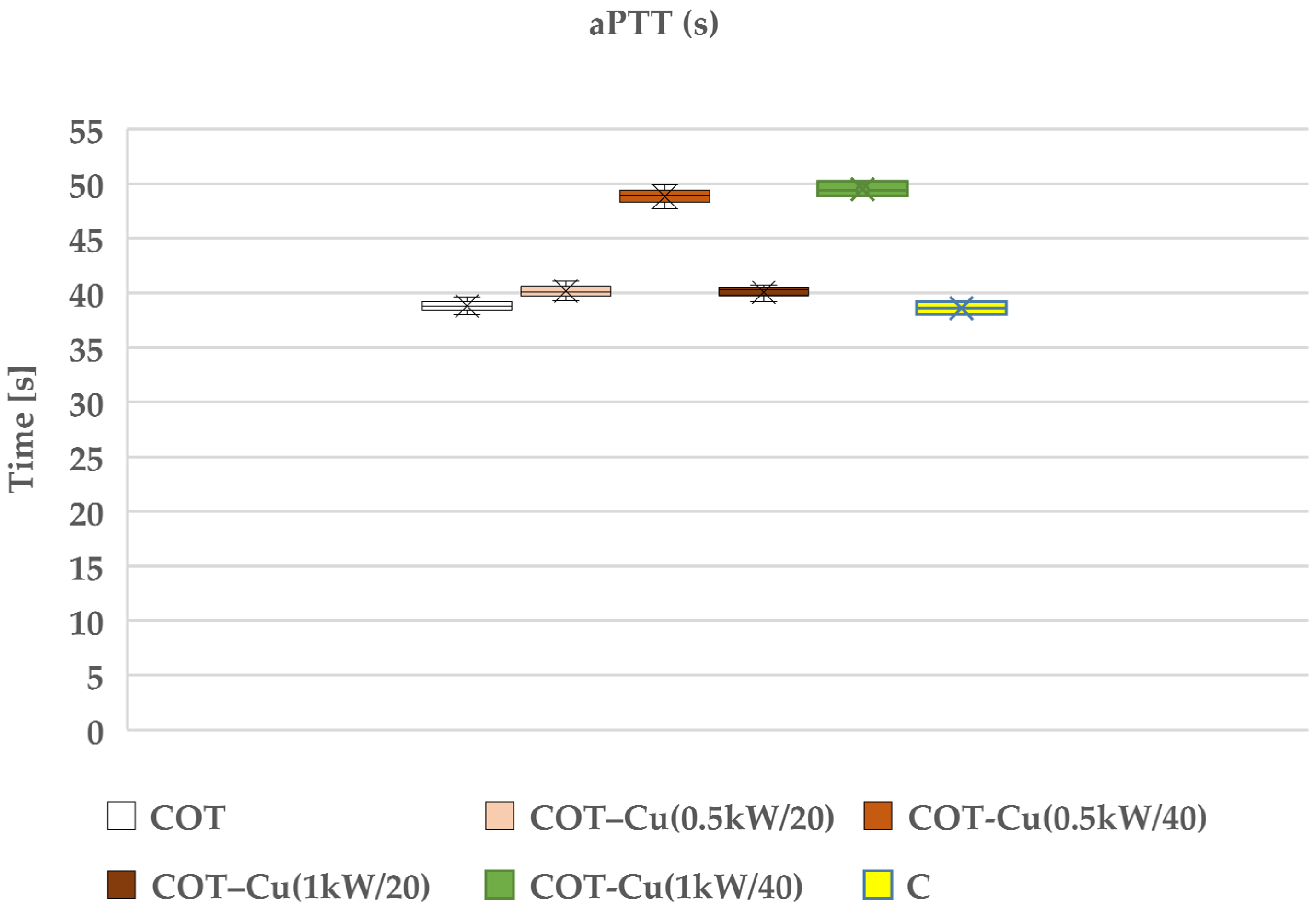
Our research aimed to understand the potential impact of sputtered copper on cotton fabric, particularly on the blood clotting process. We examined the effects of such modifications on prothrombin time (PT) (Figure 9) and partial thromboplastin time (aPTT) (Figure 10), which are key indicators for assessing the coagulation of blood plasma. The results of our study indicated that copper-coated fabric samples did not have a significant adverse effect on these indicators, suggesting that copper did not significantly interfere with the blood plasma coagulation process.
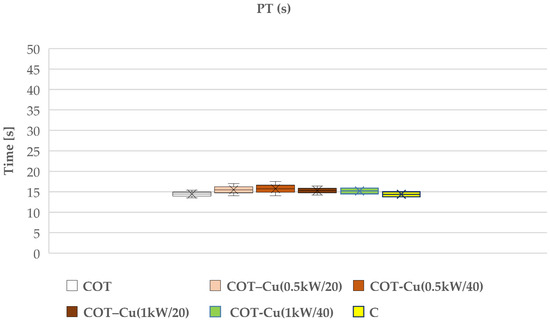
Figure 9.
Effect of the tested copper-coated cotton materials on PT: COT; COT–Cu(0.5kW/20); COT–Cu(0.5kW/40); COT–Cu(1kW/20); COT–Cu(1kW/40), and C—control sample. Results are presented as mean (×), range (bars), median (horizontal line), and interquartile range (box).
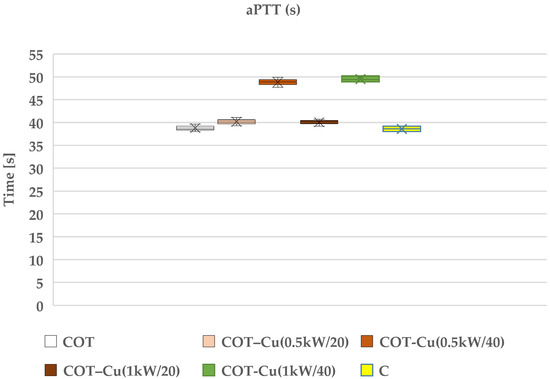
Figure 10.
Effect of the tested copper–coated cotton materials on aPTT: COT; COT–Cu(0.5kW/20); COT–Cu(0.5kW/40); COT–Cu(1kW/20); COT–Cu(1kW/40), and C—control sample. Results are presented as mean (×), range (bars), median (horizontal line), and interquartile range (box).
The aPTT time holds significance particularly in the case of bilateral samples, namely COT–Cu(0.5kW/40) and COT–Cu(1kW/40)COT-1b, indicating some inhibition of the internal (contact) coagulation process in human blood plasma. This outcome suggests that copper may influence the activation of contact factors (XI, XII, HK), leading to a reduction in their concentration in plasma and resulting in an extension of the aPTT time. Nevertheless, we did not observe analogous changes in PT, implying that copper likely affects only the contact factors and not elements of the external coagulation pathway. It is worth emphasizing that our conclusions align with previous studies that have also examined the influence of transition metals, such as copper, on the blood clotting process. These studies have shown the association between copper and aPTT through the binding of factors XI, XII, and HK in human plasma [99]. Therefore, our results are consistent with these findings and suggest that copper may impact the blood coagulation process through interactions with contact factors.
However, it should be noted that these material modifications do not have a significant negative effect on coagulation, suggesting their potential use as dressing materials. Therefore, we conclude that despite some observations regarding the effect of copper on aPTT, material modifications may be utilized in clinical practice due to additional benefits such as antibacterial properties, which were also assessed in our study.
4. Conclusions
In this study, the Cotton–copper composite material (COT–Cu) was successfully prepared using the magnetron sputtering deposition method. We investigated the microbiological and biochemical properties of the material, leading to the following key findings:
- (1)
- Modification of cotton fabrics significantly enhanced antimicrobial properties against various Gram-positive and Gram-negative bacteria as well as fungi compared to control samples made of pure cotton based on PN-EN ISO 20645:2006 [63] and EN-ISO 14119:2005 [64] standards.
- (2)
- Our findings suggest that COT–Cu materials have the potential for direct interaction with plasmid DNA, resulting in single and double DNA breaks, contributing to their antimicrobial effectiveness.
- (3)
- The Cotton–copper composite material did not significantly affect blood plasma coagulation processes, as assessed by partial thromboplastin time (aPTT) and prothrombin time (PT). The sputtering modifications do not have a significant negative effect on coagulation processes, suggesting the potential use of COT–Cu composites as dressing materials.
Author Contributions
M.Ś. developed the concept and designed experiments, performed experiments, analyzed data, and wrote the paper; Z.M. performed experiments and analyzed data; M.J. performed experiments and analyzed data; K.W. analyzed data; M.H.K. developed the concept and designed experiments, analyzed data and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly carried out within the National Science Centre (Poland), project M-ERA.NET 2022, No. 2022/04/Y/ST4/00157.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are included in the text.
Acknowledgments
The authors would like to thank Piotr Kaczmarek for his technical contributions.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zille, A. 6—Plasma Technology in Fashion and Textiles. In Sustainable Technologies for Fashion and Textiles; Nayak, R., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2020; pp. 117–142. ISBN 978-0-08-102867-4. [Google Scholar]
- Haji, A.; Kan, C.-W. Chapter 19—Plasma Treatment for Sustainable Functionalization of Textiles. In Green Chemistry for Sustainable Textiles; Ibrahim, N., Hussain, C.M., Eds.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2021; pp. 265–277. ISBN 978-0-323-85204-3. [Google Scholar]
- Eslami, E.; Jafari, R.; Momen, G. A Review of Plasma-Based Superhydrophobic Textiles: Theoretical Definitions, Fabrication, and Recent Developments. J. Coat. Technol. Res. 2021, 18, 1635–1658. [Google Scholar] [CrossRef]
- Tan, X.-Q.; Liu, J.-Y.; Niu, J.-R.; Liu, J.-Y.; Tian, J.-Y. Recent Progress in Magnetron Sputtering Technology Used on Fabrics. Materials 2018, 11, 1953. [Google Scholar] [CrossRef] [PubMed]
- Shakeri, M. Chapter 2—Cold Plasma Coating for Protective Textiles and Clothing. In Advances in Functional and Protective Textiles; Ul-Islam, S., Butola, B.S., Eds.; The Textile Institute Book Series; Woodhead Publishing: Sawston, UK, 2020. [Google Scholar]
- Ibrahim, N.A.; Eid, B.M. Plasma Treatment Technology for Surface Modification and Functionalization of Cellulosic Fabrics. In Advances in Functional Finishing of Textiles; Shahid, M., Adivarekar, R., Eds.; Springer: Singapore, 2020; pp. 275–287. ISBN 9789811536694. [Google Scholar]
- Shahidi, S.; Ghoranneviss, M.; Moazzenchi, B. New Advances in Plasma Technology for Textile. J. Fusion. Energ. 2014, 33, 97–102. [Google Scholar] [CrossRef]
- Sanders, D.; Grunden, A.; Dunn, R.R. A Review of Clothing Microbiology: The History of Clothing and the Role of Microbes in Textiles. Biol. Lett. 2021, 17, 20200700. [Google Scholar] [CrossRef] [PubMed]
- Gulati, R.; Sharma, S.; Sharma, R.K. Antimicrobial Textile: Recent Developments and Functional Perspective. Polym. Bull. 2022, 79, 5747–5771. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Dong, Q.; Chun, K.; Zhu, D.; Zhang, X.; Mao, Y.; Culver, J.N.; Tai, S.; German, J.R.; Dean, D.P.; et al. Highly Stable, Antiviral, Antibacterial Cotton Textiles via Molecular Engineering. Nat. Nanotechnol. 2023, 18, 168–176. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Biocidal Textiles Can Help Fight Nosocomial Infections. Med. Hypotheses 2008, 70, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Puvvada, R.U.; Wooding, J.P.; Bellavia, M.C.; McGuinness, E.K.; Sulchek, T.A.; Losego, M.D. Bacterial Growth and Death on Cotton Fabrics Conformally Coated with ZnO Thin Films of Varying Thicknesses via Atomic Layer Deposition (ALD). JOM 2019, 71, 178–184. [Google Scholar] [CrossRef]
- Mitchell, A.; Spencer, M.; Edmiston, C. Role of Healthcare Apparel and Other Healthcare Textiles in the Transmission of Pathogens: A Review of the Literature. J. Hosp. Infect. 2015, 90, 285–292. [Google Scholar] [CrossRef]
- Halepoto, H.; Gong, T.; Memon, H. A Bibliometric Analysis of Antibacterial Textiles. Sustainability 2022, 14, 11424. [Google Scholar] [CrossRef]
- Xu, Q.; Gu, J.; Zhao, Y.; Ke, X.; Liu, X. Antibacterial Cotton Fabric with Enhanced Durability Prepared Using L-Cysteine and Silver Nanoparticles. Fibers Polym. 2017, 18, 2204–2211. [Google Scholar] [CrossRef]
- Perelshtein, I.; Applerot, G.; Perkas, N.; Wehrschuetz-Sigl, E.; Hasmann, A.; Guebitz, G.; Gedanken, A. CuO–Cotton Nanocomposite: Formation, Morphology, and Antibacterial Activity. Surf. Coat. Technol. 2009, 204, 54–57. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial Applications of Copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Montero, D.A.; Arellano, C.; Pardo, M.; Vera, R.; Gálvez, R.; Cifuentes, M.; Berasain, M.A.; Gómez, M.; Ramírez, C.; Vidal, R.M. Antimicrobial Properties of a Novel Copper-Based Composite Coating with Potential for Use in Healthcare Facilities. Antimicrob. Resist. Infect. Control 2019, 8, 3. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact Killing and Antimicrobial Properties of Copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef]
- Ramos-Zúñiga, J.; Bruna, N.; Pérez-Donoso, J.M. Toxicity Mechanisms of Copper Nanoparticles and Copper Surfaces on Bacterial Cells and Viruses. Int. J. Mol. Sci. 2023, 24, 10503. [Google Scholar] [CrossRef]
- Alselami, A.; Drummond, R.A. How Metals Fuel Fungal Virulence, yet Promote Anti-Fungal Immunity. Dis. Model Mech. 2023, 16, dmm050393. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper as a Biocidal Tool. Curr. Med. Chem. 2005, 12, 2163–2175. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper, An Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic Copper as an Antimicrobial Surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef]
- Chyderiotis, S.; Legeay, C.; Verjat-Trannoy, D.; Le Gallou, F.; Astagneau, P.; Lepelletier, D. New Insights on Antimicrobial Efficacy of Copper Surfaces in the Healthcare Environment: A Systematic Review. Clin. Microbiol. Infect. 2018, 24, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Ermini, M.L.; Voliani, V. Antimicrobial Nano-Agents: The Copper Age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.C.; Teodora, M.; Lucian, M. Copper Nanoparticles: Synthesis and Characterization, Physiology, Toxicity and Antimicrobial Applications. Appl. Sci. 2022, 12, 141. [Google Scholar] [CrossRef]
- Maliki, M.; Ifijen, I.H.; Ikhuoria, E.U.; Jonathan, E.M.; Onaiwu, G.E.; Archibong, U.D.; Ighodaro, A. Copper Nanoparticles and Their Oxides: Optical, Anticancer and Antibacterial Properties. Int. Nano Lett. 2022, 12, 379–398. [Google Scholar] [CrossRef]
- Li, X.; Cong, Y.; Ovais, M.; Cardoso, M.B.; Hameed, S.; Chen, R.; Chen, M.; Wang, L. Copper-Based Nanoparticles against Microbial Infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2023, 15, e1888. [Google Scholar] [CrossRef] [PubMed]
- Yimeng, S.; Huilun, X.; Ziming, L.; Kejun, L.; Chaima, M.; Xiangyu, Z.; Yinchun, H.; Yan, W.; Di, H. Copper-Based Nanoparticles as Antibacterial Agents. Eur. J. Inorg. Chem. 2023, 26, e202200614. [Google Scholar] [CrossRef]
- Cortes, A.A.; Zuñiga, J.M. The Use of Copper to Help Prevent Transmission of SARS-Coronavirus and Influenza Viruses. A General Review. Diagn. Microbiol. Infect. Dis. 2020, 98, 115176. [Google Scholar] [CrossRef] [PubMed]
- Puchkova, L.V.; Kiseleva, I.V.; Polishchuk, E.V.; Broggini, M.; Ilyechova, E.Y. The Crossroads between Host Copper Metabolism and Influenza Infection. Int. J. Mol. Sci. 2021, 22, 5498. [Google Scholar] [CrossRef]
- Rani, I.; Goyal, A.; Bhatnagar, M.; Manhas, S.; Goel, P.; Pal, A.; Prasad, R. Potential Molecular Mechanisms of Zinc- and Copper-Mediated Antiviral Activity on COVID-19. Nutr. Res. 2021, 92, 109–128. [Google Scholar] [CrossRef]
- Tortella, G.R.; Pieretti, J.C.; Rubilar, O.; Fernández-Baldo, M.; Benavides-Mendoza, A.; Diez, M.C.; Seabra, A.B. Silver, Copper and Copper Oxide Nanoparticles in the Fight against Human Viruses: Progress and Perspectives. Crit. Rev. Biotechnol. 2022, 42, 431–449. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Vasyukova, I.A.; Gusev, A.A. Metal-Based Nanoparticles for the Diagnostics, Therapy, and Prevention of Viral Infections. Nanotechnol. Russ. 2023, 18, 165–188. [Google Scholar] [CrossRef]
- Gerwien, F.; Skrahina, V.; Kasper, L.; Hube, B.; Brunke, S. Metals in Fungal Virulence. FEMS Microbiol. Rev. 2018, 42, fux050. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.R.; Isikhuemhen, O.S.; Anike, F.N. Fungal-Metal Interactions: A Review of Toxicity and Homeostasis. J. Fungi 2021, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Carreira, T.S.; Alves, N.; Sousa, Â.; Valente, J.F.A. Metallic Structures: Effective Agents to Fight Pathogenic Microorganisms. Int. J. Mol. Sci. 2022, 23, 1165. [Google Scholar] [CrossRef] [PubMed]
- Madkhali, O.A. A Comprehensive Review on Potential Applications of Metallic Nanoparticles as Antifungal Therapies to Combat Human Fungal Diseases. Saudi Pharm. J. 2023, 31, 101733. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.N.; Khaffaga, M.M.; Ali, N.M.; Hassan, M.S.; El-Naggar, A.W.M.; Rabie, A.G.M. Antibacterial Functionalization of Cotton and Cotton/Polyester Fabrics Applying Hybrid Coating of Copper/Chitosan Nanocomposites Loaded Polymer Blends via Gamma Irradiation. Int. J. Biol. Macromol. 2021, 183, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Mary, G.; Bajpai, S.K.; Chand, N. Copper (II) Ions and Copper Nanoparticles-Loaded Chemically Modified Cotton Cellulose Fibers with Fair Antibacterial Properties. J. Appl. Polym. Sci. 2009, 113, 757–766. [Google Scholar] [CrossRef]
- Pérez-Alvarez, M.; Cadenas-Pliego, G.; Pérez-Camacho, O.; Comparán-Padilla, V.E.; Cabello-Alvarado, C.J.; Saucedo-Salazar, E. Green Synthesis of Copper Nanoparticles Using Cotton. Polymers 2021, 13, 1906. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liang, Q.; Lu, Y. Microstructure and Properties of Copper Plating on Citric Acid Modified Cotton Fabric. Fibers Polym. 2015, 16, 593–598. [Google Scholar] [CrossRef]
- Sedighi, A.; Montazer, M.; Hemmatinejad, N. Copper Nanoparticles on Bleached Cotton Fabric: In Situ Synthesis and Characterization. Cellulose 2014, 21, 2119–2132. [Google Scholar] [CrossRef]
- Liao, Q.; Kim, E.J.; Tang, Y.; Xu, H.; Yu, D.-G.; Song, W.; Kim, B.J. Rational Design of Hyper-Crosslinked Polymers for Biomedical Applications. J. Polym. Sci. 2024, 62, 1517–1535. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, J.; Yao, Y.; Peters, G.; Macdonald, B.; La Rosa, A.D.; Wang, Z.; Scherer, L. Environmental Impacts of Cotton and Opportunities for Improvement. Nat. Rev. Earth Environ. 2023, 4, 703–715. [Google Scholar] [CrossRef]
- Brunšek, R.; Kopitar, D.; Schwarz, I.; Marasović, P. Biodegradation Properties of Cellulose Fibers and PLA Biopolymer. Polymers 2023, 15, 3532. [Google Scholar] [CrossRef] [PubMed]
- Daria, M.; Krzysztof, L.; Jakub, M. Characteristics of Biodegradable Textiles Used in Environmental Engineering: A Comprehensive Review. J. Clean. Prod. 2020, 268, 122129. [Google Scholar] [CrossRef]
- Rana, S.; Pichandi, S.; Parveen, S.; Fangueiro, R. Natural Plant Fibers: Production, Processing, Properties and Their Sustainability Parameters. In Roadmap to Sustainable Textiles and Clothing: Eco-Friendly Raw Materials, Technologies, and Processing Methods; Muthu, S.S., Ed.; Springer: Singapore, 2014; pp. 1–35. ISBN 978-981-287-065-0. [Google Scholar]
- Szostak-Kotowa, J. Biodeterioration of Textiles. Int. Biodeterior. Biodegrad. 2004, 53, 165–170. [Google Scholar] [CrossRef]
- Egan, J.; Salmon, S. Strategies and Progress in Synthetic Textile Fiber Biodegradability. SN Appl. Sci. 2021, 4, 22. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhao, H.; Pan, C.; Wang, Z. Corrosion Behavior of Copper in Extremely Harsh Marine Atmosphere in Nansha Islands, China. Trans. Nonferrous Met. Soc. China 2021, 31, 703–714. [Google Scholar] [CrossRef]
- She, X.; Peng, J.; Qiang, Y.; Zhou, Y.; Zhang, S. Recent Advances in Protective Technologies against Copper Corrosion. J. Mater. Sci. Technol. 2024, 201, 75–94. [Google Scholar] [CrossRef]
- Fateh, A.; Aliofkhazraei, M.; Rezvanian, A.R. Review of Corrosive Environments for Copper and Its Corrosion Inhibitors. Arab. J. Chem. 2020, 13, 481–544. [Google Scholar] [CrossRef]
- Fang, L.; Zhang, X.; Sun, D. Chemical Modification of Cotton Fabrics for Improving Utilization of Reactive Dyes. Carbohydr. Polym. 2013, 91, 363–369. [Google Scholar] [CrossRef]
- Ling, C.; Guo, L.; Wang, Z. A Review on the State of Flame-Retardant Cotton Fabric: Mechanisms and Applications. Ind. Crops Prod. 2023, 194, 116264. [Google Scholar] [CrossRef]
- Szadkowski, B.; Śliwka-Kaszyńska, M.; Marzec, A. Bioactive and Biodegradable Cotton Fabrics Produced via Synergic Effect of Plant Extracts and Essential Oils in Chitosan Coating System. Sci. Rep. 2024, 14, 8530. [Google Scholar] [CrossRef] [PubMed]
- Tomšič, B.; Klemenčič, D.; Simončič, B.; Orel, B. Influence of Antimicrobial Finishes on the Biodeterioration of Cotton and Cotton/Polyester Fabrics: Leaching versus Bio-Barrier Formation. Polym. Degrad. Stab. 2011, 96, 1286–1296. [Google Scholar] [CrossRef]
- Román, L.E.; Gomez, E.D.; Solís, J.L.; Gómez, M.M. Antibacterial Cotton Fabric Functionalized with Copper Oxide Nanoparticles. Molecules 2020, 25, 5802. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kang, S.H.; Choi, Y.; Lee, W.; Kim, N.; Tanaka, M.; Kang, S.H.; Choi, J. Antibacterial and Biofilm-Inhibiting Cotton Fabrics Decorated with Copper Nanoparticles Grown on Graphene Nanosheets. Sci. Rep. 2023, 13, 11947. [Google Scholar] [CrossRef] [PubMed]
- Copper and Copper Alloys. In Corrosion and Corrosion Control; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 367–381. ISBN 978-0-470-27727-0.
- ISO 15632:2012; Microbeam Analysis—Selected Instrumental Performance Parameters for the Specification and Checking of Energy-Dispersive X-ray Spectrometers for Use in Electron Probe Microanalysis. International Organization for Standardization: Geneva, Switzerland, 2012.
- EN ISO 20645:2006; Textile Fabrics. Determination of Antibacterial Activity—Agar Diffusion Plate Test. International Organization for Standardization: Geneva, Switzerland, 2006.
- EN 14119: 2005 Point 10.5 (B2); Testing of Textiles. Evaluation of the Action of Microfungi. Visual Method. International Organization for Standardization: Geneva, Switzerland, 2005.
- Juszczak, M.; Das, S.; Kosińska, A.; Rybarczyk-Pirek, A.J.; Wzgarda-Raj, K.; Tokarz, P.; Vasudevan, S.; Chworos, A.; Woźniak, K.; Rudolf, B. Piano-Stool Ruthenium(II) Complexes with Maleimide and Phosphine or Phosphite Ligands: Synthesis and Activity against Normal and Cancer Cells. Dalton Trans. 2023, 52, 4237–4250. [Google Scholar] [CrossRef] [PubMed]
- EN ISO 9237:1998; Textiles—Determination of the Permeability of Fabrics to Air. International Organization for Standardization: Geneva, Switzerland, 1998.
- Garg, R.; Gonuguntla, S.; Sk, S.; Iqbal, M.S.; Dada, A.O.; Pal, U.; Ahmadipour, M. Sputtering Thin Films: Materials, Applications, Challenges and Future Directions. Adv. Colloid Interface Sci. 2024, 330, 103203. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, J.T. Physics and Technology of Magnetron Sputtering Discharges. Plasma Sources Sci. Technol. 2020, 29, 113001. [Google Scholar] [CrossRef]
- Braun, M. Magnetron Sputtering Technique. In Handbook of Manufacturing Engineering and Technology; Nee, A.Y.C., Ed.; Springer: London, UK, 2015; pp. 2929–2957. ISBN 978-1-4471-4670-4. [Google Scholar]
- Gudmundsson, J.T.; Lundin, D. 1—Introduction to Magnetron Sputtering. In High Power Impulse Magnetron Sputtering; Lundin, D., Minea, T., Gudmundsson, J.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–48. ISBN 978-0-12-812454-3. [Google Scholar]
- Hotová, G.; Slovák, V.; Zelenka, T.; Maršálek, R.; Parchaňská, A. The Role of the Oxygen Functional Groups in Adsorption of Copper (II) on Carbon Surface. Sci. Total Environ. 2020, 711, 135436. [Google Scholar] [CrossRef]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact Killing of Bacteria on Copper Is Suppressed If Bacterial-Metal Contact Is Prevented and Is Induced on Iron by Copper Ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore Characterization of Different Types of Coal from Coal and Gas Outburst Disaster Sites Using Low Temperature Nitrogen Adsorption Approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Ding, J.; Liu, T.; Shi, G.; Li, X.; Dang, W.; Cheng, Y.; Guo, R. Pore Structure and Fractal Characteristics of Different Shale Lithofacies in the Dalong Formation in the Western Area of the Lower Yangtze Platform. Minerals 2020, 10, 72. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Mrozińska, Z.; Kudzin, M.H.; Ponczek, M.B.; Kaczmarek, A.; Król, P.; Lisiak-Kucińska, A.; Żyłła, R.; Walawska, A. Biochemical Approach to Poly(Lactide)–Copper Composite—Impact on Blood Coagulation Processes. Materials 2024, 17, 608. [Google Scholar] [CrossRef] [PubMed]
- Lowell, S.; Shields, J.E.; Thomas, M.A.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-1-4020-2303-3. [Google Scholar]
- Gregg, S.J.; Sing, K.S.W.; Salzberg, H.W. Adsorption Surface Area and Porosity. J. Electrochem. Soc. 1967, 114, 279Ca. [Google Scholar] [CrossRef]
- Medrano, V.G.B.; Celis, V.N.; Giraldo, R.I. Systematic Analysis of the Nitrogen Adsorption-Desorption Isotherms Recorded for a Series of Microporous—Mesoporous Amorphous Aluminosilicates Using Classical Methods. Mater. Sci. 2022; in print. [Google Scholar]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of Copper Oxides in Contact Killing of Bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef]
- Popov, S.; Saphier, O.; Popov, M.; Shenker, M.; Entus, S.; Shotland, Y.; Saphier, M. Factors Enhancing the Antibacterial Effect of Monovalent Copper Ions. Curr. Microbiol. 2020, 77, 361–368. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive Oxygen Species Generation by Copper(II) Oxide Nanoparticles Determined by DNA Damage Assays and EPR Spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef]
- Applerot, G.; Lellouche, J.; Lipovsky, A.; Nitzan, Y.; Lubart, R.; Gedanken, A.; Banin, E. Understanding the Antibacterial Mechanism of CuO Nanoparticles: Revealing the Route of Induced Oxidative Stress. Small 2012, 8, 3326–3337. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-W.; Cao, A.; Jiang, Y.; Zhang, X.; Liu, J.-H.; Liu, Y.; Wang, H. Superior Antibacterial Activity of Zinc Oxide/Graphene Oxide Composites Originating from High Zinc Concentration Localized around Bacteria. ACS Appl. Mater. Interfaces 2014, 6, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Weng, Z.; Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Biomedical Applications of Functionalized ZnO Nanomaterials: From Biosensors to Bioimaging. Adv. Mater. Interfaces 2016, 3, 1500494. [Google Scholar] [CrossRef]
- Su, Y.; Cockerill, I.; Wang, Y.; Qin, Y.-X.; Chang, L.; Zheng, Y.; Zhu, D. Zinc-Based Biomaterials for Regeneration and Therapy. Trends. Biotechnol. 2019, 37, 428–441. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-Light Activation of TiO2 Photocatalysts: Advances in Theory and Experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Bono, N.; Ponti, F.; Punta, C.; Candiani, G. Effect of UV Irradiation and TiO2-Photocatalysis on Airborne Bacteria and Viruses: An Overview. Materials 2021, 14, 1075. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Zhang, L.; Zhu, J.; Wu, H.; Li, W.; Sun, Q. Antibacterial Properties and Mechanism of Biopolymer-Based Films Functionalized by CuO/ZnO Nanoparticles against Escherichia coli and Staphylococcus aureus. J. Hazard Mater. 2021, 402, 123542. [Google Scholar] [CrossRef] [PubMed]
- Mrozińska, Z.; Ponczek, M.; Kaczmarek, A.; Boguń, M.; Sulak, E.; Kudzin, M.H. Blood Coagulation Activities of Cotton-Alginate-Copper Composites. Mar. Drugs 2023, 21, 625. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Xie, X.; Liu, Y.; Zhu, Z.; Sun, L. Nanoscale Study of DNA–Cu2+ Interactions by Liquid-Cell Electron Microscopy. ACS Omega 2023, 8, 26325–26331. [Google Scholar] [CrossRef]
- Erxleben, A. Interactions of Copper Complexes with Nucleic Acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- de Souza, Í.P.; Machado, B.d.P.; de Carvalho, A.B.; Binatti, I.; Krambrock, K.; Molphy, Z.; Kellett, A.; Pereira-Maia, E.C.; Silva-Caldeira, P.P. Exploring the DNA Binding, Oxidative Cleavage, and Cytotoxic Properties of New Ternary Copper(II) Compounds Containing 4-Aminoantipyrine and N,N-Heterocyclic Co-Ligands. J. Mol. Struct. 2019, 1178, 18–28. [Google Scholar] [CrossRef]
- Pham, A.N.; Xing, G.; Miller, C.J.; Waite, T.D. Fenton-like Copper Redox Chemistry Revisited: Hydrogen Peroxide and Superoxide Mediation of Copper-Catalyzed Oxidant Production. J. Catal. 2013, 301, 54–64. [Google Scholar] [CrossRef]
- Whitaker, A.M.; Schaich, M.A.; Smith, M.R.; Flynn, T.S.; Freudenthal, B.D. Base Excision Repair of Oxidative DNA Damage: From Mechanism to Disease. Front. Biosci. (Landmark Ed.) 2017, 22, 1493–1522. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-Y.; Kim, H.-S. Oxidative Stress and the Antioxidant Enzyme System in the Developing Brain. Korean J. Pediatr. 2013, 56, 107–111. [Google Scholar] [CrossRef]
- Mutch, N.J.; Waters, E.K.; Morrissey, J.H. Immobilized Transition Metal Ions Stimulate Contact Activation and Drive Factor XII-mediated Coagulation. J. Thromb. Haemost. 2012, 10, 2108–2115. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).