Effect of Sulfuric Acid Immersion on Electrical Insulation and Surface Composition of Amorphous Carbon Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition Conditions for Amorphous Carbon Films
2.2. Spectroscopic Ellipsometric Analysis
2.3. Sulfuric Acid Immersion Conditions
2.4. Surface Morphology Analysis
2.5. Structural Analysis
2.6. Surface Composition Analysis
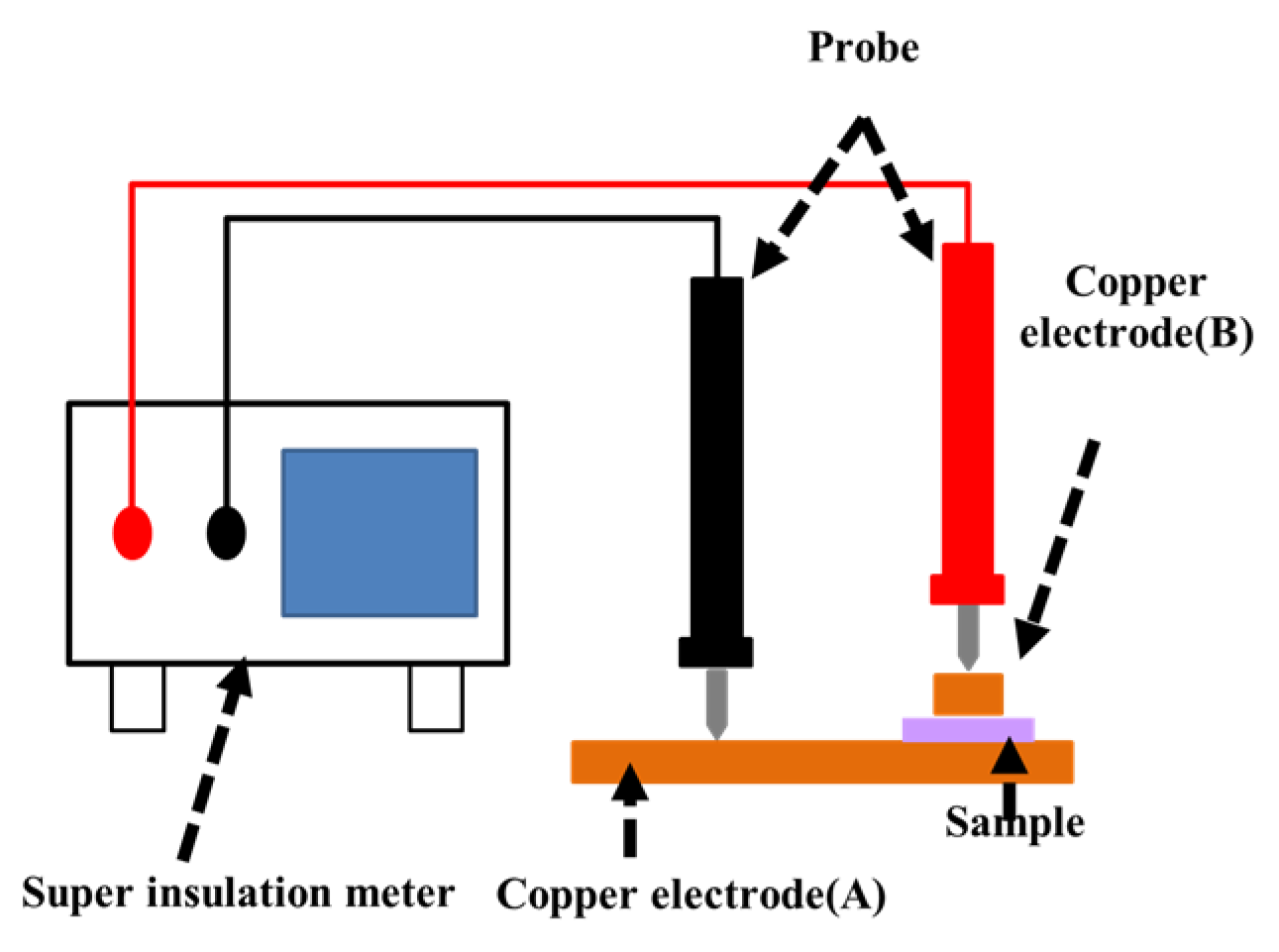
2.7. Electrical Insulation Evaluation
3. Results
3.1. Classification of Amorphous Carbon Films Based on Optical Constants
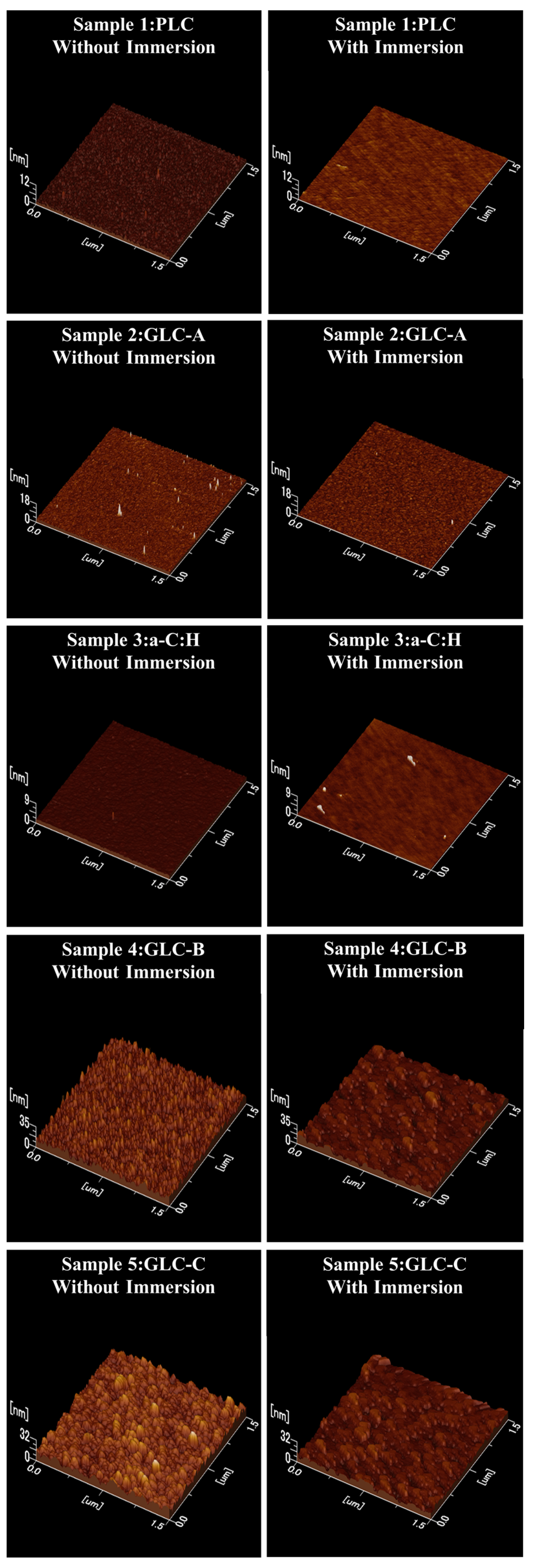
3.2. Surface Morphology of Amorphous Carbon before and after Sulfuric Acid Immersion
3.3. Effect of Sulfuric Acid Immersion on the Structure of Amorphous Carbon Films
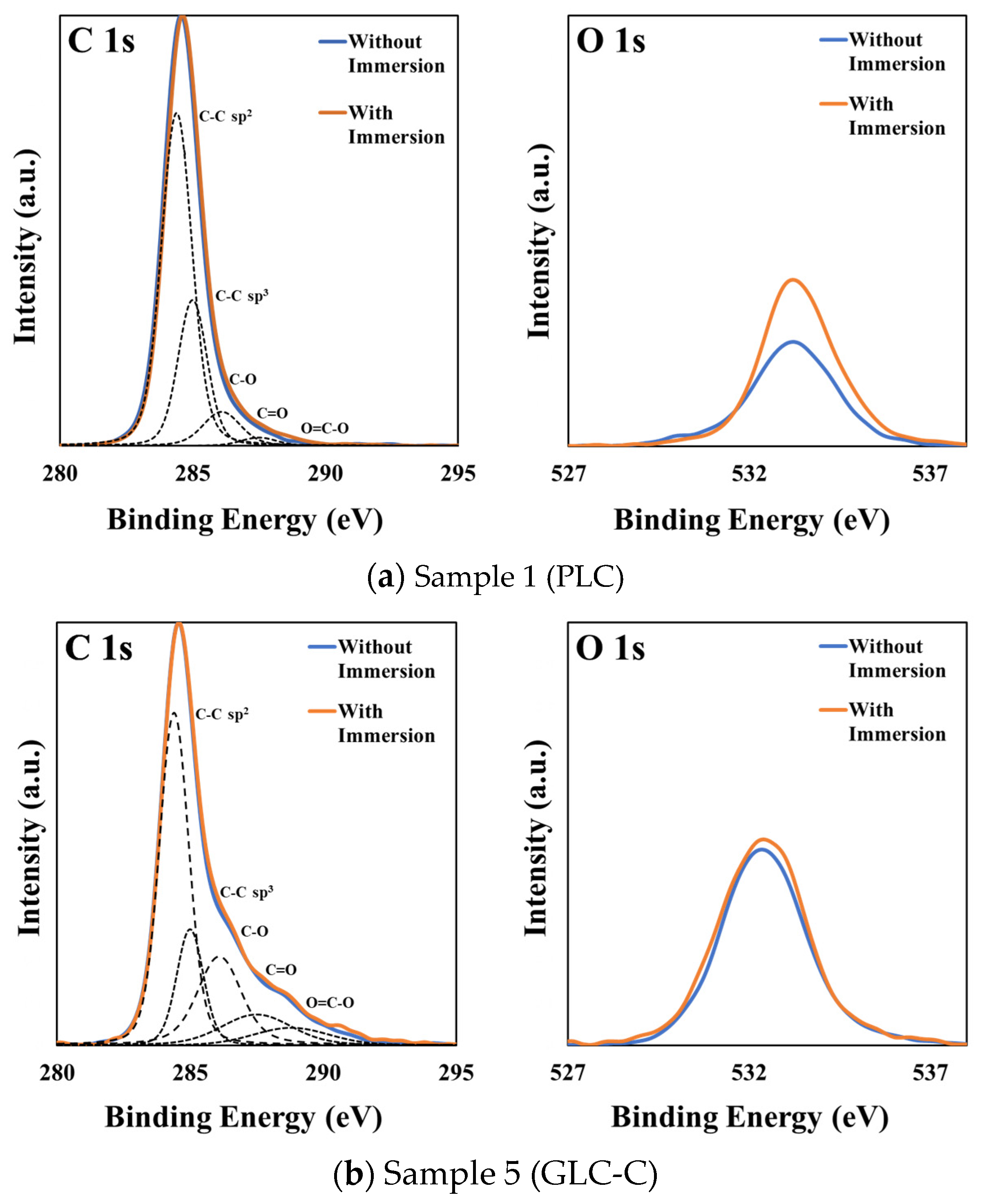
3.4. Effect of Sulfuric Acid Immersion on the Surface Composition of Amorphous Carbon Films
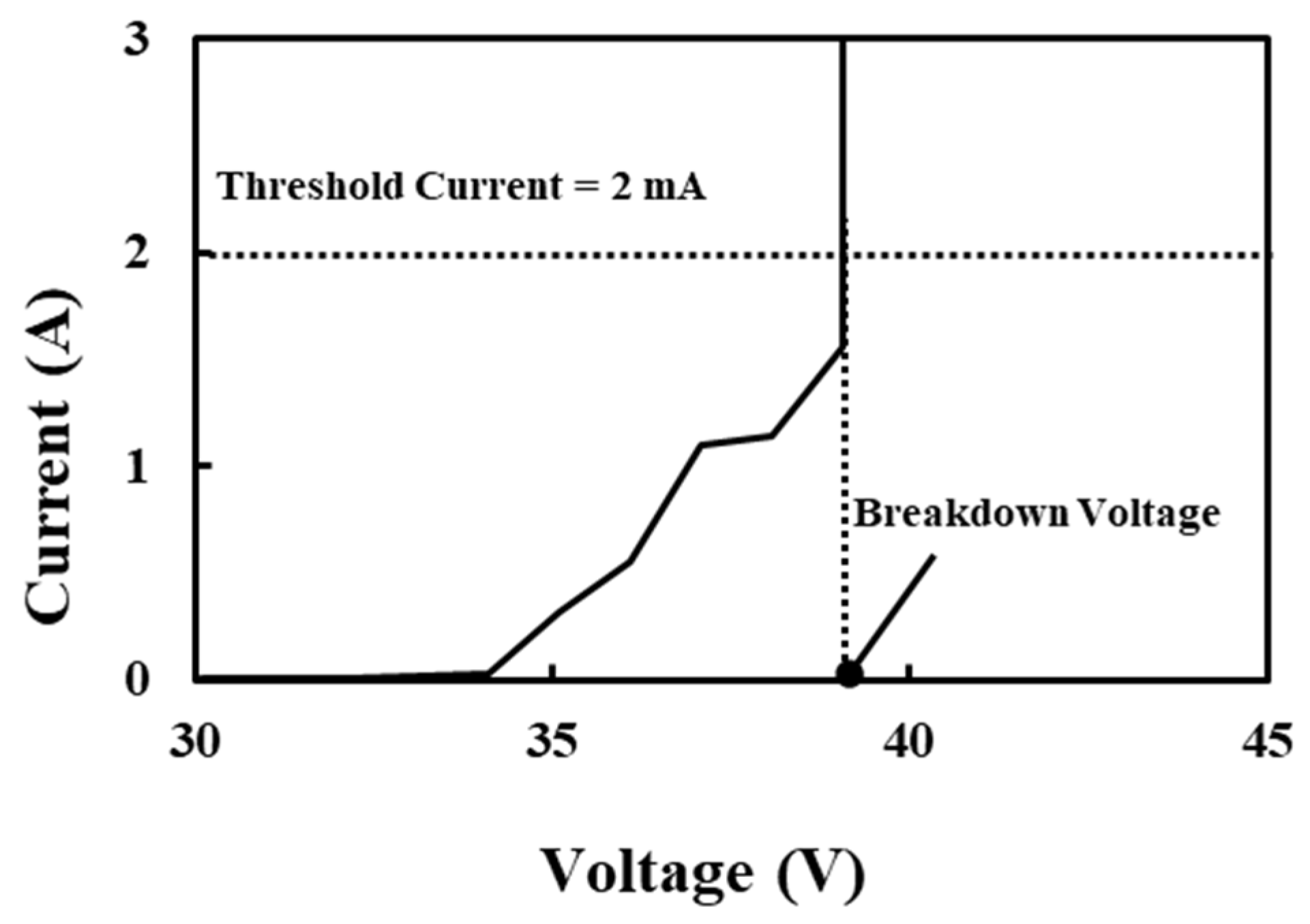
3.5. Effect of Sulfuric Acid Immersion on the Electrical Insulation Properties of Amorphous Carbon Films
3.6. Optical Classification of Amorphous Carbon Films According to Their Electrical Insulation Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Bewilogua, K.; Hofmann, D. History of diamond-like carbon films—From first experiments to worldwide applications. Surf. Coat. Technol. 2014, 242, 214–225. [Google Scholar] [CrossRef]
- ISO 23216:2021(E); Carbon Based Films—Determination of Optical Properties of Amorphous Carbon Films by Spectroscopic Ellipsometry. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/74875.html (accessed on 22 August 2022).
- Kanasugi, K.; Arimura, K.; Alanazi, A.; Ohgoe, Y.; Manome, Y.; Hiratsuka, M.; Hirakuri, K. UV sterilization effects and osteoblast proliferation on amorphous carbon films classified based on optical constants. Bioeng 2022, 9, 505. [Google Scholar] [CrossRef] [PubMed]
- Reimi, M.; Akio, N.; Ryota, Y.; Junji, T.; Keiji, K.; Hidetoshi, S. Optical constants of diamond-like carbon films formed by gas cluster ion beam assisted deposition. Appl. Phys. Exp. 2022, 15, 035501. [Google Scholar] [CrossRef]
- Nakajima, D.; Kuwabara, H.; Annaka, S.; Fujii, S.; Tanaka, Y.; Hirakuri, K. Diamond-like carbon coating for effective electrical insulation of Cu and Al wires. Diam. Relat. Mater. 2020, 103, 107731. [Google Scholar] [CrossRef]
- Nakamura, K.; Sato, Y.; Takase, T. Surface oxidation and/or corrosion behavior of vapor-grown carbon fibers (VGCFs) in nitric acid, hydrogen peroxide, and sulfuric acid solutions. Diam. Relat. Mater. 2017, 76, 108–114. [Google Scholar] [CrossRef]
- Nakamura, K.; Morooka, H.; Tanabe, Y.; Yasuda, E.; Akatsu, T.; Shindo, H. Surface oxidation and/or corrosion behavior of glass-like carbon in sulfuric and nitric acids, and in aqueous hydrogen peroxide. Corros. Sci. 2011, 53, 4010–4013. [Google Scholar] [CrossRef]
- Song, J.; Tian, H.; Li, J.; Zhang, P.; Sun, A. Effect of methane and acetylene pre-gases on the corrosion resistance of DLC coatings. Vacuum 2024, 221, 112899. [Google Scholar] [CrossRef]
- Nitta, Y.; Okamoto, K.; Nakatani, T.; Hoshi, H.; Homma, A.; Tatsumi, E.; Taenaka, Y. Diamond-like carbon thin film with controlled zeta potential for medical material application. Diam. Relat. Mater. 2008, 17, 1972–1976. [Google Scholar] [CrossRef]
- Zhou, X.; Tunmee, S.; Suzuki, T.; Phothongkam, P.; Kanda, K.; Komatsu, K.; Kawahara, S.; Ito, H.; Saitoh, H. Quantitative NEXAFS and solid-state NMR studies of sp 3 /(sp 2 + sp 3) ratio in the hydrogenated DLC films. Diam. Relat. Mater. 2017, 73, 232–240. [Google Scholar] [CrossRef]
- Moriguchi, H.; Ohara, H.; Tsujioka, M. History and applications of diamond-like carbon manufacturing processes. Sei Technol. Rev. 2016, 82, 52–55. [Google Scholar]
- Harigai, T.; Yasuoka, Y.; Nitta, N.; Furuta, H.; Hatta, A. X-ray reflectivity analysis on initial stage of diamond-like carbon film deposition on Si substrate by RF plasma CVD and on removal of the sub-surface layer by oxygen plasma etching. Diam. Relat. Mater. 2013, 38, 36–40. [Google Scholar] [CrossRef]
- Ding, X.L.; Li, Q.S.; Kong, X.H. Optical and electrical properties evolution of diamond-like carbon thin films with deposition temperature. Chin. Phys. Lett. 2009, 26, 027802. [Google Scholar] [CrossRef]
- Tomidokoro, M.; Tunmee, S.; Rittihong, U.; Euaruksakul, C.; Supruangnet, R.; Nakajima, H.; Hirata, Y.; Ohtake, N.; Akasaka, H. Electrical Conduction Properties of Hydrogenated Amorphous Carbon Films with Different Structures. Materials 2021, 14, 2355. [Google Scholar] [CrossRef] [PubMed]




| Sample No. | Deposition Method | Precursor | Substrate Bias Voltage [kV] | Target Voltage [kV] | |
|---|---|---|---|---|---|
| 1 | CVD | RF-PCVD | hydrogenated gas (C6H6) | 1.5 | - |
| 2 | Pulsed DC-PCVD | hydrogenated gas (C6H6) | 1.5 | - | |
| 3 | PVD | Ionized evaporation | hydrogenated gas (C6H6) | 1.0 | - |
| 4 | HiPIMS | graphite solid target + Ar | - | 0.75 | |
| 5 | HiPIMS | graphite solid target + Ar | −0.05 | 0.90 | |
| Sample No. | χ2 | Film Thickness (nm) | n | k | Type | Optical Surface Roughness (nm) |
|---|---|---|---|---|---|---|
| λ = 550 nm | ||||||
| 1 | 0.19 | 90 | 1.94 | 0.04 | PLC | 11 |
| 2 | 0.16 | 129 | 2.02 | 0.28 | GLC-A | 4 |
| 3 | 0.12 | 107 | 2.25 | 0.33 | a-C:H | 19 |
| 4 | 0.06 | 197 | 1.70 | 0.39 | GLC-B | 17 |
| 5 | 0.19 | 170 | 1.91 | 0.47 | GLC-C | 7 |
| Sample No. | Sulfuric Acid Immersion | Surface Roughness (nm) | D-Peak Position [cm−1] | G-Peak Position [cm−1] | ID/IG Intensity Ratio |
|---|---|---|---|---|---|
| 1 (PLC) | Without | 0.39 ± 0.04 (n = 3) | 1311.6 ± 2.4 | 1523.8 ± 0.4 | 0.31 |
| With | 0.12 ± 0.01 (n = 3) | 1309.1 ± 2.5 | 1523.1 ± 0.4 | 0.31 | |
| 2 (GLC-A) | Without | 0.33 ± 0.04 (n = 3) | 1369.1 ± 2.9 | 1541.3 ± 0.2 | 0.52 |
| With | 0.22 ± 0.01 (n = 3) | 1369.1 ± 3.0 | 1540.8 ± 0.2 | 0.52 | |
| 3 (a-C:H) | Without | 0.16 ± 0.05 (n = 3) | 1387.8 ± 3.5 | 1554.3 ± 0.2 | 0.60 |
| With | 0.12 ± 0.02 (n = 3) | 1387.4 ± 3.5 | 1555.0 ± 0.2 | 0.63 | |
| 4 (GLC-B) | Without | 3.54 ± 0.01 (n = 3) | 1366.9 ± 0.8 | 1581.2 ± 0.3 | 1.00 |
| With | 2.90 ± 0.02 (n = 3) | 1369.0 ± 0.8 | 1582.0 ± 0.3 | 1.00 | |
| 5 (GLC-C) | Without | 3.21 ± 0.36 (n = 3) | 1367.2 ± 0.9 | 1581.9 ± 0.3 | 1.10 |
| With | 2.66 ± 0.12 (n = 3) | 1374.6 ± 0.9 | 1582.8 ± 0.3 | 1.10 |
| Sample No. | Sulfuric Acid Immersion | O1s/C1s Ratio | C1s Curve Fitting Area | ||||
|---|---|---|---|---|---|---|---|
| C–C sp2 | C–C sp3 | C–O | C=O | O=C–O | |||
| 1 (PLC) | Without | 0.14 | 0.64 | 0.26 | 0.08 | 0.02 | 0.00 |
| With | 0.19 | 0.54 | 0.34 | 0.10 | 0.03 | 0.00 | |
| 2 (GLC-A) | Without | 0.12 | 0.49 | 0.34 | 0.12 | 0.03 | 0.02 |
| With | 0.15 | 0.48 | 0.34 | 0.13 | 0.03 | 0.02 | |
| 3 (a-C:H) | Without | 0.11 | 0.40 | 0.39 | 0.15 | 0.05 | 0.02 |
| With | 0.13 | 0.39 | 0.39 | 0.15 | 0.05 | 0.02 | |
| 4 (GLC-B) | Without | 0.21 | 0.44 | 0.24 | 0.16 | 0.11 | 0.04 |
| With | 0.21 | 0.43 | 0.25 | 0.17 | 0.11 | 0.04 | |
| 5 (GLC-C) | Without | 0.21 | 0.47 | 0.15 | 0.21 | 0.10 | 0.06 |
| With | 0.21 | 0.46 | 0.17 | 0.20 | 0.10 | 0.07 | |
| Sample No. | Sulfuric Acid Immersion | Electrical Insulation (n = 5) | |
|---|---|---|---|
| Insulation Voltage (V) | Electrical Insulation (kV/mm) | ||
| 1 (PLC) | Without | 40 ± 1 | 444 ± 11 |
| With | 42 ± 3 | 467 ± 33 | |
| 2 (GLC-A) | Without | 39 ± 2 | 302 ± 16 |
| With | 36 ± 1 | 279 ± 8 | |
| 3 (a-C:H) | Without | 52 ± 3 | 486 ± 28 |
| With | 53 ± 2 | 495 ± 19 | |
| 4 (GLC-B) | Without | 13 ± 0 | 66 ± 0 |
| With | 13 ± 0 | 66 ± 0 | |
| 5 (GLC-C) | Without | 16 ± 4 | 94 ± 24 |
| With | 14 ± 3 | 82 ± 29 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanasugi, K.; Ichijo, E.; Hiratsuka, M.; Hirakuri, K. Effect of Sulfuric Acid Immersion on Electrical Insulation and Surface Composition of Amorphous Carbon Films. Coatings 2024, 14, 1023. https://doi.org/10.3390/coatings14081023
Kanasugi K, Ichijo E, Hiratsuka M, Hirakuri K. Effect of Sulfuric Acid Immersion on Electrical Insulation and Surface Composition of Amorphous Carbon Films. Coatings. 2024; 14(8):1023. https://doi.org/10.3390/coatings14081023
Chicago/Turabian StyleKanasugi, Kazuya, Eito Ichijo, Masanori Hiratsuka, and Kenji Hirakuri. 2024. "Effect of Sulfuric Acid Immersion on Electrical Insulation and Surface Composition of Amorphous Carbon Films" Coatings 14, no. 8: 1023. https://doi.org/10.3390/coatings14081023





