Effect of Microcapsules of Chitosan-Coated Toddalia asiatica (L.) Lam Extracts on the Surface Coating Properties of Poplar Wood
Abstract
:1. Introduction
2. Test Materials and Methods
2.1. Materials
2.2. Preparation Method of Microcapsules
2.2.1. Preparation Method of Toddalia asiatica (L.) Lam Extracts
2.2.2. Preparation Method of Microcapsules
2.3. Painting Method for Poplar Board
2.4. Testing and Characterization
2.4.1. Performance Characterization of Microcapsules
- (1)
- Coverage rate (C): The microcapsules with a mass of M1 were weighed. M2 was the weight of the weighing filter paper. The microcapsules were soaked in ethanol and filtered and dried after 24 h. The total mass of the dried filter paper and shell material was M3. The calculation of coverage rate is shown in Formula (1) [35].
- (2)
- Yield rate (Y): The total mass of the core material, shell material, and emulsifier used for preparing microcapsule samples was denoted as M1. The mass of microcapsule powder after drying was recorded as M2. The calculation of yield rate is shown in Formula (2) [36].
- (3)
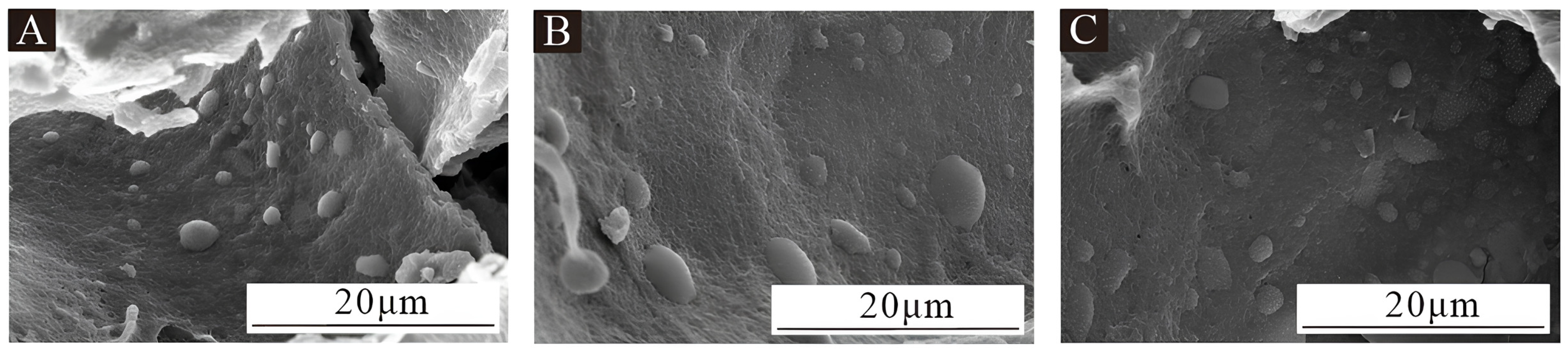
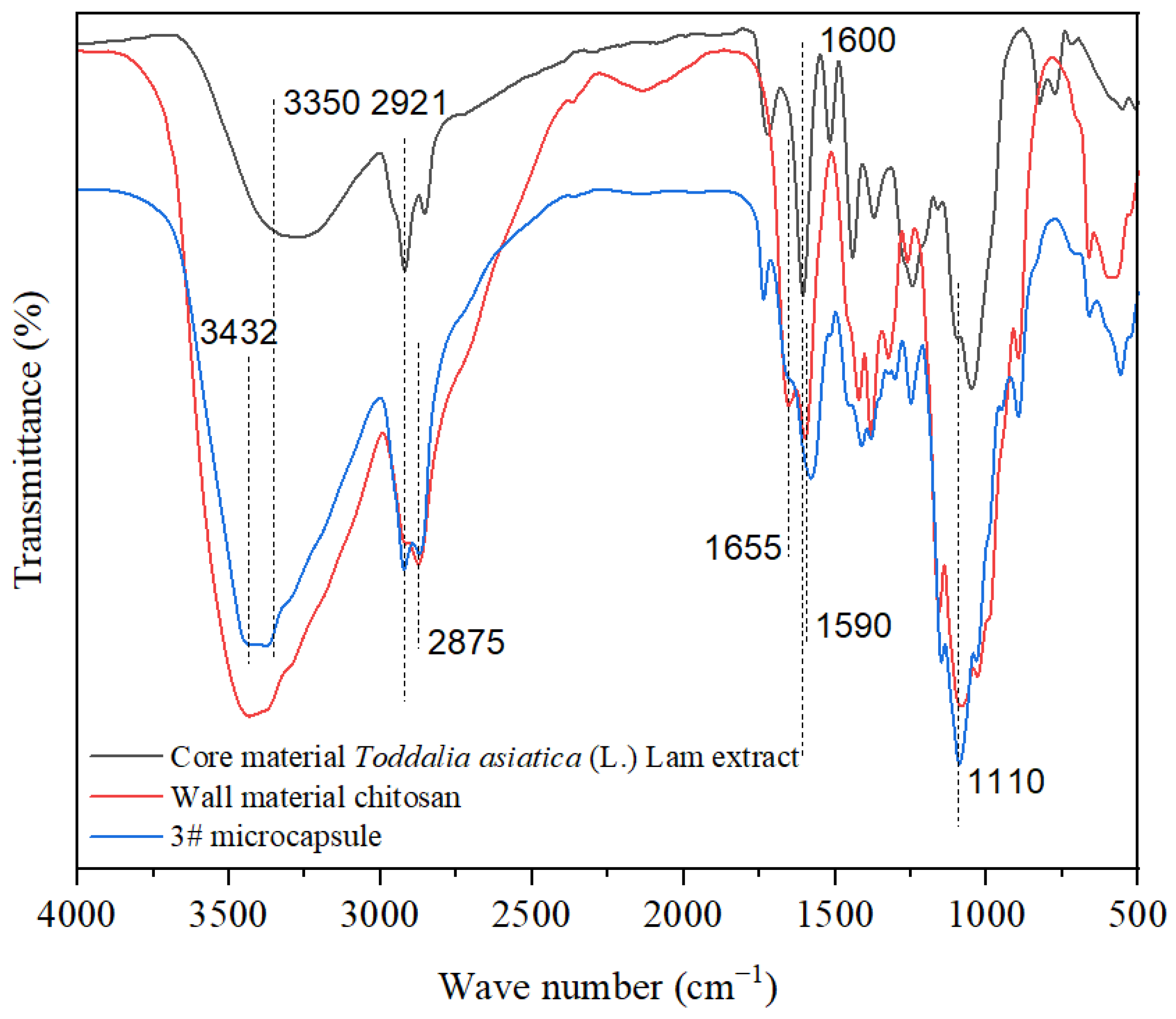
- Analysis of microstructure and chemical composition: The morphology of microcapsules was observed using a Zeiss optical microscope (OM, Carl Zeiss AG, Oberkochen, Germany). The microstructure of microcapsules and coatings was analyzed using scanning electron microscopy (SEM, Tescan, Brno, the Czech Republic). The chemical composition of microcapsules and coatings was analyzed using Fourier transform infrared spectroscopy (FTIR, Brucker AG, Karlsruhe, Germany).
2.4.2. Color Difference Testing of Coating
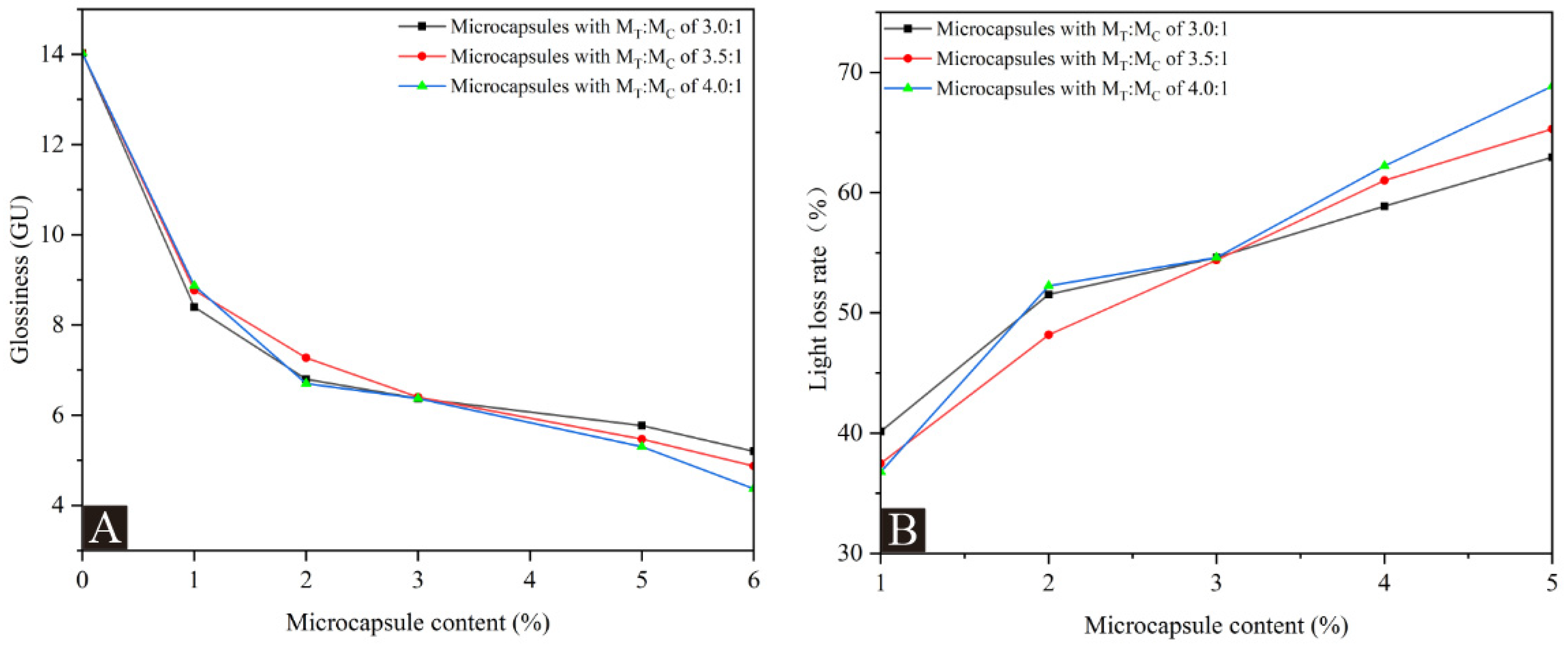
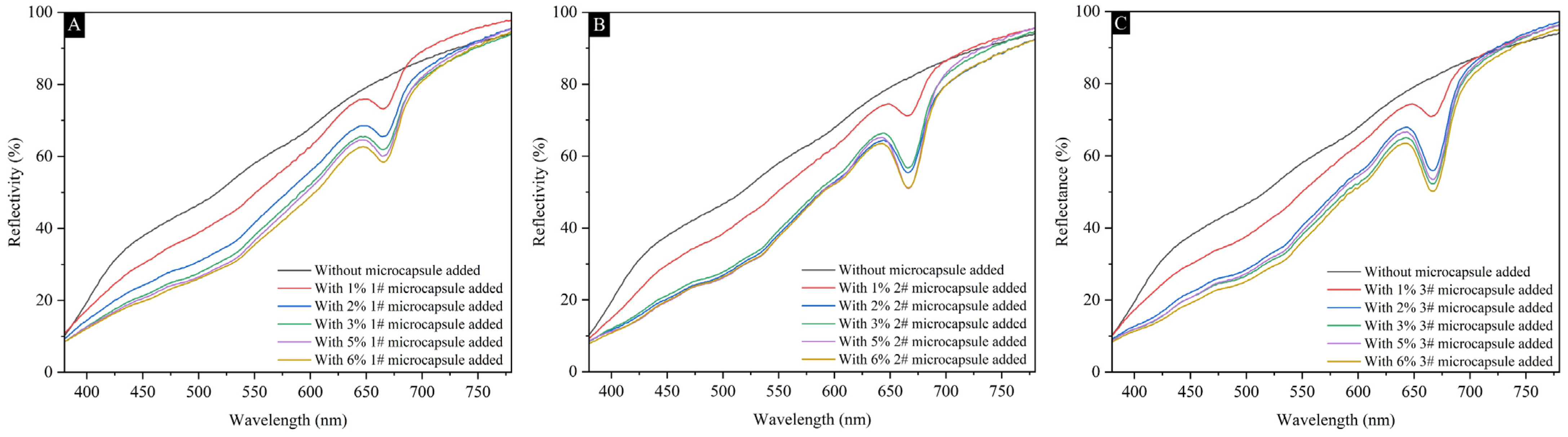
2.4.3. Glossiness and Reflectivity of Coating
2.4.4. Roughness Testing of Coating
2.4.5. Cold Liquid Resistance Test of Coating
2.4.6. Antibacterial Performance Testing of Coating
2.4.7. Hardness, Impact Resistance, and Adhesion Testing of Coatings
- (1)
- Hardness: according to GB/T 6739-2022 [45], a pencil with a hardness of 9B-9H was used and tested by a QHQ-A portable pencil hardness tester (Quzhou Aipu Measuring Instrument Co., Ltd., Quzhou, China). The pencil was inserted diagonally at a 45° angle into the pencil hardness tester with a load of 750 g for hardness testing. The pencil hardness was the coating hardness.
- (2)
- Impact resistance: according the content of GB/T 4893.9-2013 “Physical and chemical properties testing of furniture surface coating—Part 9: determination of impact resistance” [46], the impact resistance of wood surface coatings was tested with a coating impactor (Dongguan Jiaxin Measuring Instrument Co., Ltd., Dongguan, China). A magnifying glass was used to observe the number of cycles of surface rupture of the coating to evaluate its impact resistance level. Each sample was subjected to 5 impacts. The nearest integer to the arithmetic mean of an evaluation level was taken as the result of the level evaluation. The impact resistance level increased sequentially from level 5 to level 1. The evaluation for the coating impact level is shown in Table 4.
- (3)
- Adhesion: according to GB/T 4893.4-2013 [47], the adhesion of the coating was tested using a coating adhesion tester (Quzhou Aipu Measuring Instrument Co., Ltd., Quzhou, China). The coating was cross-cut with the blade at a vertical angle of 90 degrees. A 3 M adhesive tape was applied on the grid surface and quickly and smoothly peeled off at an angle close to 60°. The adhesion level decreased from level 0 to level 5.
3. Results and Discussion
3.1. Morphology and Chemical Composition Analysis of Microcapsules
3.1.1. Microscopic Morphology Analysis of Microcapsules
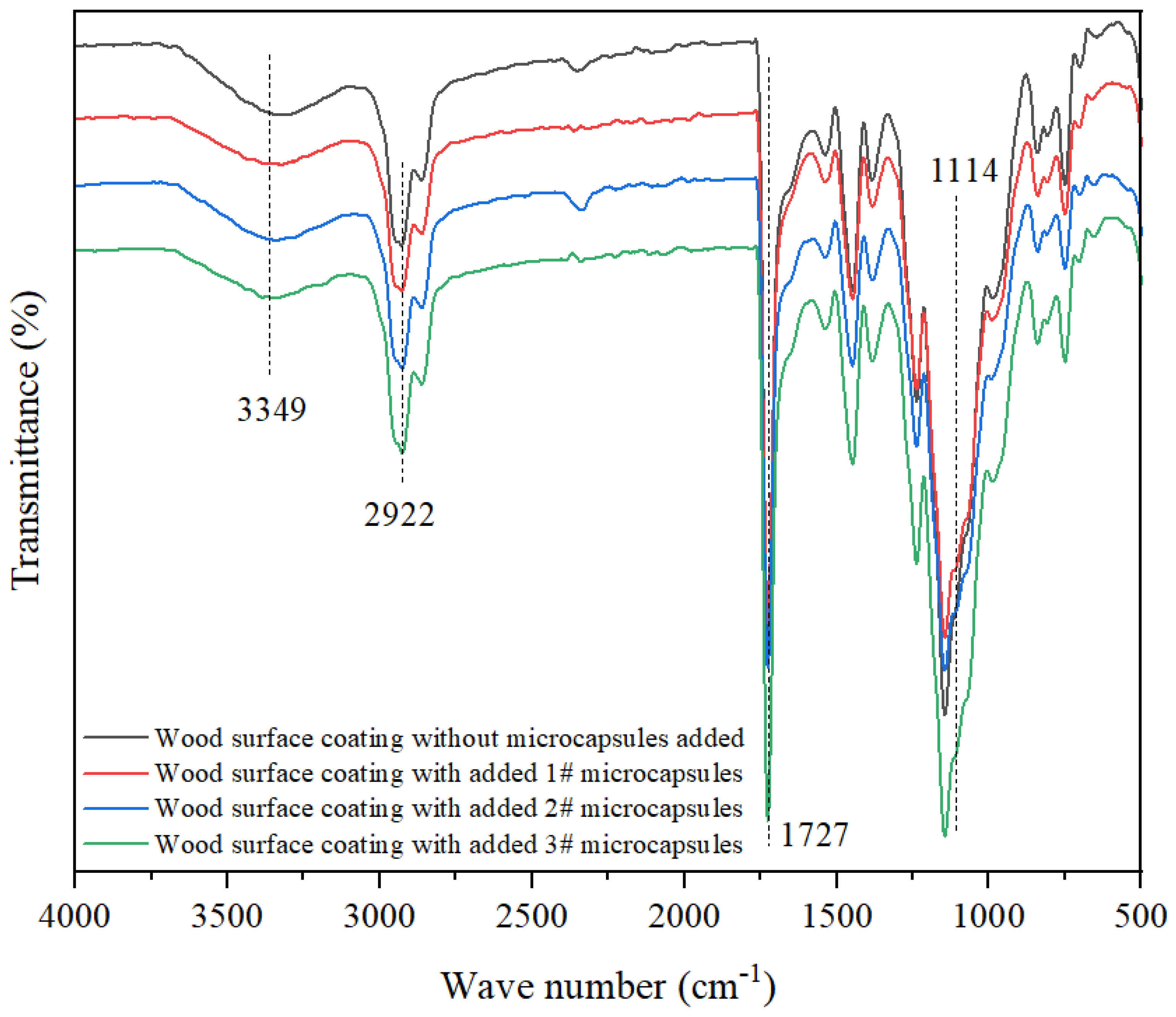
3.1.2. Chemical Composition Analysis of Microcapsules
3.2. Optical Performance Analysis of Coatings
3.3. Cold Liquid Resistance of Coatings
3.4. Mechanical Properties of Surface Coatings
3.4.1. Hardness of Surface Coating on Poplar Wood
3.4.2. Impact Resistance of Surface Coating on Poplar Wood
3.4.3. Adhesion of Surface Coating on Poplar Wood
3.4.4. Roughness of Surface Coating on Poplar Wood
3.5. Antibacterial Properties of Surface Coatings on Poplar Wood
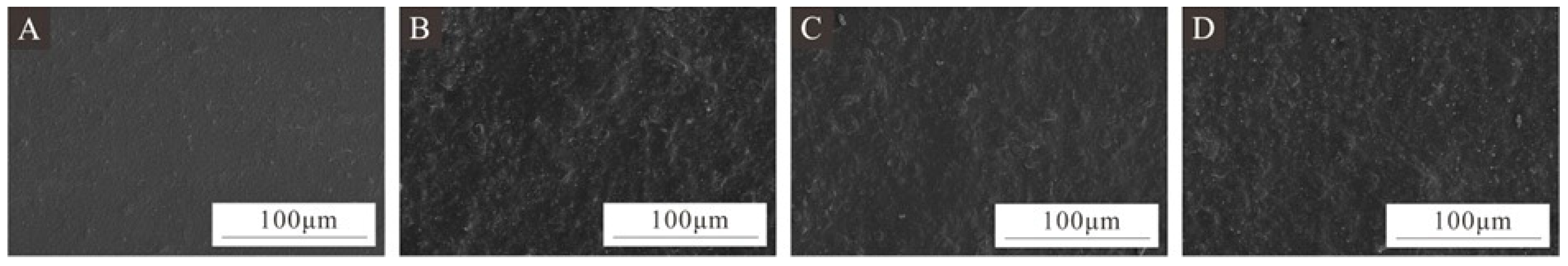
3.6. Microscopic Morphology and Chemical Composition of Surface Coating on Poplar Wood
3.7. Interface Bonding and Antibacterial Mechanism Analysis between Poplar Wood and Coating
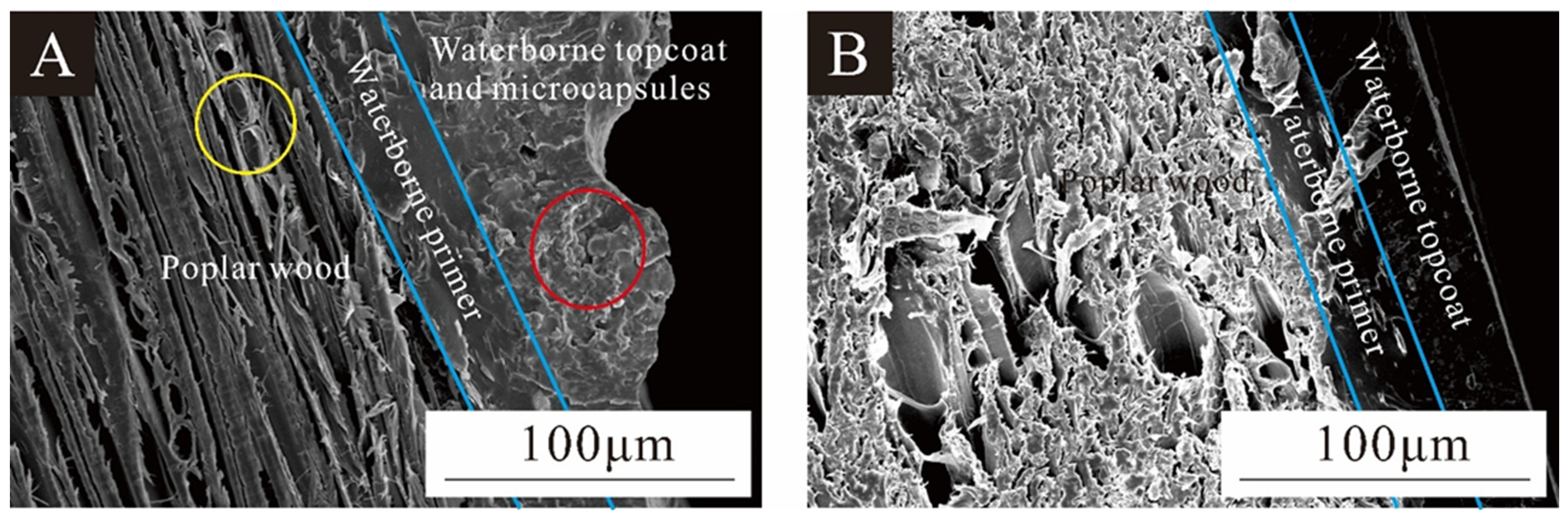
3.7.1. Interface Bonding between Poplar Wood and Coating
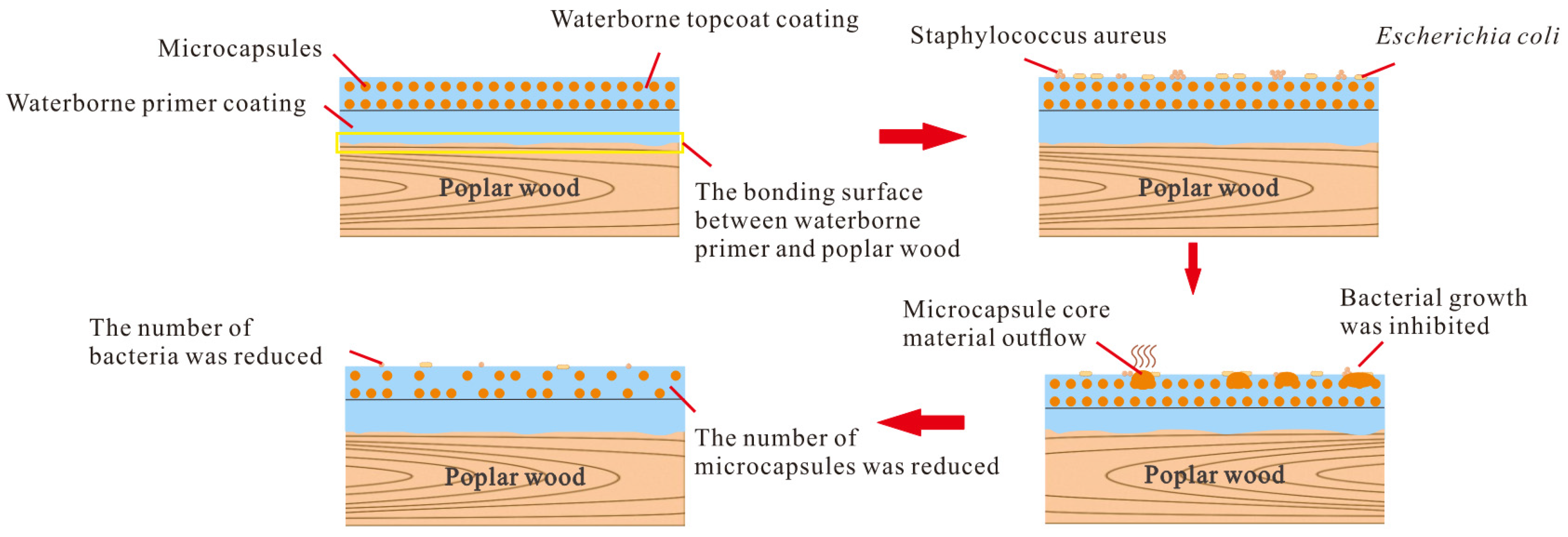
3.7.2. Analysis of Antibacterial Principle of Surface Coating on Poplar Wood
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mai, C.; Schmitt, U.; Niemz, P. A brief overview on the development of wood research. Holzforschung 2022, 75, 102–119. [Google Scholar] [CrossRef]
- Zhang, N.; Xu, W.; Tan, Y. Multi-attribute hierarchical clustering for product family division of customized wooden doors. BioResources 2023, 18, 7889–7904. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Wang, J.X.; Xu, W. Study of selective modification effect of constructed structural color layers on European beech wood surfaces. Forests 2024, 15, 261. [Google Scholar] [CrossRef]
- Yang, L.C.; Wu, Y.; Yang, F.; Wang, W.H. The effect of antibacterial and waterproof coating prepared from hexadecyltrimethoxysilane and nano-titanium dioxide on wood properties. Front. Mater. 2021, 8, 699579. [Google Scholar] [CrossRef]
- Arif, S.; Nawab, Y.; Shaker, K.; Umair, M. Mechanical performance of flame retardant and antibacterial glass-carbon/epoxy hybrid composites for furniture applications. J. Ind. Text. 2022, 51, 5822S–5846S. [Google Scholar] [CrossRef]
- Hu, W.G.; Liu, M.; Xu, L.; Guan, H.Y. Study on cold/warm sensation of materials used in desktop of furniture. Wood Res. 2020, 65, 497–506. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Sun, Y.; Zhou, Z.W.; Huang, Y.; Li, J.X.; Liu, G.Y. Response surface optimization based on freeze-thaw cycle pretreatment of poplar wood dyeing effect. Wood Res. 2023, 68, 293–305. [Google Scholar] [CrossRef]
- Hu, W.G.; Liu, N.; Guan, H.Y. Experimental and numerical study on methods of testing withdrawal resistance of mortise-and-tenon joint for Wood products. Forests 2020, 11, 280. [Google Scholar] [CrossRef]
- Hu, W.G.; Li, S.; Liu, Y. Vibrational characteristics of four wood species commonly used in wood products. BioResources 2021, 16, 7100–7110. [Google Scholar] [CrossRef]
- Jian, H.; Liang, Y.Q.; Deng, C.; Xu, J.X.; Liu, Y.; Shi, J.Y.; Wen, M.Y.; Park, H.J. Research progress on the improvement of flame retardancy, hydrophobicity, and antibacterial properties of wood surfaces. Polymers 2023, 15, 951. [Google Scholar] [CrossRef]
- Gu, Y.T.; Zhang, J.L. Tensile properties of natural and synthetic rattan strips used as furniture woven materials. Forests 2020, 11, 129. [Google Scholar] [CrossRef]
- Wu, S.S.; Zhou, L.C.; Xu, W. A convenient approach to manufacturing lightweight and high-sound-insulation plywood using furfuryl alcohol/multilayer graphene oxide as a shielding layer. Wood Mater. Sci. Eng. 2024, 1–8. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Zhou, Z.W.; Xu, R.; Dong, Y.T.; Liu, M.J.; Shen, L.M.; Han, J.L. Research on the dyeing properties of Chinese fir using ultrasonic-assisted mulberry pigment dyeing. Forests 2023, 14, 1832. [Google Scholar] [CrossRef]
- Qi, Y.R.; Wen, L.X.; Dong, Y.Y.; Gong, R.Z.; Wang, X.W.; Yao, F.B.; Liu, Y.L.; Kong, L.L.; Dong, X.Y.; Li, Y.F. AgCu nanoparticles as an antibacterial coating for wood. ACS Appl. Nano Mater. 2024, 7, 5339–5347. [Google Scholar] [CrossRef]
- Hu, W.G.; Wan, H. Comparative study on weathering durability properties of phenol formaldehyde resin modified sweetgum and southern pine specimens. Maderas-Cienc. Tecol. 2022, 24. [Google Scholar] [CrossRef]
- Sang, R.J.; Yang, F. Effect of TiO2@CaCO3 waterborne primer on the coloring performance of inkjet-printed wood product coatings. Coatings 2023, 13, 2071. [Google Scholar] [CrossRef]
- Sang, R.J.; Yang, F.; Fan, Z.X. The effect of water-based primer pretreatment on the performance of water-based inkjet coatings on wood surfaces. Coatings 2023, 13, 1649. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Zhou, Z.W.; Li, J.X.; Shen, L.M.; Han, J.L. Study on the dyeing properties of Chinese fir with Cinnamomum camphor pigment by premordant dyeing. Wood Mater. Sci. Eng. 2024, 1–11. [Google Scholar] [CrossRef]
- Jalaie, A.; Afshaar, A.; Mousavi, S.B.; Heidari, M. Investigation of the release rate of biocide and corrosion resistance of Vinyl-, Acrylic-, and Epoxy-Based antifouling paints on steel in marine infrastructures. Polymers 2023, 15, 3948. [Google Scholar] [CrossRef]
- Hu, W.G.; Zhang, J.L. Bolt-bearing yield strength of three-layered cross-laminated timber treated with phenol formaldehyde resin. Forests 2020, 11, 551. [Google Scholar] [CrossRef]
- Sang, R.J.; Yang, S.Q.; Fan, Z.X. Effects of MDF substrate surface coating process on UV inkjet print quality. Coatings 2023, 13, 970. [Google Scholar] [CrossRef]
- Cheng, L.S.; Ren, S.B.; Lu, X.N. Application of eco-Friendly waterborne polyurethane composite coating incorporated with nano cellulose crystalline and silver nano particles on wood antibacterial board. Polymers 2020, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Egghe, T.; Morent, R.; Hoogenboom, R.; De Geyter, N. Substrate-independent and widely applicable deposition of antibacterial coatings. Trends Biotechnol. 2023, 41, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Zigon, J.; Moghaddam, M.S.; Walinder, M.E.P. Wettability and surface interactions of natural and thermally modified beech wood with water and water-based coatings: The effect of surface pre-treatment type. Eur. J. Wood Wood Prod. 2023, 81, 73–88. [Google Scholar] [CrossRef]
- Veeramani, N.; Samikannu, R.; Deshpande, A.P.; Varghese, S.; Moses, V. Effects of polymeric microcapsules on self-healing composites reinforced with carbon fibers: A comparative study. Int. Polym. Proc. 2023, 38, 483–495. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Ni, Y.Z.; Chen, K.L.; Lin, Z.H.; Jia, Z.H.; Qiu, H. Double-shell lignin microcapsules were prepared by one- step method for fabric coatings with UV resistance and durable antibacterial activity. Prog. Org. Coat. 2023, 179, 107518. [Google Scholar] [CrossRef]
- Hu, W.G.; Zhang, J.L. Effect of growth rings on acoustic emission characteristic signals of southern yellow pine wood cracked in mode Ⅰ. Constr. Build. Mater. 2022, 329, 127092. [Google Scholar] [CrossRef]
- Qi, Y.Q.; Zhang, Z.Q.; Sun, Y.; Shen, L.M.; Han, J.L. Study on the Process Optimization of Peanut Coat Pigment Staining of Poplar Wood. Forests 2024, 15, 504. [Google Scholar] [CrossRef]
- Cao, C.X.; Du, P.X.; Zhu, X.M.; Yan, H.J.; Song, X.Y.; Zhu, H.; Geng, Y.L.; Wang, D.J. Rapid screening and purification of potential alkaloid neuraminidase inhibitors from Toddalia asiatica (Linn.) Lam. roots via ultrafiltration liquid chromatography combined with stepwise flow rate counter-current chromatography. J. Sep. Sci. 2019, 42, 2621–2627. [Google Scholar] [CrossRef]
- Li, X.; Qiu, Z.D.; Jin, Q.H.; Chen, G.L.; Guo, M.Q. Cell cycle arrest and apoptosis in HT-29 cells induced by dichloromethane fraction from Toddalia asiatica (L.) Lam. Front. Pharmacol. 2018, 9, 629. [Google Scholar] [CrossRef]
- Raj, M.K.; Balachandran, C.; Duraipandiyan, V.; Agastian, P.; Ignacimuthu, S. Antimicrobial activity of Ulopterol isolated from Toddalia asiatica (L.) Lam.: A traditional medicinal plant. J. Ethnopharmacol. 2012, 140, 161–165. [Google Scholar]
- Wang, Y.; Yan, X.X. Preparation of Toddalia asiatica (L.) Lam. Extract Microcapsules and Their Effect on Optical, Mechanical and Antibacterial Performance of Waterborne Topcoat Paint Films. Coatings 2024, 14, 655. [Google Scholar] [CrossRef]
- Qin, H.G.; Zhou, K.; Song, H.H.; Fang, G.; Chen, Q.; Pang, Y.Z. Toddalia asiatica extract attenuates adjuvant-induced arthritis by modulating colon Th17/Treg balance and colony homeostasis. J. Ethnopharmacol. 2023, 313, 116542. [Google Scholar] [CrossRef]
- Meng, Q.Y.; Zhong, S.L.; Wang, J.; Gao, Y.; Cui, X.J. Advances in chitosan-based microcapsules and their applications. Carbohydr. Polym. 2023, 300, 120265. [Google Scholar] [CrossRef]
- Chang, Y.J.; Yan, X.X. Preparation and self-repairing properties of MF-coated shellac water-based microcapsules. Coatings 2020, 10, 778. [Google Scholar] [CrossRef]
- Beglarigale, A.; Eyice, D.; Seki, Y.; Yalcinkaya, C.; Çopuroglu, O.; Yazici, H. Sodium silicate/polyurethane microcapsules synthesized for enhancing self-healing ability of cementitious materials: Optimization of stirring speeds and evaluation of self-healing efficiency. J. Build. 2021, 39, 102279. [Google Scholar] [CrossRef]
- GB/T 11186.3-1989; Methods for Measuring the Color of Coatings—Part 3: Calculation of Color Difference. Standardization Administration of the People’s Republic of China: Beijing, China, 1989.
- Azamatov, M.K.; Gainutdinov, I.S.; Sabirov, R.S.; Safin, R.G.; Mikhailov, A.V. Determining the color difference of interference coatings. J. Opt. Techol. 2007, 74, 205–207. [Google Scholar] [CrossRef]
- GB/T 4893.6-2013; Testing of Physical and Chemical Properties of Furniture Surface Paint Films—Part 6: Gloss Determination Method. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- Takebayashi, H.; Tanabe, J.; Aoyama, T.; Sonoda, T.; Nakanishi, Y. Using field measurements to assess aging of self-cleaning high-reflectance paint. Int. J. Thermophys. 2017, 38, 119. [Google Scholar] [CrossRef]
- GB/T 4893.1-2021; Test of Surface Coatings of Furniture—Part 1: Determination of Surface Resistance to Cold Liquids. Standardization Administration of the People’s Republic of China: Beijing, China, 2021.
- GB/T 21866-2008; Test Method and Effect for Antibacterial Capability of Paints Film. Standardization Administration of the People’s Republic of China: Beijing, China, 2008.
- GB/T 4789.2-2022; Microbiological Examination of Food Hygiene—Aerobic Plate Count. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- Shen, Y.; Shan, X.F.; Etim, I.P.; Siddiqui, M.A.; Yang, Y.; Shi, Z.W.; Su, X.P.; Chen, J.X. Comparative study of the effects of nano ZnO and CuO on the biodegradation, biocompatibility, and antibacterial properties of micro-arc oxidation coating of magnesium alloy. Acta Metall. Sin.-Engl. Lett. 2024, 37, 242–254. [Google Scholar] [CrossRef]
- GB/T 6739-2022; Paints and Varnishes—Determination of Film Hardness by Pencil Test. Standardization Administration of the People’s Republic of China: Beijing, China, 2022.
- GB/T 4893.9-2013; Test of Surface Coatings of Furniture—Part 9: Determination of Resistance to Impact. Standardization Administration of the People’s Republic of China: Beijing, China, 2013.
- GB/T 4893.4-2023; Test of Surface Coatings of Furniture—Part 4: Determination of Adhesion—Cross Cut. Standardization Administration of the People’s Republic of China: Beijing, China, 2023.
- Han, Y.; Yan, X.X.; Tao, Y. Effect of number of impregnations of Microberlinla sp. with microcapsule emulsion on the performance of self-repairing coatings on wood surfaces. Coatings 2022, 12, 989. [Google Scholar] [CrossRef]
- Varankovich, N.; Martinez, M.F.; Nickerson, M.T.; Korber, D.R. Survival of probiotics in pea protein-alginate microcapsules with or without chitosan coating during storage and in a simulated gastrointestinal environment. Food Sci. Biotechnol. 2017, 26, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, N.; Sargin, I.; Arslan, G.; Arslan, U.; Okudan, A. Ascorbic Acid Adsorption-Release Performance and Antibacterial Activity of Chitosan-ter(GMA-MA-NTBA) Polymer Microcapsules. J. Polym. Environ. 2020, 28, 2277–2288. [Google Scholar] [CrossRef]
- Zhou, J.C.; Xu, W. A fast method to prepare highly isotropic and optically adjustable transparent wood-based composites based on interface optimization. Ind. Crops Prod. 2024, 218, 118898. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.Y. Optical properties and lampshade design applications of PLA 3D printing materials. BioResources 2023, 18, 1545–1553. [Google Scholar] [CrossRef]
- Hu, W.G.; Chen, B.R.; Zhang, T.X. Experimental and numerical studies on mechanical behaviors of beech wood under compressive and tensile states. Wood Res. 2021, 66, 27–37. [Google Scholar] [CrossRef]
- Wang, Y.; Gala, S.; Huang, J.T. Fabrication of Flexible Thin Veneer for Electromagnetic Interference Shielding and Decoration through Simple Electroless Plating. Bioresources 2020, 15, 5737–5748. [Google Scholar] [CrossRef]
- Liu, Q.Q.; Gao, D.; Xu, W. Effect of Paint Process on the Performance of Modified Poplar Wood Antique. Coatings 2021, 11, 1174. [Google Scholar] [CrossRef]
- Feng, D.X.; Zhang, A.H.; Yang, Y.; Yang, P. Coumarin-containing hybrids and their antibacterial activities. Arch. Pharm. 2020, 353, e1900380. [Google Scholar] [CrossRef]




| Test Material | Purity | Manufacturer |
|---|---|---|
| Chitosan with deacetylation degree of 80.0%–95.0% | AR | Shandong Haiyi Chemical Technology Co., Ltd., Binzhou, China |
| Acetic acid | AR | Henan Maigao Chemical Co., Ltd., Zhengzhou, China |
| Tripolyphosphate (TPP) | AR | Sichuan Blue Sword Chemical Group Co., Ltd., Chengdu, China |
| Tween 80 | AR | Jinan HSBC Chemical Co., Ltd., Jinan, China |
| X-100 | AR | Shanghai Maokang Biotechnology Co., Ltd., Shanghai, China |
| NaOH | AR | Shandong Xuanhai Chemical Co., Ltd., Weifang, China |
| Anhydrous ethanol | AR | Jinan Hongrun Chemical Co., Ltd., Jinan, China |
| Nutrient agar medium | - | Huankai Microbial Technology Co., Ltd., Guangzhou, China |
| Nutrient broth | - | Qingdao Haibo Biotechnology Co., Ltd., Qingdao, China |
| Staphylococcus aureus | - | Shanghai Shifeng Biotechnology Co., Ltd., Shanghai, China |
| Escherichia coli | - | Shanghai Shifeng Biotechnology Co., Ltd., Shanghai, China |
| Cleaning agent | AR | Guangdong Baiyun Cleaning Group Co., Ltd., Guangzhou, China |
| Citric acid | AR | Shandong Lemon Biochemical Co., Ltd., Anqiu, China |
| Sample (#) | MT:MC | Chitosan (g) | Toddalia asiatica (L.) Lam Extracts (g) | Ethanol (g) | Tween 80 (g) | X-100 (g) | Deionized Water (g) | TPP (g) | Deionized Water (g) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.0:1 | 0.20 | 0.60 | 11.40 | 0.10 | 1.10 | 38.80 | 0.50 | 9.50 |
| 2 | 3.5:1 | 0.20 | 0.70 | 13.30 | 0.12 | 1.28 | 45.27 | 0.50 | 9.50 |
| 3 | 4.0:1 | 0.20 | 0.80 | 15.20 | 0.13 | 1.47 | 51.73 | 0.50 | 9.50 |
| Microcapsule Content (%) | Quality of Primer (g) | Quality of Microcapsules (g) | Quality of Waterborne Topcoat (g) |
|---|---|---|---|
| 0 | 0.720 | 0 | 0.720 |
| 1.0 | 0.720 | 0.007 | 0.713 |
| 2.0 | 0.720 | 0.015 | 0.705 |
| 3.0 | 0.720 | 0.022 | 0.698 |
| 5.0 | 0.720 | 0.036 | 0.684 |
| 6.0 | 0.720 | 0.043 | 0.677 |
| Level | Changes in Coating on Wood Surface |
|---|---|
| 1 | No visible changes (no damage). |
| 2 | No cracks on the surface of the coating, but visible impact marks. |
| 3 | There are mild cracks on the surface of the coating, usually 1–2 circular or arc cracks. |
| 4 | There are moderate to severe cracks on the surface of the coating, usually 3–4 circular or arc cracks. |
| 5 | The surface of the coating is severely damaged, usually with more than 5 cycles of ring cracks, arc cracks, or coating detachment. |
| Sample (#) | MT:MC | Microcapsule Content (%) | Glossiness (GU) | Light Loss Rate (%) | ||
|---|---|---|---|---|---|---|
| 20° | 60° | 85° | ||||
| No microcapsules | - | 0 | 2.20 | 14.03 | 33.23 | - |
| 1# microcapsules added | 3.0:1 | 1.0 | 1.47 | 8.40 | 17.63 | 40.13 |
| 2.0 | 1.30 | 6.80 | 13.77 | 51.53 | ||
| 3.0 | 1.27 | 6.37 | 9.93 | 54.60 | ||
| 5.0 | 1.13 | 5.77 | 8.33 | 58.87 | ||
| 6.0 | 1.00 | 5.20 | 8.00 | 62.94 | ||
| 2# microcapsules added | 3.5:1 | 1.0 | 1.73 | 8.77 | 17.17 | 37.49 |
| 2.0 | 1.33 | 7.27 | 12.23 | 48.18 | ||
| 3.0 | 1.27 | 6.40 | 10.53 | 54.38 | ||
| 5.0 | 1.07 | 5.47 | 8.90 | 61.01 | ||
| 6.0 | 0.90 | 4.87 | 7.63 | 65.29 | ||
| 3# microcapsules added | 4.0:1 | 1.0 | 1.67 | 8.87 | 19.13 | 36.78 |
| 2.0 | 1.33 | 6.70 | 12.10 | 52.25 | ||
| 3.0 | 1.23 | 6.37 | 11.27 | 54.60 | ||
| 5.0 | 1.10 | 5.30 | 7.43 | 62.22 | ||
| 6.0 | 0.87 | 4.37 | 5.03 | 68.85 | ||
| MT: MC | Microcapsule Content (%) | Chromaticity Parameter | ΔE | |||||
|---|---|---|---|---|---|---|---|---|
| L1 | a1 | b1 | L2 | a2 | b2 | |||
| No microcapsules | 0 | 80.35 | 5.30 | 29.95 | 80.25 | 5.55 | 29.85 | - |
| 3.0:1 | 1.0 | 74.50 | 9.85 | 34.85 | 73.30 | 9.15 | 33.75 | 8.81 |
| 2.0 | 70.85 | 15.40 | 31.50 | 70.15 | 14.90 | 31.35 | 13.90 | |
| 3.0 | 66.55 | 17.70 | 33.40 | 65.50 | 16.05 | 31.25 | 18.52 | |
| 5.0 | 66.20 | 17.70 | 31.75 | 64.20 | 16.95 | 31.70 | 19.34 | |
| 6.0 | 61.90 | 17.25 | 34.75 | 63.85 | 16.60 | 34.20 | 21.37 | |
| 3.5:1 | 1.0 | 73.20 | 10.65 | 26.80 | 72.95 | 10.35 | 25.90 | 9.53 |
| 2.0 | 66.40 | 15.25 | 27.35 | 66.10 | 15.15 | 25.80 | 17.45 | |
| 3.0 | 68.15 | 13.95 | 33.45 | 67.50 | 13.70 | 33.35 | 15.45 | |
| 5.0 | 66.15 | 15.70 | 28.90 | 64.50 | 15.25 | 28.50 | 18.09 | |
| 6.0 | 63.85 | 16.80 | 30.80 | 63.50 | 16.70 | 30.50 | 20.13 | |
| 4.0:1 | 1.0 | 76.35 | 11.25 | 29.30 | 76.15 | 10.55 | 28.95 | 6.86 |
| 2.0 | 65.75 | 15.00 | 26.20 | 65.25 | 14.30 | 24.95 | 17.98 | |
| 3.0 | 67.45 | 14.70 | 32.95 | 66.95 | 13.40 | 32.55 | 15.96 | |
| 5.0 | 61.40 | 18.20 | 32.60 | 61.30 | 18.05 | 31.85 | 22.93 | |
| 6.0 | 61.55 | 18.55 | 31.75 | 60.75 | 18.50 | 30.80 | 23.25 | |
| Microcapsule Content (%) | Reflectance R Value of Surface Coating on Poplar Wood | ||
|---|---|---|---|
| 3.0:1 | 3.5:1 | 4.0:1 | |
| 0 | 0.6193 | 0.6193 | 0.6193 |
| 1.0 | 0.5821 | 0.5703 | 0.5715 |
| 2.0 | 0.5214 | 0.4823 | 0.5091 |
| 3.0 | 0.4938 | 0.4967 | 0.4900 |
| 5.0 | 0.4886 | 0.4853 | 0.4975 |
| 6.0 | 0.4772 | 0.4750 | 0.4758 |
| Sample (#) | MT: MC | Microcapsule Content (%) | Cold Liquid Resistance Level of Surface Coating on Poplar Wood | ||
|---|---|---|---|---|---|
| Citric Acid | Ethanol | Cleaning Agents | |||
| No microcapsules | - | 0 | 2 | 3 | 3 |
| 1# microcapsules added | 3.0:1 | 1.0 | 2 | 2 | 1 |
| 2.0 | 3 | 2 | 1 | ||
| 3.0 | 3 | 2 | 1 | ||
| 5.0 | 4 | 2 | 2 | ||
| 6.0 | 4 | 2 | 2 | ||
| 2# microcapsules added | 3.5:1 | 1.0 | 2 | 2 | 1 |
| 2.0 | 2 | 2 | 1 | ||
| 3.0 | 3 | 2 | 1 | ||
| 5.0 | 4 | 2 | 2 | ||
| 6.0 | 4 | 2 | 2 | ||
| 3# microcapsules added | 4.0:1 | 1.0 | 2 | 2 | 1 |
| 2.0 | 2 | 2 | 2 | ||
| 3.0 | 3 | 2 | 2 | ||
| 5.0 | 4 | 2 | 2 | ||
| 6.0 | 4 | 2 | 2 | ||
| Microcapsule Content (%) | Hardness | ||
|---|---|---|---|
| 3.0:1 | 3.5:1 | 4.0:1 | |
| 0 | B | B | B |
| 1.0 | 2B | 2B | B |
| 2.0 | 2B | 2B | B |
| 3.0 | B | B | B |
| 5.0 | HB | B | HB |
| 6.0 | HB | HB | H |
| Microcapsule Content (%) | Impact Resistance Level | ||
|---|---|---|---|
| 3.0:1 | 3.5:1 | 4.0:1 | |
| 0 | 5 | 5 | 5 |
| 1.0 | 4 | 4 | 4 |
| 2.0 | 4 | 4 | 4 |
| 3.0 | 4 | 4 | 4 |
| 5.0 | 3 | 3 | 3 |
| 6.0 | 3 | 3 | 3 |
| Microcapsule Content (%) | Adhesion Level | ||
|---|---|---|---|
| 3.0:1 | 3.5:1 | 4.0:1 | |
| 0 | 0 | 0 | 0 |
| 1.0 | 1 | 1 | 1 |
| 2.0 | 1 | 1 | 1 |
| 3.0 | 1 | 1 | 1 |
| 5.0 | 1 | 2 | 2 |
| 6.0 | 1 | 2 | 2 |
| Microcapsule Content (%) | Roughness (µm) | ||
|---|---|---|---|
| 3.0:1 | 3.5:1 | 4.0:1 | |
| 0 | 0.260 | 0.260 | 0.260 |
| 1.0 | 0.369 | 0.743 | 0.439 |
| 2.0 | 1.336 | 0.945 | 0.756 |
| 3.0 | 1.566 | 1.464 | 1.190 |
| 5.0 | 2.073 | 1.610 | 2.022 |
| 6.0 | 2.713 | 1.959 | 2.825 |
| MT:MC | Microcapsule Content (%) | Average Number of Recovered Escherichia coli (CFU·piece−1) | Average Number of Recovered Staphylococcus aureus (CFU·piece−1) | Antibacterial Rate of Escherichia coli (%) | Antibacterial Rate of Staphylococcus aureus (%) |
|---|---|---|---|---|---|
| No microcapsules | 0 | 371 | 389 | - | - |
| 3.0:1 | 1.0 | 186 | 183 | 49.87 | 52.96 |
| 2.0 | 153 | 135 | 58.76 | 65.30 | |
| 3.0 | 144 | 84 | 61.19 | 78.41 | |
| 5.0 | 93 | 79 | 74.93 | 79.69 | |
| 6.0 | 88 | 65 | 76.28 | 83.29 | |
| 3.5:1 | 1.0 | 194 | 172 | 47.71 | 55.78 |
| 2.0 | 148 | 127 | 60.11 | 67.35 | |
| 3.0 | 125 | 119 | 66.31 | 69.41 | |
| 5.0 | 109 | 96 | 70.62 | 75.32 | |
| 6.0 | 98 | 82 | 73.58 | 78.92 | |
| 4.0:1 | 1.0 | 179 | 150 | 51.75 | 61.44 |
| 2.0 | 135 | 124 | 63.61 | 68.12 | |
| 3.0 | 126 | 74 | 66.04 | 80.98 | |
| 5.0 | 96 | 70 | 74.12 | 82.01 | |
| 6.0 | 79 | 68 | 78.71 | 82.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y.; Wang, Y.; Yan, X. Effect of Microcapsules of Chitosan-Coated Toddalia asiatica (L.) Lam Extracts on the Surface Coating Properties of Poplar Wood. Coatings 2024, 14, 1013. https://doi.org/10.3390/coatings14081013
Zhu Y, Wang Y, Yan X. Effect of Microcapsules of Chitosan-Coated Toddalia asiatica (L.) Lam Extracts on the Surface Coating Properties of Poplar Wood. Coatings. 2024; 14(8):1013. https://doi.org/10.3390/coatings14081013
Chicago/Turabian StyleZhu, Ye, Ying Wang, and Xiaoxing Yan. 2024. "Effect of Microcapsules of Chitosan-Coated Toddalia asiatica (L.) Lam Extracts on the Surface Coating Properties of Poplar Wood" Coatings 14, no. 8: 1013. https://doi.org/10.3390/coatings14081013




