Structure, Mechanical Properties and Water Vapor Corrosion Resistance of AlCrNbSiTiN High-Entropy Nitride Coatings Deposited by RF Magnetron Sputtering
Abstract
1. Introduction
2. Experimental Details
2.1. Coatings Preparation
2.2. Coatings Characterization
3. Results and Discussion
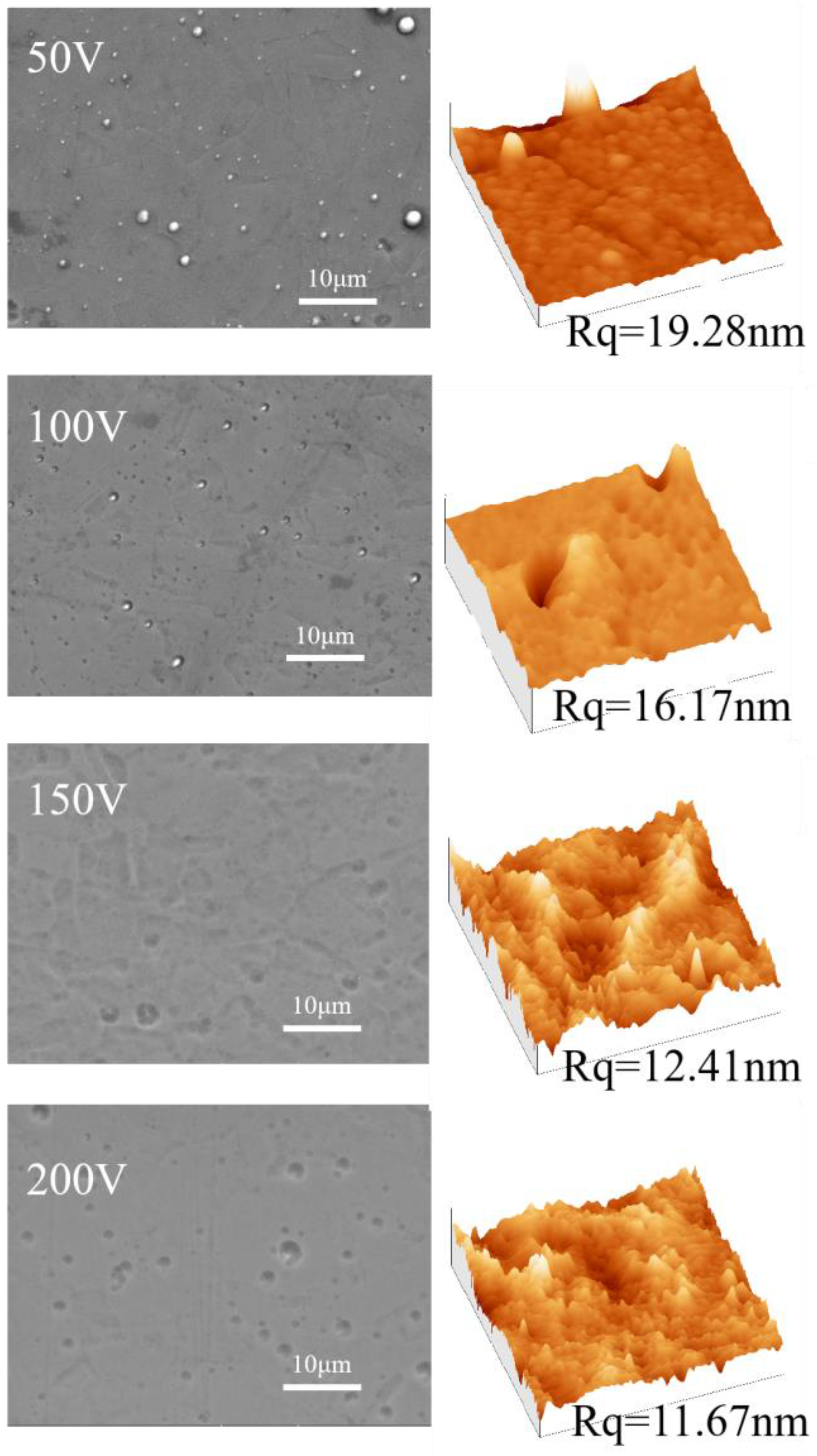
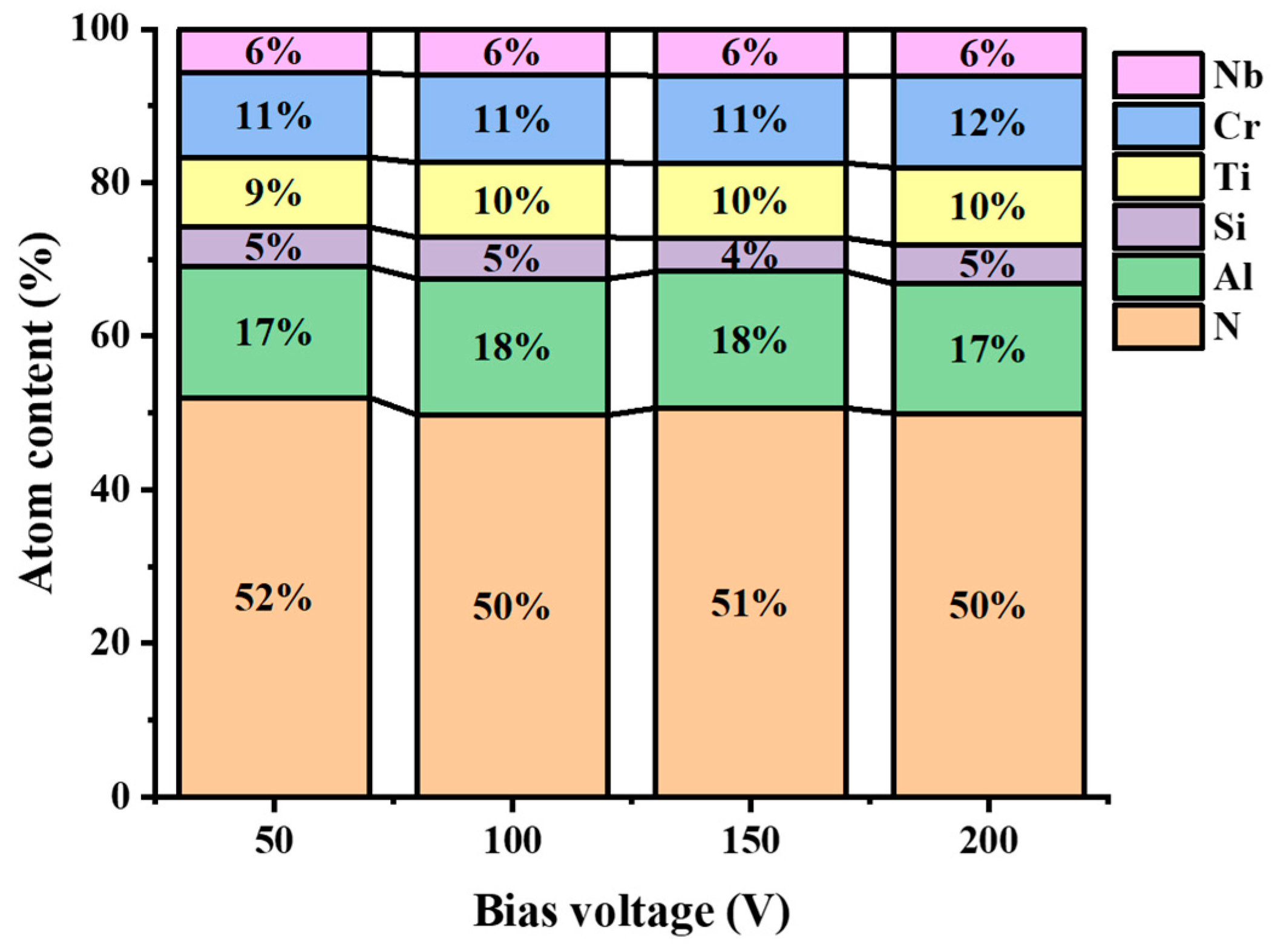
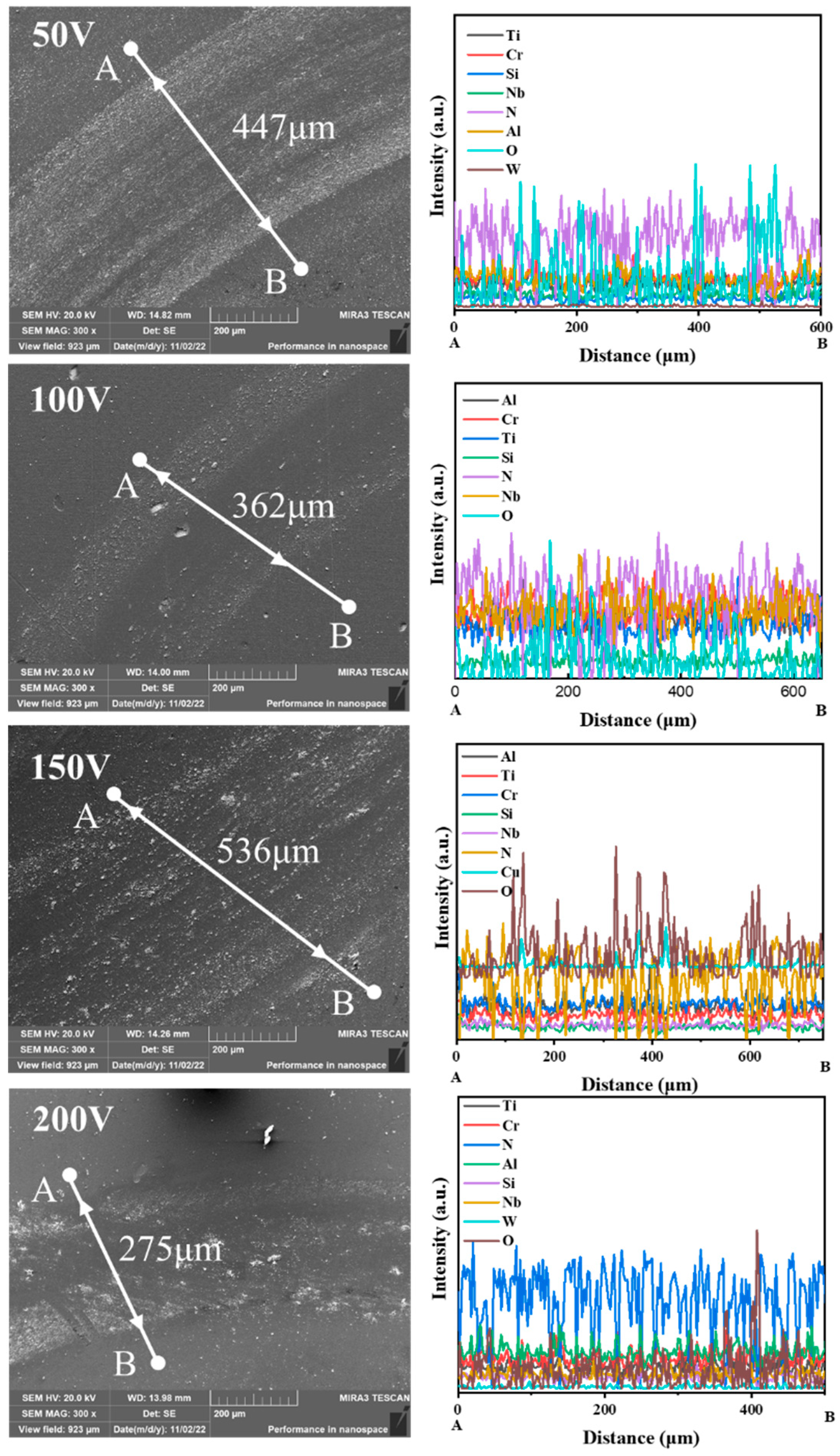
3.1. Morphology and Chemical Composition Analysis
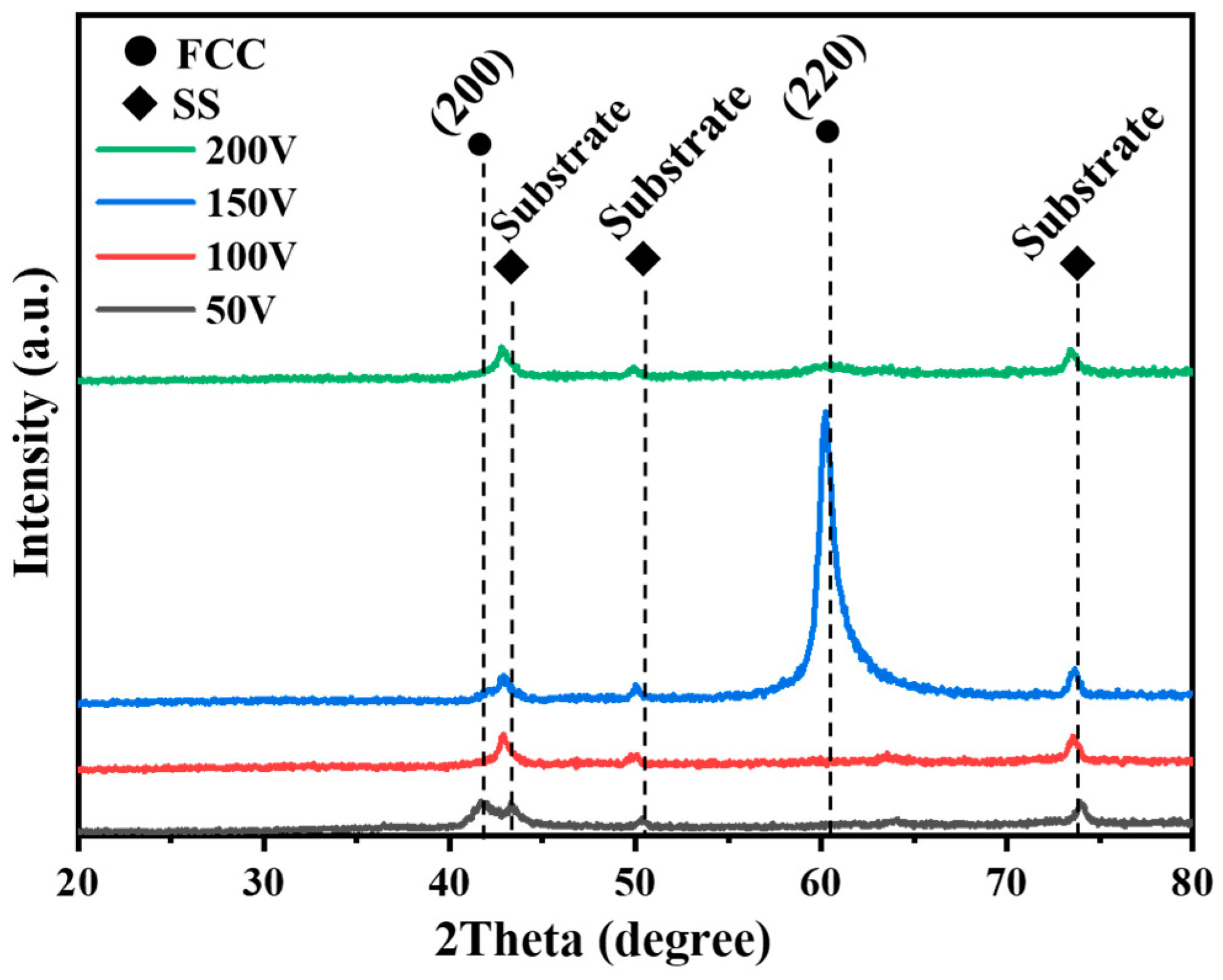
3.2. XRD Analysis
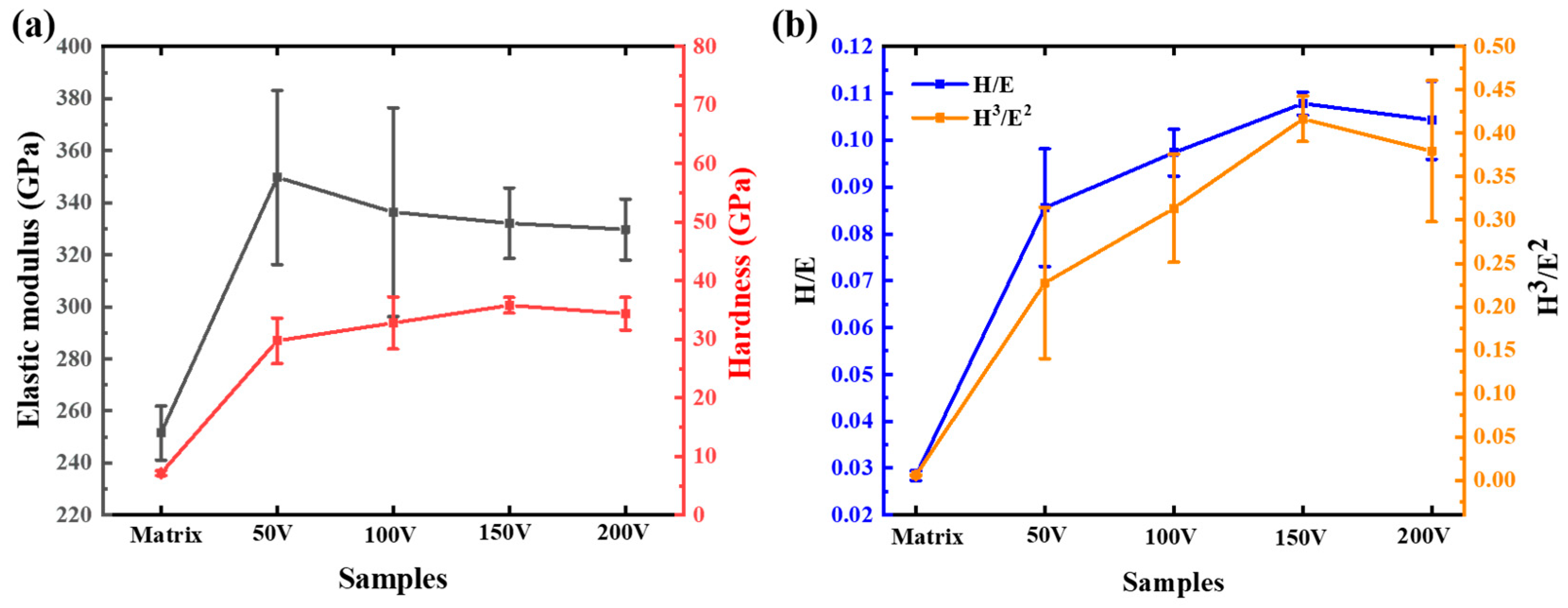
3.3. Mechanical and Tribological Properties
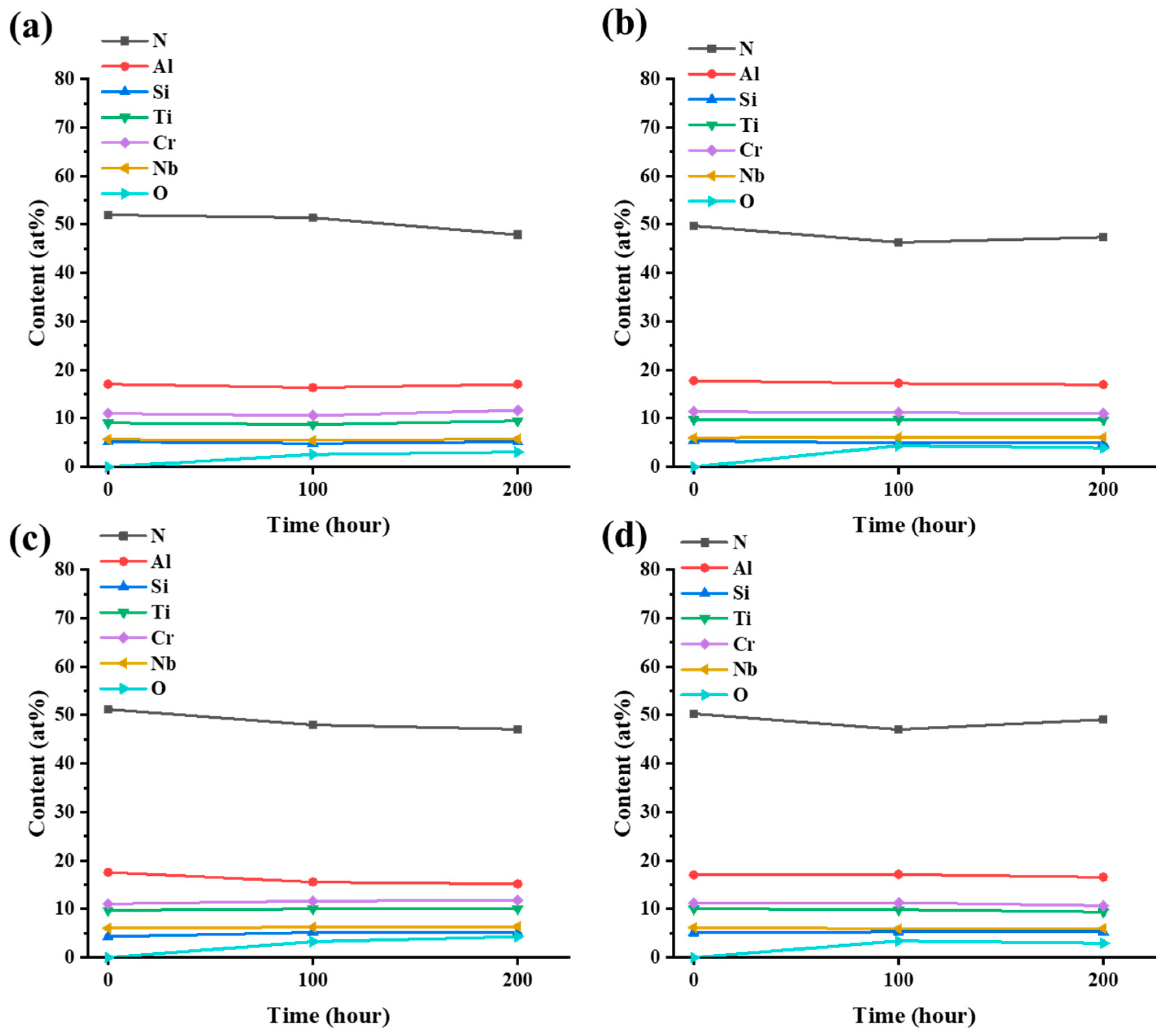
3.4. Water Vapor Corrosion Resistance Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kathrein, M.; Michotte, C.; Penoy, M.; Polcik, P.; Mitterer, C. Multifunctional multi-component PVD coatings for cutting tools. Surf. Coat. Technol. 2005, 200, 1867–1871. [Google Scholar] [CrossRef]
- Mayrhofer, P.H.; Mitterer, C.; Hultman, L.; Clemens, H. Microstructural design of hard coatings. Prog. Mater. Sci. 2006, 51, 1032–1114. [Google Scholar] [CrossRef]
- Krajinovic, I.; Daves, W.; Tkadletz, M.; Teppernegg, T.; Klunsner, T.; Schalk, N.; Mitterer, C.; Tritremmel, C.; Ecker, W.; Czettl, C. Finite element study of the influence of hard coatings on hard metal tool loading during milling. Surf. Coat. Technol. 2016, 304, 134–141. [Google Scholar] [CrossRef]
- Schalk, N.; Tkadletz, M.; Mitterer, C. Hard coatings for cutting applications: Physical vs. chemical vapor deposition and future challenges for the coatings community. Surf. Coat. Technol. 2022, 429, 127949. [Google Scholar] [CrossRef]
- Keunecke, M.; Stein, C.; Bewilogua, K.; Koelker, W.; Kassel, D.; van den Berg, H. Modified TiAlN coatings prepared by d.c. pulsed magnetron sputtering. Surf. Coat. Technol. 2010, 205, 1273–1278. [Google Scholar] [CrossRef]
- Soleimani, M.; Fattah-alhosseini, A.; Elmkhah, H.; Babaei, K.; Imantalab, O. A comparison of tribological and corrosion behavior of PVD-deposited CrN/CrAlN and CrCN/CrAlCN nanostructured coatings. Ceram. Int. 2023, 49, 5029–5041. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Vaidya, M.; Trubel, S.; Murty, B.S.; Wilde, G.; Divinski, S.V. Ni tracer diffusion in CoCrFeNi and CoCrFeMnNi high entropy alloys. J. Alloys Compd. 2016, 688, 994–1001. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.Q.; Manladan, S.M.; Geng, K.P.; Hu, S.S. Effect of heat treatment on the FeCoCrNiMnAl high-entropy alloy cladding layer. Surf. Eng. 2021, 37, 1532–1540. [Google Scholar] [CrossRef]
- Cai, Y.C.; Zhu, L.S.; Cui, Y.; Geng, K.P.; Han, T.F.; Manladan, S.M.; Luo, Z.; Han, J. Influence of high-temperature condition on the microstructure and properties of FeCoCrNiAl0.3 and FeCoCrNiAl0.7 high-entropy alloy coatings. Surf. Eng. 2021, 37, 179–187. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Raza, A.; Abdulahad, S.; Kang, B.; Ryu, H.J.; Hong, S.H. Corrosion resistance of weight reduced AlxCrFeMoV high entropy alloys. Appl. Surf. Sci. 2019, 485, 368–374. [Google Scholar] [CrossRef]
- Xu, Z.L.; Zhang, H.; Du, X.J.; He, Y.Z.; Luo, H.; Song, G.S.; Mao, L.; Zhou, T.W.; Wang, L.L. Corrosion resistance enhancement of CoCrFeMnNi high-entropy alloy fabricated by additive manufacturing. Corros. Sci. 2020, 177, 108954. [Google Scholar] [CrossRef]
- Nagase, T.; Rack, P.D.; Noh, J.H.; Egami, T. In-situ TEM observation of structural changes in nano-crystalline CoCrCuFeNi multicomponent high-entropy alloy (HEA) under fast electron irradiation by high voltage electron microscopy (HVEM). Intermetallics 2015, 59, 32–42. [Google Scholar] [CrossRef]
- Li, Y.H.; Meng, F.P.; Ge, F.F.; Huang, F. Improved oxidation resistance through an in-situ formed diffusion barrier: Oxidation behavior of amorphous multi-component FeCrAlMoSiY-coated Zr in high-temperature steam. Corros. Sci. 2021, 189, 109566. [Google Scholar] [CrossRef]
- Ye, Q.F.; Feng, K.; Li, Z.G.; Lu, F.G.; Li, R.F.; Huang, J.; Wu, Y.X. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Wu, Y.D.; Cai, Y.H.; Wang, T.; Si, J.J.; Zhu, J.; Wang, Y.D.; Hui, X.D. A refractory Hf25Nb25Ti25Zr25 high-entropy alloy with excellent structural stability and tensile properties. Mater. Lett. 2014, 130, 277–280. [Google Scholar] [CrossRef]
- Deng, Y.; Tasan, C.C.; Pradeep, K.G.; Springer, H.; Kostka, A.; Raabe, D. Design of a twinning-induced plasticity high entropy alloy. Acta Mater. 2015, 94, 124–133. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, L.H.; Shen, S.C.; Zhou, C.S. FeCrNiMnAl high-entropy alloy coating by spray deposition and thermite reaction. Surf. Eng. 2019, 35, 809–815. [Google Scholar] [CrossRef]
- Wang, H.D.; Liu, J.N.; Xing, Z.G.; Ma, G.Z.; Cui, X.F.; Jin, G.; Xu, B.S. Microstructure and corrosion behaviour of AlCoFeNiTiZr high-entropy alloy films. Surf. Eng. 2020, 36, 78–85. [Google Scholar] [CrossRef]
- Xie, B.Q.; Bao, Y.F.; Zhong, C.H.; Song, Q.N.; Yang, K.; Jiang, Y.F. Cavitation erosion resistance of high-entropy FeCoCrNiMoXB0.2 coatings cladded by laser. Surf. Eng. 2021, 37, 1606–1611. [Google Scholar] [CrossRef]
- Kuang, S.H.; Zhou, F.; Liu, W.C.; Liu, Q.B. Al2O3/MC particles reinforced MoFeCrTiWNbx high-entropy-alloy coatings prepared by laser cladding. Surf. Eng. 2022, 38, 158–165. [Google Scholar] [CrossRef]
- Tang, Z.; Yuan, T.; Tsai, C.W.; Yeh, J.W.; Lundin, C.D.; Liaw, P.K. Fatigue behavior of a wrought Al0.5CoCrCuFeNi two-phase high-entropy alloy. Acta Mater. 2015, 99, 247–258. [Google Scholar] [CrossRef]
- Tsai, D.; Chang, Z.; Kuo, L.; Lin, T.; Lin, T.; Shieu, F. Solid solution coating of (TiVCrZrHf)N with unusual structural evolution. Surf. Coat. Technol. 2013, 217, 84–87. [Google Scholar] [CrossRef]
- Yu, W.; Li, W.; Liu, P.; Zhang, K.; Ma, F.; Chen, X.; Feng, R.; Liaw, P. Silicon-content-dependent microstructures and mechanical behavior of (AlCrTiZrMo)-Six-N high-entropy alloy nitride films. Mater. Des. 2021, 203, 109553. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, X.; Zeng, X.; Xiong, Z.; Liu, Y.; Lei, Y.; Yang, B. Effect of bias voltages on microstructure and mechanical properties of (AlCrNbSiTi)N high entropy alloy nitride coatings deposited by arc ion plating. Surf. Eng. 2023, 39, 495–505. [Google Scholar] [CrossRef]
- Zhang, X.; Pelenovich, V.; Liu, Y.; Ke, X.; Zhang, J.; Yang, B.; Ma, G.; Li, M.; Wang, X. Effect of bias voltages on microstructure and properties of (TiVCrNbSiTaBY)N high entropy alloy nitride coatings deposited by RF magnetron sputtering. Vacuum 2022, 195, 110710. [Google Scholar] [CrossRef]
- Oliver, W.C.; Pharr, G.M. An improved technique for determining hardness and elastic-modulus using load and displacement sensing indentation experiments. J. Mater. Res. 1992, 7, 1564–1583. [Google Scholar] [CrossRef]
- Wang, X.; Zeng, Z.; Wang, H.; Bai, H.; Li, W.; Li, Y.; Wang, Z.; Chen, Y.; Yang, B. Effect of Bias Voltage on Structure, Mechanical Properties, and High-Temperature Water Vapor Corrosion of AlCrNbSiTi High Entropy Alloy Coatings. Coatings 2023, 13, 1948. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, X.; Tu, M.; Hu, Y.; Wang, H.; Zhang, J.; Li, Z.; Zeng, X.; Wan, Q.; Vasiliy, P.; et al. Structure and mechanical properties of multi-principal-element (AlCrNbSiTi)N hard coating. Surf. Coat. Technol. 2022, 433, 128113. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Liu, Y.; Pelenovich, V.; Zeng, X.-M.; Liu, J.; Wan, Q.; Pogrebnjak, A.; Xue, L.-J.; Chen, Z.-W.; Lei, Y.; et al. Self-assembled high-entropy nitride multilayer coating. Rare Met. 2024, 43, 2876–2883. [Google Scholar] [CrossRef]
- Kong, Q.; Ji, L.; Li, H.; Liu, X.; Wang, Y.; Chen, J.; Zhou, H. Influence of substrate bias voltage on the microstructure and residual stress of CrN films deposited by medium frequency magnetron sputtering. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2011, 176, 850–854. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Li, G.; Gao, F.; Xia, Y. Effect of bias voltage on the growth of super-hard (AlCrTiVZr)N high-entropy alloy nitride films synthesized by high power impulse magnetron sputtering. Appl. Surf. Sci. 2021, 564, 150417. [Google Scholar] [CrossRef]
- Wang, Y.X.; Zhang, S.; Lee, J.W.; Lew, W.S.; Li, B. Influence of bias voltage on the hardness and toughness of CrAlN coatings via magnetron sputtering. Surf. Coat. Technol. 2012, 206, 5103–5107. [Google Scholar] [CrossRef]
- Aouadi, K.; Tlili, B.; Nouveau, C.; Besnard, A.; Chafra, M.; Souli, R. Influence of Substrate Bias Voltage on Corrosion and Wear Behavior of Physical Vapor Deposition CrN Coatings. J. Mater. Eng. Perform. 2019, 28, 2881–2891. [Google Scholar] [CrossRef]
- Veprek, S. Plasma-induced and plasma-assisted chemical vapor-deposition. Thin Solid Film. 1985, 130, 135–154. [Google Scholar] [CrossRef]
- Petrov, I.; Hultman, L.; Helmersson, U.; Sundgren, J.E.; Greene, J.E. Microstructure modification of tin by ion-bombardment during reactive sputter deposition. Thin Solid Film. 1989, 169, 299–314. [Google Scholar] [CrossRef]
- Oh, U.C.; Je, J.H. Effects of strain-energy on the preferred orientation of tin thin-films. J. Appl. Phys. 1993, 74, 1692–1696. [Google Scholar] [CrossRef]
- Zhao, J.P.; Wang, X.; Chen, Z.Y.; Yang, S.Q.; Shi, T.S.; Liu, X.H. Overall energy model for preferred growth of TiN films during filtered arc deposition. J. Phys. D-Appl. Phys. 1997, 30, 5–12. [Google Scholar] [CrossRef]
- Li, J.C.; Chen, Y.J.; Zhao, Y.M.; Shi, X.W.; Wang, S.; Zhang, S. Super-hard (MoSiTiVZr)Nx high-entropy nitride coatings. J. Alloys Compd. 2022, 926, 166807. [Google Scholar] [CrossRef]
- Maksakova, O.V.; Simoes, S.; Pogrebnjak, A.D.; Bondar, O.V.; Kravchenko, Y.O.; Koltunowicz, T.N.; Shaimardanov, Z.K. Multilayered ZrN/CrN coatings with enhanced thermal and mechanical properties. J. Alloys Compd. 2019, 776, 679–690. [Google Scholar] [CrossRef]
- Abadias, G. Stress and preferred orientation in nitride-based PVD coatings. Surf. Coat. Technol. 2008, 202, 2223–2235. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Lin, S.-J.; Yeh, J.-W. (AlCrNbSiTi)N/TiN multilayer films designed by a hybrid coating system combining high-power impulse magnetron sputtering and cathode arc deposition. Surf. Coat. Technol. 2023, 468, 129757. [Google Scholar] [CrossRef]
- Wan, Q.; Jia, B.Y.; Liu, P.; Luo, Y.; Chen, J.; Zhang, X.Y.; Xiao, Y.Y.; Abdelkader, T.K.; Refai, M.; Zhang, J.; et al. Microstructure and mechanical properties of FeCoCrNiAl0.1N high entropy alloy nitride coatings synthesized by cathodic arc ion plating using alloy target. Surf. Coat. Technol. 2023, 457, 129305. [Google Scholar] [CrossRef]
- Asteman, H.; Svensson, J.E.; Johansson, L.G. Evidence for chromium evaporation influencing the oxidation of 304L: The effect of temperature and flow rate. Oxid. Met. 2002, 57, 193–216. [Google Scholar] [CrossRef]
- Ehlers, J.; Young, D.J.; Smaardijk, E.J.; Tyagi, A.K.; Penkalla, H.J.; Singheiser, L.; Quadakkers, W.J. Enhanced oxidation of the 9%Cr steel P91 in water vapour containing environments. Corros. Sci. 2006, 48, 3428–3454. [Google Scholar] [CrossRef]


| Layer | Bias Voltage, V | Gas/ Pressure, Pa | Target/ Current (Power) | Duration/ min |
|---|---|---|---|---|
| Ar+ etch | −150 | Ar/0.5 | Ti/150 A | 15 |
| Cr | −150 | Ar/0.5 | Cr/65 A | 5 |
| CrN | −150 | Ar+N2 (1:1)/1 | Cr/65 A | 5 |
| AlCrNbSiTiN | −50, −100, −150, −200 | Ar+N2 (1:1)/1 | AlCrNbSiTi /700 W | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, J.; Liu, Y.; Li, W.; Chen, Y.; Yang, B. Structure, Mechanical Properties and Water Vapor Corrosion Resistance of AlCrNbSiTiN High-Entropy Nitride Coatings Deposited by RF Magnetron Sputtering. Coatings 2024, 14, 1006. https://doi.org/10.3390/coatings14081006
Wang X, Liu J, Liu Y, Li W, Chen Y, Yang B. Structure, Mechanical Properties and Water Vapor Corrosion Resistance of AlCrNbSiTiN High-Entropy Nitride Coatings Deposited by RF Magnetron Sputtering. Coatings. 2024; 14(8):1006. https://doi.org/10.3390/coatings14081006
Chicago/Turabian StyleWang, Xuanzheng, Jie Liu, Yingfan Liu, Wentao Li, Yanming Chen, and Bing Yang. 2024. "Structure, Mechanical Properties and Water Vapor Corrosion Resistance of AlCrNbSiTiN High-Entropy Nitride Coatings Deposited by RF Magnetron Sputtering" Coatings 14, no. 8: 1006. https://doi.org/10.3390/coatings14081006
APA StyleWang, X., Liu, J., Liu, Y., Li, W., Chen, Y., & Yang, B. (2024). Structure, Mechanical Properties and Water Vapor Corrosion Resistance of AlCrNbSiTiN High-Entropy Nitride Coatings Deposited by RF Magnetron Sputtering. Coatings, 14(8), 1006. https://doi.org/10.3390/coatings14081006





