Effects of ALD Deposition Cycles of Al2O3 on the Morphology and Performance of FTO-Based Dye-Sensitized Solar Cells
Abstract
:1. Introduction
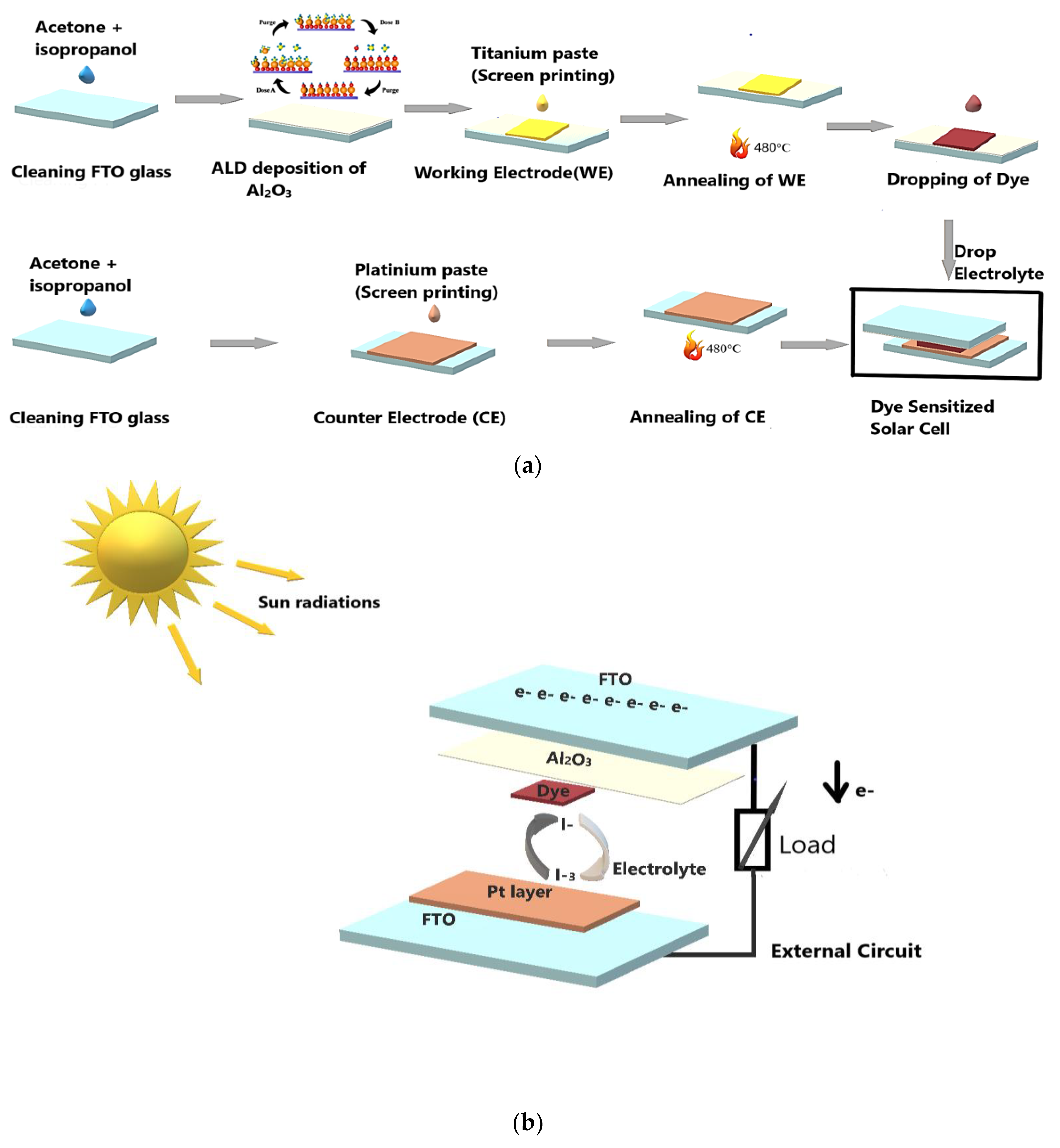
2. Materials and Methods
3. Results and Discussion
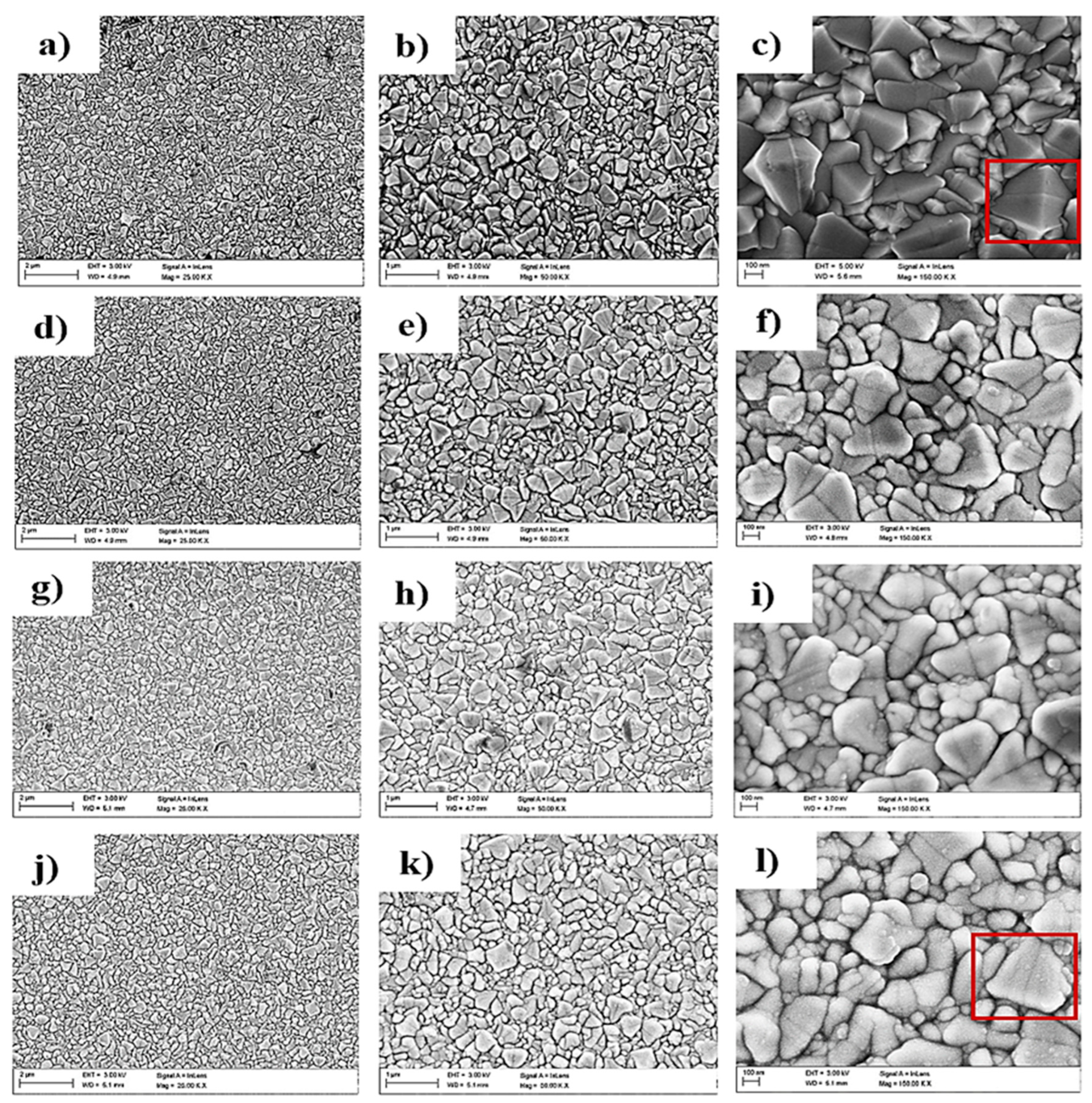
3.1. Surface Morphology
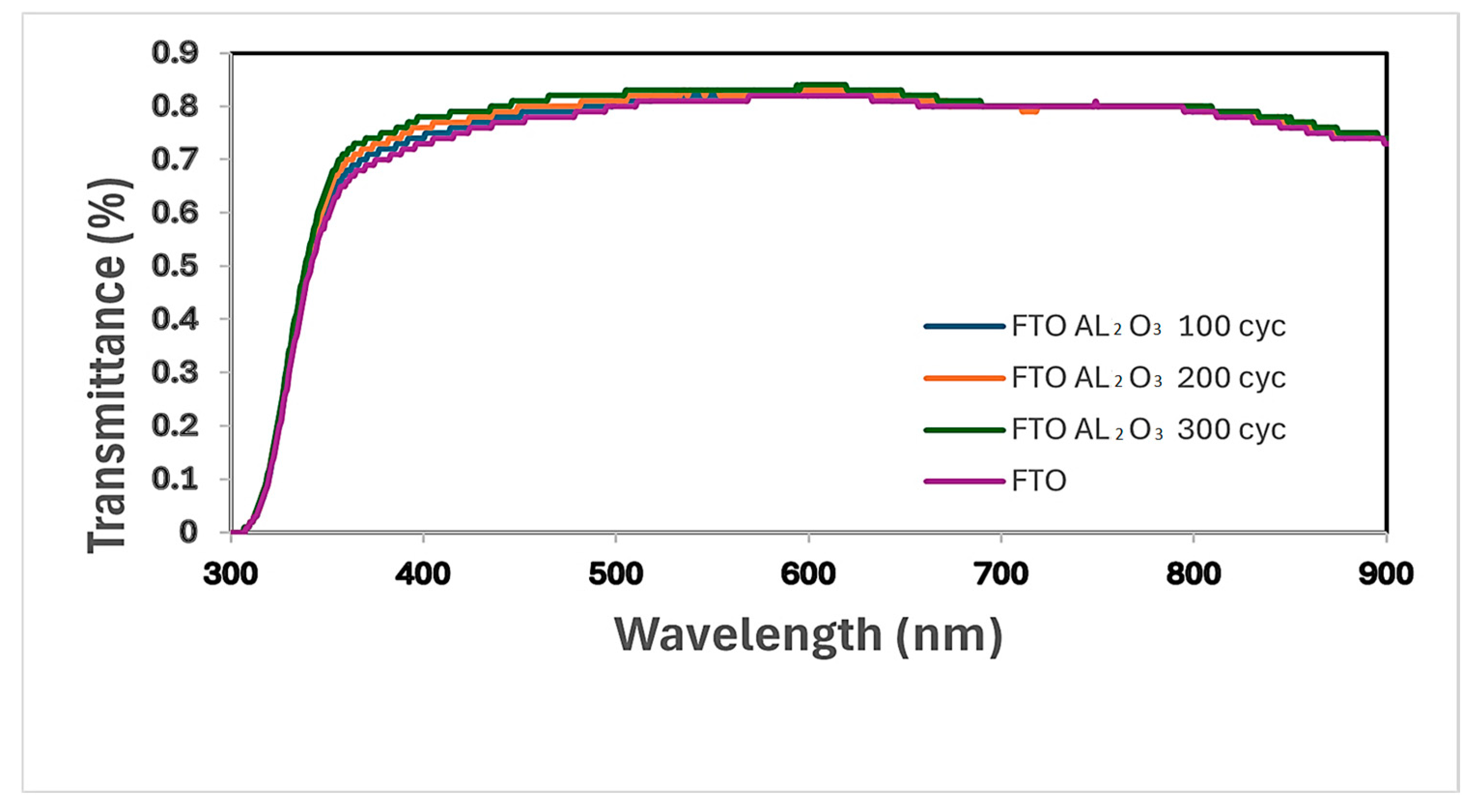
3.2. Light Transmittance Analysis
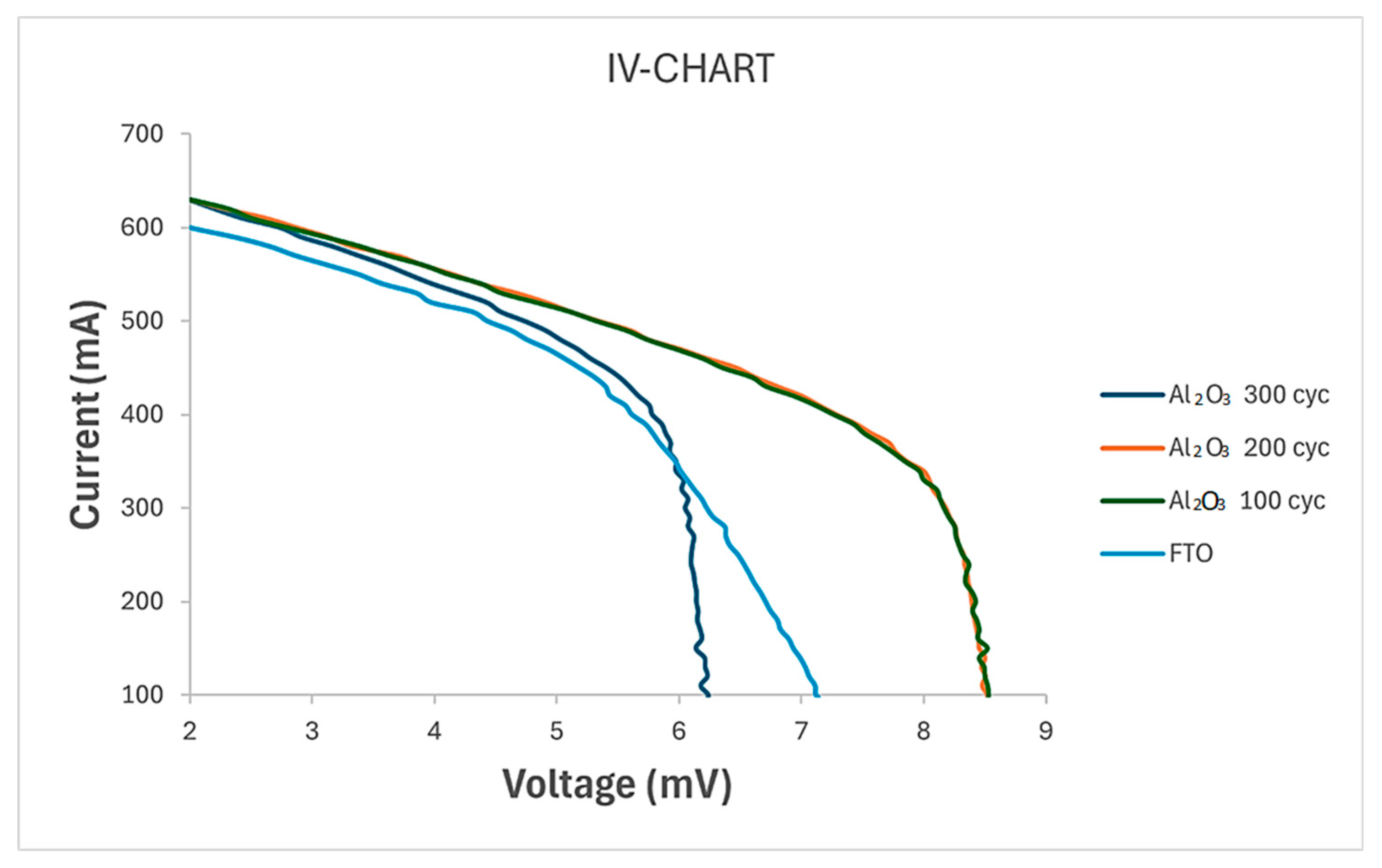
3.3. Solar Simulation Analysis and Electrical Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Al2O3 | Aluminum oxide |
| ALD | Atomic layer deposition |
| DSSCs | Dye-sensitized solar cells |
| FTO | Fluorine Tin Oxide |
| IPA | Isopropyl alcohol |
| Pt | Platinum |
| TBA+ | Tetrabutylammonium ions |
| TCOs | Transparent Conducting Oxides |
| TiO2 | Titanium dioxide |
| TMA | Trimethylaluminum |
References
- Kumavat, P.P.; Sonar, P.; Dalal, D.S. An Overview on Basics of Organic and Dye Sensitized Solar Cells, Their Mechanism and Recent Improvements. Renew. Sustain. Energy Rev. 2017, 78, 1262–1287. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-Sensitized Solar Cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- O’Regan, B.; Grätzel, M. A Low-Cost, High-Efficiency Solar Cell Based on Dye-Sensitized Colloidal TiO2 Films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- McConnell, R.D. Assessment of the Dye-Sensitized Solar Cell. Renew. Sustain. Energy Rev. 2002, 6, 271–293. [Google Scholar] [CrossRef]
- Mehmood, U.; Rahman, S.; Harrabi, K.; Hussein, I.; Reddy, B. Recent Advances in Dye Sensitized Solar Cells. Adv. Mater. Sci. Eng. 2014, 2014, 974782. [Google Scholar] [CrossRef]
- Sci, A.; Tsegaye, A.; Demiss, S. Review on Dye-Sensitized Solar Cells (DSSCs). Appl. Sci. 2018, 2, 130. [Google Scholar] [CrossRef]
- Grant, F.A. Properties of Rutile (Titanium Dioxide). Rev. Mod. Phys. 1959, 31, 646–674. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Grätzel, M. Heterogeneous Photocatalysis with Semiconductor Particulate Systems. In Chemistry and Physics of Solid Surfaces V; Vanselow, R., Howe, R., Eds.; Springer: Berlin/Heidelberg, Germany, 1984; pp. 111–139. ISBN 978-3-642-82253-7. [Google Scholar]
- Oskam, G.; Bergeron, B.; Meyer, G.; Searson, P. Pseudohalogens for Dye-Sensitized TiO2 Photoelectrochemical Cells. J. Phys. Chem. B 2001, 105, 6867–6873. [Google Scholar] [CrossRef]
- Nusbaumer, H.; Moser, J.-E.; Zakeeruddin, S.; Nazeeruddin, M.; Graetzel, M. Co II (Dbbip)22+ Complex Rivals Triiodide/Iodide Redox Mediator in Dye-Sensitized Photovoltaic Cells. J. Phys. Chem. B 2001, 105, 10461–10464. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Shi, D.; Zhang, J.; Wang, M.; Jing, X.; Humphry-Baker, R.; Wang, P.; Zakeeruddin, S.M.; Grätzel, M. Enhance the Optical Absorptivity of Nanocrystalline TiO2 Film with High Molar Extinction Coefficient Ruthenium Sensitizers for High Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2008, 130, 10720–10728. [Google Scholar] [CrossRef]
- Wisz, G.; Sawicka-Chudy, P.; Sibiński, M.; Yavorskyi, R.; Łabuz, M.; Płoch, D.; Bester, M. Formation and Characterization of Stable TiO2/CuxO-Based Solar Cells. J. Mater. 2023, 16, 5683. [Google Scholar] [CrossRef] [PubMed]
- Chavan, G.T.; Kim, Y.; Khokhar, M.Q.; Hussain, S.Q.; Cho, E.-C.; Yi, J.; Ahmad, Z.; Rosaiah, P.; Jeon, C.-W. A Brief Review of Transparent Conducting Oxides (TCO): The Influence of Different Deposition Techniques on the Efficiency of Solar Cells. Nanomaterials 2023, 13, 1226. [Google Scholar] [CrossRef] [PubMed]
- Miettunen, K.; Asghar, I.; Mastroianni, S.; Halme, J.; Barnes, P.R.F.; Rikkinen, E.; O’Regan, B.C.; Lund, P. Effect of Molecular Filtering and Electrolyte Composition on the Spatial Variation in Performance of Dye Solar Cells. J. Electroanal. Chem. 2012, 664, 63–72. [Google Scholar] [CrossRef]
- Kasimakhunova, A.M.; Olimov, S.A.; Nurdinova, R.; Iqbal, T.; Mamadalieva, L.K. Highly Efficient Conversion of Solar Energy by the Photoelectric Converter and a Thermoelectric Converter. J. Appl. Math. Phys. 2018, 06, 520–529. [Google Scholar] [CrossRef]
- Graetzel, M.; Moser, J.-E. Solar Energy Conversion. In Electron Transfer in Chemistry; Wiley-VCH: Weiheim, Germany, 2008; pp. 588–644. ISBN 978-3-527-61824-8. [Google Scholar]
- Nazeeruddin, M.K.; Kay, A.; Rodicio, I.; Humphry-Baker, R.; Mueller, E.; Liska, P.; Vlachopoulos, N.; Graetzel, M. Conversion of Light to Electricity by Cis-X2bis(2,2′-Bipyridyl-4,4′-Dicarboxylate)Ruthenium(II) Charge-Transfer Sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on Nanocrystalline Titanium Dioxide Electrodes. J. Am. Chem. Soc. 1993, 115, 6382–6390. [Google Scholar] [CrossRef]
- Fung, A.K.M.; Chiu, B.K.W.; Lam, M.H.W. Surface Modification of TiO2 by a Ruthenium(II) Polypyridyl Complex via Silyl-Linkage for the Sensitized Photocatalytic Degradation of Carbon Tetrachloride by Visible Irradiation. Water Res. 2003, 37, 1939–1947. [Google Scholar] [CrossRef]
- Wang, P.; Zakeeruddin, S.M.; Moser, J.E.; Nazeeruddin, M.K.; Sekiguchi, T.; Grätzel, M. A Stable Quasi-Solid-State Dye-Sensitized Solar Cell with an Amphiphilic Ruthenium Sensitizer and Polymer Gel Electrolyte. Nat. Mater. 2003, 2, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Senthil, T.S. Effect of Surface Morphology on the Performance of Natural Dye Sensitized TiO2 Thin Film Solar Cell. Adv. Mater. Res. 2013, 678, 326–330. [Google Scholar] [CrossRef]
- Eftekhari, L.; Ghasemi, M. Analysing the Surface Morphology of Annealed FTO/ZnS Bilayer Films: Stereometric, Fractal, and Wettability Approaches. Sci. Rep. 2024, 14, 14262. [Google Scholar] [CrossRef]
- Abedi, H.R.; Hoseinpour Gollo, M. An Experimental Study of the Effects of Surface Roughness and Coating of Cr2O3 Layer on the Laser-Forming Process. Opt. Laser Technol. 2019, 109, 336–347. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, D.-E. Effect of Surface Roughness of Top Cover Layer on the Efficiency of Dye-Sensitized Solar Cell. Sol. Energy 2012, 86, 2049–2055. [Google Scholar] [CrossRef]
- Optical Scattering and Surface Roughness—Eckhardt Optics. Available online: https://www.eckop.com/resources/scatterometer-resources/optical-scattering-versus-surface-roughness/ (accessed on 28 May 2024).
- Lee, K.-M.; Suryanarayanan, V.; Ho, K.-C. The Influence of Surface Morphology of TiO2 Coating on the Performance of Dye-Sensitized Solar Cells. Sol. Energy Mater. Sol. Cells 2006, 90, 2398–2404. [Google Scholar] [CrossRef]
- Tseng, T.-L.; Konada, U.; Kwon, Y. A Novel Approach to Predict Surface Roughness in Machining Operations Using Fuzzy Set Theory. J. Comput. Des. Eng. 2016, 3, 1–13. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, F.; Meng, W.; Huang, Y. Plasmonic Nanoparticles Enhanced Dye-Sensitized Solar Cells. In Proceedings of the SPIE Micro+Nano Materials, Devices, and Applications, Devices, VIC, Australia, 8–11 December 2013; SPIE—The International Society for Optical Engineering: Bellingham, WA, USA, 2013; Volume 8923. [Google Scholar] [CrossRef]
- Yang, R.-Y.; Chen, H.-Y.; Lai, F.-D. Performance Degradation of Dye-Sensitized Solar Cells Induced by Electrolytes. Adv. Mater. Sci. Eng. 2012, 902146. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.H.; Kim, C.S.; Choi, H.W. Achieving Enhanced Dye-Sensitized Solar Cell Performance by TiCl4/Al2O3 Doped TiO2 Nanotube Array Photoelectrodes. J. Nanomater. 2015, 2015, e545818. [Google Scholar] [CrossRef]
- Zhang, H.; Banfield, J. Understanding Polymorphic Phase Transformation Behavior During Growth of Nanocrystalline Aggregates: Insights from TiO2. J. Phys. Chem. B 2000, 104, 3481–3487. [Google Scholar] [CrossRef]
- Ren, F. Alumina Coatings on Fluorine-Doped Tin Oxide Titanium Dioxide as Photoanode for Dye-Sensitized Solar Cells. Electrochim. Acta 2015, 173, 534–539. [Google Scholar]
- Roelofs, K.; Brennan, T.; Dominguez, J.; Bailie, C.; Margulis, G.; Hoke, E.; Mcgehee, M.; Bent, S. Effect of Al2O3 Recombination Barrier Layers Deposited by Atomic Layer Deposition in Solid-State CdS Quantum Dot-Sensitized Solar Cells. J. Phys. Chem. C 2013, 117, 5584–5592. [Google Scholar] [CrossRef]
- Wu, M.-S.; Tsai, C.-H.; Jow, J.-J.; Wei, T. Enhanced Performance of Dye-Sensitized Solar Cell via Surface Modification of Mesoporous TiO2 Photoanode with Electrodeposited Thin TiO2 Layer. Electrochim. Acta 2011, 56, 8906–8911. [Google Scholar] [CrossRef]
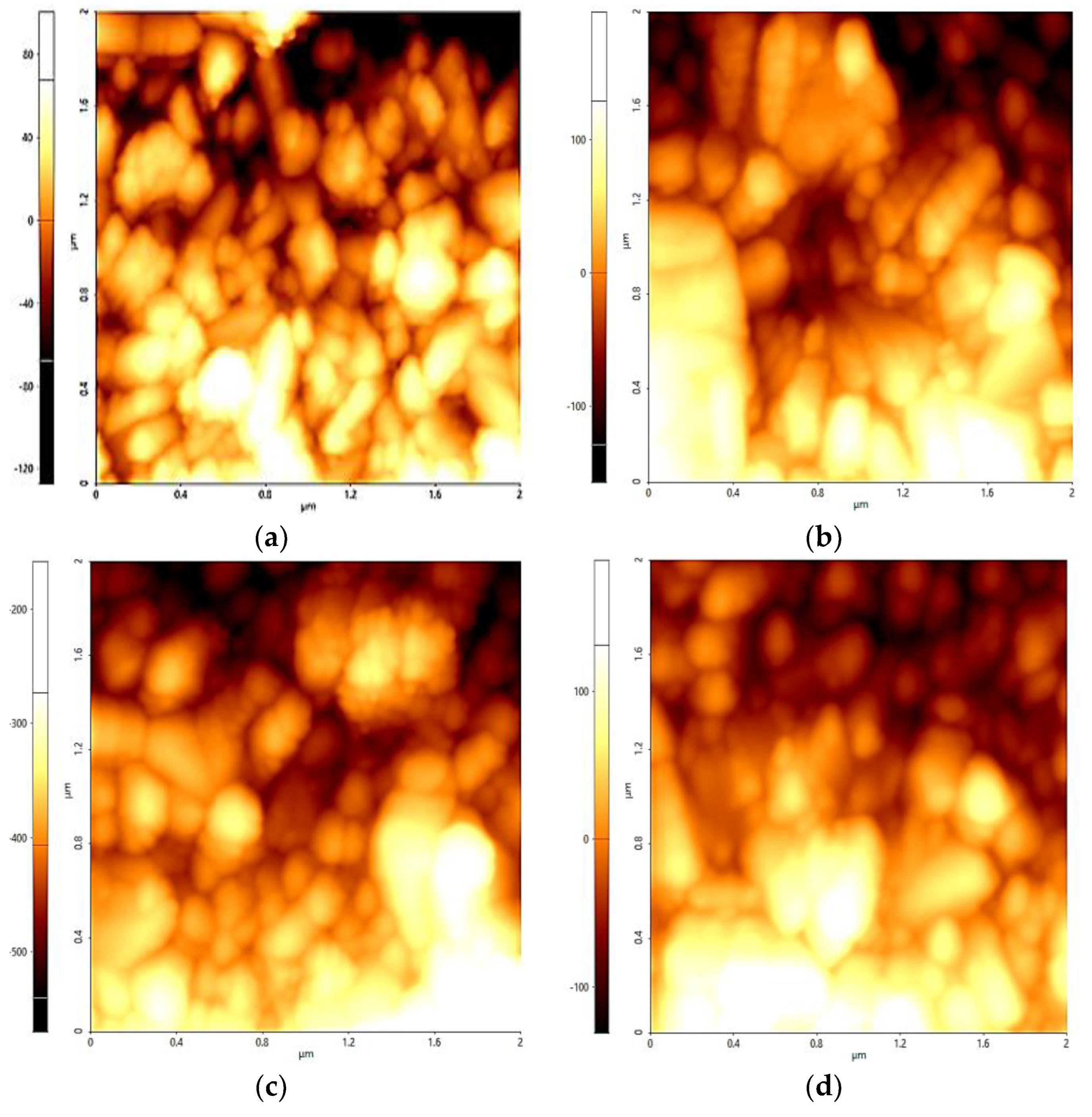
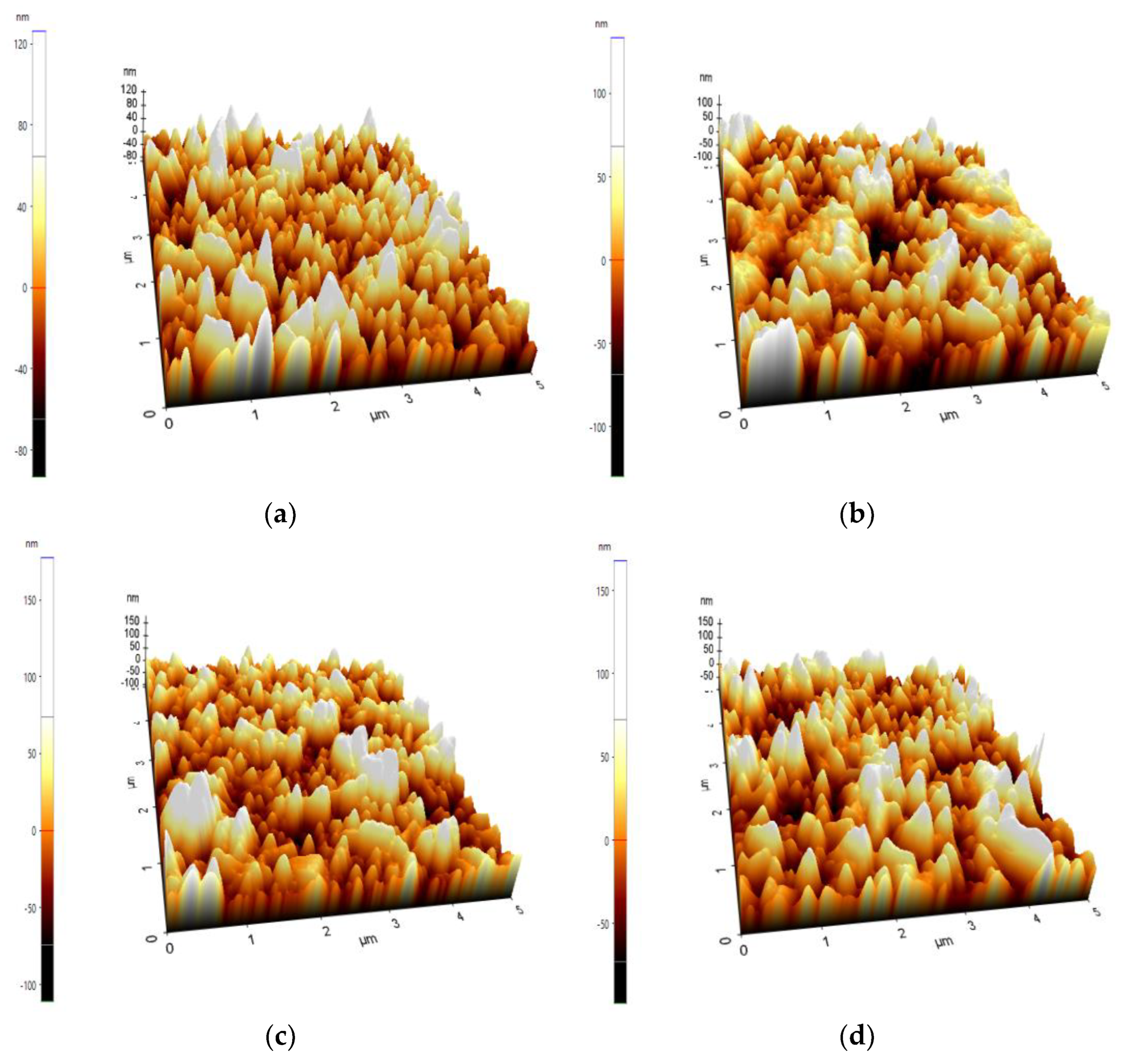
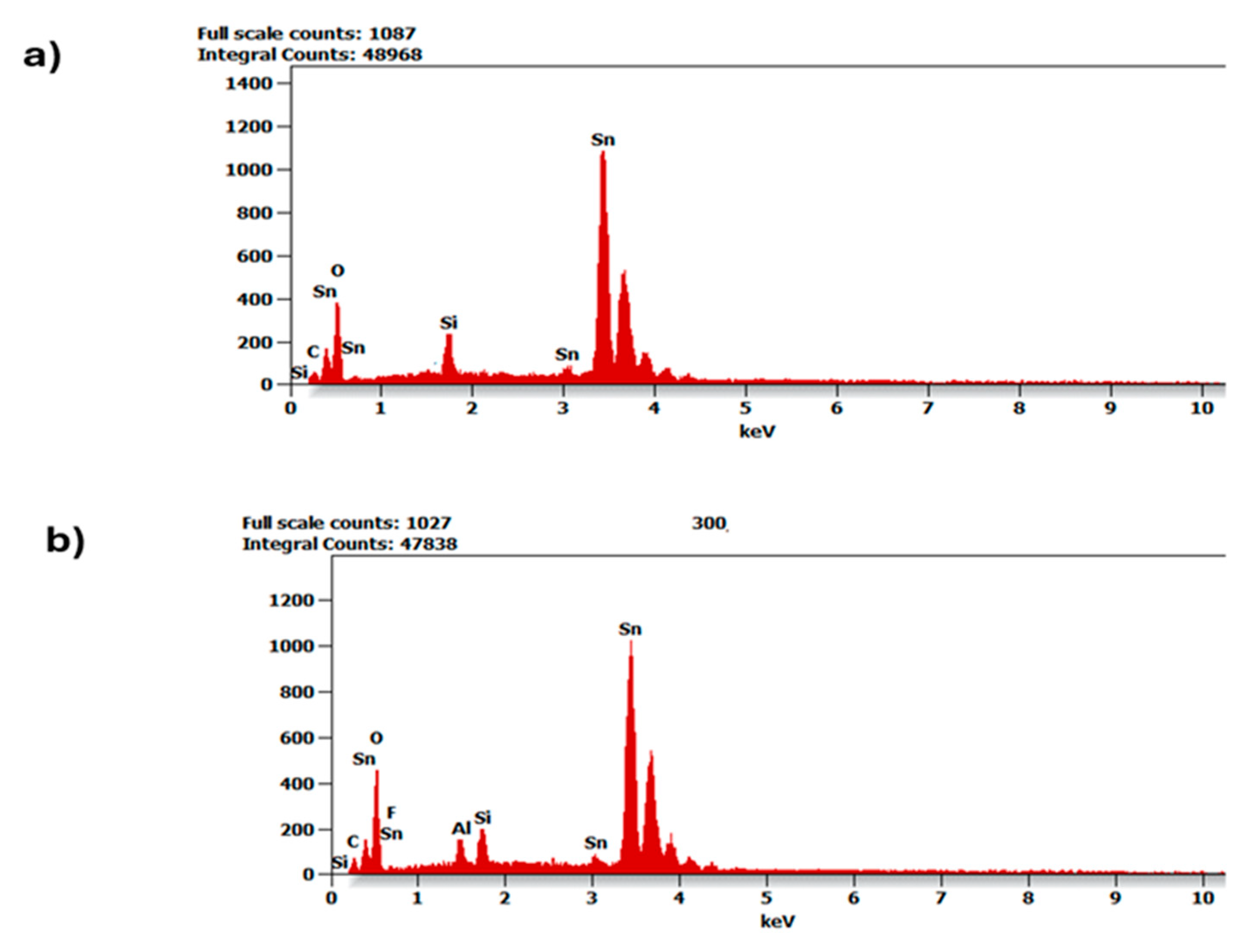
| Region Whole (µm) | Max (nm) | Min (nm) | Rq (nm) | Ra (nm) |
|---|---|---|---|---|
| Pure FTO (2x2) | 17.42 | −3.06 | 1.65 | 0.97 |
| FTO-Al2O3 100 cycles 2x2 | 125.30 | −114.51 | 41.16 | 33.98 |
| FTO-Al2O3 200 cycles 2x2 | 122.55 | −114.21 | 41.54 | 34.26 |
| FTO-Al2O3 300 cycles 2x2 | 115.02 | −95.67 | 30.58 | 23.92 |
| Sample | Isc (mA) | Voc (mV) | Imax (mA) | Vmax (mV) | Pmax (mW) | FF (-) | Efficiency (%) |
|---|---|---|---|---|---|---|---|
| Pure FTO | 7.604 | 629.999 | 5.203 | 447.278 | 2.327 | 0.49 | 3.43 |
| FTO-Al2O3 100 cycles | 8.594 | 629.622 | 6.988 | 416.957 | 2.914 | 0.54 | 4.27 |
| FTO-Al2O3 200 cycles | 8.629 | 629.618 | 7.017 | 418.249 | 2.935 | 0.54 | 4.31 |
| FTO-Al2O3 300 cycles | 6.382 | 630.025 | 5.317 | 457.963 | 2.435 | 0.61 | 3.87 |
| Sample | Sheet Resistance (Ω/sq) | Resistivity (nΩ.m) | Conductivity (MS/m) | Outer Probe Voltage (mV) | Outer Probe Current (mA) | Inner Probe Voltage (mV) |
|---|---|---|---|---|---|---|
| Pure FTO | 8.0078 | 800.78 | 1.2488 | −486.49 | −12.134 | −21.84 |
| FTO-Al2O3 100 cycles | 8.0243 | 802.43 | 1.2464 | −487.65 | −11.051 | −19.931 |
| FTO-Al2O3 200 cycles | 7.6176 | 761.176 | 1.3127 | −284.73 | −13.61 | −23.302 |
| FTO-Al2O3 300 cycles | 8.0300 | 803.00 | 1.2453 | −386.64 | −11.971 | −21.606 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Addae, E.A.; Sitek, W.; Szindler, M.; Fijalkowski, M.; Matus, K. Effects of ALD Deposition Cycles of Al2O3 on the Morphology and Performance of FTO-Based Dye-Sensitized Solar Cells. Coatings 2024, 14, 1019. https://doi.org/10.3390/coatings14081019
Addae EA, Sitek W, Szindler M, Fijalkowski M, Matus K. Effects of ALD Deposition Cycles of Al2O3 on the Morphology and Performance of FTO-Based Dye-Sensitized Solar Cells. Coatings. 2024; 14(8):1019. https://doi.org/10.3390/coatings14081019
Chicago/Turabian StyleAddae, Elizabeth Adzo, Wojciech Sitek, Marek Szindler, Mateusz Fijalkowski, and Krzysztof Matus. 2024. "Effects of ALD Deposition Cycles of Al2O3 on the Morphology and Performance of FTO-Based Dye-Sensitized Solar Cells" Coatings 14, no. 8: 1019. https://doi.org/10.3390/coatings14081019






