Ultra-Structural Surface Characteristics of Dental Silane Monolayers
Abstract
1. Introduction
2. Materials and Methods
2.1. Silicon Wafer Substrate
2.2. Silanization
2.3. Surface Characterization
2.3.1. Ellipsometry
2.3.2. Contact Angle and Surface Free Energy
2.3.3. Atomic Force Microscopy (AFM)
2.3.4. X-ray Photoelectron Spectroscopy (XPS)
3. Results
3.1. Ellipsometry
3.2. Contact Angle and Surface Free Energy
3.3. Atomic Force Microscopy (AFM)
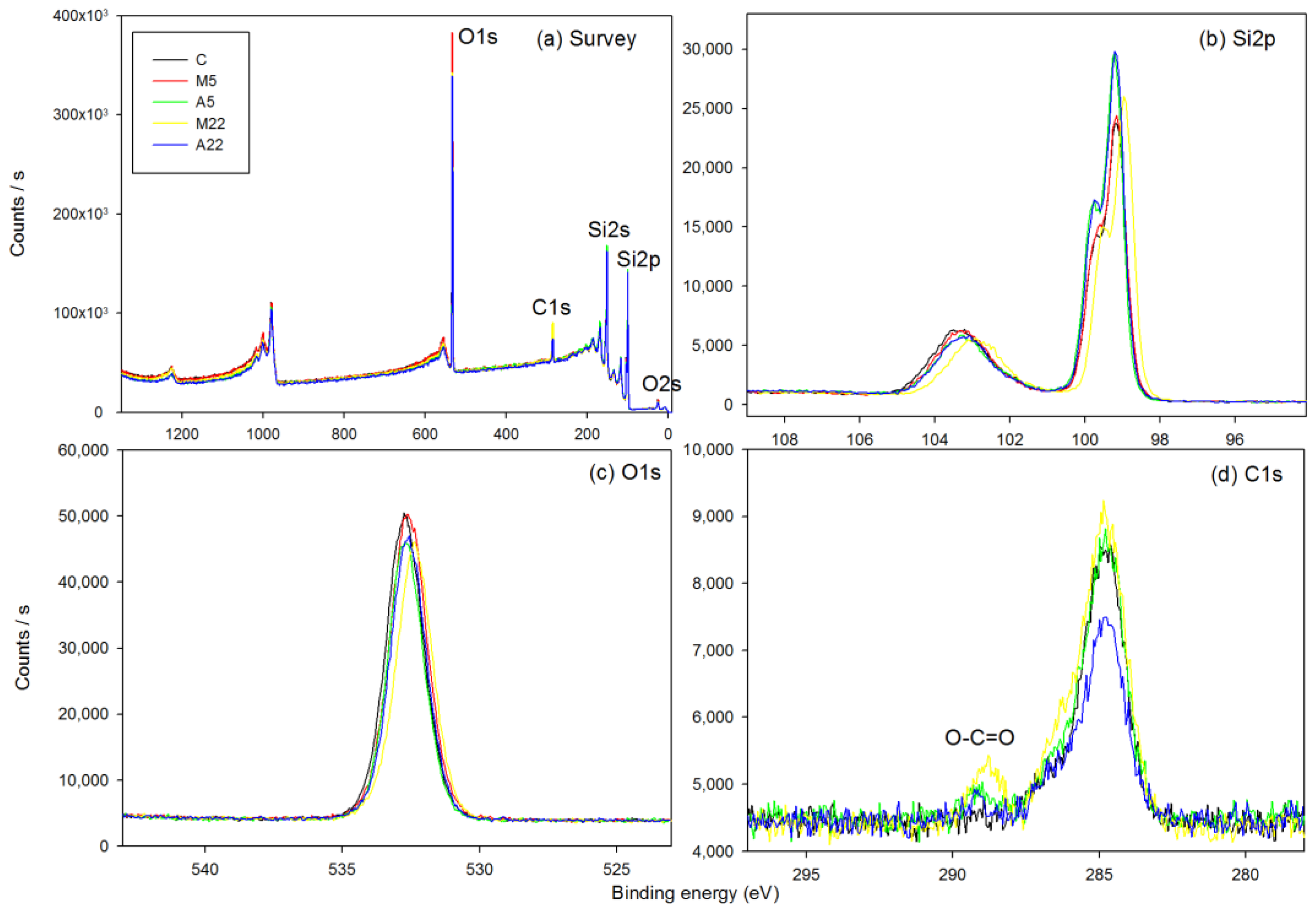
3.4. X-ray Photoelectron Spectroscopy (XPS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- German, M.J. Developments in resin-based composites. Brit. Dent. J. 2022, 232, 638–643. [Google Scholar] [CrossRef] [PubMed]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Turssi, C.; Ferracane, J.; Vogel, K. Filler features and their effects on wear and degree of conversion of particulate dental resin composites. Biomaterials 2005, 26, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Saito, O.; Mizuno, M.; Tanaka, H. Changes in translucency and color of particulate filler composite resins. Int. J. Prosthodont. 2002, 15, 494–499. [Google Scholar] [PubMed]
- Bai, X.; Chen, Y.; Zhou, T.; Pow, E.H.N.; Tsoi, J.K.H. The chemical and optical stability evaluation of injectable restorative materials under wet challenge. J. Dent. 2024, 146, 105031. [Google Scholar] [CrossRef] [PubMed]
- Shortall, A.C.; Palin, W.M.; Burtscher, P. Refractive index mismatch and monomer reactivity influence composite curing depth. J. Dent. Res. 2008, 87, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Staxrud, F.; Dahl, J.E. Silanising agents promote resin-composite repair. Int. Dent. J. 2015, 65, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Tsoi, J.K.-H.; Pow, E.H.-N.; Wong, H.M. Influence of different etching protocols on the reliability of resin bonding to CAD/CAM feldspathic porcelain. Int. J. Adhes. Adhes. 2015, 62, 18–24. [Google Scholar] [CrossRef]
- Tian, T.; Tsoi, J.K.-H.; Matinlinna, J.P.; Burrow, M.F. Aspects of bonding between resin luting cements and glass ceramic materials. Dent. Mater. 2014, 30, 147–162. [Google Scholar] [CrossRef]
- Li, J.; Bai, H.; Feng, Z. Advances in the Modification of Silane-Based Sol-Gel Coating to Improve the Corrosion Resistance of Magnesium Alloys. Molecules 2023, 28, 2563. [Google Scholar] [CrossRef]
- Zhang, C.; Li, T.; Wu, X.; Li, W.; Guo, Y.; Zhang, S.; Zhang, L. Ni-rich cathode materials with enhanced kinetics and hydrophobicity endowed by reactive silane coating. Chem. Eng. J. 2023, 473, 145309. [Google Scholar] [CrossRef]
- Passaro, J.; Bifulco, A.; Calabrese, E.; Imparato, C.; Raimondo, M.; Pantani, R.; Aronne, A.; Guadagno, L. Hybrid Hemp Particles as Functional Fillers for the Manufacturing of Hydrophobic and Anti-icing Epoxy Composite Coatings. ACS Omega 2023, 8, 23596–23606. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.M.; Alhalabi, F.; Alshehri, A.; Salem, M.A.; Robaian, A.; Alghannam, S.; Alayad, A.S.; Almutairi, B.; Alrahlah, A. Silane-Containing Universal Adhesives Influence Resin-Ceramic Microtensile Bond Strength. Coatings 2023, 13, 477. [Google Scholar] [CrossRef]
- Garcia, I.M.; de Souza, V.S.; Balhaddad, A.A.; Mokeem, L.; de Melo, M.A.S.; Scholten, J.D.; Collares, F.M. Ionic Liquid-Based Silane for SiO Nanoparticles: A Versatile Coupling Agent for Dental Resins. ACS Appl. Mater. Interfaces 2024, 16, 34057–34068. [Google Scholar] [CrossRef] [PubMed]
- Drummond, J. Degradation, fatigue, and failure of resin dental composite materials. J. Dent. Res. 2008, 87, 710–719. [Google Scholar] [CrossRef] [PubMed]
- Antonucci, J.M.; Dickens, S.H.; Fowler, B.O.; Xu, H.H.K.; McDonough, W.G. Chemistry of silanes: Interfaces in dental polymers and composites. J. Res. Natl. Inst. Stand. Technol. 2005, 110, 541–558. [Google Scholar] [CrossRef]
- Kannengiesser, J.F.; Morgenstern, B.; Janka, O.; Kickelbick, G. Oligo-Condensation Reactions of Silanediols with Conservation of Solid-State-Structural Features. Chem. A Eur. J. 2024, 30, 202303343. [Google Scholar] [CrossRef] [PubMed]
- Hoikkanen, M.; Honkanen, M.; Vippola, M.; Lepistö, T.; Vuorinen, J. Effect of silane treatment parameters on the silane layer formation and bonding to thermoplastic urethane. Prog. Org. Coatings 2011, 72, 716–723. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Arkles, B.; Steinmetz, J.R.; Zazyczny, J.; Mehta, P. Factors Contributing to the Stability of Alkoxysilanes in Aqueous-Solution. J Adhes. Sci. Technol. 1992, 6, 193–206. [Google Scholar] [CrossRef]
- Pantoja, M.; Díaz-Benito, B.; Velasco, F.; Abenojar, J.; del Real, J. Analysis of hydrolysis process of γ-methacryloxypropyltrimethoxysilane and its influence on the formation of silane coatings on 6063 aluminum alloy. Appl. Surf. Sci. 2009, 255, 6386–6390. [Google Scholar] [CrossRef]
- Bel-Hassen, R.; Boufi, S.; Salon, M.B.; Abdelmouleh, M.; Belgacem, M.N. Adsorption of silane onto cellulose fibers. II. The effect of pH on silane hydrolysis, condensation, and adsorption behavior. J. Appl. Polym. Sci. 2008, 108, 1958–1968. [Google Scholar] [CrossRef]
- Ben Ali, M.; Bessueille, F.; Chovelon, J.; Abdelghani, A.; Jaffrezic-Renault, N.; Maaref, M.; Martelet, C. Use of ultra-thin organic silane films for the improvement of gold adhesion to the silicon dioxide wafers for (bio)sensor applications. Mater. Sci. Eng. C 2008, 28, 628–632. [Google Scholar]
- Villard, N.; Seneviratne, C.; Tsoi, J.K.H.; Heinonen, M.; Matinlinna, J. Candida albicans aspects of novel silane system-coated titanium and zirconia implant surfaces. Clin. Oral Implant. Res. 2015, 26, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Monticelli, F.; Toledano, M.; Osorio, R.; Ferrari, M. Effect of temperature on the silane coupling agents when bonding core resin to quartz fiber posts. Dent. Mater. 2006, 22, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, X.; Luo, J.; Lu, X. Fabrication and tribological properties of super-hydrophobic surfaces based on porous silicon. Appl. Surf. Sci. 2009, 255, 9430–9438. [Google Scholar] [CrossRef]
- Gunda, N.S.K.; Singh, M.; Norman, L.; Kaur, K.; Mitra, S.K. Optimization and characterization of biomolecule immobilization on silicon substrates using (3-aminopropyl)triethoxysilane (APTES) and glutaraldehyde linker. Appl. Surf. Sci. 2014, 305, 522–530. [Google Scholar] [CrossRef]
- Cloarec, J.P.; Deligianis, N.; Martin, J.R.; Lawrence, I.; Souteyrand, E.; Polychronakos, C.; Lawrence, M.F. Immobilization of homooligonucleotide probe layers onto Si/SiO substrates: Characterization by electrochemical impedance measurements and radiolabelling. Biosens. Bioelectron. 2002, 17, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Clergereaux, R.; Calafat, M.; Benitez, F.; Escaich, D.; de Larclause, I.S.; Raynaud, P.; Esteve, J. Comparison between continuous and microwave oxygen plasma post-treatment on organosilicon plasma deposited layers: Effects on structure and properties. Thin Solid Films 2007, 515, 3452–3460. [Google Scholar] [CrossRef]
- Queiroz, J.R.C.; Benetti, P.; Özcan, M.; de Oliveira, L.F.C.; Della Bona, A.; Takahashi, F.E.; Bottino, M.A. Surface characterization of feldspathic ceramic using ATR FT-IR and ellipsometry after various silanization protocols. Dent. Mater. 2012, 28, 189–196. [Google Scholar] [CrossRef][Green Version]
- Hooshmand, T.; Keshvad, A.; van Noort, R. XPS Analysis of Silane Films on the Surface of a Dental Ceramic. J. Adhes. Sci. Technol. 2009, 23, 1085–1095. [Google Scholar] [CrossRef]
- Nakamura, Y.; Gotoh, T.; Honda, H.; Fujii, S.; Nagata, K. AFM Observation of a Mica Surface Treated with Silane Coupling Agent Having a Mercapto Group. Compos. Interfaces 2010, 17, 395–404. [Google Scholar] [CrossRef]
- Stalder, A.F.; Melchior, T.; Müller, M.; Sage, D.; Blu, T.; Unser, M. Low-bond axisymmetric drop shape analysis for surface tension and contact angle measurements of sessile drops. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 364, 72–81. [Google Scholar] [CrossRef]
- Tan, C.S.; Fan, A.; Chen, K.N.; Reif, R. Low-temperature thermal oxide to plasma-enhanced chemical vapor deposition oxide wafer bonding for thin-film transfer application. Appl. Phys. Lett. 2003, 82, 2649–2651. [Google Scholar] [CrossRef]
- Borges-Muñoz, A.C.; Miller, D.P.; Zurek, E.; Colón, L.A. Silanization of superficially porous silica particles with-aminophenyltrimethoxysilane. Microchem. J. 2019, 147, 263–268. [Google Scholar] [CrossRef]
- Yang, Y.; Bittner, A.M.; Baldelli, S.; Kern, K. Study of self-assembled triethoxysilane thin films made by casting neat reagents in ambient atmosphere. Thin Solid Films 2008, 516, 3948–3956. [Google Scholar] [CrossRef]
- Banga, R.; Yarwood, J.; Morgan, A.M.; Evans, B.; Kells, J. Ftir and Afm Studies of the Kinetics and Self-Assembly of Alkyltrichlorosilanes and (Perfluoroalkyl)Trichlorosilanes onto Glass and Silicon. Langmuir 1995, 11, 4393–4399. [Google Scholar] [CrossRef]
- Hidzir, N.M.; Radzali, N.A.M.; Rahman, I.A.; Shamsudin, S.A. Gamma irradiation-induced grafting of 2-hydroxyethyl methacrylate (HEMA) onto ePTFE for implant applications. Nucl. Eng. Technol. 2020, 52, 2320–2327. [Google Scholar] [CrossRef]
- Yun, J.; Burrow, M.F.; Matinlinna, J.P.; Ding, H.; Chan, S.M.; Tsoi, J.K.; Wang, Y. Design of Multi-Functional Bio-Safe Dental Resin Composites with Mineralization and Anti-Biofilm Properties. J. Funct. Biomater. 2024, 15, 120. [Google Scholar] [CrossRef]
- Ericson, T.; Pruitt, K.M.; Arwin, H.; Lundstrom, I. Ellipsometric Studies of Film Formation on Tooth Enamel and Hydrophilic Silicon Surfaces. Acta Odontol. Scand. 1982, 40, 197–201. [Google Scholar] [CrossRef]
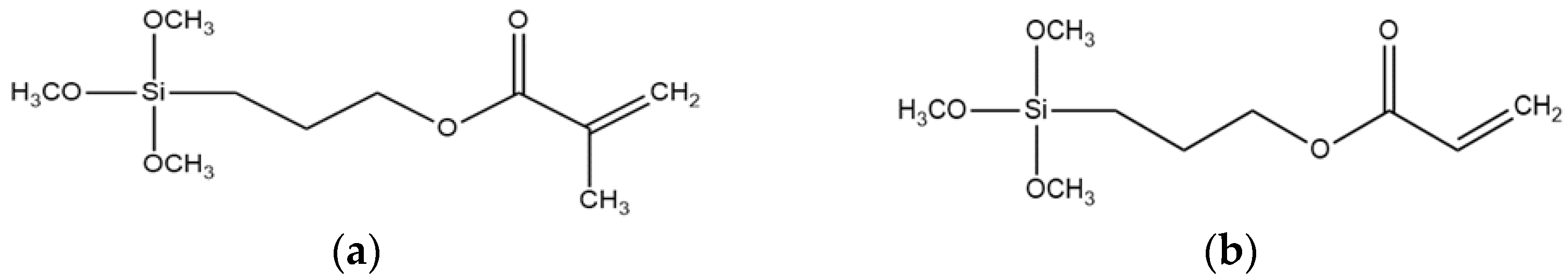



| Silanization Protocol | M5 | M22 | A5 | A22 |
|---|---|---|---|---|
| Film thickness (nm) | 1.16 | 1.00 | 1.22 | 0.85 |
| Solvent | C | M5 | M22 | A5 | A22 |
|---|---|---|---|---|---|
| Water (°) | 29.6 ± 0.2 | 70.1 ± 0.7 | 71.5 ± 0.1 | 60.7 ± 1.5 | 70.5 ± 1.4 |
| 1-Bromonaphthalene (°) | 24.2 ± 1.1 | 34.6 ± 0.4 | 29.3 ± 0.3 | 23.3 ± 0.6 | 27.1 ± 0.4 |
| Diiodomethane (°) | 31.5 ± 0.5 | 38.8 ± 0.2 | 38.7 ± 0.3 | 39.2 ± 0.6 | 40.4 ± 1.0 |
| Formamide (°) | 11.5 ± 3.4 | 48.5 ± 0.1 | 60.0 ± 0.4 | 36.3 ± 0.5 | 61.1 ± 0.2 |
| SFE (mN/m) | 46.3 | 41.5 | 40.0 | 44.5 | 40.1 |
| Peak | Position (eV) | Atomic Percentage (%) | ||||
|---|---|---|---|---|---|---|
| C | M5 | A5 | M22 | A22 | ||
| C1s | 284.8 | 8.90 | 12.97 | 9.29 | 16.72 | 10.47 |
| O1s | 532.6 | 39.52 | 40.27 | 37.66 | 36.75 | 37.60 |
| Si2p | 99.0 | 51.58 | 46.76 | 53.05 | 46.54 | 51.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Shum, W.W.-Y.; Tsoi, J.K.-H. Ultra-Structural Surface Characteristics of Dental Silane Monolayers. Coatings 2024, 14, 1005. https://doi.org/10.3390/coatings14081005
Liu X, Shum WW-Y, Tsoi JK-H. Ultra-Structural Surface Characteristics of Dental Silane Monolayers. Coatings. 2024; 14(8):1005. https://doi.org/10.3390/coatings14081005
Chicago/Turabian StyleLiu, Xiaotian, Winnie Wing-Yee Shum, and James Kit-Hon Tsoi. 2024. "Ultra-Structural Surface Characteristics of Dental Silane Monolayers" Coatings 14, no. 8: 1005. https://doi.org/10.3390/coatings14081005
APA StyleLiu, X., Shum, W. W.-Y., & Tsoi, J. K.-H. (2024). Ultra-Structural Surface Characteristics of Dental Silane Monolayers. Coatings, 14(8), 1005. https://doi.org/10.3390/coatings14081005








