Use of 2D Sulfide and Oxide Compounds as Functional Semiconducting Pigments in Protective Organic Coatings Containing Zinc Dust
Abstract
:1. Introduction
2. Materials
3. Experimental
3.1. Characterization of the Binder and Pigments by Methods Used in the Coatings Field
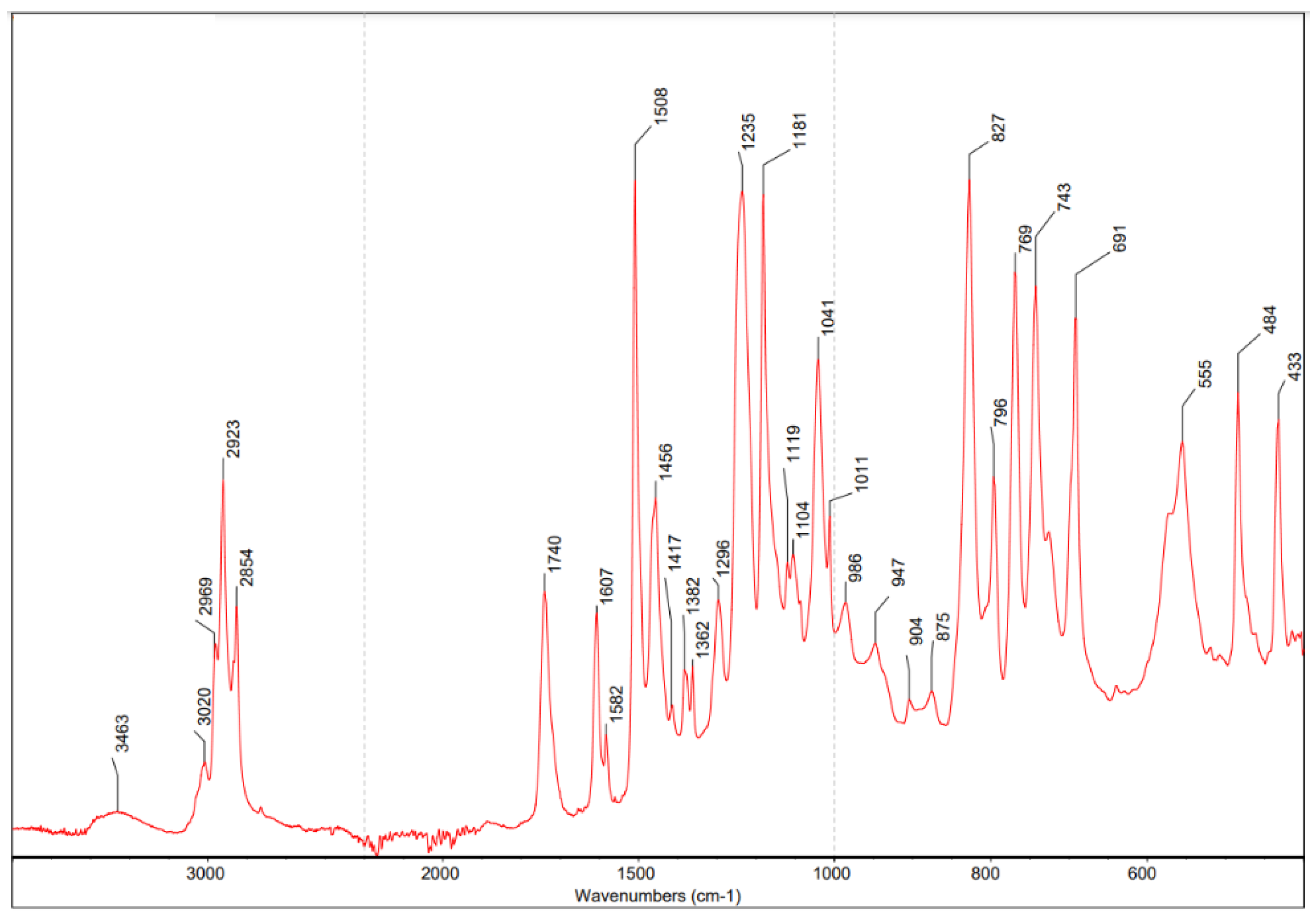
3.1.1. Characterization and Specification of the Epoxy Ester Resin
3.1.2. Determination of Physico-Chemical Properties of Studied Inorganic Pigments
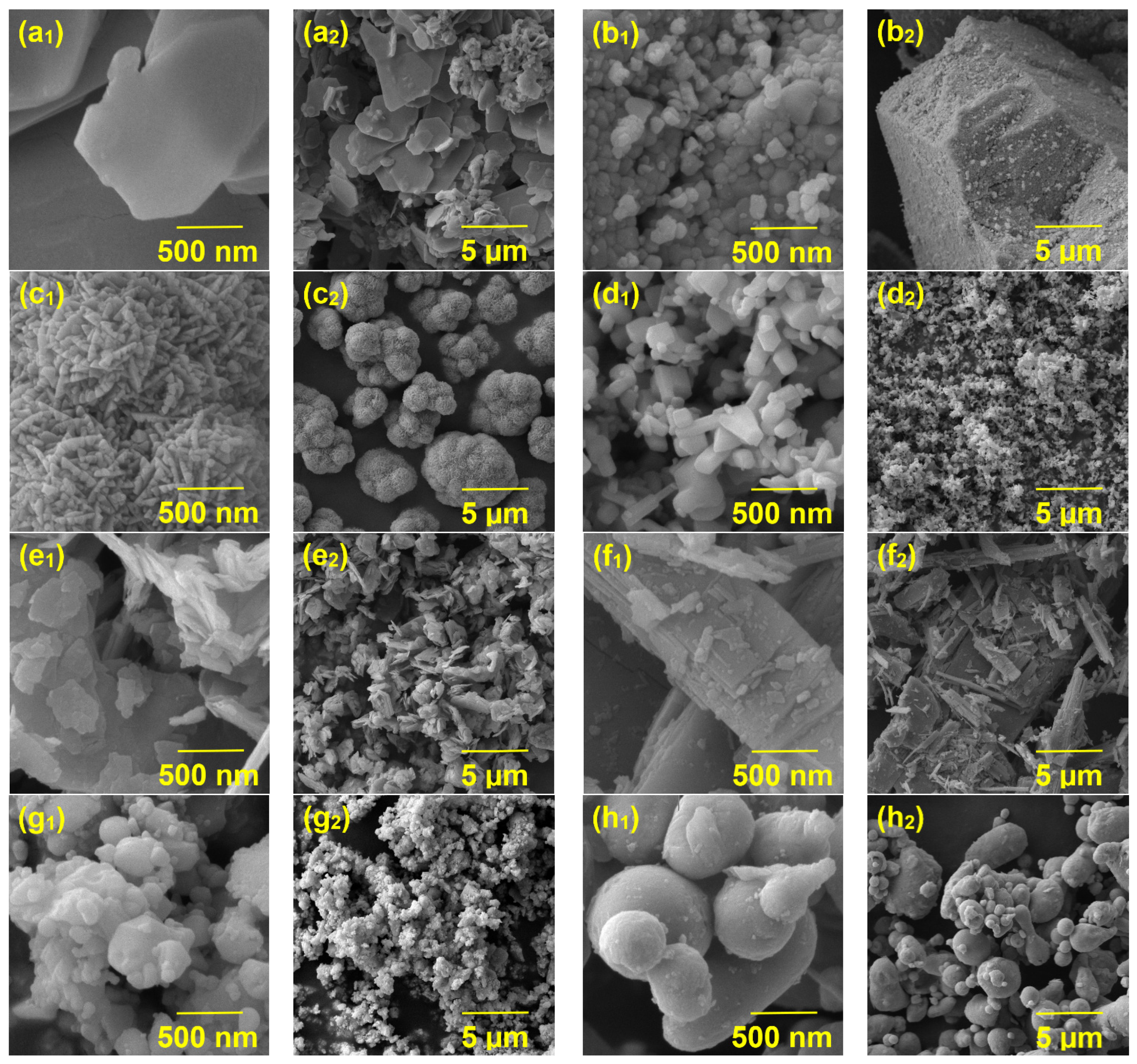
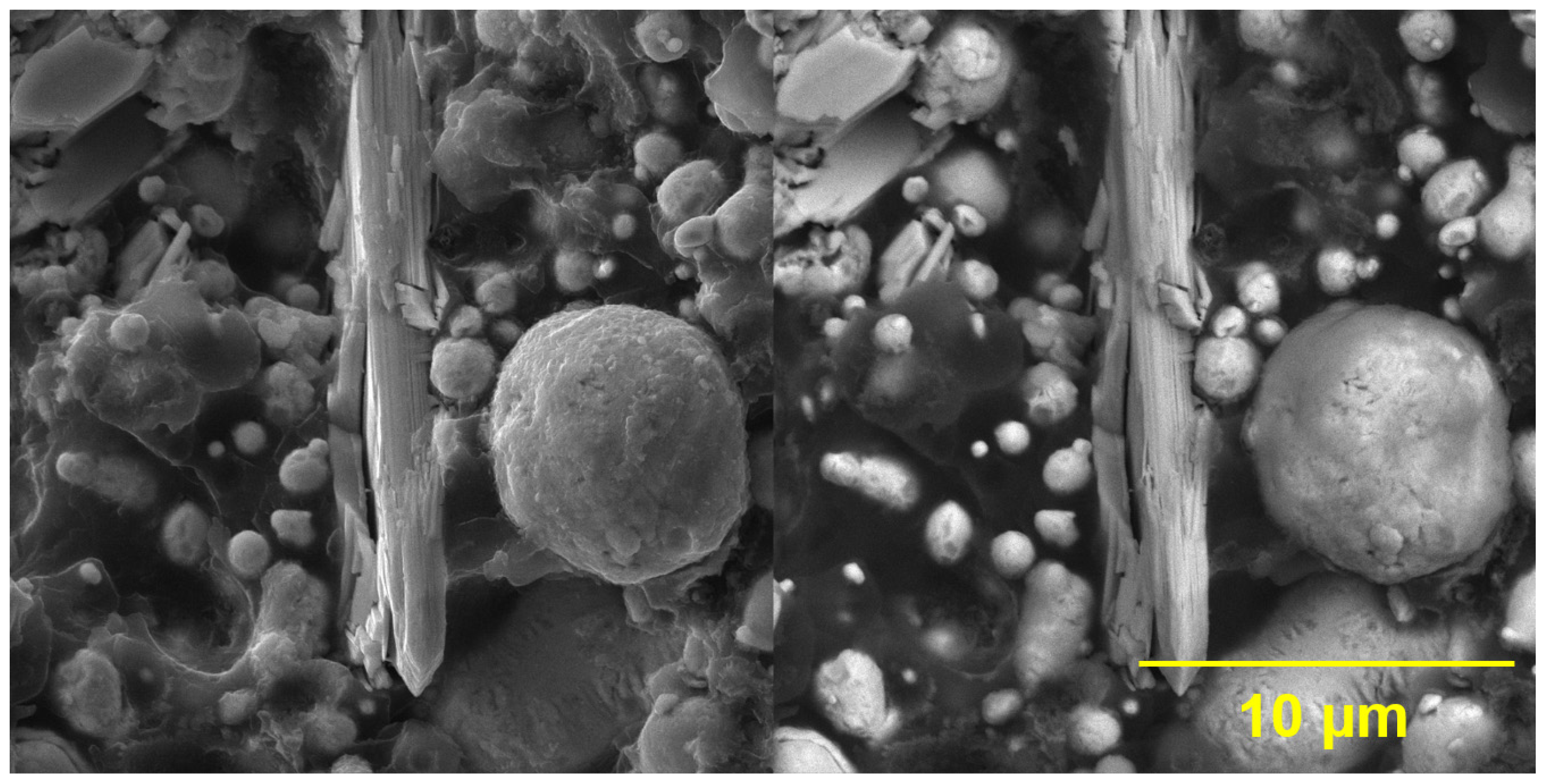
3.1.3. SEM and EDX Measurements of Pigments
3.2. Preparation of Formulated Model Coating Systems and Their Application
3.3. Study of the Physical–Mechanical and Mechanical Properties of the Tested Organic Coatings
3.4. Corrosion Test Procedures and Evaluation of Results after Corrosion Tests
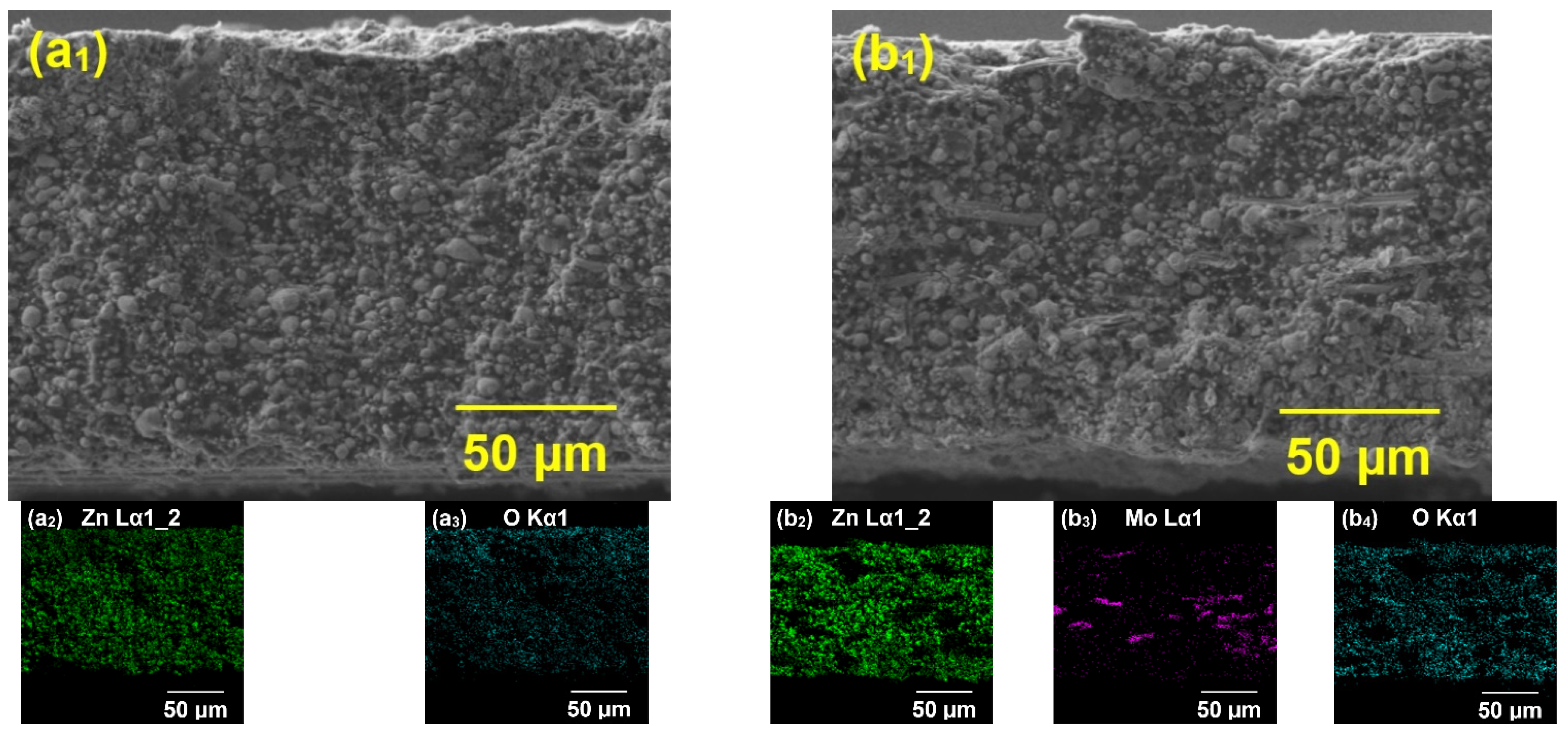
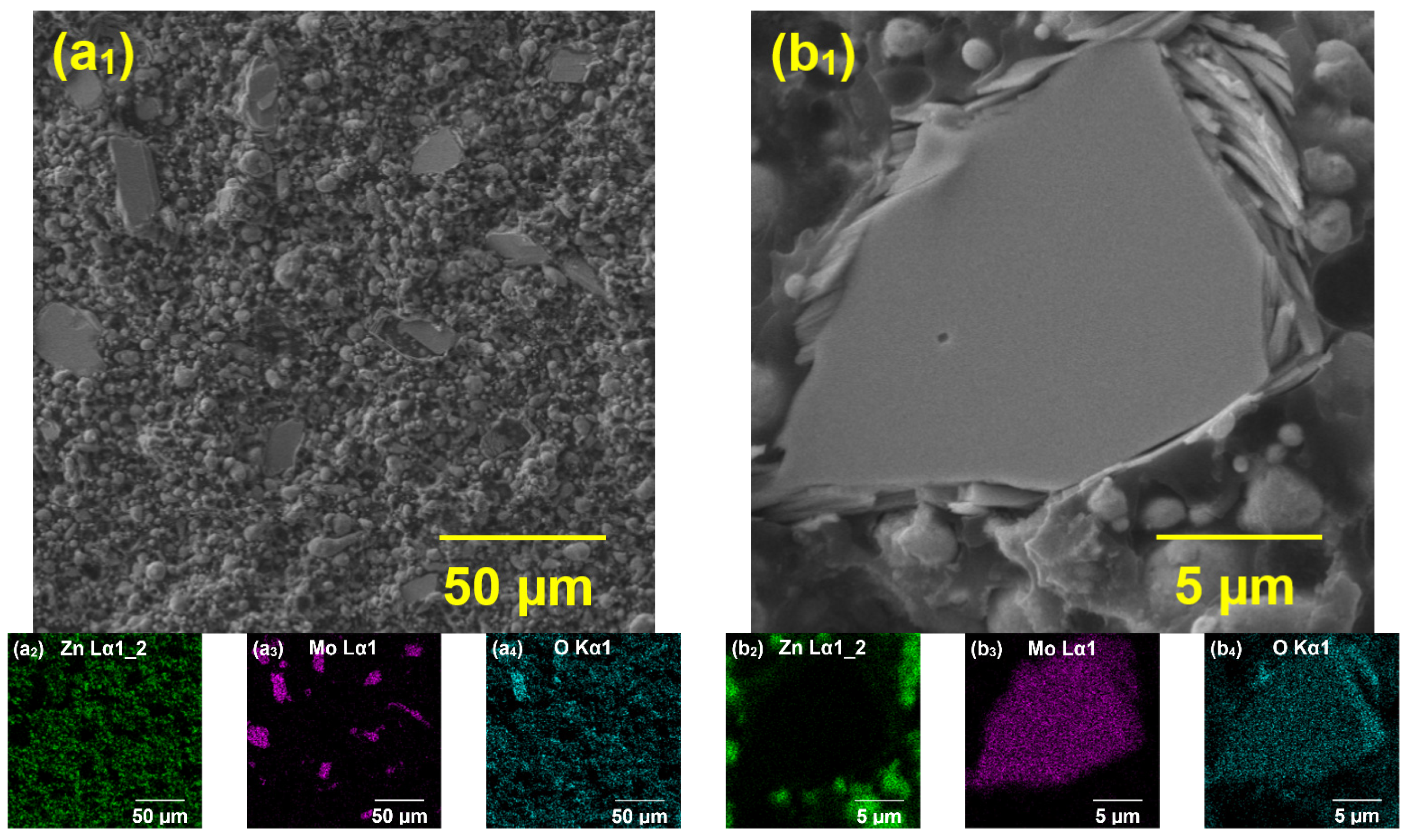
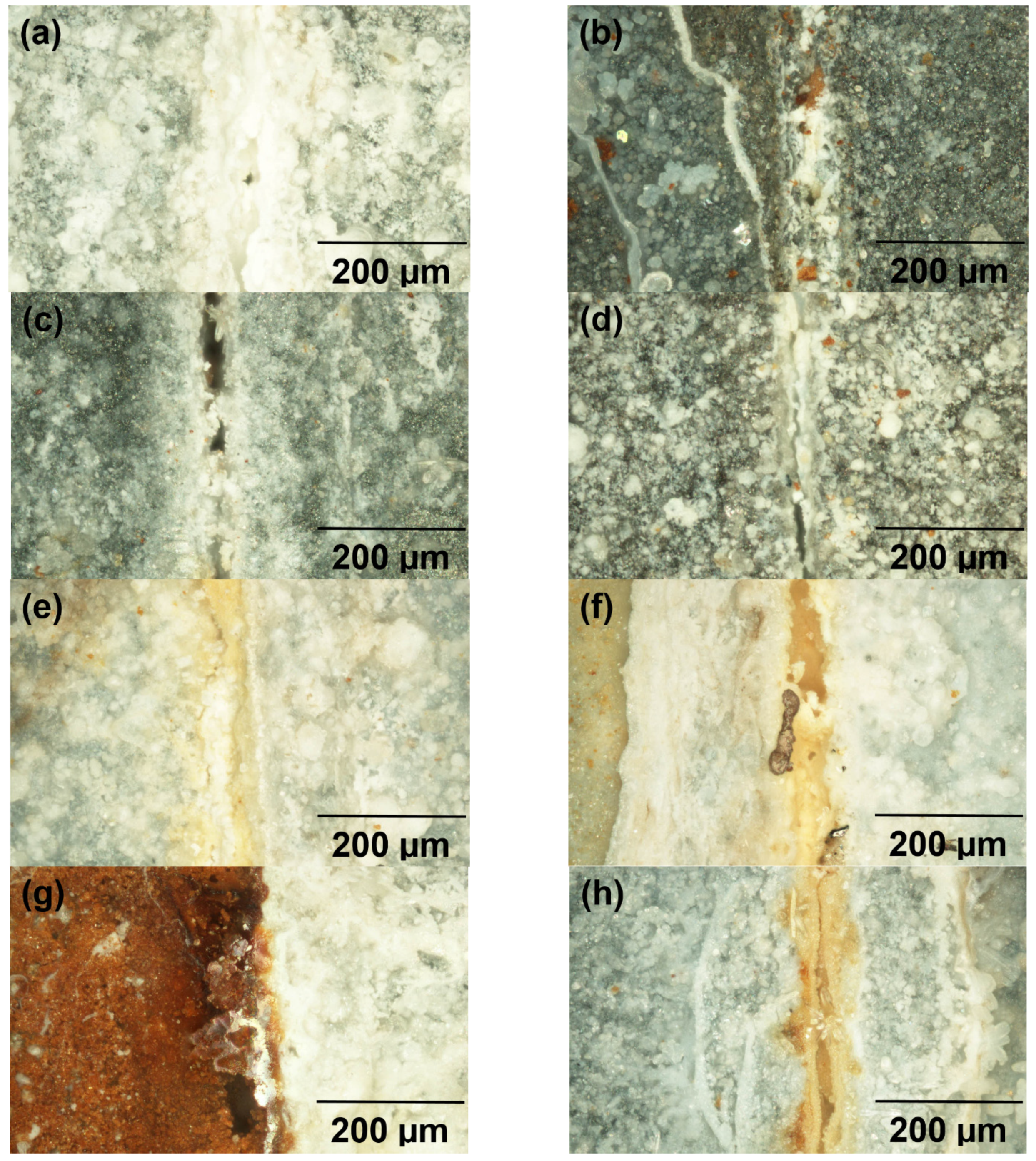
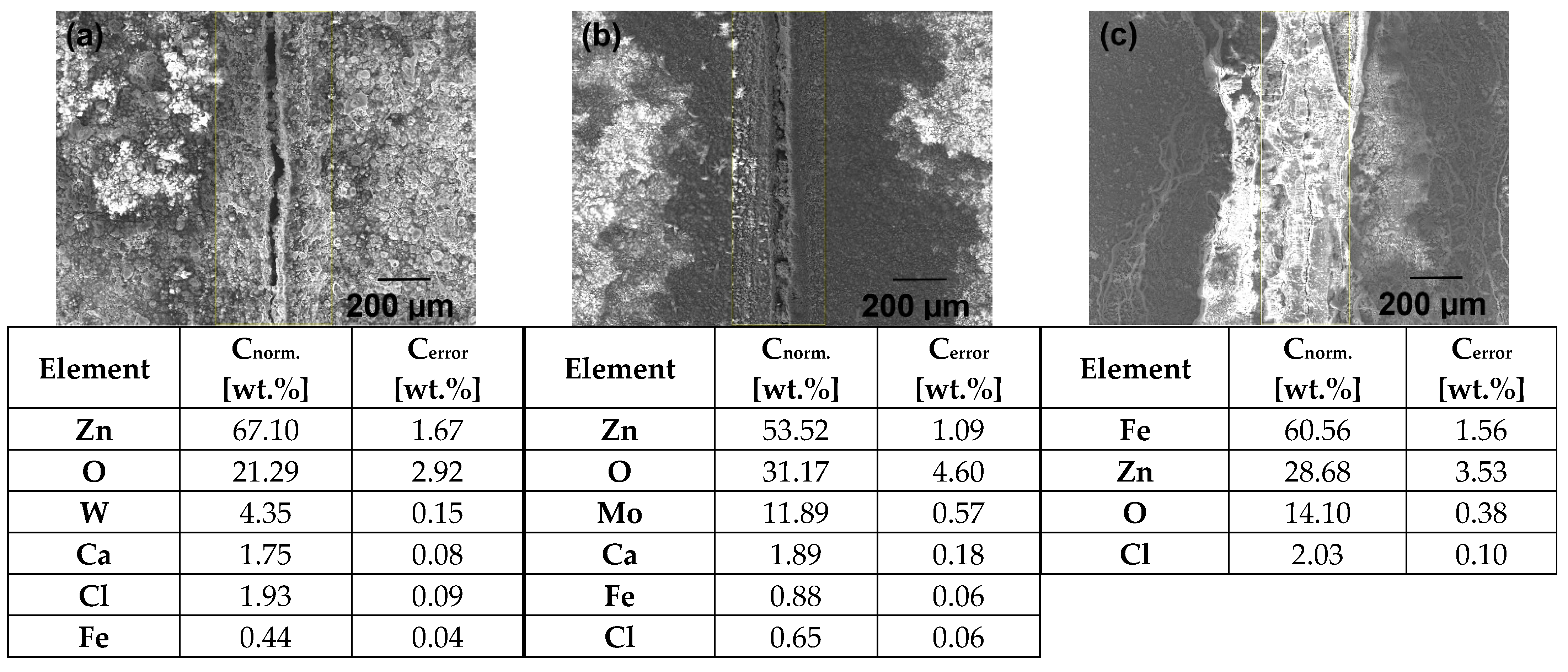
Electron Microanalysis Studied Organic Coatings
3.5. Electrochemical Measurement Linear Polarization
3.6. Determination of pH and Specific Electrical Conductivity and Corrosion Loss from Aqueous Extracts of Pigments and Paint Films
4. Results and Discussion
4.1. Specification of the Used Binder
4.2. Characterization of the Studied Inorganic Pigments
The Results of the EDX Analysis of the Studied
4.3. Results of Mechanical Properties of the Protective Coatings
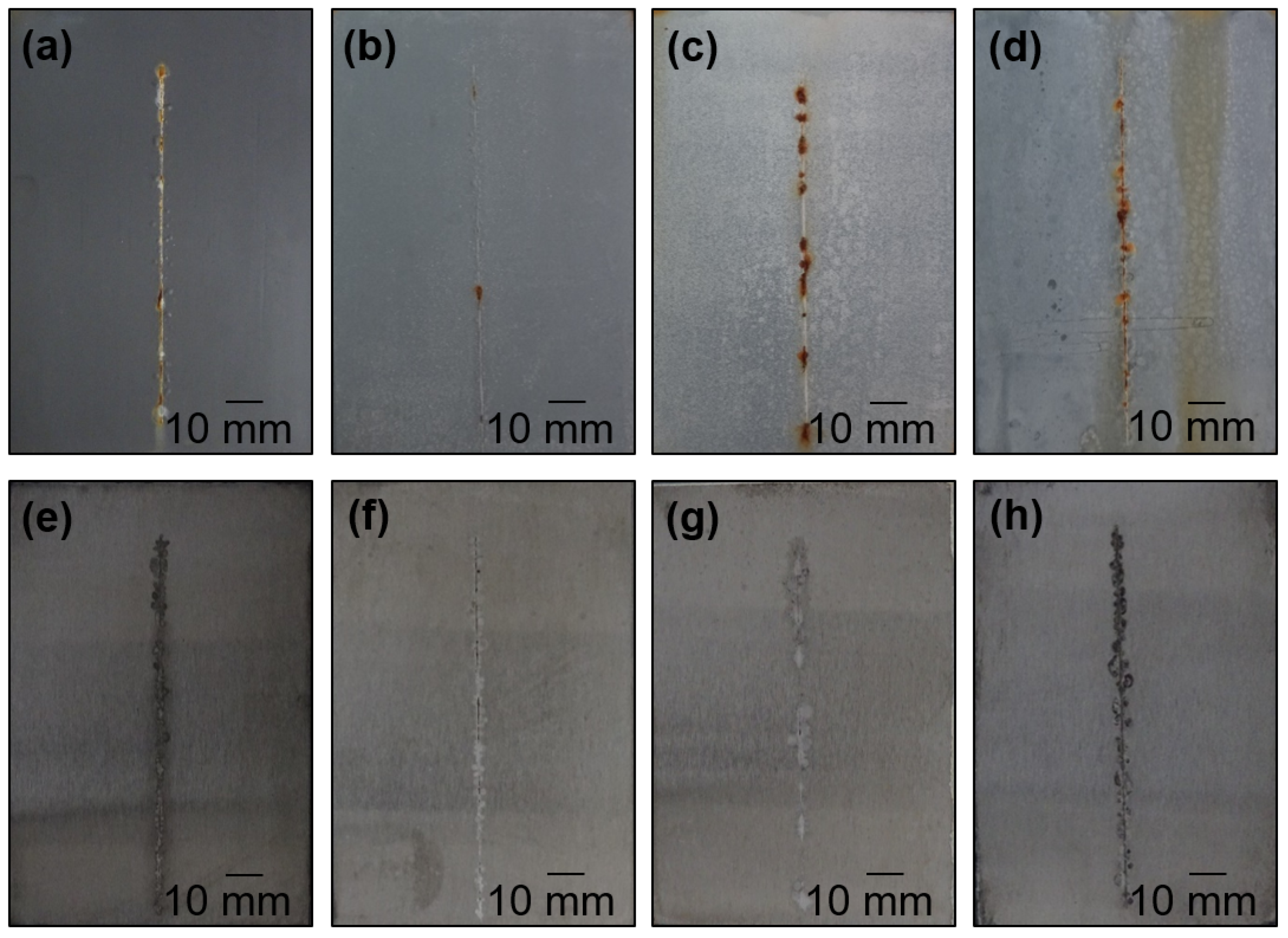
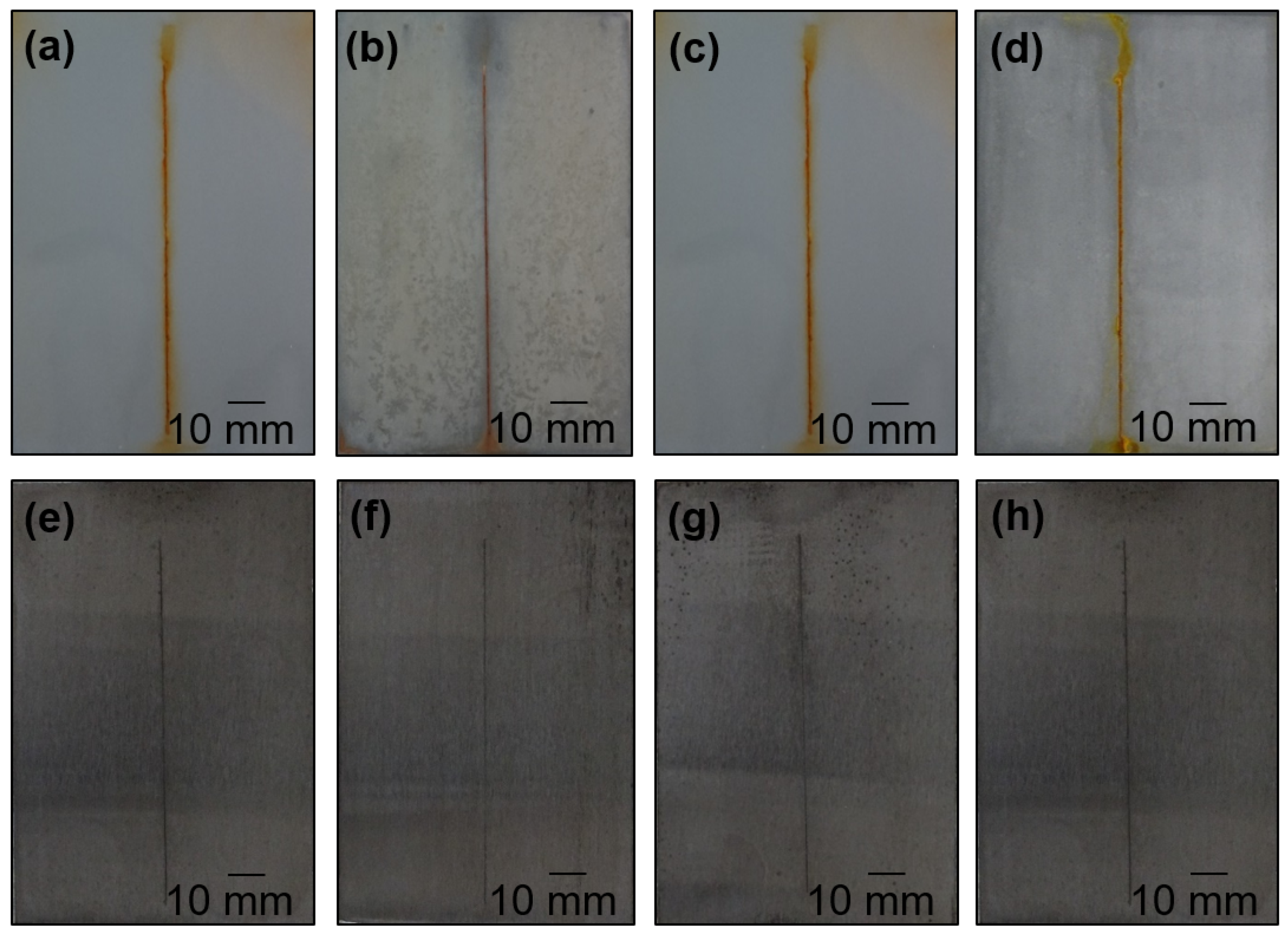
4.4. Anti-Corrosion Efficiency of Pigmented Epoxy Ester Coatings in an Atmosphere Containing Salt Electrolyte
4.5. Anti-Corrosion Efficiency of Pigmented Epoxy Ester Coatings in an Atmosphere Containing SO2
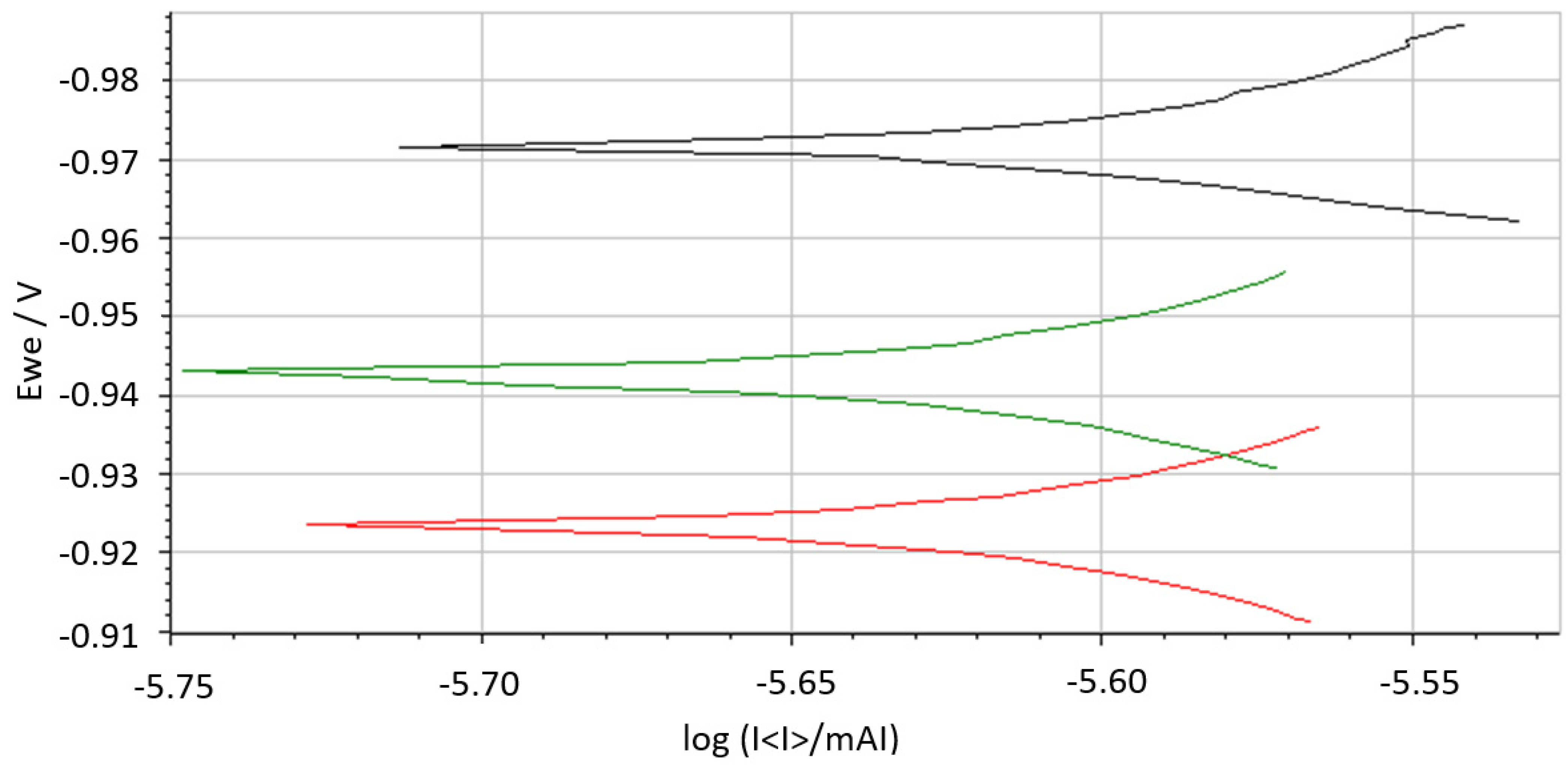
4.6. Study of Anti-Corrosion Properties of Organic Coatings Using the Linear Polarization
4.7. Determination of pH and Specific Electrical Conductivity and Corrosion Loss from Aqueous Extracts of Loose Paint Films
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Su, J.; Xiao, X.; Wang, N.; Meng, G.; Gu, L. Graphene-like two-dimensional nanosheets-based anticorrosive coatings: A review. J. Mater. Sci. Technol. 2022, 129, 139–162. [Google Scholar] [CrossRef]
- Stejskal, J.; Trchová, M.; Brodinová, J.; Kalenda, P.; Fedorová, S.; Prokeš, J.; Zemek, J. Coating of zinc ferrite particles with a conducting polymer, polyaniline. J. Colloid Interface Sci. 2006, 298, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Rasouli, S.; Ramezanzadeh, B.; Askari, A. Electrochemical investigation of the properties of Co doped ZnO nanoparticle as a corrosion inhibitive pigment for modifying corrosion resistance of the epoxy coating. Corros. Sci. 2014, 88, 387–399. [Google Scholar] [CrossRef]
- Nikravesh, B.; Ramezanzadeh, B.; Sarabi, A.A.; Kasiriha, S.M. Evaluation of the corrosion resistance of an epoxy-polyamide coating containing different ratios of micaceous iron oxide/Al pigments. Corros. Sci. 2011, 53, 1592–1603. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D.; Kohl, M.; Stejskal, J. Anticorrosion efficiency of zinc-filled epoxy coatings containing conducting polymers and pigments. Prog. Org. Coat. 2015, 78, 1–20. [Google Scholar] [CrossRef]
- Havlík, J.; Kalendová, A.; Veselý, D. Electrochemical, chemical and barrier action of zinc dust/anticorrosive pigments containing coatings. J. Phys. Chem. Solids 2007, 68, 1101–1105. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research progress in organic zinc rich primer coatings for cathodic protection of metals—A comprehensive review. Prog. Org. Coat. 2021, 153, 106040. [Google Scholar] [CrossRef]
- Ahmadzadeh, M.; Shahrabi, T.; Izadi, M.; Mohammadi, I.; Hoseinieh, S.M.; Barnoush, A. Calcareous scales deposited in the organic coating defects during artificial seawater cathodic protection: Effect of zinc cations. J. Alloys Compd. 2019, 784, 744–755. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, L.; Yang, Z.; Ma, Q.; He, D.; Xu, K.; Zhang, H.; Zhang, P.; Sun, W.; Liu, G. Effect of ZnO as corrosion product on corrosion behavior of zinc-iron corrosion protection systems. Corros. Sci. 2024, 227, 111802. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, L.; Sun, W.; Yang, Z.; Wang, S.; Liu, G. Effect of chemical conversion induced by self-corrosion of zinc powders on enhancing corrosion protection performance of zinc-rich coatings. Corros. Sci. 2022, 194, 109942. [Google Scholar] [CrossRef]
- Arman, S.Y.; Ramezanzadeh, B.; Farghadani, S.; Mehdipour, M.; Rajabi, A. Application of the electrochemical noise to investigate the corrosion resistance of an epoxy zinc-rich coating loaded with lamellar aluminum and micaceous iron oxide particles. Corros. Sci. 2013, 77, 118–127. [Google Scholar] [CrossRef]
- Müller, B.; Oughourlian, C.; Schubert, M. Amphiphilic copolymers as corrosion inhibitors for zinc pigment. Corros. Sci. 2000, 42, 577–584. [Google Scholar] [CrossRef]
- Kalantar-zadeh, K.; Ou, J.Z.; Daeneke, T.; Mitchell, A.; Sasaki, T.; Fuhrer, M.S. Two dimensional and layered transition metal oxides. Appl. Mater. Today 2016, 5, 73–89. [Google Scholar] [CrossRef]
- Wert, S.; Iffelsberger, C.; Novčić, K.A.; Pumera, M. Corrosion of catalyst in high resolution: Layered transition metal dichalcogenides electrocatalyse water splitting and corrode during the process. J. Catal. 2022, 416, 85–91. [Google Scholar] [CrossRef]
- Sinha, A.; Dhanjai; Tan, B.; Huang, Y.; Thao, H.; Dang, X.; Chen, J.; Jain, R. MoS2 nanostructures for electrochemical sensing of multidisciplinary targets: A review. TrAC Trends Anal. Chem. 2018, 102, 75–90. [Google Scholar] [CrossRef]
- Naguib, M.; Gogotsi, Y. Synthesis of two-dimensional materials by selective extraction. Acc. Chem. Res. 2015, 48, 128–135. [Google Scholar] [CrossRef]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Sadanandan, A.; Thomas, S.A.; Khan, M.E.; Alomar, M.S.; Pallavolu, M.R.; Cherusseri, J. A critical review on two-dimensional Ti3C2Tx MXenes for anti-corrosion coatings. Prog. Org. Coat. 2023, 183, 107757. [Google Scholar] [CrossRef]
- Xia, Z.; Liu, G.; Dong, Y.; Zhang, Y. Anticorrosive epoxy coatings based on polydopamine modified molybdenum disulfide. Prog. Org. Coat. 2019, 133, 154–160. [Google Scholar] [CrossRef]
- Lv, R.; Robinson, J.A.; Schaak, R.E.; Sun, D.; Sun, Y.; Mallouk, T.E.; Terrones, M. Transition Metal Dichalcogenides and Beyond: Synthesis, Properties, and Applications of Single- and Few-Layer Nanosheets. Acc. Chem. Res. 2015, 48, 56–64. [Google Scholar] [CrossRef]
- Joswig, J.O.; Lorenz, T.; Wendumu, T.B.; Gemming, S.; Seifert, G. Optics, Mechanics, and Energetics of Two-Dimensional MoS2 Nanostructures from a Theoretical Perspective. Acc. Chem. Res. 2015, 48, 48–55. [Google Scholar] [CrossRef]
- Eksik, O.; Gao, J.; Shojaee, S.A.; Thomas, A.; Chow, P.; Bartolucci, S.F.; Lucca, D.A.; Karotkar, N. Epoxy Nanocomposites with Two-Dimensional Transition Metal Dichalcogenide Additives. ACS Nano 2014, 8, 5282–5289. [Google Scholar] [CrossRef]
- Tan, W.; Zhao, W. Designing WS2@Ti3C2Tx heterojunction nanofillers via electrostatic self-assembly for achieving long term corrosion resistance under AHP environment. Mater. Today Nano 2022, 20, 100259. [Google Scholar] [CrossRef]
- Gnanaprakasam, P.; Mangalaraja, R.V.; Salvo, C. Microwave driven synthesis of tungsten sulfide nanosheets: An efficient electrocatalyst for oxygen reduction reaction. Mater. Sci. Semicond. Process. 2022, 137, 106213. [Google Scholar] [CrossRef]
- Rana, D.S.; Thakur, N.; Singh, D.; Sonia, P. Molybdenum and tungsten disulfide based nanocomposites as chemical sensor: A review. Mater. Proc. 2022, 62, 2755–2761. [Google Scholar] [CrossRef]
- Joseph, A.; Eapen, M.K.; Mampillly, M.E.; Sajith, V. Comparison of Corrosion Resistance Properties of Electrophoretically Deposited MoS2 and WS2 Nanosheets Coatings on Mild Steel. Metall. Mater. Trans. A 2021, 52, 3689–3693. [Google Scholar] [CrossRef]
- Hwnag, H.Y.; Isawa, Y.; Kawasaki, M.; Keimer, B.; Nagaosa, N.; Tokura, Y. Emergent phenomena at oxide interfaces. Nat. Mater. 2012, 11, 103–113. [Google Scholar] [CrossRef]
- Alsaif, M.; Balendhran, S.; Field, M.R.; Latham, K.; Wlodarski, W.; Ou, J.Z.; Kalantar-zadeh, K. Two dimensional α-MoO3 nanoflakes obtained using solvent-assisted grinding and sonication method: Application for H2 gas sensing. Sens. Actuators B Chem. 2014, 192, 196–204. [Google Scholar] [CrossRef]
- Scarminio, J.; Lourenço, A.; Gorenstein, A. Electrochromism and photochromism in amorphous molybdenum oxide films. Thin Solid Film. 1997, 302, 66–70. [Google Scholar] [CrossRef]
- Mutschall, D.; Holzner, K.; Obermeier, E. Sputtered molybdenum oxide thin films for NH3 detection. Sens. Actuators B 1996, 36, 320–324. [Google Scholar] [CrossRef]
- Razzaq, M.; Khan, M.J.; Imran, Z.; Ahmad, M.; Rasool, S.; Rehan, M.; Iqbal, S.; Anjum, M.A.R.; Mehboob, S.; Saifullah, M. Enhanced electrochemical performance of WO3 thin films prepared from polyvinyl alcohol-modified nanoparticle ink. Solid State Ion. 2023, 397, 116246. [Google Scholar] [CrossRef]
- Lee, S.H.; Cheong, P.L.; Smith, D.; Tracy, C.E.; Mascarenhas, A.; Pitts, J.R.; Deb, S.K. Raman spectroscopic studies of gasochromic a-WO3 thin films. Electrochim. Acta 2001, 46, 1995–1999. [Google Scholar] [CrossRef]
- Yin, X.-T.; Lv, P.; Li, J.; Jafari, A.; Wu, F.-Y.; Wang, Q.; Dastan, D.; Shi, Z.; Yu, S.; Germestani, H. Nanostructured tungsten trioxide prepared at various growth temperatures for sensing applications. J. Alloys Compd. 2020, 825, 154105. [Google Scholar] [CrossRef]
- KKapp, R. Molybdenum, Encyclopedia of Toxicology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 145–148. ISBN 978-0-12-369400-3. [Google Scholar]
- Diaz-Algara, J.; Rendón-Angeles, J.C.; Matamoros-Veloza, Z.; Yanagisawa, K.; Rodriguez-Galicia, J.L.; Rivera-Cobo, J.M. Single-step synthesis of SrMoO4 particles from SrSO4 and their anti-corrosive activity. J. Alloys Compd. 2014, 607, 73–84. [Google Scholar] [CrossRef]
- Ingrid, M. Corrosion inhibition of aluminium alloys by molybdate ions: A critical review of the chemistry, mechanisms and applications. Corros. Sci. 2024, 229, 111854. [Google Scholar] [CrossRef]
- Brahim, E.I.; Abdelaziz, A.A.; Lei, G.; Savaş, K. Chapter 15—Molybdates as corrosion inhibitors. In Inorganic Anticorrosive Materials, Past, Present and Future Perspectives; Elsevier: Amsterdam, The Netherlands, 2022; pp. 297–321. [Google Scholar] [CrossRef]
- Jabeera, B.; Shibli, S.; Anirudhan, T. Synergistic inhibitive effect of tartarate and tungstate in preventing steel corrosion in aqueous media. Appl. Surf. Sci. 2006, 252, 3520–3524. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Kang, Y.; Xiao, H.-M.; Mei, S.-G.; Zhang, G.-L.; Fu, S.Y. Preparation and characterization of transparent Al doped ZnO/epoxy composite as thermal-insulating coating. Compos. Part B Eng. 2011, 42, 2176–2180. [Google Scholar] [CrossRef]
- Kalendová, A.; Veselý, D. Study of the anticorrosive efficiency of zincite and periclase-based core–shell pigments in organic coatings. Prog. Org. Coat. 2009, 64, 5–19. [Google Scholar] [CrossRef]
- Shahine, I.; Beydoun, N.; Gaumet, J.J.; Bendeif, E.-E.; Rinnert, H.; Magri, P.; Naciri, A.E.; Miska, P.; Jradi, S.; Akil, S. Pure, Size Tunable ZnO Nanocrystals Assembled into Large Area PMMA Layer as Efficient Catalyst. Catalysts 2019, 9, 162. [Google Scholar] [CrossRef]
- El Saeed, A.M.; El-Fattah, M.A.; Azzam, A.M. Synthesis of ZnO nanoparticles and studying its influence on the antimicrobial, anticorrosion and mechanical behavior of polyurethane composite for surface coating. Dye. Pigment. 2015, 121, 282–289. [Google Scholar] [CrossRef]
- Hone, F.G.; Dejene, F.B.; Chenene, M.L.; Machava, A. Chemical bath pH influence on the structural, morphological and optical properties of zinc sulphide thin film prepared from acidic baths. Inorg. Chem. Commun. 2019, 108, 107519. [Google Scholar] [CrossRef]
- Patil, J.S.; Dhasade, S.S.; Babar, A.R.; Patil, S.; Fulari, V.J. Synthesis of nanoflakes-like shapes of zinc sulfide grown at room temperature by electrodeposition method. Superlattices Microstruct. 2015, 83, 565–574. [Google Scholar] [CrossRef]
- Guo, M.; Song, M.; Li, S.; Yin, Z.; Xinyu, S.; Bu, Y. Facile and economical synthesis of ZnS nanotubes and their superior adsorption performance for organic dyes. CrystEngComm 2017, 19, 2380–2393. [Google Scholar] [CrossRef]
- Zhang, X.G. Zinc-Rich Coatings. In Corrosion and Electrochemistry of Zinc; Springer: Boston, MA, USA, 1996; pp. 337–349. [Google Scholar] [CrossRef]
- Cao, X.; Huang, F.; Huang, C.; Liu, J.; Cheng, Y.F. Preparation of graphene nanoplate added zinc-rich epoxy coatings for enhanced sacrificial anode-based corrosion protection. Corros. Sci. 2019, 159, 108120. [Google Scholar] [CrossRef]
- Li, X.; Cubides, Y.; He, Z.; Soucek, M.D.; Castaneda, H. Corrosion assessment of zinc-rich primers containing polyaniline and the effect of acid as a dopant. Corrosion 2018, 74, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Kalendová, A.; Stejskal, J. The effect of polyaniline phosphate on mechanical and corrosive properties of protective organic coatings containing high amounts of zinc metal particles. Prog. Org. Coat. 2014, 77, 512–517. [Google Scholar] [CrossRef]
- Kohl, M.; Kalendová, A.; Deshpande, P.P.; Schmidová, E. Effects of conductive polymers (type and concentration) in coatings with zinc particles of different shapes. J. Coat. Technol. Res. 2019, 16, 949–962. [Google Scholar] [CrossRef]
- Kohl, M.; Alafid, F.; Boštíková, K.; Bouška, M.; Krejčová, A.; Svoboda, J.; Slang, S.; Michalíčková, L.; Kalendová, A.; Hrdina, R.; et al. New Azo Dyes-Based Mg Complex Pigments for Optimizing the Anti-Corrosion Efficiency of Zinc-Pigmented Epoxy Ester Organic Coatings. Coatings 2023, 13, 1276. [Google Scholar] [CrossRef]
- Giudice, C.A. Tecnología de Pinturas y Recubrimientos: Componentes, Formulación, Manufactura y Calidad/Carlos A. Giudice y Andrea M. Pereyra, 1st ed.; Edutecne: Buenos Aires, Argentina, 2009; ISBN 978-987-25360-2-2. [Google Scholar]
- Buxbaum, G. Industrial Inorganic Pigments/Edited by Gunter Buxbaum, 2nd completely rev. Weinheim; Wiley-VCH: New York, NY, USA, 1998; ISBN 3-527-28878-3. [Google Scholar]
- Chunping, Q.; Kim, D.J.; Claus, E.W.; Hao, W. Synthesis of micro-structured zinc particles by thermal evaporation and their application in zinc containing coatings for steel corrosion protection. Prog. Org. Coat. 2024, 187, 108143. [Google Scholar]
- ISO 4630:2015; Clear Liquids—Estimation of Colour by the Gardner Colour Scale. International Organization for Standardization: Geneva, Switzerland, 2015.
- ISO 2114:2020; Plastics (Polyester Resins) and Paints and Varnishes (Binders)—Determination of Partial Acid Value and Total Acid Value. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 1514:2024; Paints and Varnishes—Standard Panels for Testing. International Organization for Standardization: Geneva, Switzerland, 2024.
- ISO 2808:2019; Paints and Varnishes—Determination of Film Thickness. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 2409:2020; Paints and Varnishes—Cross-Cut Test. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 12944-6:2018; Paints and Varnishes—Corrosion Protection of Steel Structures by Protective Paint Systems, Part 6: Laboratory Performance Test Methods. International Organization for Standardization: Geneva, Switzerland, 2018.
- ISO 1522:2022; Paints and Varnishes—Pendulum Damping Test. International Organization for Standardization: Geneva, Switzerland, 2022.
- ISO 6272-1:2011; Paints and Varnishes—Rapid-Deformation (Impact Resistance) Tests, Part 1: Falling-weight Test, Large-Area Indenter. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 1519:2011; Paints and Varnishes—Bend Test (Cylindrical Mandrel). International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 1520:2006; Paints and Varnishes—Cupping Test. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 4624:2023; Paints and Varnishes—Pull-Off Test for Adhesion. International Organization for Standardization: Geneva, Switzerland, 2023.
- ASTM G85-19; Standard Practice for Modified Salt Spray (Fog) Testing. ASTM International: West Conshohocken, PA, USA, 2019.
- ISO 22479:2019; Corrosion of Metals and Alloys—Sulfur Dioxide Test in a Humid Atmosphere (Fixed Gas Method). International Organization for Standardization: Geneva, Switzerland, 2019.
- ASTM D714-02(2017); Standard Test Method for Evaluating Degree of Blistering of Paints. ASTM International: West Conshohocken, PA, USA, 2017.
- ASTM D610-08(2019); Standard Practice for Evaluating Degree of Rusting on Painted Steel Surfaces. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D1654-08(2016); Standard Test Method for Evaluation of Painted or Coated Specimens Subjected to Corrosive Environments. ASTM International: West Conshohocken, PA, USA, 2016.
- ISO 787-9:2019; General Methods of Test for Pigments and Extenders, Part 9: Determination of pH Value of an Aqueous Suspension. International Organization for Standardization: Geneva, Switzerland, 2019.
- ISO 787-14:2019; General Methods of Test for Pigments and Extenders, Part 14: Determination of Resistivity of Aqueous Extract. International Organization for Standardization: Geneva, Switzerland, 2019.
- Technical Leaflet, Chemical Raw Materials, WorléeDur D 46, Art.-No. 111005-00263, Worlée Seit 1851, 2022. Available online: https://www.worlee.de/en/chemical-raw-materials/portfolio/product-search/detailed-overview/product/71/ (accessed on 8 June 2024).
- Kalendová, A.; Veselý, D.; Kalenda, P. Properties of paints with hematite coated muscovite and talc particles. Appl. Clay Sci. 2010, 48, 581–588. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, B.; Jin, Z.; Chen, C.; Zhu, Q.; Hou, B. Epoxy coating modified by 2D MoS2/SDBS: Fabrication, anticorrosion behaviour and inhibition mechanism. RSC Adv. 2016, 6, 97512–97522. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; He, Y.; Chen, C.; Zhang, C.; Xie, P.; Zhong, F.; Li, H.; Chen, J.; Li, Z. APTES Modification of Molybdenum Disulfide to Improve the Corrosion Resistance of Waterborne Epoxy Coating. Coatings 2021, 11, 178. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, Z.Y.; Liu, M.R.; Pan, C. Synergistic Effect of NaCl and SO2 on the Initial Atmospheric Corrosion of Zinc Under Wet–Dry Cyclic Conditions. Acta Metall. Sin. (Engl. Lett.) 2019, 32, 780–796. [Google Scholar] [CrossRef]
- Oesch, S.; Faller, M. Environmental effects on materials: The effect of the air pollutants SO2, NO2, NO and O3 on the corrosion of copper, zinc and aluminium. A short literature survey and results of laboratory exposures. Corros. Sci. 1997, 39, 1505–1530. [Google Scholar] [CrossRef]
- Christiansen, F.A.; Fjellvåg, H.; Kjekshus, A.; Klewe, B. Synthesis and characterization of molybdenum(VI) oxide sulfates and crystal structures of two polymorphs of MoO2(SO4). J. Chem. Soc. Dalton Trans. 2001, 6, 806–815. [Google Scholar] [CrossRef]
- Badawy, W.A.; Feky, H.E.; Helal, N.H.; Mohammed, H.H. Hydrogen production on molybdenum in H2SO4 solutions. J. Power Sources 2014, 271, 480–488. [Google Scholar] [CrossRef]
- Manyepedza, T.; Courtney, J.M.; Snowden, A.; Jones, C.R.; Rees, N.V. Impact Electrochemistry of MoS2: Electrocatalysis and Hydrogen Generation at Low Overpotentials. J. Phys. Chem. C 2022, 126, 17942–17951. [Google Scholar] [CrossRef] [PubMed]
- Anita, N.; Joany, R.M.; Dorothy, R.; Aslam, J.; Rajendran, S.; Subramania, A.; Singh, G.; Verma, C. Chapter 4—Linear polarization resistance (LPR) technique for corrosion measurements. In Electrochemical and Analytical Techniques for Sustainable Corrosion Monitoring, Advances, Challenges and Opportunities; Elsevier: Amsterdam, The Netherlands, 2023; pp. 59–80. [Google Scholar] [CrossRef]
- Sun, H.; Su, G.; Zhang, Y.; Ren, J.; Chen, X.; Hou, H.; Ding, Z.; Zhang, T.; Liu, W. First-principles modeling of the anodic and cathodic polarization to predict the corrosion behavior of Mg and its alloys. Acta Mater. 2023, 244, 118562. [Google Scholar] [CrossRef]
- Rocchini, G. Corrosion rate monitoring by the linear polarization method. Corros. Sci. 1993, 34, 2031–2044. [Google Scholar] [CrossRef]
- Zhang, X.L.; Jiang, Z.H.; Yao, Z.P.; Song, Y.; Wu, Z.D. Effects of scan rate on the potentiodynamic polarization curve obtained to determine the Tafel slopes and corrosion current density. Corros. Sci. 2009, 51, 581–587. [Google Scholar] [CrossRef]
- Flitt, H.J.; Schweinsberg, D.P. Evaluation of corrosion rate from polarisation curves not exhibiting a Tafel region. Corros. Sci. 2005, 47, 3034–3052. [Google Scholar] [CrossRef]
- Plyasunov, A.V. An experimental study of the solubility and speciation of MoO3(s) in hydrothermal fluids at temperatures up to 350 °C-a discussion. Econ. Geol. 2020, 115, 1871. [Google Scholar] [CrossRef]



| Binder | Dry Matter [%] | Content | Acid Value [mg KOH·g−1] | Color [-] | Viscosity [mPa·s−1] | Forms | |
|---|---|---|---|---|---|---|---|
| Oil [%] | EP-Resin [%] | ||||||
| Epoxy ester resin | 60 | 40 | 60 | 3.9 | 10 | 4200 | 60% in xylene |
| Pigment | Density [g·cm−3] | Oil Absorption [g/100 g] | CPVC [-] | Size of Primary Particles [nm] | Particle Shape [-] | Assay [%] |
|---|---|---|---|---|---|---|
| WS2 | 6.79 ± 0.02 | 19.4 ± 0.2 | 41.4 | 2966 ± 1954 | sheet-like | 99.0 |
| WO3 | 7.27 ± 0.02 | 11.2 ± 0.2 | 53.3 | 187 ± 71 | spherical | 99.0 |
| ZnS | 4.05 ± 0.02 | 15.9 ± 0.2 | 59.2 | 80 ± 19 | tetrahedral | 99.9 |
| ZnO | 5.68 ± 0.02 | 14.3 ± 0.2 | 53.4 | 286 ± 129 | oval/rectangular | 99.9 |
| MoS2 | 4.67 ± 0.02 | 30.7 ± 0.2 | 39.3 | 1154 ± 696 | sheet-like | 98.0 |
| MoO3 | 4.67 ± 0.02 | 15.5 ± 0.2 | 56.3 | 3055 ± 2391 | sheet-like | 99.5 |
| ZnS/BaSO4 | 4.31 ± 0.02 | 13.4 ± 0.2 | 61.7 | 216 ± 155 | oval/spherical | 98.0 |
| Zn | 7.14 ± 0.02 | 6.7 ± 0.2 | 66.0 | 1438 ± 1054 | spherical | 99.0 |
| Inorganic Pigment | Element [Atomic %] | |||||
|---|---|---|---|---|---|---|
| W | Zn | Mo | Ba | S | O | |
| WS2 | 29.3 ± 0.9 | - | - | - | 55.4 ± 0.9 | 15.3 ± 1.4 |
| WO3 | 20.6 ± 0.3 | - | - | - | - | 79.4 ± 0.3 |
| ZnS | - | 46.4 ± 0.1 | - | - | 48.4 ± 0.6 | 5.2 ± 0.7 |
| ZnO | - | 49.9 ± 0.5 | - | - | - | 50.1 ± 0.5 |
| MoS2 | - | - | 32.2 ± 0.3 | - | 67.8 ± 0.3 | - |
| MoO3 | - | - | 23.4 ± 0.4 | - | - | 76.6 ± 0.4 |
| ZnS/BaSO4 | - | 14.2 ± 0.1 | - | 12.7 ± 0.3 | 21.5 ± 0.5 | 51.6 ± 0.6 |
| Zn | - | 90.8 ± 0.3 | - | - | - | 9.2 ± 0.3 |
| Pigment | PVC [%] | Adhesion Test 5 × 2 mm [dg.] | Blistering | Corrosion | ||
|---|---|---|---|---|---|---|
| In the Cut [dg.] | On the Film Area [dg.] | Metal Base [%] | In the Cut [mm] | |||
| WS2 | 3 | 3 | 6D | 8M | 50 | 3.5–4 |
| 5 | 2 | 6MD | 8F | 33 | 1.5–2 | |
| 10 | 1 | 8M | 8F | 3 | 1–1.5 | |
| WO3 | 3 | 3 | 8M | 8M | 50 | 1–1.5 |
| 5 | 2 | 6F | 8M | 16 | 1.5–2 | |
| 10 | 2 | 4M | 8M | 33 | 2–2.5 | |
| ZnS | 3 | 3 | 6M | 8F | 50 | 3–3.5 |
| 5 | 2 | 8M | 8F | 1 | 1.5–2 | |
| 10 | 1 | 8M | 8F | 0.3 | 1.5–2 | |
| ZnO | 3 | 3 | 6M | 6M | 50 | 2.5–3 |
| 5 | 1 | 8M | 8M | 33 | 1.5–2 | |
| 10 | 1 | 8F | 8F | 3 | 1–1.5 | |
| MoS2 | 3 | 1 | 8F | - | 0.01 | 0.5–1 |
| 5 | 0 | 8F | - | 0 | 0.5–1 | |
| 10 | 0 | 8M | - | 0 | 0.5–1 | |
| MoO3 | 3 | 1 | 8F | - | 0.01 | 0.5–1 |
| 5 | 0 | 8F | - | 0 | 0.5–1 | |
| 10 | 0 | - | - | 0 | 0–0.5 | |
| ZnS/BaSO4 | 3 | 3 | 8MD | 8F | 33 | 1–1.5 |
| 5 | 2 | 8M | 8F | 16 | 1–1.5 | |
| 10 | 2 | 8M | 8F | 10 | 1–1.5 | |
| Zn | PVC/CPVC = 0.6 | 1 | 8D | 8F | 0.1 | 2–2.5 |
| Pigment | PVC [%] | Adhesion Test 5 × 2 mm [dg.] | Blistering | Corrosion | ||
|---|---|---|---|---|---|---|
| In the Cut [dg.] | On the Film Area [dg.] | Metal Base [%] | In the Cut [mm] | |||
| WS2 | 3 | 1 | - | 8F | 1 | 0.5–1 |
| 5 | 1 | - | 8F | 0.3 | 0.5–1 | |
| 10 | 1 | - | 8F | 0.1 | 0.5–1 | |
| WO3 | 3 | 2 | - | 8M | 3 | 0.5–1 |
| 5 | 1 | - | - | 0.3 | 0–0.5 | |
| 10 | 1 | - | - | 0.3 | 0–0.5 | |
| ZnS | 3 | 5 | - | 8M | 1 | 0.5–1 |
| 5 | 5 | - | 8M | 0.3 | 0.5–1 | |
| 10 | 2 | - | 8F | 0.1 | 0.5–1 | |
| ZnO | 3 | 5 | 8F | 8F | 1 | 0.5–1 |
| 5 | 5 | 8F | 8M | 1 | 0.5–1 | |
| 10 | 5 | 8F | 8F | 1 | 0.5–1 | |
| MoS2 | 3 | 0 | - | - | 0.03 | 0–0.5 |
| 5 | 0 | - | - | 0.01 | 0–0.5 | |
| 10 | 0 | - | - | 0.01 | 0–0.5 | |
| MoO3 | 3 | 0 | - | - | 0.1 | 0–0.5 |
| 5 | 0 | - | - | 0.1 | 0–0.5 | |
| 10 | 0 | - | - | 0.1 | 0–0.5 | |
| ZnS/BaSO4 | 3 | 2 | - | 8F | 0.1 | 0.5–1 |
| 5 | 1 | - | 8F | 0.1 | 0–0.5 | |
| 10 | 1 | - | 8F | 0.1 | 0–0.5 | |
| Zn | PVC/CPVC = 0.6 | 2 | - | 8F | 0.3 | 0–0.5 |
| System | PVC [%] | Ecor [mV] | Icor [µA] | βa [mV] | βc [mV] | Rp [Ω] | CR [mm/Year] |
|---|---|---|---|---|---|---|---|
| WS2 | 3 | −962 | 7.24 × 10−6 | 33.7 | 25.1 | 8.63 × 108 | 1.07 × 10−7 |
| 5 | −956 | 7.20 × 10−6 | 33.4 | 25.2 | 8.66 × 108 | 1.06 × 10−7 | |
| 10 | −958 | 7.14 × 10−6 | 33.6 | 25.1 | 8.76 × 108 | 1.05 × 10−7 | |
| WO3 | 3 | −966 | 5.66 × 10−6 | 34.1 | 25.7 | 1.12 × 109 | 8.36 × 10−8 |
| 5 | −934 | 3.13 × 10−6 | 34.0 | 24.9 | 1.99 × 109 | 4.62 × 10−8 | |
| 10 | −935 | 3.16 × 10−6 | 34.1 | 25.0 | 1.98 × 109 | 4.67 × 10−8 | |
| ZnS | 3 | −960 | 6.74 × 10−6 | 33.8 | 24.9 | 9.24 × 108 | 9.96 × 10−8 |
| 5 | −952 | 6.88 × 10−6 | 33.4 | 24.5 | 8.92 × 108 | 1.02 × 10−7 | |
| 10 | −956 | 7.06 × 10−6 | 33.5 | 24.2 | 8.64 × 108 | 1.04 × 10−7 | |
| ZnO | 3 | −969 | 7.24 × 10−6 | 32.8 | 25.1 | 8.53 × 108 | 1.07 × 10−7 |
| 5 | −948 | 7.28 × 10−6 | 32.6 | 24.9 | 8.42 × 108 | 1.08 × 10−7 | |
| 10 | −950 | 7.31 × 10−6 | 32.8 | 25.2 | 8.47 × 108 | 1.08 × 10−7 | |
| MoS2 | 3 | −962 | 2.82 × 10−6 | 33.4 | 24.6 | 2.18 × 109 | 4.17 × 10−8 |
| 5 | −950 | 2.80 × 10−6 | 33.3 | 24.2 | 2.17 × 109 | 4.14 × 10−8 | |
| 10 | −959 | 2.07 × 10−6 | 32.7 | 24.1 | 3.02 × 109 | 3.06 × 10−8 | |
| MoO3 | 3 | −971 | 1.92 × 10−6 | 34,6 | 25,1 | 3.29 × 109 | 2.84 × 10−8 |
| 5 | −923 | 1.84 × 10−6 | 34.7 | 24.4 | 3.38 × 109 | 2.72 × 10−8 | |
| 10 | −944 | 1.74 × 10−6 | 34.3 | 25.2 | 3.63 × 109 | 2.57 × 10−8 | |
| ZnS/BaSO4 | 3 | −967 | 6.31 × 10−6 | 33.8 | 25.1 | 9.89 × 108 | 9.32 × 10−8 |
| 5 | −946 | 6.23 × 10−6 | 33.6 | 24.9 | 9.97 × 108 | 9.20 × 10−8 | |
| 10 | −951 | 6.21 × 10−6 | 31.8 | 25.2 | 9.83 × 108 | 9.17 × 10−8 | |
| Zn | PVC/CPVC = 0.6 | −980 | 6.70 × 10−6 | 33.7 | 25.2 | 9.34 × 108 | 9.90 × 10−8 |
| System | PVC [%] | [µS·cm−1] | [µS·cm−1] | [-] | [-] | KMf [g·m−2] | XHf [%] | URf [mm] | Vkf [g·m−2·d−1] |
|---|---|---|---|---|---|---|---|---|---|
| 3 | 68.2 | 84.0 | 7.2 | 7.0 | 0.42 | 89.4 | 5.4 × 10−5 | 8.4 × 10−2 | |
| WS2 | 5 | 79.2 | 87.0 | 7.4 | 7.1 | 0.35 | 74.5 | 4.5 × 10−5 | 7.0 × 10−2 |
| 10 | 68.2 | 89.0 | 7.6 | 7.1 | 0.32 | 68.1 | 4.1 × 10−5 | 6.4 × 10−2 | |
| 3 | 50.2 | 66.0 | 7.3 | 7.0 | 0.36 | 76.6 | 4.6 × 10−5 | 7.2 × 10−2 | |
| WO3 | 5 | 57.2 | 72.0 | 7.2 | 7.1 | 0.33 | 70.2 | 4.2 × 10−5 | 6.6 × 10−2 |
| 10 | 62.6 | 74.0 | 7.4 | 7.1 | 0.38 | 80.9 | 4.8 × 10−5 | 7.6 × 10−2 | |
| 3 | 67.6 | 87.0 | 7.8 | 7.0 | 0.31 | 66.0 | 3.9 × 10−5 | 6.2 × 10−2 | |
| ZnS | 5 | 64.6 | 96.0 | 7.3 | 7.1 | 0.38 | 80.9 | 4.8 × 10−5 | 7.6 × 10−2 |
| 10 | 63.8 | 97.0 | 7.2 | 7.0 | 0.34 | 72.3 | 4.3 × 10−5 | 6.8 × 10−2 | |
| 3 | 70.0 | 84.0 | 7.1 | 6.9 | 0.33 | 70.2 | 4.2 × 10−5 | 6.6 × 10−2 | |
| ZnO | 5 | 68.4 | 89.0 | 7.2 | 7.0 | 0.32 | 68.1 | 4.1 × 10−5 | 6.4 × 10−2 |
| 10 | 60.8 | 91.0 | 7.3 | 7.0 | 0.38 | 80.9 | 4.8 × 10−5 | 7.6 × 10−2 | |
| 3 | 62.4 | 86.0 | 6.8 | 7.0 | 0.35 | 74.5 | 4.5 × 10−5 | 7.0 × 10−2 | |
| MoS2 | 5 | 70.8 | 13.0 | 6.9 | 7.0 | 0.38 | 80.9 | 4.8 × 10−5 | 7.6 × 10−2 |
| 10 | 74.8 | 151.0 | 7.1 | 7.0 | 0.37 | 78.7 | 4.7 × 10−5 | 7.4 × 10−2 | |
| 3 | 377.0 | 572.0 | 6.9 | 6.8 | 0.26 | 55.3 | 3.3 × 10−5 | 5.2 × 10−2 | |
| MoO3 | 5 | 600.0 | 1086.0 | 6.8 | 6.8 | 0.22 | 46.8 | 2.8 × 10−5 | 4.4 × 10−2 |
| 10 | 784.0 | 1309.0 | 6.6 | 6.7 | 0.09 | 19.1 | 1.1 × 10−5 | 1.8 × 10−2 | |
| 3 | 67.6 | 138.0 | 6.7 | 7.1 | 0.32 | 68.1 | 4.1 × 10−5 | 6.4 × 10−2 | |
| ZnS/BaSO4 | 5 | 83.2 | 129.0 | 7.1 | 6.9 | 0.35 | 74.5 | 4.5 × 10−5 | 7.0 × 10−2 |
| 10 | 97.6 | 127.0 | 7.1 | 6.9 | 0.35 | 74.5 | 4.5 × 10−5 | 7.0 × 10−2 | |
| Zn | PVC/CPVC =0.6 | 57.7 | 57.0 | 7.2 | 7.3 | 0.45 | 95.7 | 5.7 × 10−5 | 9.0 × 10−2 |
| Distilled water | - | 1.5 | 13.1 | 6.9 | 7.1 | 0.47 | 100 | 6.0 × 10−5 | 9.4 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kohl, M.; Boštíková, K.; Slang, S.; Schmidová, E.; Kalendová, A. Use of 2D Sulfide and Oxide Compounds as Functional Semiconducting Pigments in Protective Organic Coatings Containing Zinc Dust. Coatings 2024, 14, 1009. https://doi.org/10.3390/coatings14081009
Kohl M, Boštíková K, Slang S, Schmidová E, Kalendová A. Use of 2D Sulfide and Oxide Compounds as Functional Semiconducting Pigments in Protective Organic Coatings Containing Zinc Dust. Coatings. 2024; 14(8):1009. https://doi.org/10.3390/coatings14081009
Chicago/Turabian StyleKohl, Miroslav, Karolína Boštíková, Stanislav Slang, Eva Schmidová, and Andréa Kalendová. 2024. "Use of 2D Sulfide and Oxide Compounds as Functional Semiconducting Pigments in Protective Organic Coatings Containing Zinc Dust" Coatings 14, no. 8: 1009. https://doi.org/10.3390/coatings14081009






